Abstract
Myocardial strain is a principle for quantification of left ventricular (LV) function which is now feasible with speckle-tracking echocardiography. The best evaluated strain parameter is global longitudinal strain (GLS) which is more sensitive than left ventricular ejection fraction (LVEF) as a measure of systolic function, and may be used to identify sub-clinical LV dysfunction in cardiomyopathies. Furthermore, GLS is recommended as routine measurement in patients undergoing chemotherapy to detect reduction in LV function prior to fall in LVEF. Intersegmental variability in timing of peak myocardial strain has been proposed as predictor of risk of ventricular arrhythmias. Strain imaging may be applied to guide placement of the LV pacing lead in patients receiving cardiac resynchronization therapy. Strain may also be used to diagnose myocardial ischaemia, but the technology is not sufficiently standardized to be recommended as a general tool for this purpose. Peak systolic left atrial strain is a promising supplementary index of LV filling pressure. The strain imaging methodology is still undergoing development, and further clinical trials are needed to determine if clinical decisions based on strain imaging result in better outcome. With this important limitation in mind, strain may be applied clinically as a supplementary diagnostic method.
Keywords: Left ventricular function, Heart failure, Strain imaging, Left atrial strain, Ventricular arrhythmia, Chemotherapy, Cardiomyopathy, Hypertrophic cardiomyopathy
Principles of strain
In echocardiography, the term ‘strain’ is used to describe local shortening, thickening and lengthening of the myocardium as a measure of regional LV function. The term originates from the field of continuum mechanics and is used to describe a general 3D deformation of a small cube during a short time interval. The strain tensor has six components (numbers), three of them giving the shortening along three orthogonal axes (x, y, z) in an external coordinate system, and three share strain numbers giving the skew in the x–y, x–z, and y–z planes. By dividing the myocardium into a large number of cubes, the complex and detailed deformation can be described by one strain tensor for each small cube at each time during the cardiac cycle.1 This description is, however, too detailed for practical use in echocardiography, where there is a need for a limited number of measurable parameters representing the average deformation within a segment of the myocardium. It is more convenient to use an internal coordinate system aligned with the three cardiac axes: longitudinal, circumferential, and radial, and to measure the shortening and elongation in the three directions through the cardiac cycle, with reference to the size at the time of the QRS-complex.
If we denote L(t) as the segment length along one of these directions at any time t in the cardiac cycle and L0 as initial length, 1D strain is defined as ɛ(t)= (L(t)−L0)/L0. This is also called Lagrange strain, and it is measured by the distance between two material points in the myocardium, both following the motion during contraction and relaxation. Note that positive strain means elongation, whereas negative strain is shortening. To avoid confusion when communicating about strain, it is recommended to refer an increase or decrease in the absolute value of strain.
Strain ɛ(t) in the myocardium can be measured by tissue Doppler imaging (TDI) or by speckle-tracking echocardiography (STE). The theoretical basis for measuring strain by TDI is that myocardial velocity gradient is an estimate of strain rate, and natural strain ɛN can be calculated as the temporal integral of strain rate. Lagrange strain can then be found by the formula ɛ = exp(ɛN) − 1.2,3 Speckle-tracking echocardiography utilizes the phenomenon in which natural acoustic markers in grey scale ultrasound images form interference patterns (speckles) within myocardial tissue. These patterns are quite stable over the short time period between two consecutive frames, and the 2D displacement for each point in the myocardium is found by automatic search for similar patterns in the two frames (block matching). This process is repeated for all frames through the cardiac cycle to produce a 2D displacement curve for each point in the myocardium. Subsequently, strains are calculated from each LV segment in circumferential, longitudinal, or radial directions.4 In 2D STE, only two directions of strain can be measured at a time; e.g. longitudinal/radial from long-axis views, and circumferential/radial from short-axis views. In 3D STE, strain in all three directions is available from the same 3D recording, but with lower temporal and spatial resolution.5 Currently, 3D strain is undergoing development and is yet not ready for clinical routine use.
Note that strain measurement by STE is only possible for muscular structures which are larger than the resolution cell in the ultrasound image. Thin structures such as the atria will not give reliable regional strain information. However, successful tracking of the cavity boarder with STE can be used to estimate the percentage change in cavity circumference. In this case, average longitudinal strain should correlate well with, e.g. percentage left atrial (LA) circumference change.
Quantification of global and regional left ventricular function
Myocardial strain and strain rate using TDI was introduced in the late 1990s,2,3 but has several limitations, including angle dependency. Currently, the most widely used strain modality is STE which can track speckles essentially independent of angle. Importantly, the measurements are still angle dependent because radial strain has opposite polarity of longitudinal and circumferential strains. Therefore, with increasing deviation from the major axis, there will be progressive reduction in absolute strain. To avoid underestimation, it is important to minimize foreshortening when using apical views, and in the short axis one should try to obtain circular LV images. Due to poorer spatial resolution in the lateral direction, best strain performance is always achieved in the direction of the ultrasound beam. Furthermore, similar to all measures of myocardial fibre shortening, systolic strain is load dependant and therefore blood pressure should be considered when interpreting measurements of strain.3,6 Other sources of variation in strain are age and gender.7
Strain rate may also be measured by STE and has a strong relationship to contractility,8,9 but is limited by signal noise and relatively low frame rate which implies that information may be lost. Left ventricular twisting motion may also be measured by STE but this modality needs further development and is not ready for use in clinical routine.
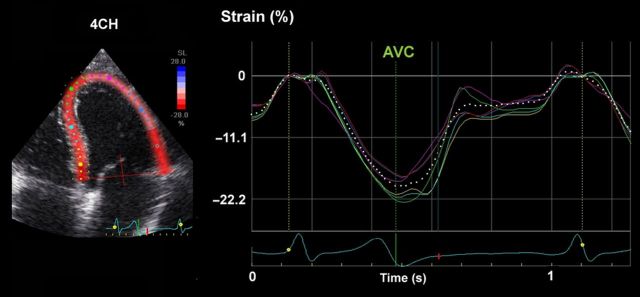
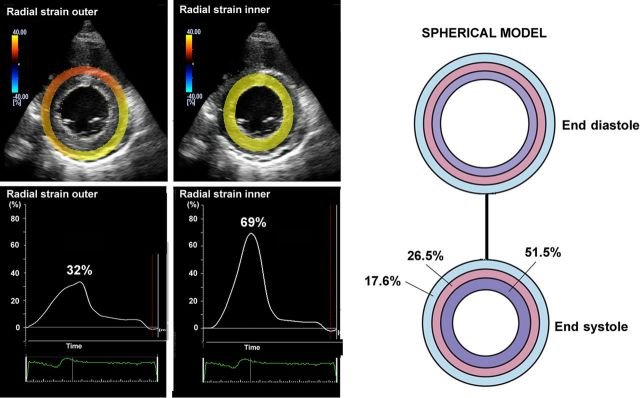
Most laboratories record LV strain in the long axis and use global longitudinal strain (GLS) calculated as the average from all segments, as a measure of global LV function. Figure 1 shows typical segmental strain traces in a normal heart. Images for GLS are made in standard apical two-, three-, and four-chamber views and aortic valve closure (AVC) is used for timing of end-systole.10 When regional speckle tracking is suboptimal and recordings need to be rejected in more than two myocardial segments in a single view, the calculation of GLS should be avoided10 and other methods for measuring global LV function should be used. Normal GLS for most echocardiography systems is reported between 18 and 25% in healthy individuals, a variation, which in part may be explained by inter-software and inter-vendor variability. Technically, good recordings can be achieved along any axis, but interpretation of radial and circumferential strains are complicated by substantial transmural non-uniformity in the normal left ventricle. Figure 2 illustrates and explains this phenomenon which is entirely a geometric effect and does not imply differences in contractility between LV wall layers. For longitudinal strain, however, such geometrical effects are of less magnitude.
