Abstract
Glioma stem cells (GSCs) constitute a slow-dividing, small population within a heterogeneous glioblastoma. They are able to self-renew, recapitulate a whole tumor, and differentiate into other specific GBM subpopulations. Therefore, they have been held responsible for malignant relapse after primary standard therapy and the poor prognosis of recurrent GBM. The failure of current therapies to eliminate specific GSC subpopulations has been considered a major factor contributing to the inevitable recurrence in GBM patients following treatment. Here, we discuss the molecular mechanisms of chemoresistance of GSCs and the reasons why complete eradication of GSCs is so difficult to achieve. We will also describe the targeted therapies currently available towards GSCs and possible mechanisms to overcome such chemoresistance and avoid therapeutic relapse.
Keywords: Glioma stem cells, malignant glioma, recurrence, mechanisms, chemoresistance, plasticity
1. Introduction
Gliomas are primary brain tumors that are derived from genetic alterations in neural stem cells or neural progenitor cells. According to the World Health Organization (WHO), they are classified into four grades of ascending malignancy [1]. The higher the tumor grade, the poorer is the overall prognosis. Glioblastoma multiforme (GBM), or grade IV glioma, is the most common type of primary malignant brain tumor, accounting for 55% of all cases [2] (Figure 1). Even after gold-standard treatment with successful tumor resection, radiotherapy, and chemotherapy (temozolomide), the tumor always recurs, resulting in a very poor outcome. The median survival only reaches up to 18 months and approximately 30% of patients achieve 2-year survival [3,4]. Massive research has been applied for discovery of new molecular targets to stop therapy resistance and GBM recurrence. However, currently available therapies have only a palliative effect.
Figure 1. Histological differences between GBM and low-grade glioma (LGG).
GBM (left) has a central area with densely cellular tumor that exhibits mitoses and endothelial/microvascular proliferation, with infiltration between normal neurons at the periphery of the lesion. (A) Staining for IDH1 and (B) nuclear labeling for p53. LGG (right) H&E staining, with (C) positive IDH1 and (D) positive nuclear p53. Based on the immunoprofile, this particular GBM likely arose as secondary GBM out of a diffuse astrocytoma. 100X magnification.
Malignant recurrence is characterized by the presence of tumor cells that possess specific genetic and epigenetic alterations that render them able to survive the insults of the current standard of care and lead to full regrowth of the tumor. It is believed that this happens as a consequence of a combined selection of previously resistant malignant subpopulations and acquired epigenetic/genetic alterations of naïve tumor cells into a more aggressive phenotype after primary therapy. This resistance is observed over time, being marked by transitory disease control, and invariably renders additional rounds of chemo or radiotherapies for recurrent tumors ineffective.
The heterogeneity of GBM has been appointed as one of the main causes of therapeutic resistance and malignant relapse. According to this concept, tumors are composed by genetically and phenotypically heterogeneous clones [5–8]. Genetic heterogeneity is acquired by an evolutionary process, in which malignant tissues arise from a single mutated cell. Tumor progression would be a result of random accumulations of somatic mutations in genetically unstable malignant cells followed by selection of tumorigenic subclones by environmental cues [9,10]. The observation of subclones of tumor cells with different karyotypes first validated the model of genetic heterogeneity in GBM [11]. This concept was further endorsed as a mechanism of resistance to therapy by the recent description of glioma subclones carrying genetic amplifications of distinctive receptor tyrosine kinases (RTK), such as EGFR and PDGFRA [12,13]. Therapeutic agents that specifically target a single RTK would not be able to eradicate the whole tumor and, as a consequence, non-targeted subclones would lead to tumor relapse.
The presence of phenotypically heterogeneous clones of tumor cells introduced the concept of functional heterogeneity. According to this model, there would exist a hierarchical model of tumorigenesis, in which only a very small GBM subpopulation, the glioma stem cells (GSCs), would be capable of demonstrating self-renewal capacity, multi-potency, and induction of tumorigenesis [14–17]. The two main outcomes of the division of GSCs would be the differentiation into two heterogeneous GBM cells or self-renewal to sustain the GSC pool. Therefore, GSCs have been directly linked as a major cause of therapeutic relapse in GBM [18–20]. Recognition of both genetic and phenotypic heterogeneity in GBM has opened doors for a better understanding of the specific subpopulations of GBM that are responsible for resistance to therapy and the development of new and combined therapeutic targets. The goal of this review is to discuss the role of GSCs in resistance to chemotherapy and malignant recurrence. We will discuss the molecular mechanisms of chemoresistance of GSCs and the reason why complete eradication of GSCs is so difficult to achieve. We will also describe the targeted therapies currently available towards GSCs and possible mechanisms to overcome such chemoresistance and avoid tumor relapse.
2. Glioma stem cell biology and its role in GBM
GBM is renowned for aggressive behavior, resistance to therapy and near pathognomonic heterogeneity. GBM is heterogeneous in many ways, including appearance, genetic composition and the variety of mechanisms by which it evades therapy [21]. Extensive study of GBM has borne out the concept that within tumors, cells exist both in differentiated and undifferentiated, progenitor-like states [20]. Glioma stem cells (GSCs) have emerged as a target of both continued study and directed therapy as evidence of their role in GBM pathophysiology continues to grow. GSCs represent a subpopulation of relatively undifferentiated cells capable of self-renewal while also generating clonal populations of differentiated tumor cells. GSCs are increasingly recognized as a driving force supporting glioma genesis, resistance to therapy and aggressive recurrence [20]. Recent studies support transformation of GSCs from neural stem cells (NSCs) as a possible sentinel event in glioma formation [22]. Treatment resistance in GSCs is a multi-faceted, redundant process that nearly ensures poor efficacy [23–27]. GSCs exist throughout the tumor and are able to migrate along white matter pathways, frequently escaping even gross-total resection [28]. They additionally harbor genetic and cellular anomalies that directly counter current standard of care therapy [23–25,29]. Ultimately, GSCs are able to utilize the same advantages that enable them to drive initial tumor formation to support recurrent growth. Recurrent tumors are comprised largely of cells that resisted initial treatment efforts, presenting a more formidable therapeutic challenge and expected survival of mere months [30].
Pursuant to their critical role in GBM, it is imperative to efficiently isolate GSCs for study. To date, many characteristics and markers have been identified with varying degrees of specificity for GSCs and contributions to the GSC phenotype [15,31,32]. The hallmarks of GSC growth are neurosphere formation in appropriate culture conditions, and the in vivo recapitulation of aggressive, infiltrative tumors in animal models [20,30]. Neurosphere formation is a basic but reliable method of isolating the GSC population from tumor tissue. Sorting cells based on markers is also utilized to identify and separate GSCs from tumor tissue. CD133, a transmembrane glycoprotein, is the most widely recognized and reliable marker [15]. Cells expressing CD133 are easily isolated, and routinely display the qualities characteristic of GSCs. In extensive preclinical studies these cells are able to grow in neurospheres and recapitulate human tumors after injection of low cell numbers in animal models [15]. CD133-negative GSCs, however, have been isolated from patient-derived GBM samples, highlighting the work still to be done in this regard [31]. As such other markers have been pursued both as independent identifiers of GSCs, and those that when combined with CD133, improve the likelihood of obtaining a pure population of GSCs. Promising examples of other markers include CD15/SSEA, A2B5, Notch, CD44, EZH2, STAT3, and a host of other surface moieties and transcription factors [15,31,32]. More accurate and efficient isolation of GSCs will allow focus on their specific biology and where it is most vulnerable to future therapeutic efforts.
