Abstract
Aims
Coronary plaque characteristics are associated with ischaemia. Differences in plaque volumes and composition may explain the discordance between coronary stenosis severity and ischaemia. We evaluated the association between coronary stenosis severity, plaque characteristics, coronary computed tomography angiography (CTA)-derived fractional flow reserve (FFRCT), and lesion-specific ischaemia identified by FFR in a substudy of the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps).
Methods and results
Coronary CTA stenosis, plaque volumes, FFRCT, and FFR were assessed in 484 vessels from 254 patients. Stenosis >50% was considered obstructive. Plaque volumes (non-calcified plaque [NCP], low-density NCP [LD-NCP], and calcified plaque [CP]) were quantified using semi-automated software. Optimal thresholds of quantitative plaque variables were defined by area under the receiver-operating characteristics curve (AUC) analysis. Ischaemia was defined by FFR or FFRCT ≤0.80. Plaque volumes were inversely related to FFR irrespective of stenosis severity. Relative risk (95% confidence interval) for prediction of ischaemia for stenosis >50%, NCP ≥185 mm3, LD-NCP ≥30 mm3, CP ≥9 mm3, and FFRCT ≤0.80 were 5.0 (3.0–8.3), 3.7 (2.4–5.6), 4.6 (2.9–7.4), 1.4 (1.0–2.0), and 13.6 (8.4–21.9), respectively. Low-density NCP predicted ischaemia independent of other plaque characteristics. Low-density NCP and FFRCT yielded diagnostic improvement over stenosis assessment with AUCs increasing from 0.71 by stenosis >50% to 0.79 and 0.90 when adding LD-NCP ≥30 mm3 and LD-NCP ≥30 mm3 + FFRCT ≤0.80, respectively.
Conclusion
Stenosis severity, plaque characteristics, and FFRCT predict lesion-specific ischaemia. Plaque assessment and FFRCT provide improved discrimination of ischaemia compared with stenosis assessment alone.
Keywords: Coronary plaque, Computed tomography angiography, Computational fluid dynamics, Fractional flow reserve, Ischaemia
See page 1228 for the editorial comment on this article (doi:10.1093/eurheartj/ehv748)
Introduction
Traditionally, the presence of severe coronary stenosis has been interpreted as indicative of myocardial ischaemia. However, it is increasingly recognized that disconnect between stenosis severity and the presence of ischaemia is common. Approximately half of obstructive lesions by coronary computed tomography angiography (CTA) or invasive coronary angiography (ICA) cause ischaemia.1,2 On the other hand, also non-obstructive lesions may be ischaemia-causing.3–5 Recently, it has been demonstrated by coronary CTA, applying elaborate manual segmentation, and by intravascular ultrasound, that atherosclerotic plaque characteristics, such as necrotic core, spotty calcification, or positive remodelling, are associated with the presence of ischaemia independent of the degree of luminal stenosis.5–10 Therefore, composition of coronary atherosclerotic plaques has been proposed as a potential missing link between stenosis and ischaemia.11
Fractional flow reserve (FFR) derived from coronary CTA (FFRCT) is a promising non-invasive maker of coronary physiology.12–15 The diagnostic performance of FFRCT is high and superior to coronary stenosis assessment alone when compared with measured FFR. Like ICA and FFR, FFRCT is coupled with coronary CTA, and thus represents a hybrid anatomical–physiological diagnostic strategy. Moreover, coronary CTA can assess plaque burden and composition comparable with intravascular ultrasound.16 Thus, added to non-invasive, semi-automated plaque assessment, potentially allowing for rapid and reproducible segmentation, we hypothesized that non-invasive physiological assessment with FFRCT would contribute with valuable diagnostic information. Accordingly, the aim of this study was to investigate the associations between coronary stenosis severity, semi-automated assessment of atherosclerotic plaques, FFRCT, and lesion-specific ischaemia using FFR as the reference standard.
Methods
Study population
This was a pre-specified post hoc substudy comprising all patients from the HeartFlow analysis of coronary blood flow using CT angiography: NeXt sTeps (NXT) trial (NCT01757678).15,17 Patients suspected of stable coronary artery disease (CAD) were included. Coronary CTA was performed ≤60 days prior to clinically indicated non-emergent ICA. Exclusion criteria included prior stent implantation or coronary bypass surgery, contraindications to beta-blockers, nitrates or adenosine, suspicion of acute coronary syndrome, significant arrhythmia, and body mass index >35 kg/m2.15,17 The study complied with the Declaration of Helsinki. The local ethics committees approved the study protocol. All patients provided written informed consent.
Invasive coronary angiography and fractional flow reserve measurements
Angiography and FFR were performed according to standard practice.15,17 The FFR pressure-wire was positioned minimum 20 mm distal to the stenosis in vessel segments ≥2 mm. Hyperaemia was induced by intravenous adenosine (140–180 μg/kg/min). Fractional flow reserve ≤0.80 defined lesion-specific ischaemia.
Coronary computed tomography angiography acquisition
Coronary CTA was performed using CT scanners ≥64 detector rows.15,17 Beta-blockers were administered if necessary targeting a heart rate of <60 b.p.m. Sublingual nitrates were administered prior to scanning in all patients. Stenosis severity was categorized as 0, 1–29, 30–50, 51–70, 71–90, 91–99, or 100% in coronary segments ≥2 mm by experienced local investigators.18 Coronary stenosis >50% was considered obstructive.