Figure 1.
Segmental strains in apical four-chamber view, showing normal contractions. The color of each trace corresponds to anatomical points on the 2-D color image to the left. The white dotted line represents average strain.
Figure 2.
Left panels: left ventricular short-axis view from a healthy individual showing higher radial strains in inner than outer layer. Right panel: Transmural difference in radial strain is a pure geometrical effect, since reduction in external diameter of a passive circular structure leads to more thickening of inner than outer layers. The figure simulates reduction of inner radius by 25% and the numbers indicate the resulting thickening in inner, mid and outer wall layers.11
Coronary artery disease: detection of myocardial ischaemia and viability
Strain imaging may be applied to diagnose ischaemia by showing reduction in peak systolic strain, but equally important is demonstration of systolic lengthening and post-systolic shortening which are characteristic features of ischaemic dysfunction12–14 (Figure 3). When reporting values for peak strain from ischaemic segments, it is important to take timing into account, since marked post-systolic shortening may result in near normal peak strain, as illustrated in Figure 3. Therefore, end-systolic strain and not peak strain should be used to measure systolic function.
Figure 3.
Left panel: Strain by sonomicrometry in an anaesthetized dog model showing normal contraction in the upper left corner. In the upper right corner, a recording during coronary stenosis showing reduced systolic shortening in combination with marked post-systolic shortening, which implies active contraction and therefore viable myocardium. The two lower recordings illustrate dyskinesia during coronary occlusion. The lower left shows early-systolic lengthening, followed by late and post-systolic shortening, consistent with some degree of active contraction. The lower right recording shows myocardium with no active contraction and reflects the effect of the time-varying left ventricular pressure on the passively behaving myocardium ED = end diastole. Modified from Lyseggen14 and Skulstad.15 Right panel: myocardial strain by tissue Doppler imaging in a patient with acute anterior myocardial infarction. A series of recordings along the septum are displayed. The color of each trace corresponds to anatomical points on the 2-D images to the left. These traces have features similar to the recordings by sonomicrometry in the left panel, illustrating the ability of strain by echocardiography to reflect myocardial segmental contraction Courtsey of Erik Lyseggen.
Left ventricular systolic strain is displayed as bull's eye plot in Figure 4 from a patient with acute myocardial infarction. Suboptimal images with noise artefacts complicate data interpretation and the study can be non-conclusive, but in the majority of patients image quality is satisfactory. This implies, however, that there is some degree of subjectivity in the interpretation of strain images. When the issue is whether a patient has ischaemia, the finding of typical strain features of ischaemia in more than just a single segment favours ischaemic dysfunction rather than noise artefacts. This is illustrated in Figure 5 which shows mild reduction in systolic shortening and post-systolic shortening in several adjacent segments.
Figure 4.
Patient with anterior myocardial infarction. Each trace represents one LV segment. Apical segments are dyskinetic (blue colour in bull's eye plot) while other segments are hypokinetic.
Figure 5.
Strain imaging in patient with atypical symptoms, no chest pain and no signs of ischaemia in electrocardiogram. Each trace represents one LV segment. Possible inferior wall hypokinesia on grey scale imaging. Strain imaging showed moderately reduced systolic shortening and marked post-systolic shortening in the inferior wall (red circle). The patient was referred for angiography which revealed a subtotal stenosis of the right coronary artery (right panel) and was successfully treated with percutaneous coronary intervention. ES = end systole.
An interesting application of GLS is in the evaluation of patients with suspected stable angina pectoris where it was shown to be an independent predictor of significant coronary heart disease, at rest and during dobutamine stress echocardiography.16,17 Another promising application of strain imaging is identification of the relatively large subgroup of non ST-elevation myocardial infarction patients with total coronary occlusion, who needs urgent revascularization.18 Lack of ST elevation in these patients reflects limited sensitivity of electrocardiogram (ECG) in identifying patients with coronary occlusion.19
Post-systolic strain has been proposed as a marker of viability, but should not be used as a stand-alone index since post-systolic shortening also occurs also in myocardium with transmural necrosis or scar (Figure 6). In the latter case, there is typically systolic lengthening, and post-systolic shortening is due to passive recoil when LV pressure is falling during isovolumic relaxation. Furthermore, load-dependent interactions with non-ischaemic myocardium can modify systolic strain.20,21 When there is systolic hypokinesia or akinesia indicating some degree of active contraction, post-systolic shortening is attributed to active contraction and reflects viable myocardium.14 Importantly, a segment which is entirely passive during the first few hours after coronary occlusion, may yet not be irreversibly injured and may recover with reperfusion.15 In the chronic phase after an infarct, however, an entirely passive strain curve is most likely a sign of scarring. There are limited clinical data to verify these concepts which have been well documented in experimental models.
Figure 6.
Longitudinal strain by speckle-tracking echocardiography (two-chamber view) in a patient with acute myocardial infarction the day after percutaneous coronary intervention of an occluded left anterior descending coronary artery. Follow-up late enhancement cardiac magnetic resonance (lower left) showed myocardial scarring represented by the white area in apex and anterior wall. Strain curves display typical features of ischaemic dysfunction, ranging from lengthening throughout systole in a segment with transmural infarction and different degrees of dysfunction in other segments. The color of each trace corresponds to anatomical points on the 2-D color image and the white dotted line shows the average of the six strain curves. The yellow curve shows normal contraction in a non-infarcted segment.
More documentation of added clinical value is needed, however, before strain imaging can be recommended for routine use in the evaluation of patients with chest pain. In spite of promising reports,22–25 strain imaging is yet not ready for routine assessment of viability. In some cases, however, the additional diagnostic information provided by strain imaging can be helpful, in particular when other non-invasive tests are non-conclusive. But even for this limited application, interpretations are subjective and the experience which comes from frequent use of the technology is essential.