GSCs have a synergistic, codependent relationship with their environment within the tumor, occupying specialized niches that have now been well characterized. GSCs are most frequently found in the perivascular and hypoxic regions of tumors [33,34]. The perivascular niche is that surrounding the blood vessels infiltrating the tumor, which are frequently poorly formed, leaky and friable [33]. Vessels with poor integrity create a microenvironment with high interstitial pressure and poor delivery of oxygen and other nutrients. Drug penetration into the tumor is therefore also limited, representing the most basic means of GSC resistance to therapy [35]. GSCs have a complex relationship with tumor-associated endothelial cells, mutually supporting each other through intercellular signaling [33]. GSCs are a prominent factor in the recruitment of blood vessels to support continued tumor growth [36]. Evidence also increasingly suggests that GSCs not only stimulate endothelial cell growth and proliferation associated with neo-angiogenesis, but that GSCs can directly form endothelial cells [36].
A relatively low tissue oxygen tension is a characteristic in GBM as a function of the poor vasculature and dense cellularity, and GSCs are frequently found in regions of mild to moderate hypoxia [37]. The hypoxic niche supports GSC survival and proliferation via a unique transcription factor profile. The hypoxia-inducible factors (HIFs) are induced both upon cellular recognition of hypoxia and in GSCs at baseline [37]. GSCs utilize HIF signaling to maintain stemness and promote survival and expansion [37]. HIFs enact these changes via induction of GSC-associated genes (c-MYC, OCT4, NESTIN, NANOG) and up-regulation of downstream effectors (NOTCH, Prostatic Acid Phosphatase, AKT). The hypoxic niche also protects GSCs by way of limited drug penetration and decreased potential for generation of reactive oxygen species (ROS), processes crucial to current treatment paradigms. Hypoxia has a direct role in chemotherapeutic resistance as well, inducing MGMT expression in GSC cell lines [38]. This niche offers many mechanisms as targets for future translational efforts in therapy.
The immunosuppressive niche is one created by GBM cells, with GSCs being a key mediator. Via secreted factors and cell surface alterations, GSCs manipulate the tumor microenvironment [27]. GBM first recruits native microglia and peripheral macrophages, then induces an immunosuppressive phenotype in both [39]. In regard to adaptive immunity, GSCs induce tumor antigen anergy through ineffective processing and presentation to antigen presenting cells. In addition, direct GSC-mediated induction of effector T cell death and Treg proliferation further limits the potential host response [27]. Immune therapy therefore must overcome multiple hurdles: presentation of antigens in an effective manner, inducing an adequate anti-tumor effector response, and overcoming the preponderance of immunosuppressive mechanisms.
The unique biology of GSCs is crucial to creating a population within GBM tumors that is continually resistant to the best therapy available. GSCs represent the cells most likely to persist through standard of care treatment, and are the cells demonstrated to be the most potent and tumorigenic in the first place. They are capable of generating many populations of cells with each clone prepared to combat therapy in various ways. The cells surviving therapy have already been selected as the ones best equipped to form tumors, survive in a hostile environment, and preserve the most malignant characteristics. It comes as no surprise then, that patients harboring tumors with a high proportion of GSCs may have poorer prognoses.
3. Why is it difficult to attack glioma stem cells?
Despite gross-total resection at surgery, good response to irradiation, and first-round chemotherapy with the best chemotherapeutic agent available in the market, temozolomide (TMZ), recurrence of GBM is still inevitable in nearly all cases [40]. Given the intrinsic genetic and phenotypic heterogeneity of GBM, it is possible that the current standard of care is only eradicating specific and more susceptible GBM subpopulations, while the most resistant ones survive and repopulate the tumor. This leads to a more aggressive recurrent tumor that does not respond to initial therapy and significantly impairs patient prognosis. Here, we will discuss the main mechanisms and characteristics of GSCs that render this subpopulation such a difficult target for primary chemotherapies.
Primarily, in order to penetrate the brain, all systemically administered drugs need to cross the blood brain barrier (BBB). The BBB is a highly selective structure separating the circulating blood from the parenchyma and cerebrospinal fluid (CSF) in the central nervous system (CNS). It is mainly composed of capillary endothelial cells connected by tight junctions, which significantly impair the efficiency of chemotherapeutic agents by decreasing their concentration in the brain parenchyma [41]. Although TMZ does not present a first-pass effect, which would decrease its final concentration in the blood due to intestinal and hepatic degradation, previous studies have shown a significant decrease of the drug concentration in the brain. The final concentration of TMZ in the plasma of human patients is 50uM, while in the CSF it is only 5uM [42–44]. Although it has been shown in vitro that the concentration of 50uM is able to deplete 50% of clonigenic GBM cells, the concentration of 5uM had no effect on cell death in vitro, regardless of MGMT status [45–47]. The real concentrations of TMZ in the plasma and in the brain parenchyma are important factors that will decide if the amount of drug that gets to the tumor site is enough to eradicate the remaining malignant cells that were not affected by surgery and radiation therapy. Additional studies suggest that tumor cells in contrast-enhancing areas, which present a disrupted BBB, are likely exposed to plasma concentrations of the drug (50uM), while infiltrative tumor cells in the normal brain parenchyma are exposed to 5uM [44]. Therefore, it is natural to conclude that new therapeutic agents need to be added to the current standard of care in order to deplete chemoresistant infiltrative GSC subpopulations. Similar studies are not available for BCNU (bis-chloroethylnitrosourea), another alkylating agent used in GBM chemotherapy.
Invariable GBM recurrence after TMZ therapy indicates the presence of chemoresistant malignant cells [48]. TMZ achieves significant cytotoxic effect in GBM cells by methylating the O6 position of guanine in DNA. This methyl residue can be removed from DNA by O6-methylguanine-DNA-methyltransferase (MGMT), a repair enzyme that has variable expressions in GBM. It is believed that greater MGMT promoter methylation leads to its inactivation, therefore rendering higher activity to chemotherapeutic alkylating agents, such as TMZ [49–51]. CD133+ GSCs were shown to contribute to the resistance to TMZ through increased expression of MGMT, BCRP1 (Breakpoint Cluster Region Pseudogene 1), and anti-apoptosis proteins [48]. Similar results were observed in another study using a mouse model of genetically engineered glioma. There, Bleau et al. noticed an increased side population after TMZ therapy, which is an indication of increased GSC population [52]. In addition, TMZ was shown incapable of blocking self-renewal of GSCs that express MGMT [45]. TMZ therapy, however, was effective in eliminating MGMT-negative GSCs [53]. Chemoresistance of cancer stem cells have also been reported by several other mechanisms, such as increased expression of ABC transporters [54,55], which will be explained in further detail in topic 5.
GSC plasticity was recently shown as an important mechanism of chemoresistance. The cancer stem cell hypothesis postulates that a complete elimination of GSCs would stop the continuous growth of GBM and its recurrence by halting tumorigenesis. However, this hierarchical model has been questioned by recent discoveries that niche factors such as hypoxia and acidic stress are able to induce stemness of non-GSCs [37,38,56]. Additional lines of research have demonstrated that CD133- glioma cells are able to acquire CD133+ properties in nude rats [57]. Similar conversion of non-GSCs into GSCs was determined after primary chemotherapy in a recurrent GBM model [19]. In line with these results, Chen et al. reported the existence of multiple types of CSCs within a single cell line [58]. This newly discussed concept of cellular plasticity as a mechanism of resistance to therapy was also confirmed by Dahan et al. [59]. The authors demonstrated the dedifferentiation of human-derived non-GSCs into GSCs following ionizing radiation via a survivin-dependent pathway. Taken together, these data suggest that GSC plasticity through generation of GSCs from non-GSCs may represent a new mechanism of therapeutic resistance following selective elimination of GSCs.