Coronary plaque analysis
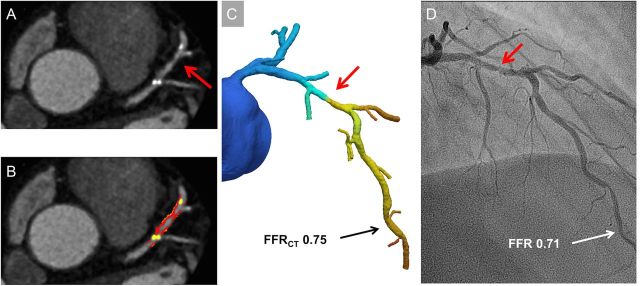
Coronary segments ≥2 mm with plaque were analysed using semi-automated software (AutoPlaq version 9.7, Cedars-Sinai Medical Center, Los Angeles, CA, USA). Two experienced readers (S.G. and K.A.Ø.) blinded to the coronary CTA readings, FFRCT, and FFR results performed the analyses using multiplanar coronary CTA images. Scan-specific thresholds for non-calcified plaque (NCP) and calcified plaque (CP) were automatically generated.16 Plaque components were quantified within the manually designated area using adaptive algorithms.16 Adjustments were made if necessary. Aggregate plaque volume (APV %) was computed as (total plaque volume/vessel volume)*100%.19 Low-density non-CP (LD-NCP) was defined as plaque with attenuation <30 Hounsfield units. Remodelling index was calculated as maximum lesion vessel area/area of a proximal normal reference point.19 Positive remodelling was defined by remodelling index >1.1.5 Spotty calcification was visually identified as calcifications comprising <90° of the vessel circumference and <3 mm in length.5 Plaque analysis was performed on a per-vessel basis (detailed description provided in Supplementary Material). A case example is shown in Figure 1.
Figure 1.
Case example. (A) Multiplanar reconstruction showing 50–70% stenosis (red arrow) in the left anterior descending artery. (B) Plaque analysis of the proximal portion of left anterior descending artery: non-calcified plaque 201 mm3 (red plus orange), low-density non-calcified plaque 35 mm3 (orange), and calcified plaque 41 mm3 (yellow). Total per-vessel plaque volumes: non-calcified plaque 454 mm3, low-density non-calcified plaque 85 mm3, and calcified plaque 50 mm3. (C) Fractional flow reserve derived from coronary computed tomography angiography in the distal left anterior descending artery was 0.75. (D) Invasive coronary angiogram with a 60% stenosis in the proximal portion of left anterior descending artery (red arrow). Measured fractional flow reserve was 0.71.
Computation of fractional flow reserve derived from coronary computed tomography angiography
Computation of FFRCT was performed centrally (HeartFlow, Inc., Redwood City, CA, USA) by independent blinded analysts (software version 1.4). The FFRCT computation process has previously been described.12 FFRCT was computed throughout the coronary tree; however, only values corresponding anatomically to the measured FFR were included in the analysis.15,17 FFRCT ≤0.80 was considered diagnostic of lesion-specific ischaemia.13–15
Statistical analysis
Continuous variables are presented as means ± standard deviation (SD) or medians (interquartile range) as appropriate, and categorical variables as numbers and percentages. Data were compared using Student's t-test, one-way ANOVA, Mann–Whitney U-test, Kruskal–Wallis test, or Pearson's χ2 test as appropriate. Plaque variables were dichotomized using area under the receiver-operating characteristics curve (AUC) analysis to define the optimal thresholds for discrimination of FFR ≤0.80.20 The thresholds were validated by bootstrapping with 10 000 samples. Relative risk of ischaemia (FFR ≤0.80) in dichotomous analysis was estimated by the log-binomial regression model or the least square method as appropriate. The latter estimates were adjusted for clustering effects by robust variance estimation. Incremental discrimination of ischaemia was assessed by AUC analysis with confidence intervals (CI) adjusted for clustering effects by bootstrapping. The AUC analyses were performed for both dichotomous and continuous variables, the latter supplemented by restricted cubic spline to adjust for non-linearity.21 The ability to predict ischaemia in a new patient sample was assessed by enhanced bootstrapping.21 Models comprising increasing numbers of predictors were compared by the Wald test. The calibration of the final model was assessed by calibration-in-the-large and calibration slope.22 Interobserver variability of plaque characteristics was assessed by Bland–Altman analysis in a consecutive selection of 10% of patients. Two-sided P-values <0.05 were considered statistically significant. Statistical analyses were performed with Stata software version 12 (StataCorp, College Station, TX, USA).
Results
The study population comprised 254 patients, in whom 484 vessels were interrogated by FFR (left anterior descending artery 41%, left circumflex artery 30%, and right coronary artery 29%). Baseline characteristics of the study population have previously been described.15 In brief, mean (SD) age was 64 (10) years, 64% (162) were male, 87% (220) had intermediate (20–80%) pre-test risk by Diamond Forrester risk score, and mean (SD, range) Agatston score was 302 (468, 0–3599). Mean (SD) FFR was 0.87 (0.13). Fractional flow reserve was ≤0.80 in 100 (21%) vessels.