Cardiomyopathies and sub-clinical left ventricular dysfunction
When there is an overt clinical cardiomyopathy with typical findings by conventional echocardiography, strain imaging is usually not needed. In early stages of disease, however, strain imaging can be of considerable help in the diagnostic evaluation and to define prognosis. In a number of cardiac disorders, the ability of GLS to predict cardiovascular outcome may be superior to LVEF.26 The reason why GLS appears to be more sensitive than LVEF to detect myocardial dysfunction may in part reflect the limited ability of LVEF to assess systolic function in ventricles with hypertrophy. This is well known from patients with hypertrophic cardiomyopathy (HCM) in whom LVEF may be normal or even supernormal when systolic function is markedly reduced. The same principle applies to other conditions with hypertrophy because hypertrophic ventricles thicken more in absolute terms, which reduces cavity volumes more than in ventricles with normal wall thickness.27 As an example, a normal ventricle with end-diastolic wall thickness 10 mm and radial strain of 40% and a ventricle with 20 mm wall thickness and with reduced systolic function and reduced radial strain to 20%, will both thicken by 4 mm. This principle is one reason why LVEF may overestimate systolic function in hypertrophic ventricles. Furthermore, since longitudinal myocardial fibres are located predominantly in the subendocardium, which is the wall layer which is most susceptible to ischemia, reductions in longitudinal strain may be found prior to reduction in EF.26
Prognosis in HCM is closely related to LV function and morphology.28 In contrast to LVEF which very often is normal in HCM, longitudinal strains are reduced in early stages of the disease, whereas radial and circumferential strains may be preserved (Figure 7). Longitudinal strain is reduced at the site of hypertrophy, commonly in the interventricular septum. Current clinical practice guidelines for management of HCM29 include strain echocardiography for evaluating longitudinal function in early disease. Longitudinal function can be abnormal even before development of increased wall thickness in mutation positive family members29 (Figure 7). The finding of reduced longitudinal strain, particularly in the presence of an abnormal ECG, increases the probability of disease.
Figure 7.
Longitudinal strain curves from apical four-chamber view in a 28-year-old male who was positive for a hypertrophic cardiomyopathy-related mutation in the MYBPC3 gene detected by family genetic screening. The color of each trace corresponds to anatomical points on the 2-D color image to the left. Average strain from four-chamber view was 14% (white dotted trace) and global longitudinal strain was 16%, indicating reduced longitudinal function. Ejection fraction was 57%. He was asymptomatic and had no hypertrophy by echocardiography, cardiac magnetic resonance nor by electrocardiography. Green vertical line indicates timing of AVC.
The diagnosis of pathological hypertrophy in young healthy athletes is challenging and preliminary data suggest that strain imaging may be of help as reduction in systolic strain, which is typical for HCM, is not found in physiological hypertrophy.30 Further validation is needed, however, before this application of strain imaging can be recommended as routine. Of the echocardiographic parameters, the single most important discriminator is LV end-diastolic diameter since hypertrophy in HCM occurs at the expense of cavity size, resulting in a small LV cavity (<45 mm), whereas almost all athletes with physiological LV hypertrophy have concomitant enlargement of the LV cavity (between 55 and 65 mm).31 Reduced early diastolic mitral annular velocity by tissue Doppler is also consistent with pathological hypertrophy, but cannot be used confirm or refute the diagnosis of physiological hypertrophy in every case.32
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is diagnosed according to the 2010 Task Force Criteria33 using different modalities including imaging by echocardiography and cardiac magnetic resonance (CMR). Reduced RV function by reduced RV-free wall strain and a dyssynchronous RV contraction pattern have been shown to be early markers of ARVC,34,35 and may help diagnosis in early phases of disease.
Uncomplicated diabetes mellitus is associated with reduced LV longitudinal strain, but preserved radial strain,36–38 and reduced GLS may be a marker of risk of diabetic cardiomyopathy.39 The role of strain imaging in routine evaluation of patients with diabetes remains to be defined.
Cardiotoxicity during chemotherapy
Myocardial toxic effects from chemotherapy has become a leading cause of morbidity and mortality in cancer survivors. Therefore, protection of the heart during chemotherapy by monitoring cardiac function and administration of appropriate therapy has become a major clinical issue. There is, however, no high-level evidence to guide choice of imaging method, how frequently measurements should be done, and there is limited data on efficacy of medical therapy to prevent or reverse LV dysfunction due to chemotherapy. It is general agreement that LVEF should be measured prior to chemotherapy using preferentially echocardiography. Radionuclide imaging is also used, but gives less diagnostic information and is associated with significant ionizing radiation. Patients who develop heart failure during chemotherapy are treated with standard guideline-based heart failure therapy just as any other heart failure patient.40 For patients who develop asymptomatic LV dysfunction, however, there is not sufficient evidence to give firm recommendations with regard to medical therapy.
When reduction in LVEF during chemotherapy is established, it may be too late for treatment.41 Importantly, reduction in myocardial strain precede significant change in LVEF.42 In a recent consensus document from the ASE and EACVI, a practical guide is given for how to apply strain imaging in the evaluation of adult patients who receive cancer therapy, and GLS by STE was the recommended strain parameter for early detection of sub-clinical LV dysfunction.43 A relative decrease in GLS >15% compared with baseline is likely to be of clinical significance, whereas a decrease <8% is not43 (Figure 8). However, although strain imaging may detect sub-clinical myocardial changes, the value of these changes in predicting clinical outcome is still unknown. A combination of strain imaging with ultrasensitive troponin has been proposed.
Figure 8.
Strain imaging for early detection of sub-clinical left ventricular dysfunction during chemotherapy. Modified from Plana et al.43 *The data supporting the initiation of cardioprotection for the treatment of sub-clinical left ventricular dysfunction is limited.
It is recommended to measure LVEF at least prior to chemotherapy, at completion of therapy and 6 months later. For laboratories with competence in strain imaging, it is recommended to measure GLS in addition to LVEF, which will be helpful in cases when LVEF is in the lower normal range and it is difficult to conclude about systolic function. In such cases, the finding of subnormal strain should result in closer monitoring of cardiac function. Not all sub-clinical reduction in LV function may progress to significant dysfunction or heart failure, and there is need for studies which can help to define criteria for clinically relevant changes in strain.
Due to lack of standardization of methodology between vendors, it is essential that each echocardiography laboratory defines a normal range of strain values and ensures high degree of reproducibility. Furthermore, when doing serial evaluations, similar equipment and algorithms for calculating strain should be used.
Risk assessment and prognosis
Prognosis in cardiac disease is closely related to systolic function which is commonly measured as LVEF. An increasing number of studies have suggested that GLS is superior to EF as a measure of LV function and as predictor mortality and cardiac events44–46 (Figure 9). No absolute values for GLS, that indicate high risk are established, but we suggest that absolute GLS <12% represent severe systolic dysfunction and adverse prognosis,44,46 and <15–16% seems to represent risk in patients with relatively preserved EF.44,47
Figure 9.