Finally, recent genome-wide expression profiling and DNA methylation analysis followed by clinical assessment have demonstrated the existence of three main high-grade glioma phenotypes: proneural, mesenchymal, and classical [60–62]. The proneural phenotype is mostly associated with IDH1 mutation or PDGFRa amplification, p53 mutation, and either positive or negative CpG island methylator phenotype (CIMP) [63,64]. Patients bearing proneural tumors present a better prognosis, with slow progression from low-grade glioma to GBM [58,60] (Figures 1 and 3). The mesenchymal group retains wild-type IDH1 and is associated with deregulated expression of NF1 gene. The classical subtype, in turn, possesses an increased activity of EGFR pathway. Patients from both classical and mesenchymal groups harbor a poor prognosis [62] (see Table 1). It was recently shown that, typically, GBM recurrence after failure of primary therapies is characterized by a phenotypic shift from proneural to mesenchymal subtypes [60]. Mao et al. described a similar epithelial-mesenchymal transition (EMT) shift as being a major contributor to the maintenance of GSCs [65]. The observation that a small subset of GSCs in proneural GBM tumors displays a mesenchymal phenotype reveals that both proneural and mesenchymal signatures may co-exist within the same tumor [65]. The idea of intratumoral heterogeneity of GSC subpopulations warrants special care towards the development of specific therapies aiming one particular subgroup. In this scenario, the depletion of one GSC subpopulation may lead to the enrichment of the other, leading to therapeutic resistance and aggressive malignant phenotype.
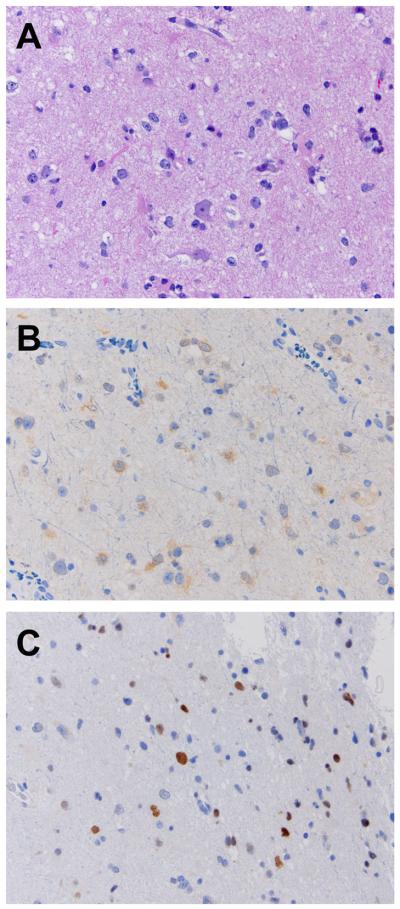
Figure 3. Representative immunohistochemistry of LGG.
LGG (diffuse astrocytoma) (A) H&E staining, (B) positive IDH1 and (C) positive nuclear p53. 200X magnification.
Table 1.
Distinct characteristics between the three main high-grade glioma phenotypes.
| Glioma subtype | Prognosis | Frequency | Resistance to therapy | Gene mutation | Altered signaling pathway |
|---|---|---|---|---|---|
| Proneural - IDH1 | Slow progression | 12% | No | IDH1, TP53, ATRX, CIMP+ | IDH1 |
| Proneural - PDGFRA | Slow progression | 14% | No | PDGFRA amplification, TP53, CDK2A deletion, CIMP− | RTK |
| Mesenchymal | Poor | 35% | Yes | NF1 | STAT3 |
| Classical | Poor | 39% | Yes | EGFR amplification or EGFRvIII mutation | RTK-PI3K-AKT-mTOR |
Abbreviations: IDH1, isocitrate dehydrogenase 1; TP53, tumor protein p53; ATRX, transcriptional regulator ATRX; PDGFRA, platelet-derived growth factor receptor alpha; CDK2A, Cyclin-dependent kinase 2; RTK, receptor tyrosine kinases; NF1, neurofibromin 1; STAT3, signal transducer and activator of transcription 3; EGFR, epidermal growth factor receptor; EGFRvIII, epidermal growth factor receptor variant III; PI3K, Phosphoinositide 3-kinase; AKT, protein kinase B; mTOR, mammalian target of rapamycin; CIMP, CpG island methylator phenotype.
4. Molecular mechanisms of glioma stem cell resistance to chemotherapy
GBM maintains status as one of the most feared malignancies based on various mechanisms of resistance against current therapeutic measures. GSCs are crucial to the dismal prognosis associated with GBM on account of their tumorigenic properties and enhanced mechanisms of treatment resistance. The myriad cellular mechanisms by which GSCs circumvent chemo- and radiotherapy include changes in DNA analysis and processing, cell cycle regulation, limitation of drug accumulation in tumor cells, and continued regeneration of a progenitor cell population (Table 2). DNA repair and cell cycle regulation are the primary line of defense against current chemo- and radiotherapy, which targets DNA replication in proliferating cells [23,29]. Drug concentration in the tumor is limited at best, influenced by the blood-brain barrier, tumor vascular supply, and active drug efflux via MDR channels [35]. While GSCs retain the potential to proliferate indefinitely, they do so relatively slowly, further rendering them insensitive to cell cycle regulation [29]. All mechanisms of treatment resistance work in concert to severely limit efficacy of current therapeutic practices.
Table 2.
Mechanisms of Resistance in GSCs
| Mechanism | Pathway | Reference |
|---|---|---|
| MGMT | • Reverses O-6 guanine alklation | [48] |
| • Allows DNA synthesis and cell cycle progression | ||
| MMR | • Recognition of mismatches, additions, deletions | [66] |
| • Attempted repair by MutSα/MutLα | ||
| • MutSα/MutLα deficient in glioma | ||
| • Induction of p53 mediated-apoptosis | ||
| BER | • Excision of methylated, deaminated, hydroxylated bases via APE1 | [67] |
| • Repair via DNA polymerase and ligase | ||
| • APE1 upregulated in glioma | ||
| Chk1 | • Recognition of DNA damage during cell cycle | [69] |
| • Arrest of cell cycle at S, G2 | ||
| • Improved DNA repair in glioma | ||
| Chk2 | • Activated after ATM recognition of DS-DNA breaks | [69] |
| • Arrest of cell cycle at any stage | ||
| • Improved DNA repair in glioma | ||
| ATP-binding cassette transporters | • Chemotherapy efflux | [26,52,71,73] |
| • Increased migration, invasion via MMP | ||
| • Maintenance of stem-state, MDR activation - SHH, Bcl-2, Akt, Survivin |
Abbreviations: APE1, human AP endonuclease; DS-DNA, double-stranded deoxyribonucleic acid; ATM, ataxia telangiectasia mutated; MMP, matrix metalloproteinase; CDK2, Cyclin-dependent kinase 2; MDR, multidrug resistance; Bcl-2, B-cell lymphoma 2; AKT, protein kinase B; ATP, adenosine triphosphate.
Extensive DNA repair aberrations are prominent in GSCs and account for frequent treatment failure and dismal survival expectation. TMZ and other alkylating agents cause base pair changes that are ideal targets for the most common DNA repair pathways [25]. GSCs benefit from enhanced DNA repair based on MGMT expression, which is increased in comparison to differentiated GBM cells [48]. The mismatch repair (MMR) system is integral to induction of cytotoxicity in TMZ treatment via recognition of alkylated base pairs, but is frequently inactive or grossly dysfunctional in GSCs [29]. In sensitive cells, MMR recognizes base-pair mismatches as well as addition and deletions, including the O6-methylguanine produced by TMZ [66]. Cells with irreparable DNA damage develop double-stranded DNA breaks and undergo apoptosis [66]. Aberrant MMR activity allows continued replication of cells with genetic changes otherwise inconsistent with progression of the cell cycle. Cells are not only therapy-resistant, but have the potential to develop additional advantageous mutations as a result of therapy.
Base excision repair (BER) aids in correcting the base pair anomalies recognized by the MMR system [67]. The BER system, in normal cells, is capable of recognizing and excising a variety of base alterations, including those induced by common chemo- and radiotherapy [67]. Damaged base pairs are removed by the corresponding DNA glycosylase and/or apurinic/apyrimidinic endonuclease (APE1), followed by DNA polymerase and ligase mediated strand repair [24]. Not only is the BER system, and APE1 specifically, hyperactive in malignancy but it negatively regulates the MMR system [24]. Poly ADP-ribose polymerase (PARP-1), an effector of apoptosis, plays a key role in cancer biology by detecting DNA damage, and activating repair systems. PARP-1 affects both MMR and BER, and is upregulated in glioma [68]. The cumulative effect of the different systems creates a cell population well-suited to withstand, and in some cases benefit from, the nucleic acid damage induced by both chemo- and radiotherapy.