Relationship between coronary stenosis severity and lesion-specific ischaemia
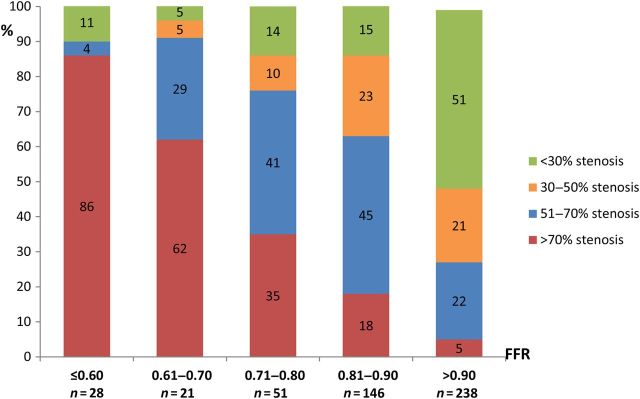
The relationship between stenosis severity and FFR is illustrated in Figure 2. Obstructive lesions were present in 239 (49%) vessels. Fractional flow reserve was ≤0.80 in 83 (35%) vessels with obstructive lesions and in 17 (7%) vessels without obstructive lesions (P < 0.001; Table 1). In the event of >50% stenosis compared with the absence of stenosis, there was a five-fold increase in vessels with FFR ≤0.80 (Table 2).
Figure 2.
Distribution of coronary stenosis severity in relation to fractional flow reserve. N = 484 vessels. Values shown are percentages within the fractional flow reserve groups, P < 0.001 for <30% stenosis, 51–70% stenosis, and >70% stenosis and P = 0.006 for the 30–50% stenosis category.
Table 1.
Plaque characteristics and FFRCT according to coronary stenosis severity and lesion-specific ischaemia (FFR ≤0.80)
| Overall |
Stenosis >50% (N = 239) | Stenosis ≤50% (N = 245) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FFR >0.80 (n = 384) | FFR ≤0.80 (n = 100) | P-value | FFR >0.80 (n = 156) | FFR ≤0.80 (n = 83) | P-value | FFR >0.80 (n = 228) | FFR ≤0.80 (n = 17) | P-value | |
| NCP, mm3 | 145 ± 144 | 265 ± 148 | <0.0001 | 210 ± 163 | 274 ± 140 | 0.003 | 101 ± 108 | 223 ± 181 | <0.0001 |
| LD-NCP, mm3 | 23 ± 27 | 54 ± 46 | <0.0001 | 35 ± 31 | 56 ± 47 | <0.0001 | 15 ± 19 | 44 ± 41 | <0.0001 |
| CP, mm3 | 23 ± 43 | 29 ± 41 | 0.011 | 31 ± 52 | 29 ± 41 | 0.832 | 17 ± 35 | 29 ± 41 | 0.040 |
| Total plaque volume, mm3 | 168 ± 170 | 294 ± 167 | <0.0001 | 241 ± 194 | 303 ± 159 | 0.014 | 117 ± 130 | 252 ± 204 | 0.0001 |
| APV, % | 46 ± 27 | 55 ± 21 | 0.002 | 55 ± 21 | 57 ± 20 | 0.454 | 40 ± 28 | 47 ± 23 | 0.333 |
| Remodelling index | 1.3 ± 0.7 | 1.5 ± 0.4 | 0.014 | 1.6 ± 0.7 | 1.6 ± 0.4 | 0.698 | 1.2 ± 0.6 | 1.3 ± 0.5 | 0.269 |
| Plaque length, mm | 25 ± 21 | 42 ± 22 | <0.0001 | 33 ± 22 | 42 ± 20 | 0.002 | 19 ± 18 | 41 ± 28 | <0.0001 |
| Spotty calcification, n (%) | 228 (59) | 67 (67) | 0.164 | 93 (59) | 54 (65) | 0.410 | 135 (59) | 13 (76) | 0.160 |
| Agatston scorea | 89 ± 173 | 123 ± 164 | 0.023 | 134 ± 227 | 128 ± 168 | 0.817 | 60 ± 117 | 92 ± 141 | 0.119 |
| FFRCT | 0.88 ± 0.07 | 0.69 ± 0.12 | <0.0001 | 0.85 ± 0.08 | 0.68 ± 0.12 | <0.0001 | 0.91 ± 0.05 | 0.74 ± 0.12 | <0.0001 |
If not otherwise stated, values are mean ± SD. N = 484 vessels.
FFRCT, fractional flow reserve derived from coronary computed tomography angiography; FFR, fractional flow reserve; NCP, non-calcified plaque; LD-NCP, low-density non-calcified plaque; CP, calcified plaque; APV, aggregate plaque volume.
aAgatston score available in 214 patients (333 vessels).
Table 2.
Univariable analyses of coronary stenosis severity, plaque characteristics, and FFRCT for prediction of lesion-specific ischaemia (FFR ≤0.80; N = 484 vessels).
