For the last ten years our laboratory has been studying the proliferation, migration and differentiation of neuronal progenitor cells located in the anterior part of the postnatal forebrain subventricular zone (SVZa). SVZa-derived cells possess a number of proliferative characteristics that distinguish them from the other progenitor cells in the central nervous system (CNS). This review summarizes our recent findings, in which we compared the pattern of cell cycle inhibitory proteins expressed by the neonatal SVZa to that of telencephalic ventricular zone (VZ) cells.
The neurons of the mammalian forebrain arise from the progenitor cells within the ventricular and subventricular zones. During embryogenesis, the neuronal progenitor cells of the developing cerebral cortex reside in the VZ that lines the lateral ventricles, and the immature neurons generated at the ventricular surface migrate through the overlying intermediate zone (IZ) to reach their destinations in the cortical plate (CP). Once the immature neurons of the VZ cease proliferation and begin to differentiate and migrate, they remain forever postmitotic.
Not all neuronal progenitor cells of the CNS obey the order of “proliferation arrest prior to migration and differentiation”. The neuronal progenitor cells, which concurrently undergo division while they migrate even though they express a neuronal phenotype, are located within a distinct region of the anterior part of the postnatal forebrain subventricular zone (the SVZa; Luskin, 1993; Menezes et al., 1995). SVZa-derived cells migrate along a highly restricted pathway, the rostral migratory stream (RMS), to reach the subependymal zone in the middle of the olfactory bulb and then migrate radially to their final destinations in granule cell and glomerular layers where they become postmitotic interneurons. Thus, unlike the progenitor cells of the embryonic telencephalic VZ, SVZa-derived cells initiate differentiation without becoming postmitotic.
The decision to proliferate or become postmitotic is made in the G1 phase of the cell cycle, where intrinsic and extrinsic signals come together to act on different groups of proteins that inhibit or facilitate cell cycle progression (Sherr, 1994). Transition from G1 to S phase is negatively regulated by two families of cyclin dependent kinase inhibitors (CKIs): the CIP/KIP family (including p21CIP1, p27KIP1, p57KIP2) and the INK4 family (including p15INK4b p16INK4a, p18INK4c and p19INK4d) (Elledge and Harper, 1994). Among these CKIs, p18INK4c, p19INK4d and p27KIP1 are expressed in the CNS during development (Zindy et al., 1997a; van Lookeren Campagne and Gill, 1998) and may play pivotal roles in neurogenesis.
As indicated above, SVZa-derived cells can undergo cell division despite the initiation of differentiation and migration, unlike the progenitor cells of the embryonic telencephalic VZ. This unique property of the SVZa cells prompted us to examine whether they regulate their cell cycle in a different manner from other CNS progenitors. To determine whether the differential regulation of the G1-S progression can account for the unusual proliferative behavior of SVZa progenitor cells, we have compared the pattern of p19INK4d expression by the cells of the neonatal SVZa to that of telencephalic VZ cells.
p19INK4d is expressed by postmitotic immature neurons of the telencephalic VZ
VZ progenitors of the developing cerebral cortex withdraw from the cell cycle at the ventricular surface and the newly-generated postmitotic neurons initiate differentiation and migrate to their final destinations in the CP (Bayer et al., 1991). Our staining of the developing cerebral cortex with an antibody to p19INK4d revealed that the newly generated neurons at the ventricular surface express p19INK4d, while the progenitor cell that are in the S phase at the basal border of the VZ are devoid of p19INK4d immunoreactivity (Coskun and Luskin, 2001). This suggests that p19INK4d plays a role in regulating the timely withdrawal of VZ progenitors from the cell cycle.
Based on the p19INK4d expression pattern, the VZ can be divided into a p19INK4d(−) upper (VZu) and p19INK4d(+) lower (VZl) subdivision. Furthermore, our experiments demonstrated that p19INK4d expression is downregulated while the cells ascend through VZu and re-activated upon entering the IZ. Migrating cortical neurons in the IZ start to express p19INK4d in a punctate manner in the apical domain of the cell at the cytoplasmic-nuclear border, while the neurons of the CP express p19INK4d in a more diffuse pattern (i.e., crescent shape) (Fig. 1C).