Further, the cell cycle itself and intrinsic mechanisms to ensure its integrity are altered in GSCs. DNA checkpoints, specifically Chk1 and Chk2 kinases prevent cell cycle progression on detection of DNA damage. Chk1 is labile, but active even in the absence of DNA damage in S and G2 phase [69]. Chk2 is a stable protein, activated by ATM-mediated dimerization and auto-phosphorylation in response to DNA damage detected at any point in the cell cycle [69]. Chk1 and Chk2 provide yet another mechanism for therapeutic evasion in GBM. Increased Chk1 and Chk2 activity is induced by oxidative and replicative stress [25]. Cells with p53-deletions, and therefore more susceptible to all DNA repair alterations, are selected for survival and replication. Chk1 and Chk2 activity is increased in GSCs even before treatment, contributing to the prolonged cell cycle and making them inherently resistant to cell death enacted by DNA damage [29]. Due to their overwhelming role in therapeutic resistance, cellular DNA damage response elements are widely studied with hope that they provide a future therapeutic target.
Chemotherapy is also hindered by limited bioavailability in GBM. GSCs express the ATP-binding cassette (ABC) transporter channels at an increased rate compared to differentiated tumor cells, adding to the known challenges in drug delivery presented by the BBB and irregular tumor vasculature [26,70]. ABC channels actively and expeditiously transport molecules, including TMZ, both at the BBB and the cellular level to allow some cells to completely elude therapy and promote recurrence [52,71]. Expression of these proteins, with ABCG2 being the main example, is also associated with subpopulations of cells that are stem-like and multidrug resistant [26]. The mechanisms of multidrug resistance are still vague, with many pathways (SHH, Bcl-2, Akt, Survivin, etc.) proposed to be involved, making effective targeting and regulation difficult [72–74]. The ABC protein encoded by ABCG2 appears to enhance migration and invasiveness of GSCs and general GBM cells, possibly through interaction with matrix metalloproteinases (MMP) [75]. The ABC gene family is yet another example of redundant pathways enhancing the survival and adaptation of aggressively malignant cells.
The continued study of GSCs and their poor response to therapy suggests more than just resistance as the reason they are enriched after treatment. Though GSCs do appear to respond to TMZ therapy as clonal expansion is decreased, most studies cite low overall cell death [45]. Going one step further, recent evidence supports interconversion between differentiated GBM cells and GSCs, or phenotypic plasticity. GSCs, as discussed previously, self-renew and maintain their own population while also producing clonal populations of differentiated cells throughout the tumor [20]. Studies now support retroversion of differentiated cells to a more stem-like or progenitor state, a change recently suggested to occur spontaneously in some cases, or to be induced or potentiated by TMZ therapy and possibly other cellular stressors including hypoxia and radiation [19]. HIF2α, loss of PTEN, and loss of von Hippel-Lindau have all been proposed as inciting events in GSC conversion [76,77]. Change to a stem-like phenotype from non-GSCs has been confirmed by assessing neurosphere and xenograft tumor formation, as well as cellular activity of classical GSC genes (OCT4, NANOG, c-MYC, etc.) [19,76,77]. Plasticity magnifies the ineffectiveness of current therapy, which not only spares GSCs but also replenishes those best suited to withstand subsequent therapy.
The combined mechanisms of resistance explain the incremental, inadequate progress made in GBM therapy in the last several decades. GSCs provide hope that the source of GBM, and its myriad survival mechanisms, has been discovered. As such, intense study is and will remain focused on GSC biology with the promise of new drug targets better suited for therapeutic success.
5. Targeted therapies toward glioma stem cells (that may be combined with chemo to increase therapeutic efficacy and decrease tumor recurrence)
In light of their crucial role in GBM pathogenesis, developing therapeutic agents specific to GSCs is a growing focus of GBM research. A major limitation in targeting GSCs is that they share many features with NSCs. Both NSCs and GSCs retain similar properties as undifferentiated progenitors, capable of both self-renewal and generation of clonal populations of cells [78]. NSCs also occupy a perivascular niche and rely on its cellular signaling for survival. One aspect that remains to be fully explored is the difference in expression of the same genes in both NSCs and GSCs, and if the difference is significant enough to allow a therapeutic effect while sparing normal NSCs [79]. Until this is clear, the pursuit of those molecules and pathways specific to GSCs will continue, with some potential options already being studied: EGFR/AKT, EZH2, NOTCH, Hedgehog-Gli, and STAT3 [80–82]. GSC specific targets are an important goal of continuing efforts, however, one must consider NSC off-target toxicity in the context of a malignant and uniformly fatal disease process.
GSC-specific therapeutic strategies to date have included promotion of differentiation, disruption of GSC niches, immunotherapy, and enhancing susceptibility to traditional chemotherapy (Table 3). Inducing differentiation to more terminal glioblastoma cells makes them inherently more sensitive to therapy, less capable of engraftment, and in some cases can directly induce apoptosis [83–86]. Conversion to more differentiated glioma cells also limits or in some cases reverses the negative immune effects mediated by GSCs [27]. To date inducers of cellular differentiation effective in GSCs include bone morphogenic proteins (BMPs) and post-transcriptional modification using miRNA (451, 1792, 137, 124) [83–86]. Preclinical studies of these compounds have demonstrated their ability to decrease GSC neurosphere formation and tumor engraftment while also inducing apoptosis. With improved delivery and specificity they may represent promising translational therapeutic options.
Table 3.
GSC-targeted therapies
| Therapy | Mechanism | Target(s) | Reference |
|---|---|---|---|
| Pro-differentiation | 1) Smad-dependent transcriptional regulation | 1) BMP | [83] |
| 2) miRNA | [84–86] | ||
| 2) Increased miRNA-451, inhibition of miRNA-17-92, overexpression of miRNA-124 and -137 individually caused differentiation and/or apoptosis | |||
| Niche Therapy | 1) Increased local tissue oxygen; HIF transcription factor/pathway inhibition | 1) Hypoxic | [87–89] |
| 2) Perivascular | [33,90–93] | ||
| 3) Immune | [95–98] | ||
| 2) Inhibition of VEGF, Notch, mTOR, Delta-like 4 (DLL4), laminins, nitric oxide (NO), and sonic hedgehog (SHH), stromal derived factor 1 | |||
| 3) Targeted antibodies, T and dendritic cell transfer | |||
| DNA repair/checkpoint MDR | 1) Gene knockdown | 1) Chk1, Chk2 | [99] |
| 2) Inhibitors | 2) Chk1/2, ATM | [100] | |
| 1) Down-regulation with miRNA-328, melatonin | 1) ABCG2 | [26,104,105] | |
| Cell signaling and survival pathways | 1) GSI | 1) Notch, Hedgehog-Gli | [52,53,106,107] |
| 2) Kinase inhibitors – sorafenib, etc. | 2) PI3K/Akt, MAPK |
Abbreviations: TGFβ, transforming growth factor beta 1; BMP, bone morphogenetic protein; miRNA, microRNA; HIF, hypoxia-inducible factors; MDR, multidrug resistance; Bcl-2, B-cell lymphoma 2; AKT, protein kinase B; ATP, adenosine triphosphate; PI3K, phosphoinositide 3-kinase; ATM, ataxia telangiectasia; ABCG2, ATP-binding cassette sub-family G member 2; GSI, selective inhibition of heterotrimeric Gs signaling; MAPK, mitogen-activated protein kinases.