In patients with preserved left ventricular ejection fraction (>40%) after myocardial infarction those with absolute global longitudinal strain <14% had increased risk for the combined endpoint of all-cause mortality and heart failure admission. Months on x axis. Modified from Ersboll et al.44
It was shown in patients with long QT syndrome (LQTS), that large inter-segmental variability in contraction duration, named mechanical dispersion, was associated with increased risk of ventricular arrhythmias.48 Mechanical dispersion is calculated as standard deviation of contraction duration measured from peak Q wave or start of the R wave in ECG to peak shortening strain in multiple LV segments.48 Normally, all segments have relatively similar contraction duration and therefore low values for mechanical dispersion. Patients with LQTS and high risk for arrhythmias have larger mechanical dispersion.48 The LQTS is a cardiac ion channel disease, and has been considered a purely electrical abnormality with high risk of ventricular arrhythmias and sudden cardiac death. Early papers recognized unsuspected mechanical alterations by M-mode echocardiography in LQTS, which were associated with risk of ventricular arrhythmias.49 These findings were confirmed 20 years later by tissue Doppler imaging and speckle-tracking strain echocardiography, showing that mechanical dispersion in LQTS was associated with risk of arrhythmias48,50 and that these patients had a sub-clinical impairment of myocardial function.51 Mechanical dispersion may be used as an additive parameter for risk assessment in these individuals. The current evidence, however, is not strong enough to support use of mechanical dispersion as an additional criterion when decisions are made regarding implantable cardioverter-defibrillator implantation in LQTS patients. The term mechanical dispersion has also been used to describe and quantify mechanical dyssynchrony in other diseases, including previous myocardial infarction (Figure 10) and non-ischaemic cardiomyopathy,47,52,53 and is associated with increased risk of ventricular arrhythmias.
Figure 10.
Left panel shows synchronous contraction by longitudinal strain in a patient after myocardial infarction. Mid panel shows heterogeneous timing of contraction and pronounced mechanical dispersion in a patient after myocardial infarction with ventricular arrhythmias. Right panel shows better arrhythmia free event rate in those with mechanical dispersion <75 ms.47
Mechanical dispersion is an index of inter-segmental discoordination of contraction and similar to some other velocity and strain indices which have been used to quantify LV dyssynchrony, it measures variability in time-to-peak shortening. This approach is interesting in particular because it may represent a means to identify high-risk patients with normal or preserved LVEF. In patients with heart failure and in coronary artery disease such as displayed in Figures 4–6, segmental variation in timing of peak strain is obvious and calculation of mechanical dispersion may not provide much additional information. It remains to be determined if mechanical dispersion is superior to other indices of dyssynchrony to predict risk of ventricular arrhythmias. One strength of mechanical dispersion is that the same recording may be used to measure GLS as a parameter of global systolic function.
Most likely, the aetiology of mechanical dispersion is different in LQTS and in most other conditions. In LQTS, mechanical dispersion may reflect inhomogeneous prolongation of action potential duration which in turn leads to different contraction durations. Potential aetiologies of mechanical dispersion in patients with cardiomyopathy are fibrosis or ischaemia which may cause local delays in electromechanical activation. Furthermore, non-uniform loading conditions in a diseased ventricle may impacts timing of peak shortening. Since dyssynchrony is common in heart failure and is associated with increased risk, we should focus on understanding the underlying pathophysiology.54 In this regard, indices of dyssynchrony such as mechanical dispersion and other myocardial velocity and strain indices represent important research tools.54
Heart failure with preserved ejection fraction
In principle, strain imaging is an excellent modality for evaluation of diastolic function in terms of early-diastolic strain rate which reflects myocardial lengthening rate and untwisting rate which is tightly coupled to restoring forces and diastolic suction.55–58 Due to significant signal noise and other technical limitations, however, strain rate cannot replace e′ by tissue Doppler as clinical method to estimate LV lengthening velocity.
Global longitudinal strain, however, is a very promising method to identify patients with mild systolic dysfunction which is not reflected in reduced EF. Figure 11 is from a study which compared longitudinal and circumferential global strain in patients with manifest HFpEF to hypertensives with diastolic dysfunction without heart failure and a group of normal controls, and found lower longitudinal and circumferential peak strain in HFpEF patients.59 This application of strain imaging has yet not been included in clinical practice guidelines, but it is likely to become a useful application when evaluating patients with unexplained heart failure symptoms.
Figure 11.
Left ventricular strain in hypertension and heart failure with preserved ejection fraction (HFpEF). Left panel: average longitudinal and circumferential systolic strain among normal controls (n = 50), hypertensive heart disease (n = 44) and heart failure with preserved ejection fraction (n = 219). Right panel: three categories heart failure with preserved ejection fraction based on left ventricular ejection fraction. *P < 0.0001 vs. controls and between hypertensive heart disease and heart failure with preserved ejection fraction overall for longitudinal strain and circumferential strain. #P < 0.0002 vs. controls. †Left ventricular ejection fraction-adjusted P < 0.001 compared with controls.59
Valvular heart disease
Deciding timing for surgery in asymptomatic moderate-to-severe valvular heart disease is still problematic and is based on symptoms, severity of the lesion and its impact on LV volume and function. The most solid predictor of impaired outcome in regurgitant valvular heart diseases is LV volumes. Furthermore, when LVEF is reduced, it indicates depression of myocardial contractility. Reduction in EF, however, is often a late consequence of valve dysfunction and may even imply irreversible myocardial injury. Currently, there is a shift towards interventions earlier in the disease, and emerging data suggest that strain imaging may identify myocardial injury at an early stage and prior to reduction in EF. As suggested by recent studies, quantification of myocardial function by strain imaging provides added clinical value in mitral- and aortic regurgitation and in aortic stenosis.60–62 Since current recommendations for management of valvular heart disease are based mainly on observational studies, there is need for prospective randomized controlled trials,63 and these should include strain imaging as a more sensitive measure of impaired LV function. Laboratories with experience in strain imaging, however, may already apply this methodology to conclude about LV contractility in patients with EF in the lower normal range, and GLS <16% indicates reduced contractility. Further validation is needed before firm recommendations can be made regarding routine use of strain imaging in decision making regarding timing of valve surgery and interventions.
Cardiac resynchronization therapy
Several attempts have been made to improve selection criteria for responders to cardiac resynchronization therapy (CRT), including testing of various echocardiographic indices, but none of these approaches are proven to improve responder rate.64 Therefore, current guidelines do not recommend assessment of dyssynchrony by echocardiography in the diagnostic work up for patient selection before CRT.65 Importantly, in most previous trials dyssynchrony was quantified using indices for timing of peak contraction. More recent studies have focused on the abnormal wall motion patterns which are typical for left bundle branch block using strain imaging. This pattern which includes early-systolic shortening and rebound stretch in the septum, combined with early-systolic lengthening and peak shortening after AVC in the LV lateral wall, predicts response to CRT66–68 (Figure 12). Furthermore, in patients with left bundle branch block in ECG, the absence of this contraction pattern is associated with a marked increase in risk of adverse events after CRT.68 In this subgroup which do not show the typical strain pattern, it is possible that QRS widening is caused by mechanisms such as hypertrophy and diffuse fibrosis and therefore these ventricles may not be the optimal target for pacing therapy. It remains to be determined if these and other new insights based on strain imaging may be utilized to improve responder rate to CRT. Future studies should also investigate if strain imaging may be useful in the evaluation of patients after implantation of CRT, as suggested by a recent study which showed that dyssynchrony measured several months after device implantation is associated with serious ventricular arrhythmias.53
Figure 12.