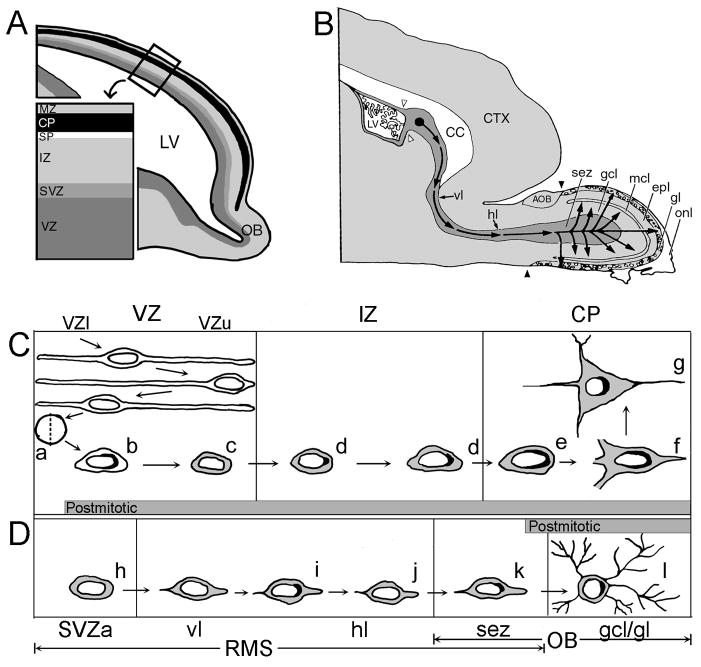
Figure 1. Summary diagram depicting the subcellular changes of p19INK4d expression by VZ cells compared to SVZa cells as they migrate to their final destinations.
(A) Sagittal view of the embryonic telencephalon illustrating the laminar subdivisions of the developing cerebral cortex. Immature neurons of the VZ migrate through the layers of the telencephalon to reach the CP, where they complete their differentiation.
(B) Sagittal view of the neonatal forebrain illustrating the migratory path of the SVZa-derived cells. The neurons that arise in the SVZa (solid black circle) migrate along the RMS to reach the olfactory bulb, where they differentiate into interneurons. The set of open arrowheads demarcates the border between the neurogenic SVZa and gliogenic posterior portion of the SVZ. The set of closed arrowheads indicates the caudal border of the main olfactory bulb.
(C) Expression of p19INK4d during the generation of a typical cortical neuron of the embryonic telencephalon. After VZ progenitor cells divide and withdraw from the mitotic cycle at the ventricular surface (a), the postmitotic progeny starts expressing p19INK4d (black) at the cytoplasmic-nuclear border in the VZl (b). p19INK4d expression is downregulated as the migrating neurons pass through the VZu (c) and is re-expressed in a punctate manner upon entering the IZ (d). Furthermore, within the CP, the p19INK4d expression persists in the differentiating neurons (e–g) in a more diffuse manner with a perinuclear crescent shaped pattern. Gray shading represents expression of neuron-specific markers (Menezes and Luskin, 1994).
(D) Expression of p19INK4d during the generation of a typical interneuron of the neonatal olfactory bulb. Unlike the cells of the telencephalic VZ, the SVZa progenitor cells and their progeny are immunoreactive for the neuron-specific antibody TuJ1 (gray shading) at virtually all times in the RMS (h–l). The SVZa neuronal progenitor cells exhibit only negligible amounts of p19INK4d in the SVZa (h). However, when in the proximal portion of the RMS, the TuJ1(+) SVZa-derived cells start to express p19INK4d at their apical pole while en route to the olfactory bulb (i). Subsequently, SVZa-derived cell downregulate their expression of p19INK4d and re-enter the cell cycle to undergo another round of cell division in the RMS (j). When in the olfactory bulb, the SVZa-derived cells terminally exit the cell cycle and migrate into one of the overlying cellular layers of the olfactory bulb. The p19INK4d persists in the postmitotic olfactory bulb interneurons within the gcl and gl (k and l). AOB, accessory olfactory bulb; CC, corpus callosum; CP, cortical plate; CTX, cerebral cortex; epl, external plexiform layer; gcl, granule cell layer; gl, glomerular layer; hl, horizontal limb of the RMS; IZ, intermediate zone; LV, lateral ventricle; mcl, mitral cell layer; MZ, marginal zone; OB, olfactory bulb; RMS, rostral migratory stream; onl, olfactory nerve layer; sez, subependymal zone; SP, subplate; SVZa, anterior part of the neonatal subventricular zone; vl, vertical limb of RMS; VZ, ventricular zone; VZl, lower portion of the ventricular zone; VZu, upper portion of the ventricular zone.
