Abstract
Objective. RA is associated with a 50–60% increase in risk of cardiovascular (CV) death. This study aimed to compare management of CV risk factors in RA and matched non-RA patients.
Methods. A retrospective cohort study was conducted using UK clinical practice data. Patients presenting with an incident RA diagnosis were matched 1:4 to non-RA patients based on a propensity score for RA, entry year, CV risk category and treatment received at index date (date of RA diagnosis). Patients tested and treated for CV risk factors as well as those attaining CV risk factor management goals were evaluated in both groups.
Results. Between 1987 and 2010, 24 859 RA patients were identified and matched to 87 304 non-RA patients. At index date, groups had similar baseline characteristics. Annual blood pressure, lipids and diabetes-related testing were similar in both groups, although CRP and ESR were higher in RA patients at diagnosis and decreased over time. RA patients prescribed antihypertensives increased from 38.2% at diagnosis to 45.7% at 5 years, from 14.0 to 20.6% for lipid-lowering treatments and from 5.1 to 6.4% for antidiabetics. Similar treatment percentages were observed in non-RA patients, although slightly lower for antihypertensives. Modest (2%) but significantly lower attainment of lipid and diabetes goals at 1 year was observed in RA patients.
Conclusion. There were no differences between groups in the frequency of testing and treatment of CV risk factors. Higher CV risk in RA patients seems unlikely to be driven by differences in traditional CV risk factor management.
Keywords: RA, cardiovascular, management, hypertension, dyslipidaemia, diabetes, goal, prescription, cohort
Rheumatology key messages
No differences were found between RA vs non-RA groups in testing or treatments for cardiovascular risk factors.
Higher cardiovascular risk in RA is not driven by the lack of cardiovascular risk factor management.
Introduction
RA is associated with increased morbidity and mortality. Meta-analyses of published literature have shown that RA is associated with a 50–60% increase in risk of CV death vs the general population [1–3]. Despite improvements in RA therapies, it appears that the mortality gap between RA patients and the general population persists, and may even be increasing [2]. Cardiovascular (CV) complications are the primary cause for this increase in mortality and also contribute to increased risk for CV events in RA patients compared with the non-RA population [4, 5].
The pathogenic mechanisms underlying increased CV risk in RA have yet to be elucidated. There is emerging evidence to suggest that traditional CV risk factors do not fully account for the increased likelihood of CV complications in RA, and the immune dysregulation, chronic high-grade inflammation and metabolic disturbances found in RA, along with RA disease activity and treatments such as corticosteroids, contribute to CV risk in RA patients [6–9]. Investigators have found that several treatment regimens for RA reduce the risk of CV events [10]. Long-term use of DMARDs may modify atherosclerosis via beneficial effects on endothelial function as well as inflammatory markers [11]. With regard to biologic DMARDs (bio-DMARDs), a meta-analysis reported that therapy with TNF-α inhibitors was associated with a reduced risk of all CV events, myocardial infarction and stroke in cohort studies [12]. A meta-analysis of randomized controlled trials also produced a point estimate indicating a lower risk of CV events with TNF-α inhibitor therapies, but this was not statistically significant [13].
Although the literature on CV risk in RA patients is extensive, there are a few limitations. The current literature does not fully address how the traditional CV risk factors of hypertension, lipids, weight and haemoglobin A1c (HbA1c) are managed in RA patients in comparison to the general population. Thus, the literature fails to inform whether the increased risk of CV events observed in RA patients could partly be due to worse CV risk management. Some studies indicate a lack of screening for CV risk factors by the rheumatologist (vs primary care providers) in RA patients and relatively low statin use among RA patients [14, 15]. However, these studies did not have a comparator non-RA group.
Although traditional CV risk factors may not fully explain the excess CV risk in RA patients, it is important to understand how these are managed in RA patients, especially with the introduction of agents such as Janus kinase inhibitors and anti-IL6 in the management of RA. These newer therapies are associated with changes in lipid levels, including increases in both low-density lipoprotein (LDL) and high-density lipoprotein (HDL) [16]. The objective of this analysis was to describe the management of traditional CV risk factors, such as lipids, blood pressure and HbA1c, in RA patients in clinical practice settings and compare this management with that of matched non-RA patients.
Methods
Study population and design
This was a retrospective cohort analysis based on the UK clinical practice database from 1987 to 2011. The database was obtained from the Clinical Practice Research Datalink (CPRD), which contains information on resources managed by general practitioners. It is one of the largest computerized databases of anonymized longitudinal medical records from primary care. The current CPRD data set includes information on around 5 million currently active patients of research standard quality from about 590 primary care practices in the UK, representing ∼8% of the UK population. The CPRD population is representative of the general UK population. Selected laboratory data are available for a subset of patients. These mainly concern CV and diabetes-related laboratory data. The CPRD has been granted multiple research ethics committee approval (05/MRE04/87) to undertake purely observational studies, with external data linkages including Hospital Episode Statistics (HES) and Office for National Statistics (ONS) mortality data. The work of CPRD is also covered by National Information Governance Board (NIGB)-Ethics and Confidentiality Committee (ECC) approval ECC 5-05 (a) 2012. This study was approved by the Independent Scientific Advisory Committee for Medicines and Healthcare products Regulatory (MHRA) database research (ISAC) under protocol number 12_079Ra.
The study population included all adult patients (age ⩾18 years) recorded in the CPRD database with records of sufficient quality, identified through the acceptable patient flag. The RA population was defined as all patients presenting at least one RA read code after 1 January 1988 (index code), with no RA or juvenile RA codes before the index code. The read codes for RA were those that were included in group 1 or 2 of Thomas et al. [17], and the list of codes was also validated by clinical experts. The index date was defined as the date of the first RA-related clinical or referral record, that is, index code. Patients were required to have at least 12 months of follow-up before the index date.