Recording of left ventricular longitudinal strain by speckle-tracking echocardiography in a patient with heart failure and left bundle branch block: there is a characteristic left bundle branch block pattern with early-systolic shortening in the septum (blue arrows), combined with early (pre-stretch) in the lateral wall (yellow arrow), and late peak contraction in the lateral wall (red arrow). AVC, aortic valve closure. Modified from Risum et al.68
Although, at the present stage, cardiac imaging has no proven value in selection of patients for CRT, there is evidence for applying strain imaging to find optimal position for the pacing lead in the LV-free wall.65 Several studies have demonstrated that a lead position which coincides with the region of latest mechanical activation yields superior outcomes (Figure 13).65 Furthermore, avoiding placing the lateral lead over a transmural scar is important, and a peak radial strain value of <10% was proposed as a marker of scar.69
Figure 13.
The figure illustrates how radial strain may be used to determine which segments have latest mechanical activation. A left ventricular parasternal short-axis recording is displayed. Strain in anteroseptal segment shows early-systolic thickening (yellow curve). Lateral (light blue), posterior (green and pink), and posterioseptal segments (blue and red) show late thickening, indicating latest activation.
Left atrial strain
Left atrial (LA) volume reflects the chronic effect of LV filling pressures over time, but may also be enlarged in healthy athletes and in patients with atrial arrhythmias when filling pressure is normal. Preliminary data suggest that peak LA strain measured by 2D STE may represent a means to evaluate instant LA pressure.70,71 Left atrial strain can be measured with high feasibility from apical views by 2D STE72 (Figure 14). Due to the exponential LA pressure–volume relationship, atrial compliance will decrease when pressure is elevated. This is why elevated LA pressure is associated with reduced atrial strain during LV systole (Figure 14). The method is somewhat limited by measurement problems related to the pulmonary vein outlets and the LA appendage.72 More evaluation is needed before LA strain may be recommended for routine clinical use.
Figure 14.
(A and B) Left atrial (LA) strain by two different speckle-tracking software. (A) Segmental traces of LA strain and average strain (white-dashed trace). Yellow arrow indicates peak strain. Modified from Cameli et al.74 (B) Relationship between LA strain and left ventricular end-diastolic pressure.73
Inter-vendor variability
A recent study conducted by the EACVI-ASE-Industry Task Force to standardize deformation imaging was recently finished.75 The study tested the variability of speckle-tracking GLS obtained using different ultrasound machines and software packages, and compared GLS measurement variability with other echocardiographic parameters. Endocardial GLS was measured as this was the only GLS parameter which could be provided by all manufactures. Reproducibility of GLS measurements was good and in many cases superior to the reproducibility of LVEF. However, there was a small but statistically significant variation among vendors (Figure 15). This implies that when measuring strain one should take into account the type of echo equipment in use when defining normal reference values. Furthermore, caution should be exerted when companies deliver upgrades of software since this may lead to changes in strain values.76
Figure 15.
Average (±standard deviation) global longitudinal strain of all study subjects presented per vendor. The study was done in individuals with normal to severely impaired left ventricular function. As shown in the table, there was a significant differences between most vendors (P < 0.00). Blue dot, P < 0.05.75
A major limitation of the current state of the technology is that segmental strains vary considerably in different publications and there is lack of sufficiently validated reference values for segmental strain. Variability in segmental strains to a large extent reflects different methodologies between vendors, but differences depending on which wall layers are incorporated in the analysis also contributes. With 3D strain, sampling rates are relatively low and this may influence measurements.
Key points
Numerous studies have shown that myocardial strain imaging provides unique diagnostic information. Global longitudinal strain by STE is more sensitive than LVEF as a marker of LV dysfunction. The strain imaging methodology is still undergoing development and further clinical trials are needed to determine if clinical decisions based on strain imaging results in better outcome. With this important limitation in mind, strain may be applied clinically as a supplementary diagnostic method and in the following conditions it appears to be useful.
– In patients with preserved or normal LVEF, reduced GLS may be used to identify systolic dysfunction.
– Strain imaging can be used to identify sub-clinical LV dysfunction in individuals who are evaluated for cardiomyopathy. This includes family screening for HCM and the finding of reduced GLS indicates early disease.
– In patients with valvular heart disease reduced GLS reflects negative impact of the valve lesion on myocardial function prior to fall in LVEF, but so far this application is not recommended for use in clinical routine.
– Strain imaging is recommended in addition to LVEF in patients undergoing chemotheraphy to identify sub-clinical LV dysfunction.
– Mechanical dispersion as a measure of dyssynchrony, can identify patients with high risk of ventricular arrhythmias, but this approach is not ready for clinical implementation.
– Strain may be used to diagnose myocardial ischaemia, but the technology is not sufficiently standardized to be recommended as a general tool for this purpose. In unclear clinical cases, however, it may be considered as a supplementary method.
– Strain imaging may be applied in patients eligible for CRT to guide placement of the LV pacing lead, but is currently not recommended for selection of CRT responders.
– Peak systolic longitudinal LA strain is a promising supplementary index of LV filling pressure, but needs further validation in prospective trials.
Directions for the future
Further development of strain imaging should be focused on even better standardization of the methodology between different vendors, and the ongoing development of 3D strain should improve the diagnostic potential from the technology. Furthermore, automated image analysis which takes into account more information than just peak strain is expected to improve the diagnostic power of strain imaging. This applies in particular to myocardial ischaemia where there are so many well-defined features in the strain trace that differs from normal myocardium and this information is wasted when using GLS as the only parameter. Since strain imaging can identify LV dysfunction earlier than conventional methods this opens a new perspective in heart failure prophylaxis and primary prevention with institution of therapeutic measures before the patients develop symptoms and irreversible myocardial dysfunction. Prospective clinical trials should be started to investigate the added clinical value that strain may represent in patient management. In the meantime, strain may be applied in routine clinical diagnostics with the limitations of the technology kept in mind.
Authors’ contributions
O.A.S.: handled funding and supervision; O.A.S., H.T., A.O., K.H.H., S.U.: acquired the data; O.A.S., H.T., A.O., K.H.H., S.U.: conceived and designed the research; O.A.S., H.T., A.O., K.H.H., S.U.: drafted the manuscript; O.A.S., H.T., A.O., K.H.H., S.U.: made critical revision of the manuscript for key intellectual content.
Funding
O.A.S. was funded by grants from the Norwegian Council on Cardiovascular Diseases, Helse Sør-Øst, the University of Oslo, Inger and John Fredriksen's Foundation and the KG Jebsen foundation. K.H.H. was supported by the Center for Cardiological Innovation, funded by the Norwegian research council. Funding to pay the Open Access publication charges for this article was provided by University of Oslo, Institute of Clinical Medicine.
Conflict of interest: K.H.H. has licensed the method of mechanical dispersion. H.T. has received personal consultant fees from GE-Vingmed Ultrasound, outside the work in this article. H.T. has a patent Ultrasound imaging for displaying strain EP 1021129 B1 issued, a patent Method and apparatus for spectral strain rate visualization US 7022078 B2 issued, a patent WO 1999017660 A1 pending, a patent US 6776759 B2 issued, and a patent US 6099471 A issued.