The suggestion has been put forward that CKIs not only coordinate cell cycle exit of the progenitor cells, but also maintain the postmitotic cells in a quiescent state (Zindy et al., 1999). In agreement with this notion, our data revealed that p19INK4d expression persists in the differentiating postmitotic neurons of the cerebral cortex. This persistent expression of p19INK4d in quiescent cells suggests that p19INK4d prevents postmitotic cells from re-entering the proliferative cycle; the neurons in p19INK4d-p27KIP1 double knockout mice undergo extra rounds of cell division in regions where only quiescent neurons reside in wild type animals (Zindy et al., 1999). Collectively, our results revealed that in the developing telencephalon, p19INK4d is expressed mainly by the postmitotic immature neurons and has a characteristics perinuclear distribution that is correlated with a cell’s laminar position and state of differentiation.
SVZa-derived cells downregulate p19INK4d expression in the RMS and re-enter the cell cycle
In order to determine whether the pattern of p19INK4d expression by SVZa neuronal progenitor cells can account for their unusual proliferative behavior, we analyzed the spatiotemporal expression pattern of p19INK4d by the cells of the rodent RMS. Our data revealed that SVZa-derived cells exhibit an anteriorhigh-posteriorlow gradient of p19INK4d expression along the RMS (Coskun and Luskin, 2001). Detection of progressively more p19INK4d-immunoreactive cells in the RMS as the olfactory bulb is approached indicates that few cells withdraw from the cell cycle in the SVZa and increasingly more as they reach the bulb.
To reconcile the observation that cells of the SVZa/RMS undergo division as they migrate and that SVZa-derived cells in the proximal portion of the RMS initiate p19INK4d expression (usually indicative of a postmitotic cell), we investigated the hypothesis that SVZa cells may downregulate the expression of p19INK4d and re-enter the cell cycle to undergo another round of cell division before reaching the subependymal zone. To test this hypothesis, we administered the proliferation marker BrdU to rat pups at 3 and 9 hr before their perfusion and examined the expression of p19INK4d by the BrdU(+) cells along the RMS. We observed that at 3 hr following BrdU administration, very few SVZa-derived cells along the RMS co-localize BrdU and p19INK4d. However, at 9 hr following BrdU administration, a significant fraction of the BrdU(+) cells were immunoreactive for both BrdU and p19INK4d. Taken together, these findings indicate that SVZa-derived cells in the RMS successively downregulate their p19INK4d expression prior to undergoing division, which may enable them to repeatedly exit and re-enter the cell cycle. Consistent with the idea that p19INK4d expression persists in postmitotic cells to maintain them in a quiescent state, postmitotic neurons at their final destinations in the olfactory bulb were also p19INK4d-immunoreactive.
Conclusions and future directions
In conclusion, based on the p19INK4d immunoreactivity and cell cycle kinetics, SVZa-derived cells appear to successively exit and re-enter the cell cycle, despite expressing a neuronal phenotype. This is in contrast to telencephalic VZ cells, in which p19INK4d expression is maintained even before they initiate differentiation. Since the SVZa-derived cells in the RMS appear to repeatedly downregulate p19INK4d expression, this might indicate that SVZa cells continue to undergo multiple rounds of de-differentiation and division. Therefore, the unique proliferation characteristics of the SVZa-derived cells can be, to some extent, attributed to the dynamic regulation of p19INK4d expression.