| Overall |
Stenosis >50% (N = 239) | Stenosis ≤50% (N = 245) | ||||
|---|---|---|---|---|---|---|
| RR (95% CI) | P-value | RR (95% CI) | P-value | RR (95% CI) | P-value | |
| Stenosis >50% | 5.0 (3.0–8.3) | <0.001 | – | – | – | – |
| NCP ≥185 mm3 | 3.7 (2.4–5.6) | <0.001 | 2.2 (1.4–3.4) | 0.001 | 3.5 (1.3–9.2) | 0.013 |
| LD-NCP ≥30 mm3 | 4.6 (2.9–7.4) | <0.001 | 2.6 (1.7–4.1) | <0.001 | 5.7 (2.1–15.6) | 0.001 |
| CP ≥9 mm3 | 1.4 (1.0–2.0) | 0.070 | 1.0 (0.7–1.4) | 0.956 | 2.2 (0.8–6.0) | 0.117 |
| Total plaque volume ≥195 mm3 | 3.4 (2.3–5.2) | <0.001 | 2.0 (1.3–3.0) | 0.001 | 4.0 (1.5–10.7) | 0.006 |
| APV ≥50% | 1.8 (1.3–2.6) | 0.001 | 1.2 (0.9–1.8) | 0.207 | 1.8 (0.7–5.1) | 0.245 |
| Remodelling index >1.1 | 3.1 (1.4–6.6) | 0.004 | 1.7 (0.8–3.9) | 0.181 | 2.2 (0.6–7.7) | 0.224 |
| Plaque length ≥30 mm | 2.7 (1.8–4.0) | <0.001 | 1.6 (1.1–2.4) | 0.016 | 3.5 (1.3–9.7) | 0.014 |
| Spotty calcification | 1.3 (0.9–2.0) | 0.211 | 1.2 (0.8–1.7) | 0.427 | 2.1 (0.7–6.5) | 0.182 |
| FFRCT ≤0.80 | 13.6 (8.4–21.9) | <0.001 | 8.3 (4.5–15.1) | <0.001 | 17.7 (7.5–42.0) | <0.001 |
FFRCT, fractional flow reserve derived from coronary computed tomography angiography; FFR, fractional flow reserve; RR, relative risk; CI, confidence interval; NCP, non-calcified plaque; LD-NCP, low-density non-calcified plaque; CP, calcified plaque; APV, aggregate plaque volume.
Relationship between plaque characteristics and lesion-specific ischaemia
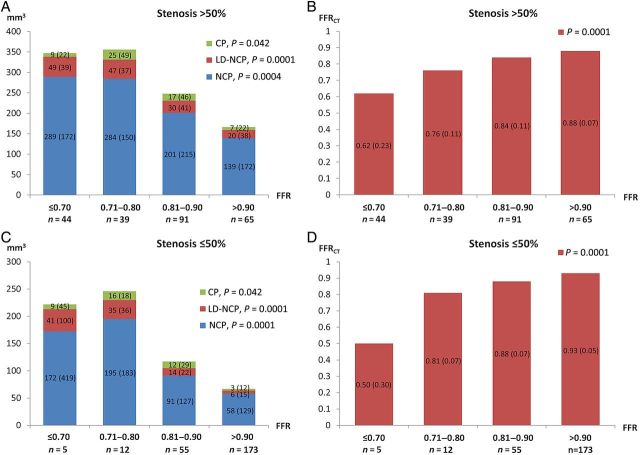
Volumes of NCP, LD-NCP, and CP were inversely related to FFR in both vessels with and without obstructive lesions (Figure 3). Table 1 summarizes the different qualitative and quantitative plaque characteristics in relation to the presence or absence of coronary stenosis and FFR ≤0.80. The optimal thresholds for detection of FFR ≤0.80 for different plaque characteristics are provided in Table 2. Irrespective of stenosis severity, LD-NCP volume ≥30 mm3, NCP volume ≥185 mm3, total plaque volume ≥195 mm3, and plaque length ≥30 mm predicted FFR ≤0.80 (Table 2). Low-density NCP volume ≥30 mm3 predicted ischaemia independent of other plaque characteristics (Table 3).
Figure 3.
Distribution of coronary plaque volumes (A + C) and fractional flow reserve derived from coronary computed tomography angiography values (B + D) in relation to fractional flow reserve. N = 484 vessels. Values shown are medians (interquartile range).
Table 3.
Multivariable analysis of coronary plaque characteristics for prediction of lesion-specific ischaemia (FFR ≤0.80; N = 484 vessels)
| RR (95% CI) adjusted for age and gender | P-value | |
|---|---|---|
| NCP ≥185 mm3 | 1.2 (0.6–2.5) | 0.610 |
| LD-NCP ≥30 mm3 | 4.3 (2.0–9.2) | <0.001 |
| Total plaque volume ≥195 mm3 | 0.9 (0.4–2.1) | 0.834 |
| APV ≥50% | 1.0 (0.7–1.5) | 0.861 |
| Remodelling index >1.1 | 1.5 (0.7–3.5) | 0.295 |
| Plaque length ≥30 mm | 0.8 (0.5–1.3) | 0.298 |
FFR, fractional flow reserve; RR, relative risk; CI, confidence interval; NCP, non-calcified plaque; LD-NCP, low-density non-calcified plaque; APV, aggregate plaque volume.
There was good interobserver agreement in plaque analysis results (see Supplementary material, Figure S1).
Relationship between fractional flow reserve derived from coronary computed tomography angiography and lesion-specific ischaemia
There was a positive relationship between FFRCT and FFR both in vessels with and without obstructive lesions (Figure 3). Mean (SD) FFRCT was 0.84 (0.11), and FFRCT was ≤0.80 in 135 (28%) vessels. Mean FFRCT according to the presence or absence of coronary stenosis and FFR is given in Table 1. Irrespective of stenosis severity, FFRCT ≤0.80 was associated with the presence of ischaemia (Tables 1 and 2).