Besides direct cellular manipulation, some studies have attempted to target GSCs by modifying the niches that they characteristically occupy. Preliminary attempts to increase local tissue oxygen have been explored to improve tumor cell stress after both chemo- and radiotherapy [87]. Much prior work has focused on targeting HIFs and their downstream effectors to disrupt GSC signaling and survival [88,89]. Notch has a critical function in the hypoxic and perivascular niches, serving to prevent neuronal differentiation and support GSC growth [33]. The relationship between GSCs and endothelial cells of the neovasculature continues to be explored and has yielded targets to disrupt the synergistic relationship. Inhibitors of mTOR, Delta-like 4 (DLL4), laminins, nitric oxide (NO), and sonic hedgehog (SHH) ligand have all been developed to affect the survival of GSCs, with various degrees of success in preclinical study and early clinical trials [90–93]. GSCs stimulation of angiogenesis is beneficial for nutrient supply in tumor growth and endothelial cell secretion of supportive factors. Anti-angiogenic therapies targeting VEGF, stromal derived factor 1, and others combined with the current standard of care may be beneficial from a multidisciplinary perspective [94].
GBM immunotherapy is an area of intense interest, and GSCs are no exception given the growing support of their integral pathological role. Antibody-directed therapy using known cell surface moieties has the potential to both disrupt GSC biology and deliver other therapy directly to GSCs. CD133 antibodies have been used for conjugated delivery of nanoparticles and chemotherapeutic agents [95,96]. Antibodies may also have utility in targeting GSC-secreted factors, such as TGFβ and Galectin-3, to hinder development of the immunosuppressive niche [27]. The most clinically advanced therapies in current use are adoptive T cell and dendritic cell transfer [97,98]. Host T and dendritic cells are isolated and exposed to tumor/GSC antigens ex vivo, away from the immunosuppressive environment, then administered back to the host. Primed T cells are then able to attack tumor cells when injected back into the host. Re-administration of dendritic cells holds the promise of inducing a sustained adaptive, anti-tumor immune response. These strategies, as well as depleting microglia, macrophages and Tregs warrant further study to optimize this approach.
Therapy directly addressing the mechanisms of therapeutic resistance discussed previously are being explored with the hope of improving cytotoxicity of current therapy. Knockdown of Chk1 and Chk2 kinases with siRNA has proven effective in vitro in preventing cell cycle arrest and inducing apoptosis [99]. Checkpoint, ATM and other inhibitors are of avid interest in decreasing cell cycle arrest, promoting apoptosis after DNA damage from alkylating chemotherapy or radiation [100–103]. Inhibition of the MDR genes such as ABCG2 with miRNA-328 and other small molecules shows potential for decreasing resistance and increasing the therapeutic impact of chemotherapy in preclinical studies [26,104]. By inhibiting or down-regulating ABCG2, concentration of TMZ and other therapeutics in the tumor can be improved while also decreasing migration and invasiveness via regulation of matrix metalloproteinase activity [70,75]. Melatonin also has a synergistic cytotoxic effect with chemotherapy in GSCs and other cell lines by down-regulating ABCG2 [105].
Cell signaling pathways that directly contribute to survival and therapeutic resistance in GSCs are current targets of investigation as well. Sorafenib is a multi-kinase inhibitor that affects the PI3K/Akt and MAPK pathways [106]. Sorafenib preferentially acts on GSCs versus differentiated cells, down-regulating genes associated with stemness, limiting growth and inducing apoptosis. Recent studies have also explored the cytotoxic effect of metformin, an oral hypoglycemic agent, specifically on GSCs [107]. Gamma secretase inhibitors (GSIs) are used to modulate Notch and Hedgehog-Gli pathways to increase GSC sensitivity to TMZ [53]. Akt is frequently mentioned in the study of GSCs and their life cycle, and attempts to downregulate Akt have met with some treatment-sensitizing success [52]. Other pathways have and will continue to emerge in similar fashion to attempt to gain an efficacious advantage [108,109]. Metformin, sorafenib, and other drugs being re-purposed in this context are intriguing options as their safety has already been evaluated in other conditions, decreasing the barriers to their use in human trials.
While they currently represent one of the most significant challenges in GBM therapy, GSCs offer a plethora of potential therapeutic targets and optimism for the future. Overall the diversity of therapeutic approaches under consideration reflect the magnitude of the clinical problem presented by GBM.
6. What does the future hold?
In this review we discussed some mechanisms believed to be associated with chemoresistance of GSCs and how this specific subpopulation would drive the recurrence of a much more aggressive GBM. We also debated the main reasons of why complete eradication of GSC subpopulations is so difficult to achieve. However, the study of cancer stem cells and their effect on malignant recurrence is still in its infancy. Therefore, many additional relevant mechanisms are expected to be discovered in a near future, together with a deeper understanding of the current paradigm. In this topic, we will discuss five main future directions that may significantly impact future studies in this subject.
First, due to the major role of chemoresistant GSCs in driving GBM recurrence, it is imperative to unveil relevant GSC-specific markers and survival pathways that may act as possible targets for new treatments. In addition, a better understanding of the intrinsic and extrinsic factors that drive self-renewal and differentiation of the GSC population will facilitate the manipulation of cell cycle checkpoints in order to sensitize GSCs to standard chemotherapies. Second, with technological advances, the advent of personalized medicine will culminate with the development of therapies focused on the molecular identity of individual patients and tumors. The discovery and isolation of specific molecular signatures in the patient's tumor will allow for the selection of a more appropriate regimen of chemo- or radiotherapy [110]. Furthermore, in-depth analysis of intratumoral heterogeneity and GSC phenotype by single cell study will enable the identification of possible mechanisms by which the tumor evades standard therapies and acquires more aggressive features.
Third, personalized medicine will take a step forward with the development of genome-wide expression analysis of each tumor. Such an individualized approach would enable the accurate prediction of patient outcome to a particular therapy based on each tumor genomic profile. The main idea is that, the more complete a picture we get from all genetic and phenotypic heterogeneities present in a tumor, the more chances we will have to find an effective treatment. The better understanding of the genetic and phenotypic characteristics each patient's tumor would allow for the discovery of new targets and the development of new GSC-specific therapies that could be combined with the current standard of care. This individualized approach would permit matching patients with new treatments that are more likely to be effective and cause fewer side effects. Fourth, targeting single GSC genes may likely be an ineffective approach to counteract GSCs' self-renewal and tumorigenicity associated with GBM recurrence. Therefore, combined poly-pharmacologic therapies may be an effective alternative to simultaneously target multiple GSC survival-promoting factors.
Last, it is imperative to understand better the mechanisms that drive GSC plasticity, so we can effectively stop it. The increased conversion of non-GSCs into GSCs after primary chemotherapy significantly increases the difficulty of targeting the GSC population responsible for malignant relapse. As a result, treatments that are intended to target GSCs may be rendered completely ineffective if non-GSCs acquire stem-like characteristics at a later time post-therapy. The recently described idea that CSCs and non-CSCs may regulate, activate, and protect each other through a mechanism that involves EGFR amplification raises an important discussion on the effectiveness of the currently available standard therapies that are devised to mostly target rapidly dividing differentiated GBM cells [111]. We believe that a simultaneous targeting of tumor-initiating and differentiated glioma cells may be a viable strategy to overcome GSC plasticity and intratumoral heterogeneity. The discovery of novel therapeutic strategies able to target undifferentiated and dormant GBM cells allied with the use of GSC-specific genomic signatures in guiding clinical treatments may present a feasible way to achieve long-lasting therapeutic results.
Expert commentary
The role of GSCs in the phenotypic and genetic heterogeneity of GBM is a new and attractive area of current research. GSCs are intrinsically plastic and their dynamic interconversion between GSCs and non-GSCs has been held responsible for the invariable malignant recurrence after primary standard therapies and the dismal prognosis of patients bearing recurrent tumors. We believe that only a better understanding of the mechanisms that drive GSC interconversion and the role of intratumoral microenvironment in the generation of the specific cues necessary for triggering this plasticity will enable us to develop effective targeted therapies against these GBM subpopulations. In addition, we believe that the use of such targeted therapies needs to be combined with poly-pharmacologic treatments that are able to target both rapidly dividing and quiescent GBM subpopulations. A combination of a deep understanding of GBM heterogeneity by both wide-genome and single cell analysis and the application of this knowledge to guide the choice of the best therapeutic regimen for each patient will open doors for more personalized medicine and long-lasting elimination of malignant relapse.