References
- 1. Waldman LK, Fung YC, Covell JW. Transmural myocardial deformation in the canine left ventricle. Normal in vivo three-dimensional finite strains. Circ Res 1985;57:152–163. [DOI] [PubMed] [Google Scholar]
- 2. Heimdal A, Stoylen A, Torp H, Skjaerpe T. Real-time strain rate imaging of the left ventricle by ultrasound. J Am Soc Echocardiogr 1998;11:1013–1019. [DOI] [PubMed] [Google Scholar]
- 3. Urheim S, Edvardsen T, Torp H, Angelsen B, Smiseth OA. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation 2000;102:1158–1164. [DOI] [PubMed] [Google Scholar]
- 4. Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, Stoylen A, Ihlen H, Lima JA, Smiseth OA, Slordahl SA. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol 2006;47:789–793. [DOI] [PubMed] [Google Scholar]
- 5. Jasaityte R, Heyde B, D'Hooge J. Current state of three-dimensional myocardial strain estimation using echocardiography. J Am Soc Echocardiogr 2013;26:15–28. [DOI] [PubMed] [Google Scholar]
- 6. Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr 2013;26:185–191. [DOI] [PubMed] [Google Scholar]
- 7. Andre F, Steen H, Matheis P, Westkott M, Breuninger K, Sander Y, Kammerer R, Galuschky C, Giannitsis E, Korosoglou G, Katus HA, Buss SJ. Age- and gender-related normal left ventricular deformation assessed by cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson 2015;17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abraham TP, Laskowski C, Zhan WZ, Belohlavek M, Martin EA, Greenleaf JF, Sieck GC. Myocardial contractility by strain echocardiography: comparison with physiological measurements in an in vitro model. Am J Physiol Heart Circ Physiol 2003;285:H2599–H2604. [DOI] [PubMed] [Google Scholar]
- 9. Greenberg NL, Firstenberg MS, Castro PL, Main M, Travaglini A, Odabashian JA, Drinko JK, Rodriguez LL, Thomas JD, Garcia MJ. Doppler-derived myocardial systolic strain rate is a strong index of left ventricular contractility. Circulation 2002;105:99–105. [DOI] [PubMed] [Google Scholar]
- 10. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 11. Hexeberg E, Homans DC, Bache RJ. Interpretation of systolic wall thickening. Can thickening of a discrete layer reflect fibre performance? Cardiovasc Res 1995;29:16–21. [PubMed] [Google Scholar]
- 12. Edvardsen T, Aakhus S, Endresen K, Bjomerheim R, Smiseth OA, Ihlen H. Acute regional myocardial ischemia identified by 2-dimensional multiregion tissue Doppler imaging technique. J Am Soc Echocardiogr 2000;13:986–994. [DOI] [PubMed] [Google Scholar]
- 13. Kukulski T, Jamal F, Herbots L, D'Hooge J, Bijnens B, Hatle L, De Scheerder I, Sutherland GR. Identification of acutely ischemic myocardium using ultrasonic strain measurements. A clinical study in patients undergoing coronary angioplasty. J Am Coll Cardiol 2003;41:810–819. [DOI] [PubMed] [Google Scholar]
- 14. Skulstad H, Edvardsen T, Urheim S, Rabben SI, Stugaard M, Lyseggen E, Ihlen H, Smiseth OA. Postsystolic shortening in ischemic myocardium: active contraction or passive recoil? Circulation 2002;106:718–724. [DOI] [PubMed] [Google Scholar]
- 15. Lyseggen E, Skulstad H, Helle-Valle T, Vartdal T, Urheim S, Rabben SI, Opdahl A, Ihlen H, Smiseth OA. Myocardial strain analysis in acute coronary occlusion: a tool to assess myocardial viability and reperfusion. Circulation 2005;112:3901–3910. [DOI] [PubMed] [Google Scholar]
- 16. Biering-Sorensen T, Hoffmann S, Mogelvang R, Zeeberg Iversen A, Galatius S, Fritz-Hansen T, Bech J, Jensen JS. Myocardial strain analysis by 2-dimensional speckle tracking echocardiography improves diagnostics of coronary artery stenosis in stable angina pectoris. Circ Cardiovasc Imaging 2014;7:58–65. [DOI] [PubMed] [Google Scholar]
- 17. Voigt JU, Exner B, Schmiedehausen K, Huchzermeyer C, Reulbach U, Nixdorff U, Platsch G, Kuwert T, Daniel WG, Flachskampf FA. Strain-rate imaging during dobutamine stress echocardiography provides objective evidence of inducible ischemia. Circulation 2003;107:2120–2126. [DOI] [PubMed] [Google Scholar]
- 18. Eek C, Grenne B, Brunvand H, Aakhus S, Endresen K, Hol PK, Smith HJ, Smiseth OA, Edvardsen T, Skulstad H. Strain echocardiography and wall motion score index predicts final infarct size in patients with non-ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging 2010;3:187–194. [DOI] [PubMed] [Google Scholar]
- 19. Grenne B, Eek C, Sjoli B, Dahlslett T, Uchto M, Hol PK, Skulstad H, Smiseth OA, Edvardsen T, Brunvand H. Acute coronary occlusion in non-ST-elevation acute coronary syndrome: outcome and early identification by strain echocardiography. Heart 2010;96:1550–1556. [DOI] [PubMed] [Google Scholar]
- 20. Kumada T, Karliner JS, Pouleur H, Gallagher KP, Shirato K, Ross J Jr. Effects of coronary occlusion on early ventricular diastolic events in conscious dogs. Am J Physiol 1979;237:H542–H549. [DOI] [PubMed] [Google Scholar]
- 21. Lew WY, Ban-Hayashi E. Mechanisms of improving regional and global ventricular function by preload alterations during acute ischemia in the canine left ventricle. Circulation 1985;72:1125–1134. [DOI] [PubMed] [Google Scholar]
- 22. Bansal M, Jeffriess L, Leano R, Mundy J, Marwick TH. Assessment of myocardial viability at dobutamine echocardiography by deformation analysis using tissue velocity and speckle-tracking. JACC Cardiovasc Imaging 2010;3:121–131. [DOI] [PubMed] [Google Scholar]
- 23. Becker M, Hoffmann R, Kuhl HP, Grawe H, Katoh M, Kramann R, Bucker A, Hanrath P, Heussen N. Analysis of myocardial deformation based on ultrasonic pixel tracking to determine transmurality in chronic myocardial infarction. Eur Heart J 2006;27:2560–2566. [DOI] [PubMed] [Google Scholar]
- 24. Delgado V, van Bommel RJ, Bertini M, Borleffs CJ, Marsan NA, Arnold CT, Nucifora G, van de Veire NR, Ypenburg C, Boersma E, Holman ER, Schalij MJ, Bax JJ. Relative merits of left ventricular dyssynchrony, left ventricular lead position, and myocardial scar to predict long-term survival of ischemic heart failure patients undergoing cardiac resynchronization therapy. Circulation 2011;123:70–78. [DOI] [PubMed] [Google Scholar]
- 25. Roes SD, Mollema SA, Lamb HJ, van der Wall EE, de Roos A, Bax JJ. Validation of echocardiographic two-dimensional speckle tracking longitudinal strain imaging for viability assessment in patients with chronic ischemic left ventricular dysfunction and comparison with contrast-enhanced magnetic resonance imaging. Am J Cardiol 2009;104:312–317. [DOI] [PubMed] [Google Scholar]