Combined assessment of coronary stenosis severity, plaque characteristics, and fractional flow reserve derived from coronary computed tomography angiography for diagnosing ischaemia
The AUCs (95% CI) for discrimination of FFR ≤0.80 were 0.71 (0.67–0.76) for coronary stenosis >50%, 0.73 (0.67–0.78) for LD-NCP ≥30 mm3, and 0.85 (0.82–0.89) for FFRCT ≤0.80. The addition of LD-NCP ≥30 mm3 to stenosis >50% provided incremental prediction of ischaemia, with further improvement by FFRCT ≤0.80 (Table 4). The full model was well calibrated (see Supplementary material, Figure S2). In subgroup analysis, FFRCT ≤0.80 provided incremental discrimination of ischaemia over LD-NCP in both vessels without stenosis (AUC [95% CI] 0.88 [0.79–0.98] vs. 0.71 [0.57–0.84]; P < 0.001) and in vessels with stenosis >50% (AUC 0.84 [0.79–0.89] vs. 0.66 [0.60–0.73]; P < 0.001).
Table 4.
Comparison of different models for discrimination of ischaemia (FFR ≤0.80; N = 484 vessels)
| Model | Wald test, P-value | AUC (95% CI) |
|---|---|---|
| Model 1: Stenosis >50% | Comparison with no effect, <0.001 | 0.71 (0.67–0.76) |
| Model 2: Stenosis >50% + LD-NCP ≥30 mm3 | Comparison with Model 1, <0.001 | 0.79 (0.74–0.84) |
| Model 3: Stenosis >50% + LD-NCP ≥30 mm3 + FFRCT ≤0.80 | Comparison with Model 2, <0.001 | 0.90 (0.87–0.93) |
FFR, fractional flow reserve; AUC, area under the receiver-operating characteristics curve; CI, confidence interval; LD-NCP, low-density non-calcified plaque; FFRCT, fractional flow reserve derived from coronary computed tomography angiography.
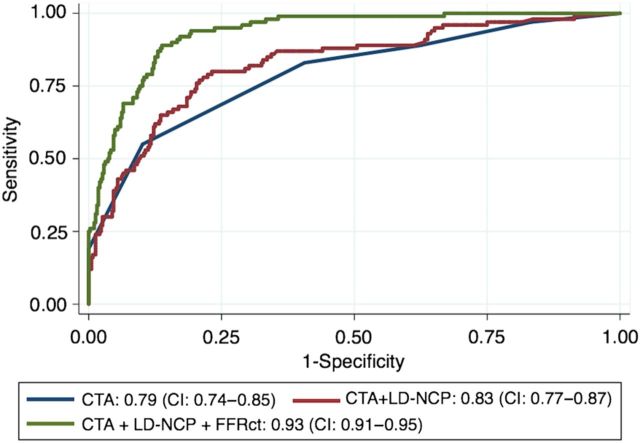
Model discrimination was modestly improved by the use of continuous variables for stenosis severity, LD-NCP volume, and FFRCT (see Supplementary material, Table S1). Applying a continuous analysis strategy, a stepwise improvement in AUC was present when information regarding LD-NCP volume and FFRCT were combined with stenosis severity (Figure 4). The addition of other plaque characteristics did not provide incremental risk prediction beyond stenosis severity and LD-NCP. The AUC of FFRCT alone (0.93 [0.91–0.95]) was not improved by the addition of stenosis severity and LD-NCP.
Figure 4.
AUCs for discrimination of fractional flow reserve ≤0.80. AUC, area under the receiver-operating characteristics curve; CI, confidence interval; CTA, stenosis severity by coronary CTA; FFRCT, fractional flow reserve derived from coronary computed tomography angiography; LD-NCP, low-density non-calcified plaque.
Discussion
In this multicentre study, we demonstrated an inverse relationship between coronary plaque volumes and lesion-specific ischaemia. Non-CP volume, plaque length, and in particular LD-NCP predicted ischaemia. These findings applied consistently to vessels with and without obstructive lesions. The assessment of LD-NCP provided incremental discrimination of ischaemia beyond stenosis severity alone, with further discrimination of ischaemia by adding information regarding FFRCT.