Five-year view
Understanding GSC plasticity is the key for the development of effective therapies targeting this population in recurrent tumors. Single-cell and wide-genome analysis of primary and recurrent tumors will show us the key differences that drive specific mutations in these different populations. This knowledge, together with the study of specific environmental cues that trigger these changes in GSC phenotype will enable the development of more personalized medicine, which will lead to effective and long-lasting therapeutic results.
Figure 2. Origin, self-renewal, and dedifferentiation of GSCs.
Image depicting GSGs originating from mutated NSCs (1). Depending on specific environmental cues (2), the “GSC of origin” will undergo either asymmetric or symmetric cell division to give rise to, respectively, self-renewed GSCs and differentiated non-GSCs (3). This will ultimately lead to a genetically and phenotypically heterogeneous GBM. The plastic phenotype is seen by the interconversion between GSCs and non-GSCs (4). Non-mutated neural progenitor cells will originate a wide range of cells in the brain, such as astrocytes, glia cell, and neurons (5).
Key issues.
Glioma stem cells develop from upon mutation of neural progenitor cells or neural stem cells. GSCs then mediate tumorigenesis, resistance to therapy and recurrence.
GSCs are inherently resistant to standard of care therapy. The mechanisms of resistance include MGMT promoter methylation status, enhanced repair of DNA damage, impaired induction of apoptosis, aberrant DNA checkpoint analysis, ABC-type transporter expression and multidrug resistance, phenotypic plasticity and specialized cyto-protective niches.
Current pharmacologic strategies to target GSCs include the induction of differentiation, disruption of GSC niches, immunotherapy, and restoration of sensitivity to current therapy.
Targeting GSCs while limiting toxicity to normal tissue is of critical current and future significance. GSC-specific microenvironments, cell markers, gene expression profiles, and signaling pathways currently serve as the best targets for a directed approach.
Similar to the combination of TMZ and radiation, future therapy for GBM is likely to require a combinatorial approach to eliminate two very different cell populations: GSCs and differentiated cells.
Progress in GBM and GSC-directed therapy will require a combination of traditional and progressive approaches, including genetic analysis of individual tumors and detailed single-cell study. Such tailored investigation and therapy will attempt to match the heterogeneity found in GBM.
Acknowledgments
This work was supported by the NCI (R01CA122930, R01CA138587), the NIH (R01NS077388), the National Institute of Neurological Disorders and Stroke (U01NS069997), and the American Cancer Society (RSG-07-276-01-MGO).
Footnotes
Financial and competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta neuropathologica. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Legler JM, Ries LA, Smith MA, et al. Cancer surveillance series [corrected]: brain and other central nervous system cancers: recent trends in incidence and mortality. Journal of the National Cancer Institute. 1999;91(16):1382–1390. doi: 10.1093/jnci/91.16.1382. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Dietrich PY, Ostermann Kraljevic S, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20(5):1375–1382. doi: 10.1200/JCO.2002.20.5.1375. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell PJ, Pleasance ED, Stephens PJ, et al. Subclonal phylogenetic structures in cancer revealed by ultra-deep sequencing. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(35):13081–13086. doi: 10.1073/pnas.0801523105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding L, Ellis MJ, Li S, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464(7291):999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navin N, Kendall J, Troge J, et al. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472(7341):90–94. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 10.Aparicio S, Caldas C. The implications of clonal genome evolution for cancer medicine. The New England journal of medicine. 2013;368(9):842–851. doi: 10.1056/NEJMra1204892. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro JR, Yung WK, Shapiro WR. Isolation, karyotype, and clonal growth of heterogeneous subpopulations of human malignant gliomas. Cancer research. 1981;41(6):2349–2359. [PubMed] [Google Scholar]
- 12.Little SE, Popov S, Jury A, et al. Receptor tyrosine kinase genes amplified in glioblastoma exhibit a mutual exclusivity in variable proportions reflective of individual tumor heterogeneity. Cancer research. 2012;72(7):1614–1620. doi: 10.1158/0008-5472.CAN-11-4069. [DOI] [PubMed] [Google Scholar]
- 13.Snuderl M, Fazlollahi L, Le LP, et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer cell. 2011;20(6):810–817. doi: 10.1016/j.ccr.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer research. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 15.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 16.Yuan X, Curtin J, Xiong Y, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23(58):9392–9400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- 17.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer research. 2004;64(19):7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 18.Huang Z, Cheng L, Guryanova OA, Wu Q, Bao S. Cancer stem cells in glioblastoma--molecular signaling and therapeutic targeting. Protein & cell. 2010;1(7):638–655. doi: 10.1007/s13238-010-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auffinger B, Tobias AL, Han Y, et al. Conversion of differentiated cancer cells into cancer stem-like cells in a glioblastoma model after primary chemotherapy. Cell death and differentiation. 2014;21(7):1119–1131. doi: 10.1038/cdd.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed AU, Auffinger B, Lesniak MS. Understanding glioma stem cells: rationale, clinical relevance and therapeutic strategies. Expert review of neurotherapeutics. 2013;13(5):545–555. doi: 10.1586/ern.13.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baronchelli S, Bentivegna A, Redaelli S, et al. Delineating the cytogenomic and epigenomic landscapes of glioma stem cell lines. PLoS One. 2013;8(2):e57462. doi: 10.1371/journal.pone.0057462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alcantara Llaguno S, Chen J, Kwon CH, et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer cell. 2009;15(1):45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 24.Bobola MS, Blank A, Berger MS, Stevens BA, Silber JR. Apurinic/apyrimidinic endonuclease activity is elevated in human adult gliomas. Clinical cancer research: an official journal of the American Association for Cancer Research. 2001;7(11):3510–3518. [PubMed] [Google Scholar]
- 25.Bartkova J, Hamerlik P, Stockhausen MT, et al. Replication stress and oxidative damage contribute to aberrant constitutive activation of DNA damage signalling in human gliomas. Oncogene. 2010;29(36):5095–5102. doi: 10.1038/onc.2010.249. [DOI] [PubMed] [Google Scholar]
- 26.Li WQ, Li YM, Tao BB, et al. Downregulation of ABCG2 expression in glioblastoma cancer stem cells with miRNA-328 may decrease their chemoresistance. Med Sci Monit. 2010;16(10):HY27–30. [PubMed] [Google Scholar]
- 27.Wei J, Barr J, Kong LY, et al. Glioma-associated cancer-initiating cells induce immunosuppression. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16(2):461–473. doi: 10.1158/1078-0432.CCR-09-1983. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Fei S, Qi X, Kedong S, Guangchun J, Jian L, Wei Q. The antitumor effect of mesenchymal stem cells transduced with a lentiviral vector expressing cytosine deaminase in a rat glioma model. Journal of cancer research and clinical oncology. 2012;138(2):347–357. doi: 10.1007/s00432-011-1104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ropolo M, Daga A, Griffero F, et al. Comparative analysis of DNA repair in stem and nonstem glioma cell cultures. Molecular cancer research: MCR. 2009;7(3):383–392. doi: 10.1158/1541-7786.MCR-08-0409. [DOI] [PubMed] [Google Scholar]