- 26. Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014;100:1673–1680. [DOI] [PubMed] [Google Scholar]
- 27. Maciver DH. The relative impact of circumferential and longitudinal shortening on left ventricular ejection fraction and stroke volume. Exp Clin Cardiol 2012;17:5–11, Spring. [PMC free article] [PubMed] [Google Scholar]
- 28. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW, American College of Cardiology Foundation/American Heart Association Task Force on Practice G, American Association for Thoracic S, American Society of E, American Society of Nuclear C, Heart Failure Society of A, Heart Rhythm S, Society for Cardiovascular A, Interventions, Society of Thoracic S. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011;124:2761–2796. [DOI] [PubMed] [Google Scholar]
- 29. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 30. Afonso L, Kondur A, Simegn M, Niraj A, Hari P, Kaur R, Ramappa P, Pradhan J, Bhandare D, Williams KA, Zalawadiya S, Pinheiro A, Abraham TP. Two-dimensional strain profiles in patients with physiological and pathological hypertrophy and preserved left ventricular systolic function: a comparative analyses. BMJ Open 2012;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caselli S, Maron MS, Urbano-Moral JA, Pandian NG, Maron BJ, Pelliccia A. Differentiating left ventricular hypertrophy in athletes from that in patients with hypertrophic cardiomyopathy. Am J Cardiol 2014;114:1383–1389. [DOI] [PubMed] [Google Scholar]
- 32. Rawlins J, Bhan A, Sharma S. Left ventricular hypertrophy in athletes. Eur J Echocardiogr 2009;10:350–356. [DOI] [PubMed] [Google Scholar]
- 33. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J 2010;31:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saberniak J, Hasselberg NE, Borgquist R, Platonov PG, Sarvari SI, Smith HJ, Ribe M, Holst AG, Edvardsen T, Haugaa KH. Vigorous physical activity impairs myocardial function in patients with arrhythmogenic right ventricular cardiomyopathy and in mutation positive family members. Eur J Heart Fail 2014;16:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sarvari SI, Haugaa KH, Anfinsen OG, Leren TP, Smiseth OA, Kongsgaard E, Amlie JP, Edvardsen T. Right ventricular mechanical dispersion is related to malignant arrhythmias: a study of patients with arrhythmogenic right ventricular cardiomyopathy and subclinical right ventricular dysfunction. Eur Heart J 2011;32:1089–1096. [DOI] [PubMed] [Google Scholar]
- 36. Fang ZY, Leano R, Marwick TH. Relationship between longitudinal and radial contractility in subclinical diabetic heart disease. Clin Sci 2004;106:53–60. [DOI] [PubMed] [Google Scholar]
- 37. Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Shanks M, Nucifora G, Smit JW, Diamant M, Romijn JA, de Roos A, Leung DY, Lamb HJ, Bax JJ. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol 2009;104:1398–1401. [DOI] [PubMed] [Google Scholar]
- 38. Vinereanu D, Nicolaides E, Tweddel AC, Madler CF, Holst B, Boden LE, Cinteza M, Rees AE, Fraser AG. Subclinical left ventricular dysfunction in asymptomatic patients with Type II diabetes mellitus, related to serum lipids and glycated haemoglobin. Clin Sci 2003;105:591–599. [DOI] [PubMed] [Google Scholar]
- 39. Ernande L, Bergerot C, Girerd N, Thibault H, Davidsen ES, Gautier Pignon-Blanc P, Amaz C, Croisille P, De Buyzere ML, Rietzschel ER, Gillebert TC, Moulin P, Altman M, Derumeaux G. Longitudinal myocardial strain alteration is associated with left ventricular remodeling in asymptomatic patients with type 2 diabetes mellitus. J Am Soc Echocardiogr 2014;27:479–488. [DOI] [PubMed] [Google Scholar]
- 40. Eschenhagen T, Force T, Ewer MS, de Keulenaer GW, Suter TM, Anker SD, Avkiran M, de Azambuja E, Balligand JL, Brutsaert DL, Condorelli G, Hansen A, Heymans S, Hill JA, Hirsch E, Hilfiker-Kleiner D, Janssens S, de Jong S, Neubauer G, Pieske B, Ponikowski P, Pirmohamed M, Rauchhaus M, Sawyer D, Sugden PH, Wojta J, Zannad F, Shah AM. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2011;13:1–10. [DOI] [PubMed] [Google Scholar]
- 41. Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, Rubino M, Veglia F, Fiorentini C, Cipolla CM. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010;55:213–220. [DOI] [PubMed] [Google Scholar]
- 42. Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol 2014;63(25 Pt A):2751–2768. [DOI] [PubMed] [Google Scholar]
- 43. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhaes A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2014;15:1063–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ersboll M, Valeur N, Mogensen UM, Andersen MJ, Moller JE, Velazquez EJ, Hassager C, Sogaard P, Kober L. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol 2013;61:2365–2373. [DOI] [PubMed] [Google Scholar]
- 45. Mignot A, Donal E, Zaroui A, Reant P, Salem A, Hamon C, Monzy S, Roudaut R, Habib G, Lafitte S. Global longitudinal strain as a major predictor of cardiac events in patients with depressed left ventricular function: a multicenter study. J Am Soc Echocardiogr 2010;23:1019–1024. [DOI] [PubMed] [Google Scholar]
- 46. Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2009;2:356–364. [DOI] [PubMed] [Google Scholar]
- 47. Haugaa KH, Grenne BL, Eek CH, Ersboll M, Valeur N, Svendsen JH, Florian A, Sjoli B, Brunvand H, Kober L, Voigt JU, Desmet W, Smiseth OA, Edvardsen T. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc Imaging 2013;6:841–850. [DOI] [PubMed] [Google Scholar]
- 48. Haugaa KH, Amlie JP, Berge KE, Leren TP, Smiseth OA, Edvardsen T. Transmural differences in myocardial contraction in long-QT syndrome: mechanical consequences of ion channel dysfunction. Circulation 2010;122:1355–1363. [DOI] [PubMed] [Google Scholar]
- 49. De Ferrari GM, Nador F, Beria G, Sala S, Lotto A, Schwartz PJ. Effect of calcium channel block on the wall motion abnormality of the idiopathic long QT syndrome. Circulation 1994;89:2126–2132. [DOI] [PubMed] [Google Scholar]
- 50. Haugaa KH, Edvardsen T, Leren TP, Gran JM, Smiseth OA, Amlie JP. Left ventricular mechanical dispersion by tissue Doppler imaging: a novel approach for identifying high-risk individuals with long QT syndrome. Eur Heart J 2009;30:330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leren IS, Hasselberg NE, Saberniak J, Haland TF, Kongsgard E, Smiseth OA, Edvardsen T, Haugaa KH. Cardiac mechanical alterations and genotype specific differences in subjects with long QT syndrome. JACC Cardiovasc Imaging 2015;8:501–510. [DOI] [PubMed] [Google Scholar]