Previous studies have demonstrated an association between coronary atherosclerotic plaque characteristics and ischaemia.5–8 Similar to our findings, myocardial perfusion imaging studies have demonstrated an association between NCP volume, positive remodelling, LD-NCP, and ischaemia.6,8 On the other hand, a study by Naya et al. (N = 73)23 reported no significant association between plaque length, plaque composition, or remodelling index by coronary CTA and the presence of ischaemia. In a study by Nakazato et al. (N = 58),7 it was demonstrated that APV% was superior and additive to luminal narrowing for the discrimination of ischaemia. In a recent substudy of the Determination of Fractional Flow Reserve by Anatomic Computed Tomographic AngiOgraphy (DeFACTO) trial (N = 252),5 APV%, LD-NCP, lesion length, and positive remodelling predicted ischaemia. Moreover, in contrast to the findings in this study, increasing numbers of adverse plaque characteristics were associated with improved prediction of ischaemia. Major differences in crucial determinants of study outcomes may explain the differences in results between studies. The prior studies evaluating coronary plaque characteristics in relation to FFR5,7 investigated plaques located upstream from the measured FFR point. Plaque analysis in this study included all coronary segments ≥2 mm. This strategy appears clinically relevant, since evaluation of coronary CTA is independent of the location of a hypothetical FFR sensor. Moreover, plaques localized downstream from the FFR sensor location may contribute to the induction of ischaemia. In contrast to previous studies, we provided optimal thresholds for quantitative plaque characteristics in order to increase clinical applicability of study results. Finally, the use of a semi-automated plaque segmentation algorithm potentially allows for rapid segmentation in a fashion that could conceivably be performed clinically with excellent correlation with intravascular ultrasound.16
Our finding of a continuous relationship between plaque volumes and FFR, irrespective of the presence or absence of obstructive disease, indicates that the presence of coronary plaques per se is associated with ischaemia. In accordance with previous findings,1–5 we found that although stenosis >50% was a predictor of FFR ≤0.80, ischaemia was present in 7% of vessels without obstructive lesions, and 24% of the vessels with FFR 0.71–0.80 had no obstructive lesions. Moreover, the finding in this study of LD-NCP providing incremental discrimination of ischaemia beyond stenosis severity is in accordance with previous results.5,8 Local impaired function of the coronary endothelium caused by the presence of high LD-NCP volume is a potential explanation for the mismatch between stenosis severity and ischaemia.11 Low-density NCP is the coronary CTA surrogate for the presence of necrotic core.8 Plaques with necrotic cores harbour abundant oxidative stress and local inflammation, and may compromise production and bioavailability of the vasodilator nitric oxide and increase levels of vasoconstrictors such as isoprostanes.11,24 This can lead to local endothelial dysfunction, which may cause a focal ‘functional stenosis’ with inability of the vessel segment to dilate adequately during stress.25 Moreover, plaques with necrotic cores are the main cause of myocardial infarction and sudden cardiovascular death.26–28 Findings in this and other studies of an association between the presence of LD-NCP and ischaemia may explain why revascularization may be safely deferred in the absence of FFR ≤0.80 even in lesions with severe stenosis.29
Over the past decades, an optimal non-invasive imaging modality combining anatomy and physiology with the ability to serve as a ‘one-stop shop’ for the diagnosis of ischaemia and gatekeeping to ICA has been requested.30 FFRCT, a novel clinical tool for non-invasive and reproducible computation of FFR from standard coronary CTA,12,31 has been evaluated in three studies using FFR as the reference standard.13–15 The most recent NXT trial performed with refined FFRCT technology demonstrated superior per-patient and per-vessel discrimination of ischaemia of FFRCT when compared with coronary CTA stenosis assessment.15,17 Moreover, it was recently demonstrated that a diagnostic strategy comprising FFRCT vs. standard practice before ICA reduces the number of subsequent ICA and the proportion of unnecessary ICA examinations without influencing the short-term clinical outcome.32 The present study adds to these studies by demonstrating that FFRCT provides incremental discrimination of lesion-specific ischaemia beyond stenosis severity and plaque assessment. In contrast to our findings, a recently published substudy of the DeFACTO trial reported improved discrimination of ischaemia by adding plaque characteristics to stenosis severity and FFRCT.9 However, the DeFACTO study was conducted with an earlier generation FFRCT analysis algorithm than in the NXT trial. Moreover, in DeFACTO, in contrast to the present trial, pre-scan administration of beta-blockers and nitroglycerine was not administered in a substantial number of patients which adversely affected CT image quality with a corresponding increase in differences between FFRCT and measured FFR.33
Our findings suggest that a comprehensive anatomical–physiological approach combining coronary CTA anatomical stenosis assessment with semi-automated quantification of plaque volumes and FFRCT computation may be a valuable strategy for non-invasive assessment of stable CAD and potentially efficient gatekeeping to the catheterization laboratory. In addition, the results in this study indicate that coronary CTA plaque assessment, by a simple and reproducible metric such as LD-NCP volume, may be beneficial for selection of patients for further diagnostic testing.
Limitations
We did not confirm plaque findings by intravascular ultrasound. However, plaque assessment by coronary CTA has been shown to highly correlate with the findings by intravascular ultrasound.16 The relationship between stenosis severity and plaque characteristics is dose-dependent, and thus, collinearity may exist. However, coexistence of various plaque features is likely to represent CAD at high risk of producing ischeamia.5 The pre-specified selection criteria for inclusion in this study resulted in a higher proportion of patients with obstructive CAD than in a non-selected coronary CTA population.15,17 The thresholds for plaque characteristics were generated from the present study data. Optimal thresholds may differ in populations with lower prevalence of disease. Patients with acute coronary syndromes or previous revascularization were excluded in this study. Thus, generalizability of results to these patient categories needs further delineation.
Conclusions
In patients suspected of CAD, coronary stenosis severity, plaque characteristics, and FFRCT predict lesion-specific ischaemia. The addition of coronary atherosclerotic plaque and FFRCT assessment improve the discrimination of ischaemia compared with stenosis evaluation alone.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors' contributions
S.G., K.A.Ø.: performed statistical analysis; S.G., K.A.Ø., H.E.B., J.M.J., E.H.C, A.K.K., H.B., J.F.L., B.S.K., B.L.I.: acquired the data; H.E.B., S.A., J.F.L., B.L.N.: handled funding and supervision; S.G., K.A.Ø., D.D., J.L., H.E.B., J.M.J., D.S.B., J.N., A.A., B.L.N.: conceived and designed the research; S.G., K.A.Ø., B.L.N.: drafted the manuscript; D.D., J.L., H.E.B., J.M.J., E.H.C., J.N., A.A., S.A., B.S.K., A.K.K., J.F.L., D.S.B., B.L.N.: made critical revision of the manuscript for key intellectual content.
Funding
Funding to pay the Open Access publication charges for this article was provided by HeartFlow, Inc.