- 30.Glas M, Rath BH, Simon M, et al. Residual tumor cells are unique cellular targets in glioblastoma. Annals of neurology. 2010;68(2):264–269. doi: 10.1002/ana.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao XG, Zhang X, Xue XY, et al. Brain Tumor Stem-Like Cells Identified by Neural Stem Cell Marker CD15. Translational oncology. 2009;2(4):247–257. doi: 10.1593/tlo.09136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Wakeman TP, Lathia JD, et al. Notch promotes radioresistance of glioma stem cells. Stem Cells. 2010;28(1):17–28. doi: 10.1002/stem.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu TS, Costello MA, Talsma CE, et al. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer research. 2011;71(18):6061–6072. doi: 10.1158/0008-5472.CAN-10-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei J, Wu A, Kong LY, et al. Hypoxia potentiates glioma-mediated immunosuppression. PLoS One. 2011;6(1):e16195. doi: 10.1371/journal.pone.0016195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosso L, Brock CS, Gallo JM, et al. A new model for prediction of drug distribution in tumor and normal tissues: pharmacokinetics of temozolomide in glioma patients. Cancer research. 2009;69(1):120–127. doi: 10.1158/0008-5472.CAN-08-2356. [DOI] [PubMed] [Google Scholar]
- 36.Filatova A, Acker T, Garvalov BK. The cancer stem cell niche(s): the crosstalk between glioma stem cells and their microenvironment. Biochimica et biophysica acta. 2013;1830(2):2496–2508. doi: 10.1016/j.bbagen.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Seidel S, Garvalov BK, Wirta V, et al. A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2 alpha. Brain: a journal of neurology. 2010;133(Pt 4):983–995. doi: 10.1093/brain/awq042. [DOI] [PubMed] [Google Scholar]
- 38.Pistollato F, Abbadi S, Rampazzo E, et al. Intratumoral hypoxic gradient drives stem cells distribution and MGMT expression in glioblastoma. Stem Cells. 2010;28(5):851–862. doi: 10.1002/stem.415. [DOI] [PubMed] [Google Scholar]
- 39.Wu A, Wei J, Kong LY, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12(11):1113–1125. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gautschi OP, Cadosch D, Collen TD, et al. Glioblastoma multiforme--new hope due to modern therapeutical approaches. Praxis. 2010;99(5):295–308. doi: 10.1024/1661-8157/a000052. [DOI] [PubMed] [Google Scholar]
- 41.Pardridge WM. Drug transport across the blood-brain barrier. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32(11):1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brada M, Judson I, Beale P, et al. Phase I dose-escalation and pharmacokinetic study of temozolomide (SCH 52365) for refractory or relapsing malignancies. British journal of cancer. 1999;81(6):1022–1030. doi: 10.1038/sj.bjc.6690802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ostermann S, Csajka C, Buclin T, et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10(11):3728–3736. doi: 10.1158/1078-0432.CCR-03-0807. [DOI] [PubMed] [Google Scholar]
- 44.Portnow J, Badie B, Chen M, Liu A, Blanchard S, Synold TW. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15(22):7092–7098. doi: 10.1158/1078-0432.CCR-09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beier D, Rohrl S, Pillai DR, et al. Temozolomide preferentially depletes cancer stem cells in glioblastoma. Cancer research. 2008;68(14):5706–5715. doi: 10.1158/0008-5472.CAN-07-6878. [DOI] [PubMed] [Google Scholar]
- 46.Blough MD, Westgate MR, Beauchamp D, et al. Sensitivity to temozolomide in brain tumor initiating cells. Neuro-oncology. 2010;12(7):756–760. doi: 10.1093/neuonc/noq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hermisson M, Klumpp A, Wick W, et al. O6-methylguanine DNA methyltransferase and p53 status predict temozolomide sensitivity in human malignant glioma cells. Journal of neurochemistry. 2006;96(3):766–776. doi: 10.1111/j.1471-4159.2005.03583.x. [DOI] [PubMed] [Google Scholar]
- 48.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Molecular cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. The New England journal of medicine. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 50.Beier D, Schulz JB, Beier CP. Chemoresistance of glioblastoma cancer stem cells--much more complex than expected. Molecular cancer. 2011;10:128. doi: 10.1186/1476-4598-10-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorensen MD, Fosmark S, Hellwege S, Beier D, Kristensen BW, Beier CP. Chemoresistance and chemotherapy targeting stem-like cells in malignant glioma. Advances in experimental medicine and biology. 2015;853:111–138. doi: 10.1007/978-3-319-16537-0_7. [DOI] [PubMed] [Google Scholar]
- 52.Bleau AM, Hambardzumyan D, Ozawa T, et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell stem cell. 2009;4(3):226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Current biology: CB. 2007;17(2):165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schatton T, Murphy GF, Frank NY, et al. Identification of cells initiating human melanomas. Nature. 2008;451(7176):345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirschmann-Jax C, Foster AE, Wulf GG, et al. A distinct "side population" of cells with high drug efflux capacity in human tumor cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(39):14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hjelmeland AB, Wu Q, Heddleston JM, et al. Acidic stress promotes a glioma stem cell phenotype. Cell death and differentiation. 2011;18(5):829–840. doi: 10.1038/cdd.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J, Sakariassen PO, Tsinkalovsky O, et al. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. International journal of cancer. Journal international du cancer. 2008;122(4):761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- 58.Chen R, Nishimura MC, Bumbaca SM, et al. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer cell. 2010;17(4):362–375. doi: 10.1016/j.ccr.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 59.Dahan P, Martinez Gala J, Delmas C, et al. Ionizing radiations sustain glioblastoma cell dedifferentiation to a stem-like phenotype through survivin: possible involvement in radioresistance. Cell death & disease. 2014;5:e1543. doi: 10.1038/cddis.2014.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer cell. 2006;9(3):157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 61.Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer cell. 2012;22(4):425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 62.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu C, Ward PS, Kapoor GS, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483(7390):474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mao P, Joshi K, Li J, et al. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(21):8644–8649. doi: 10.1073/pnas.1221478110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yip S, Miao J, Cahill DP, et al. MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15(14):4622–4629. doi: 10.1158/1078-0432.CCR-08-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johannessen TC, Bjerkvig R, Tysnes BB. DNA repair and cancer stem-like cells--potential partners in glioma drug resistance? Cancer Treat Rev. 2008;34(6):558–567. doi: 10.1016/j.ctrv.2008.03.125. [DOI] [PubMed] [Google Scholar]
- 68.Wharton SB, McNelis U, Bell HS, Whittle IR. Expression of poly(ADP-ribose) polymerase and distribution of poly(ADP-ribosyl)ation in glioblastoma and in a glioma multicellular tumour spheroid model. Neuropathology and applied neurobiology. 2000;26(6):528–535. doi: 10.1046/j.0305-1846.2000.00288.x. [DOI] [PubMed] [Google Scholar]
- 69.Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer cell. 2003;3(5):421–429. doi: 10.1016/s1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 70.Xu ZY, Wang K, Li XQ, et al. The ABCG2 transporter is a key molecular determinant of the efficacy of sonodynamic therapy with Photofrin in glioma stem-like cells. Ultrasonics. 2013;53(1):232–238. doi: 10.1016/j.ultras.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 71.Golebiewska A, Bougnaud S, Stieber D, et al. Side population in human glioblastoma is non-tumorigenic and characterizes brain endothelial cells. Brain: a journal of neurology. 2013;136(Pt 5):1462–1475. doi: 10.1093/brain/awt025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui D, Xu Q, Wang K, Che X. Gli1 is a potential target for alleviating multidrug resistance of gliomas. J Neurol Sci. 2010;288(1–2):156–166. doi: 10.1016/j.jns.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 73.Haar CP, Hebbar P, Wallace GC, et al. Drug resistance in glioblastoma: a mini review. Neurochem Res. 2012;37(6):1192–1200. doi: 10.1007/s11064-011-0701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martin V, Xu J, Pabbisetty SK, et al. Tie2-mediated multidrug resistance in malignant gliomas is associated with upregulation of ABC transporters. Oncogene. 2009;28(24):2358–2363. doi: 10.1038/onc.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gong W, Wang Z, Wan Y, Shi L, Zhou Y. Downregulation of ABCG2 protein inhibits migration and invasion in U251 glioma stem cells. Neuroreport. 2014;25(8):625–632. doi: 10.1097/WNR.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 76.Kanno H, Sato H, Yokoyama TA, Yoshizumi T, Yamada S. The VHL tumor suppressor protein regulates tumorigenicity of U87-derived glioma stem-like cells by inhibiting the JAK/STAT signaling pathway. International journal of oncology. 2013;42(3):881–886. doi: 10.3892/ijo.2013.1773. [DOI] [PubMed] [Google Scholar]