- 52. Haugaa KH, Goebel B, Dahlslett T, Meyer K, Jung C, Lauten A, Figulla HR, Poerner TC, Edvardsen T. Risk assessment of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy by strain echocardiography. J Am Soc Echocardiogr 2012;25:667–673. [DOI] [PubMed] [Google Scholar]
- 53. Haugaa KH, Smedsrud MK, Steen T, Kongsgaard E, Loennechen JP, Skjaerpe T, Voigt JU, Willems R, Smith G, Smiseth OA, Amlie JP, Edvardsen T. Mechanical dispersion assessed by myocardial strain in patients after myocardial infarction for risk prediction of ventricular arrhythmia. JACC Cardiovasc Imaging 2010;3:247–256. [DOI] [PubMed] [Google Scholar]
- 54. Kass DA. An epidemic of dyssynchrony: but what does it mean? J Am Coll Cardiol 2008;51:12–17. [DOI] [PubMed] [Google Scholar]
- 55. Helle-Valle T, Crosby J, Edvardsen T, Lyseggen E, Amundsen BH, Smith HJ, Rosen BD, Lima JA, Torp H, Ihlen H, Smiseth OA. New noninvasive method for assessment of left ventricular rotation: speckle tracking echocardiography. Circulation 2005;112:3149–3156. [DOI] [PubMed] [Google Scholar]
- 56. Opdahl A, Remme EW, Helle-Valle T, Edvardsen T, Smiseth OA. Myocardial relaxation, restoring forces, and early-diastolic load are independent determinants of left ventricular untwisting rate. Circulation 2012;126:1441–1451. [DOI] [PubMed] [Google Scholar]
- 57. Opdahl A, Remme EW, Helle-Valle T, Lyseggen E, Vartdal T, Pettersen E, Edvardsen T, Smiseth OA. Determinants of left ventricular early-diastolic lengthening velocity: independent contributions from left ventricular relaxation, restoring forces, and lengthening load. Circulation 2009;119:2578–2586. [DOI] [PubMed] [Google Scholar]
- 58. Rademakers FE, Buchalter MB, Rogers WJ, Zerhouni EA, Weisfeldt ML, Weiss JL, Shapiro EP. Dissociation between left ventricular untwisting and filling. Accentuation by catecholamines. Circulation 1992;85:1572–1581. [DOI] [PubMed] [Google Scholar]
- 59. Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, McMurray JJ, Solomon SD, Investigators P. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol 2014;63:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kusunose K, Agarwal S, Marwick TH, Griffin BP, Popovic ZB. Decision making in asymptomatic aortic regurgitation in the era of guidelines: incremental values of resting and exercise cardiac dysfunction. Circ Cardiovasc Imaging 2014;7:352–362. [DOI] [PubMed] [Google Scholar]
- 61. Witkowski TG, Thomas JD, Debonnaire PJ, Delgado V, Hoke U, Ewe SH, Versteegh MI, Holman ER, Schalij MJ, Bax JJ, Klautz RJ, Marsan NA. Global longitudinal strain predicts left ventricular dysfunction after mitral valve repair. Eur Heart J Cardiovasc Imaging 2013;14:69–76. [DOI] [PubMed] [Google Scholar]
- 62. Yingchoncharoen T, Gibby C, Rodriguez LL, Grimm RA, Marwick TH. Association of myocardial deformation with outcome in asymptomatic aortic stenosis with normal ejection fraction. Circ Cardiovasc Imaging 2012;5:719–725. [DOI] [PubMed] [Google Scholar]
- 63. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD, American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438–2488. [DOI] [PubMed] [Google Scholar]
- 64. Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J 3rd, St John Sutton M, De Sutter J, Murillo J. Results of the predictors of response to CRT (PROSPECT) trial. Circulation 2008;117:2608–2616. [DOI] [PubMed] [Google Scholar]
- 65. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE, Guidelines ESCCfP, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, Kirchhof P, Blomstrom-Lundqvist C, Badano LP, Aliyev F, Bansch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, Le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, Van Gelder IC, Wilson CM. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013;34:2281–2329. [DOI] [PubMed] [Google Scholar]
- 66. Leenders GE, De Boeck BW, Teske AJ, Meine M, Bogaard MD, Prinzen FW, Doevendans PA, Cramer MJ. Septal rebound stretch is a strong predictor of outcome after cardiac resynchronization therapy. J Card Fail 2012;18:404–412. [DOI] [PubMed] [Google Scholar]
- 67. Marechaux S, Guiot A, Castel AL, Guyomar Y, Semichon M, Delelis F, Heuls S, Ennezat PV, Graux P, Tribouilloy C. Relationship between two-dimensional speckle-tracking septal strain and response to cardiac resynchronization therapy in patients with left ventricular dysfunction and left bundle branch block: a prospective pilot study. J Am Soc Echocardiogr 2014;27:501–511. [DOI] [PubMed] [Google Scholar]
- 68. Risum N, Tayal B, Hansen TF, Bruun NE, Jensen MT, Lauridsen TK, Saba S, Kisslo J, Gorcsan J III, Sogaard P. Identification of typical left bundle branch block contraction by strain echocardiography is additive to electrocardiography in prediction of long-term outcome after cardiac resynchronization therapy. J Am Coll Cardiol 2015;66:631–641. [DOI] [PubMed] [Google Scholar]
- 69. Khan FZ, Virdee MS, Palmer CR, Pugh PJ, O'Halloran D, Elsik M, Read PA, Begley D, Fynn SP, Dutka DP. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am Coll Cardiol 2012;59:1509–1518. [DOI] [PubMed] [Google Scholar]
- 70. Flachskampf FA, Biering-Sørensen T, Solomon SD, Duvernoy O, Bjerner T, Smiseth OA. Cardiac imaging to evaluate left ventricular diastolic function: state-of-the-art. J Am Coll Cardiol Cardiovasc Imaging 2015;8:1071–1093. [DOI] [PubMed] [Google Scholar]
- 71. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 72. Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr 2011;12:167–205. [DOI] [PubMed] [Google Scholar]
- 73. Wakami K, Ohte N, Asada K, Fukuta H, Goto T, Mukai S, Narita H, Kimura G. Correlation between left ventricular end-diastolic pressure and peak left atrial wall strain during left ventricular systole. J Am Soc Echocardiogr 2009;22:847–851. [DOI] [PubMed] [Google Scholar]
- 74. Cameli M, Lisi M, Mondillo S, Padeletti M, Ballo P, Tsioulpas C, Bernazzali S, Maccherini M. Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound 2010;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Farsalinos KE, Daraban A, Ünlü S, Thomas JD, Badano LP, Voigt JU. Head-to-head comparison of global longitudinal strain measurements among nine different vendors. J Am Soc Echocardiogr 2015;Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 76. Nagata Y, Takeuchi M, Mizukoshi K, Wu VC, Lin FC, Negishi K, Nakatani S, Otsuji Y. Intervendor variability of two-dimensional strain using vendor-specific and vendor-independent software. J Am Soc Echocardiogr 2015;28:630–641. [DOI] [PubMed] [Google Scholar]