Conflict of interest: S.A. has received grants from Siemens Healthcare and Abbott Vascular. D.D. is partially supported by grants from Diane & Guilford Glazer Cardiac Imaging Research Fund and the Cardiac Imaging Research Initiative (Adelson Medical Research Foundation). D.D. and D.S.B. have received royalties for software licencing from Cedars-Sinai Medical Center and have a patent. J.L. serves as a consultant for GE Healthcare and HeartFlow. J.N. has received non-financial support from Philips Healthcare, GE Healthcare, and Panasonic Healthcare.
Supplementary Material
Acknowledgements
We thank Prof. Erik Thorlund Parner, PhD, Section for Biostatistics, Department of Public Health, Aarhus University, Denmark, for invaluable statistical assistance.
References
- 1. Meijboom WB, Van Mieghem CA, van Pelt N, Weustink A, Pugliese F, Mollet NR, Boersma E, Regar E, van Geuns RJ, de Jaegere PJ, Serruys PW, Krestin GP, de Feyter PJ. Comprehensive assessment of coronary artery stenoses: computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol 2008;52:636–643. [DOI] [PubMed] [Google Scholar]
- 2. Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O'Rourke RA, Dada M, Spertus JA, Chaitman BR, Friedman J, Slomka P, Heller GV, Germano G, Gosselin G, Berger P, Kostuk WJ, Schwartz RG, Knudtson M, Veledar E, Bates ER, McCallister B, Teo KK, Boden WE, COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008;117:1283–1291. [DOI] [PubMed] [Google Scholar]
- 3. Curzen N, Rana O, Nicholas Z, Golledge P, Zaman A, Oldroyd K, Hanratty C, Banning A, Wheatcroft S, Hobson A, Chitkara K, Hildick-Smith D, McKenzie D, Calver A, Dimitrov BD, Corbett S. Does routine pressure wire assessment influence management strategy at coronary angiography for diagnosis of chest pain? The RIPCORD study. Circ Cardiovasc Interv 2014;7:248–255. [DOI] [PubMed] [Google Scholar]
- 4. Schuijf JD, Wijns W, Jukema JW, Atsma DE, de Roos A, Lamb HJ, Stokkel MP, Dibbets-Schneider P, Decramer I, De Bondt P, van der Wall EE, Vanhoenacker PK, Bax JJ. Relationship between noninvasive coronary angiography with multi-slice computed tomography and myocardial perfusion imaging. J Am Coll Cardiol 2006;48:2508–2514. [DOI] [PubMed] [Google Scholar]
- 5. Park HB, Heo R, O Hartaigh B, Cho I, Gransar H, Nakazato R, Leipsic J, Mancini GB, Koo BK, Otake H, Budoff MJ, Berman DS, Erglis A, Chang HJ, Min JK. Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: a direct comparison to fractional flow reserve. JACC Cardiovasc Imaging 2015;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bauer RW, Thilo C, Chiaramida SA, Vogl TJ, Costello P, Schoepf UJ. Noncalcified atherosclerotic plaque burden at coronary CT angiography: a better predictor of ischemia at stress myocardial perfusion imaging than calcium score and stenosis severity. AJR Am J Roentgenol 2009;193:410–418. [DOI] [PubMed] [Google Scholar]
- 7. Nakazato R, Shalev A, Doh JH, Koo BK, Gransar H, Gomez MJ, Leipsic J, Park HB, Berman DS, Min JK. Aggregate plaque volume by coronary computed tomography angiography is superior and incremental to luminal narrowing for diagnosis of ischemic lesions of intermediate stenosis severity. J Am Coll Cardiol 2013;62:460–467. [DOI] [PubMed] [Google Scholar]
- 8. Shmilovich H, Cheng VY, Tamarappoo BK, Dey D, Nakazato R, Gransar H, Thomson LE, Hayes SW, Friedman JD, Germano G, Slomka PJ, Berman DS. Vulnerable plaque features on coronary CT angiography as markers of inducible regional myocardial hypoperfusion from severe coronary artery stenoses. Atherosclerosis 2011;219:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakazato R, Park HB, Gransar H, Leipsic JA, Budoff MJ, Mancini GB, Erglis A, Berman DS, Min JK. Additive diagnostic value of atherosclerotic plaque characteristics to non-invasive FFR for identification of lesions causing ischaemia: results from a prospective international multicentre trial. EuroIntervention 2015;11 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10. Sakurai S, Takashima H, Waseda K, Gosho M, Kurita A, Ando H, Maeda K, Suzuki A, Fujimoto M, Amano T. Influence of plaque characteristics on fractional flow reserve for coronary lesions with intermediate to obstructive stenosis: insights from integrated-backscatter intravascular ultrasound analysis. Int J Cardiovasc Imaging 2015;31:1295–1301. [DOI] [PubMed] [Google Scholar]
- 11. Ahmadi A, Kini A, Narula J. Discordance between ischemia and stenosis, or PINSS and NIPSS: are we ready for new vocabulary? JACC Cardiovasc Imaging 2015;8:111–114. [DOI] [PubMed] [Google Scholar]
- 12. Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol 2013;61:2233–2241. [DOI] [PubMed] [Google Scholar]
- 13. Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, Dunning A, DeFrance T, Lansky A, Leipsic J, Min JK. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol 2011;58:1989–1997. [DOI] [PubMed] [Google Scholar]