It had been suggested that different members of the CIP/KIP and INK4 families might cooperate to ensure timely cell cycle exit of proliferating cells (Thullberg et al., 2000). In order to determine the cooperative interactions between the CKIs in the regulation of SVZa cell proliferation and differentiation, our future experiments will determine which other CKIs are expressed by SVZa cells in addition to p19INK4d. We have also initiated studies to determine the extracellular signaling molecules that act on CKIs to regulate the proliferation of SVZa-derived cells (Coskun et al., 2001).
Acknowledgments
This work was supported in part by a grant awarded to MBL from the National Institute of Deafness and Other Communicative Disorders (RO1DC03190)
References
- Bayer SA, Altman J, Russo RJ, Dai XF, Simmons JA. Cell migration in the rat embryonic neocortex. J Comp Neurol. 1991;307:499–516. doi: 10.1002/cne.903070312. [DOI] [PubMed] [Google Scholar]
- Coskun V, Luskin MB. The expression pattern of the cell cycle inhibitor p19INK4d by progenitor cells of the rat embryonic telencephalon and neonatal anterior subventricular zone. J Neurosci. 2001;21:3092–3103. doi: 10.1523/JNEUROSCI.21-09-03092.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun V, Venkatraman G, Yang H, Rao MS, Luskin MB. Retroviral manipulation of the expression of bone morphogenetic protein receptor Ia by SVZa progenitor cells leads to changes in their p19INK4d expression but not in their neuronal commitment. Int J Dev Neurosci. 2001;19:219–227. doi: 10.1016/s0736-5748(00)00092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge SJ, Harper JW. Cdk inhibitors: on the threshold of checkpoints and development. Curr Opin Cell Biol. 1994;6:847–852. doi: 10.1016/0955-0674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Menezes JR, Luskin MB. Expression of neuron-specific tubulin defines a novel population of proliferative layers of the developing telencephalon. J Neurosci. 1994;14:5399–5416. doi: 10.1523/JNEUROSCI.14-09-05399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes JR, Smith CM, Nelson KC, Luskin MB. The division of neuronal progenitor cells during migration in the neonatal mammalian forebrain. Mol Cell Neurosci. 1995;6:496–508. doi: 10.1006/mcne.1995.0002. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Thullberg M, Bartkova J, Khan S, Hansen K, Ronnstrand L, Lukas J, Strauss M, Bartek J. Distinct versus redundant properties among members of the INK4 family of cyclin-dependent kinase inhibitors. FEBS Lett. 2000;470:161–166. doi: 10.1016/s0014-5793(00)01307-7. [DOI] [PubMed] [Google Scholar]
- van Lookeren Campagne M, Gill R. Tumor-suppressor p53 is expressed in proliferating and newly formed neurons of the embryonic and postnatal rat brain: comparison with expression of the cell cycle regulators p21Waf1/Cip1, p27Kip1, p57Kip2, p16Ink4a, cyclin G1, and the proto-oncogene Bax. J Comp Neurol. 1998;397:181–198. doi: 10.1002/(sici)1096-9861(19980727)397:2<181::aid-cne3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Zindy F, Quelle DE, Roussel MF, Sherr CJ. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997a;15:203–211. doi: 10.1038/sj.onc.1201178. [DOI] [PubMed] [Google Scholar]
- Zindy F, Soares H, Herzog KH, Morgan J, Sherr CJ, Roussel MF. Expression of INK4 inhibitors of cyclin D-dependent kinases during mouse brain development. Cell Growth Differ. 1997b;8:1139–1150. [PubMed] [Google Scholar]
- Zindy F, Cunningham JJ, Sherr CJ, Jogal S, Smeyne RJ, Roussel MF. Postnatal neuronal proliferation in mice lacking Ink4d and Kip1 inhibitors of cyclin- dependent kinases. Proc Natl Acad Sci U S A. 1999;96:13462–13467. doi: 10.1073/pnas.96.23.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]