- 77.Korkaya H, Paulson A, Charafe-Jauffret E, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS biology. 2009;7(6):e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nduom EK, Hadjipanayis CG, Van Meir EG. Glioblastoma cancer stem-like cells: implications for pathogenesis and treatment. Cancer J. 2012;18(1):100–106. doi: 10.1097/PPO.0b013e3182452e0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rheinbay E, Suva ML, Gillespie SM, et al. An aberrant transcription factor network essential for Wnt signaling and stem cell maintenance in glioblastoma. Cell reports. 2013;3(5):1567–1579. doi: 10.1016/j.celrep.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nature reviews. Cancer. 2006;6(6):425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 81.Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nature reviews. Drug discovery. 2009;8(10):806–823. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]
- 82.Park DM, Rich JN. Biology of glioma cancer stem cells. Molecules and cells. 2009;28(1):7–12. doi: 10.1007/s10059-009-0111-2. [DOI] [PubMed] [Google Scholar]
- 83.Piccirillo SG, Reynolds BA, Zanetti N, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444(7120):761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 84.Gal H, Pandi G, Kanner AA, et al. MIR-451 and Imatinib mesylate inhibit tumor growth of Glioblastoma stem cells. Biochemical and biophysical research communications. 2008;376(1):86–90. doi: 10.1016/j.bbrc.2008.08.107. [DOI] [PubMed] [Google Scholar]
- 85.Ernst A, Campos B, Meier J, et al. De-repression of CTGF via the miR-17-92 cluster upon differentiation of human glioblastoma spheroid cultures. Oncogene. 2010;29(23):3411–3422. doi: 10.1038/onc.2010.83. [DOI] [PubMed] [Google Scholar]
- 86.Silber J, Lim DA, Petritsch C, et al. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC medicine. 2008;6:14. doi: 10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liong C, Ortiz D, Ao-ieong E, Navati MS, Friedman JM, Cabrales P. Localized increase of tissue oxygen tension by magnetic targeted drug delivery. Nanotechnology. 2014;25(26):265102. doi: 10.1088/0957-4484/25/26/265102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8(20):3274–3284. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gillespie DL, Aguirre MT, Ravichandran S, et al. RNA interference targeting hypoxia-inducible factor 1alpha via a novel multifunctional surfactant attenuates glioma growth in an intracranial mouse model. Journal of neurosurgery. 2015;122(2):331–341. doi: 10.3171/2014.10.JNS132363. [DOI] [PubMed] [Google Scholar]
- 90.Teodorczyk M, Schmidt MH. Notching on Cancer's Door: Notch Signaling in Brain Tumors. Frontiers in oncology. 2014;4:341. doi: 10.3389/fonc.2014.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Galan-Moya EM, Le Guelte A, Lima Fernandes E, et al. Secreted factors from brain endothelial cells maintain glioblastoma stem-like cell expansion through the mTOR pathway. EMBO reports. 2011;12(5):470–476. doi: 10.1038/embor.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lathia JD, Li M, Hall PE, et al. Laminin alpha 2 enables glioblastoma stem cell growth. Annals of neurology. 2012;72(5):766–778. doi: 10.1002/ana.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ulasov IV, Nandi S, Dey M, Sonabend AM, Lesniak MS. Inhibition of Sonic hedgehog and Notch pathways enhances sensitivity of CD133(+) glioma stem cells to temozolomide therapy. Molecular medicine. 2011;17(1–2):103–112. doi: 10.2119/molmed.2010.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ricci-Vitiani L, Pallini R, Biffoni M, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468(7325):824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 95.Wang CH, Chiou SH, Chou CP, Chen YC, Huang YJ, Peng CA. Photothermolysis of glioblastoma stem-like cells targeted by carbon nanotubes conjugated with CD133 monoclonal antibody. Nanomedicine. 2011;7(1):69–79. doi: 10.1016/j.nano.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 96.Smith LM, Nesterova A, Ryan MC, et al. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. British journal of cancer. 2008;99(1):100–109. doi: 10.1038/sj.bjc.6604437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu ZB, Qiu C, Zhang AL, et al. Glioma-associated antigen HEATR1 induces functional cytotoxic T lymphocytes in patients with glioma. J Immunol Res. 2014;2014:131494. doi: 10.1155/2014/131494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bielamowicz K, Khawja S, Ahmed N. Adoptive cell therapies for glioblastoma. Frontiers in oncology. 2013;3:275. doi: 10.3389/fonc.2013.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu J, Lai G, Wan F, et al. Knockdown of checkpoint kinase 1 is associated with the increased radiosensitivity of glioblastoma stem-like cells. Tohoku J Exp Med. 2012;226(4):267–274. doi: 10.1620/tjem.226.267. [DOI] [PubMed] [Google Scholar]
- 100.Nadkarni A, Shrivastav M, Mladek AC, et al. ATM inhibitor KU-55933 increases the TMZ responsiveness of only inherently TMZ sensitive GBM cells. J Neurooncol. 2012;110(3):349–357. doi: 10.1007/s11060-012-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Signore M, Pelacchi F, di Martino S, et al. Combined PDK1 and CHK1 inhibition is required to kill glioblastoma stem-like cells in vitro and in vivo. Cell death & disease. 2014;5:e1223. doi: 10.1038/cddis.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vecchio D, Daga A, Carra E, et al. Predictability, efficacy and safety of radiosensitization of glioblastoma-initiating cells by the ATM inhibitor KU-60019. International journal of cancer. Journal international du cancer. 2014;135(2):479–491. doi: 10.1002/ijc.28680. [DOI] [PubMed] [Google Scholar]
- 103.Facchino S, Abdouh M, Chatoo W, Bernier G. BMI1 confers radioresistance to normal and cancerous neural stem cells through recruitment of the DNA damage response machinery. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30(30):10096–10111. doi: 10.1523/JNEUROSCI.1634-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Imai Y, Yoshimori M, Fukuda K, Yamagishi H, Ueda Y. The PI3K/Akt inhibitor LY294002 reverses BCRP-mediated drug resistance without affecting BCRP translocation. Oncology reports. 2012;27(6):1703–1709. doi: 10.3892/or.2012.1724. [DOI] [PubMed] [Google Scholar]
- 105.Martín V, Sanchez-Sanchez AM, Herrera F, et al. Melatonin-induced methylation of the ABCG2/BCRP promoter as a novel mechanism to overcome multidrug resistance in brain tumour stem cells. British journal of cancer. 2013;108(10):2005–2012. doi: 10.1038/bjc.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carra E, Barbieri F, Marubbi D, et al. Sorafenib selectively depletes human glioblastoma tumor-initiating cells from primary cultures. Cell Cycle. 2013;12(3):491–500. doi: 10.4161/cc.23372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aldea MD, Petrushev B, Soritau O, et al. Metformin plus sorafenib highly impacts temozolomide resistant glioblastoma stem-like cells. J BUON. 2014;19(2):502–511. [PubMed] [Google Scholar]
- 108.Chang CJ, Hsu CC, Yung MC, et al. Enhanced radiosensitivity and radiation-induced apoptosis in glioma CD133-positive cells by knockdown of SirT1 expression. Biochemical and biophysical research communications. 2009;380(2):236–242. doi: 10.1016/j.bbrc.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 109.Cheng L, Wu Q, Guryanova OA, et al. Elevated invasive potential of glioblastoma stem cells. Biochemical and biophysical research communications. 2011;406(4):643–648. doi: 10.1016/j.bbrc.2011.02.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scatena R, Bottoni P, Pontoglio A, Giardina B. Cancer stem cells: the development of new cancer therapeutics. Expert opinion on biological therapy. 2011;11(7):875–892. doi: 10.1517/14712598.2011.573780. [DOI] [PubMed] [Google Scholar]
- 111.Inda MM, Bonavia R, Mukasa A, et al. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes & development. 2010;24(16):1731–1745. doi: 10.1101/gad.1890510. [DOI] [PMC free article] [PubMed] [Google Scholar]