- 14. Min JK, Leipsic J, Pencina MJ, Berman DS, Koo BK, van Mieghem C, Erglis A, Lin FY, Dunning AM, Apruzzese P, Budoff MJ, Cole JH, Jaffer FA, Leon MB, Malpeso J, Mancini GB, Park SJ, Schwartz RS, Shaw LJ, Mauri L. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 2012;308:1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Norgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, Jensen JM, Mauri L, De Bruyne B, Bezerra H, Osawa K, Marwan M, Naber C, Erglis A, Park SJ, Christiansen EH, Kaltoft A, Lassen JF, Botker HE, Achenbach S, NXT Trial Study Group. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol 2014;63:1145–1155. [DOI] [PubMed] [Google Scholar]
- 16. Dey D, Schepis T, Marwan M, Slomka PJ, Berman DS, Achenbach S. Automated three-dimensional quantification of noncalcified coronary plaque from coronary CT angiography: comparison with intravascular US. Radiology 2010;257:516–522. [DOI] [PubMed] [Google Scholar]
- 17. Gaur S, Achenbach S, Leipsic J, Mauri L, Bezerra HG, Jensen JM, Botker HE, Lassen JF, Norgaard BL. Rationale and design of the HeartFlowNXT (HeartFlow analysis of coronary blood flow using CT angiography: NeXt sTeps) study. J Cardiovasc Comput Tomogr 2013;7:279–288. [DOI] [PubMed] [Google Scholar]
- 18. Raff GL, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ, Cheng V, DeFrance T, Hellinger JC, Karlsberg RP, Society of Cardiovascular Computed Tomography. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr 2009;3:122–136. [DOI] [PubMed] [Google Scholar]
- 19. Dey D, Achenbach S, Schuhbaeck A, Pflederer T, Nakazato R, Slomka PJ, Berman DS, Marwan M. Comparison of quantitative atherosclerotic plaque burden from coronary CT angiography in patients with first acute coronary syndrome and stable coronary artery disease. J Cardiovasc Comput Tomogr 2014;8:368–374. [DOI] [PubMed] [Google Scholar]
- 20. Greiner M, Sohr D, Gobel P. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J Immunol Methods 1995;185:123–132. [DOI] [PubMed] [Google Scholar]
- 21. Harrell FE., Jr Regression Modeling Strategies. 1st ed New York: Springer Series in Statistics; 2001. [Google Scholar]
- 22. Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J 2014;35:1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Naya M, Murthy VL, Blankstein R, Sitek A, Hainer J, Foster C, Gaber M, Fantony JM, Dorbala S, Di Carli MF. Quantitative relationship between the extent and morphology of coronary atherosclerotic plaque and downstream myocardial perfusion. J Am Coll Cardiol 2011;58:1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lavi S, Yang EH, Prasad A, Mathew V, Barsness GW, Rihal CS, Lerman LO, Lerman A. The interaction between coronary endothelial dysfunction, local oxidative stress, and endogenous nitric oxide in humans. Hypertension 2008;51:127–133. [DOI] [PubMed] [Google Scholar]
- 25. Ahmadi N, Ruiz-Garcia J, Hajsadeghi F, Azen S, Mack W, Hodis H, Lerman A. Impaired coronary artery distensibility is an endothelium-dependent process and is associated with vulnerable plaque composition. Clin Physiol Funct Imaging 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 26. Narula J, Nakano M, Virmani R, Kolodgie FD, Petersen R, Newcomb R, Malik S, Fuster V, Finn AV. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J Am Coll Cardiol 2013;61:1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW, PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226–235. [DOI] [PubMed] [Google Scholar]
- 28. Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, Naruse H, Ishii J, Hishida H, Wong ND, Virmani R, Kondo T, Ozaki Y, Narula J. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54:49–57. [DOI] [PubMed] [Google Scholar]
- 29. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van't Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF, FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213–224. [DOI] [PubMed] [Google Scholar]
- 30. Patel MR. Detecting obstructive coronary disease with CT angiography and noninvasive fractional flow reserve. JAMA 2012;308:1269–1270. [DOI] [PubMed] [Google Scholar]
- 31. Gaur S, Bezerra HG, Lassen JF, Christiansen EH, Tanaka K, Jensen JM, Oldroyd KG, Leipsic J, Achenbach S, Kaltoft AK, Botker HE, Norgaard BL. Fractional flow reserve derived from coronary CT angiography: variation of repeated analyses. J Cardiovasc Comput Tomogr 2014;8:307–314. [DOI] [PubMed] [Google Scholar]
- 32. Douglas PS, Pontone G, Hlatky MA, Patel MR, Norgaard BL, Byrne RA, Curzen N, Purcell I, Gutberlet M, Rioufol G, Hink U, Schuchlenz HW, Feuchtner G, Gilard M, Andreini D, Jensen JM, Hadamitzky M, Chiswell K, Cyr D, Wilk A, Wang F, Rogers C, De Bruyne B, PLATFORM Investigators. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFRCT: outcome and resource impacts study. Eur Heart J 2015;36:3359–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leipsic J, Yang TH, Thompson A, Koo BK, Mancini GB, Taylor C, Budoff MJ, Park HB, Berman DS, Min JK. CT angiography (CTA) and diagnostic performance of noninvasive fractional flow reserve: results from the Determination of Fractional Flow Reserve by Anatomic CTA (DeFACTO) study. AJR Am J Roentgenol 2014;202:989–994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.