In the CPRD database, medical diagnoses and events were identified through read codes, and medcodes were used for treatments. Lists of codes were constructed to define covariates and CV risk factors, based upon most recent values within 2 years preceding the index date. In order to create the code list for each condition, published lists of codes were used and supplemented by additional searches of the medical and product browsers. The list was then screened by an analyst in order to exclude all non-relevant codes and then a second screening was conducted by a person with medical qualification with expertise in read codes. Laboratory values were identified and calculated at the index date for baseline and updated for each subsequent year after the index date considering the most recent value, within 2 years of the date of interest. The CV risk as defined by the National Cholesterol Education Program (NCEP, 2002) is composed of four categories of low, medium, high and secondary prevention, by summing the following risk factors: dyslipidaemia (LDL ⩾4 mmol/l or HDL ⩽1 mmol/l), hypertension (>140/90 mmHg), age (>45 years for males and >55 years for females) and current smoker [18]. If patients had none of the risk factors they were considered low risk, one risk factor was medium risk and more than one risk factor was high risk. Patients with diabetes, heart disease, a history of a CV event or a history of a CV procedure were considered the target group for secondary prevention.
Matched non-RA cohort
RA patients were matched 1:4 to non-RA patients based on their year of entry in the CPRD database, CV risk category (NCEP classification), treatment status at index date and a propensity score estimating the probability of having RA [18]. The propensity score probability was based on a logistic regression model that included gender, smoking, obesity, Charlson Co-morbidity Index and family history of RA as covariates. Each RA patient was categorized into low, medium or high CV risk at the index date. CV risk categories and treatment status for CV risk were calculated for all non-RA patients every 6 months. Non-RA patients were selected as potential matches based on the CV risk category and treatment status of the closest cut-off to the case’s index date. Potential matches were also required to have entered the CPRD database during the same year as the case and to have an activity in the database within 2 months of the case’s index date. Based on the pool of potential matches, each RA patient was matched to a non-RA patient using the nearest neighbour match method of its RA risk score, and consequently removed from the pool of potential non-RA matches. This matching based on a risk score was performed in order to select patients with similar characteristics with the exception of RA diagnosis, thereby reducing bias due to confounding variables. An index date was assigned to the non-RA patient based on the closest observation date to the RA patient’s index date, and the match was confirmed based on the recalculated non-RA patient’s CV risk category at the assigned index date. The process was repeated to match a maximum of four controls to each case. Standardized differences were used to compare the measured baseline characteristics between the RA and the non-RA populations. A standardized difference of <0.1 will be considered indicative of good balance [17].
Outcomes
Management of traditional CV risk factors was evaluated in terms of the percentage of patients evaluated for CV risk factors, the percentage of patients receiving treatment with lipid-lowering therapies, antihypertensive therapies and antidiabetic therapies at index and up to 5 years after the index date. In addition, we evaluated the percentage of patients attaining blood pressure, lipids and HbA1c goals annually up to 5 years post index date. The goals were based on UK clinical guidelines; for blood pressure the goal was <140/90 mmHg, for dyslipidaemia the goal was either an LDL cholesterol of <2 mmol/l or total cholesterol of <4 mmol/l, and for diabetes the goal was HbA1c <7.5%.
Statistical analyses
Baseline characteristics between RA and matched non-RA patients were compared using standard statistical tests and standardized differences. Statistical analysis involved comparing between RA and matched non-RA patients the percentage of patients evaluated for CV risk factors, treated for CV risk factors and attaining CV risk factor management goals at baseline and at 5 years post baseline. Evaluation of CV risks was based on comparing the mean number of CV risk factor lab values (such as HDL-C, LDL-C, Total Cholesterol, Triglyceride, Diastolic BP, Systolic BP, CRP, ESR, HbA1c, Fast Glucose) during each year between the RA cohort and the matched non-RA cohort. The proportion of patients attaining blood pressure, lipid and HbA1c goals at yearly intervals up to 5 years was compared between RA and matched non-RA patients using χ2 statistics. The analysis of attainment of goals for blood pressure, lipids and HbA1c was based on a last observation carried forward approach. No additional adjustments besides imputation for missing values on laboratory tests using last observation carried forward were made because the matching was successful. The analysis was carried out using the statistical software SAS 9.2. Graphs were plotted using Excel 2007. An α level of 0.05 was used for determination of statistical significance.
Results
Between 1987 and 2010, there were 24 859 patients with RA who were identified in the CPRD data set with a mean (s.d.) follow-up of 5.8 (4.4) years, representing 144 342 patient-years. Each RA patient was matched to four non-RA patients (n = 87 304) with a mean (s.d.) follow-up of 5.7 (4.4) years. At the time of RA diagnosis, the mean (s.d.) age for the RA cohort was 60.0 (15.1) years, and 69.2% were females. In terms of CV risk stratification, 20% of RA patients were categorized as secondary prevention; 30% of the primary prevention patients were high risk, 31% were medium risk and 19% low risk. The RA and non-RA patients received similar baseline therapies to treat hypertension, diabetes or dyslipidaemia (41.8 vs 40.7%). Overall, the RA and the non-RA cohort were well matched on the baseline age and baseline CV risks. Despite statistically significant differences, the effect size is very marginal as confirmed by standardized differences close to 0. The incremental difference in Charlson Co-morbidity Index between RA and non-RA patients (1.1) can be explained by the Charlson Co-morbidity Index calculation itself, because RA activity is counted as a co-morbidity (Table 1).
Table 1.
Summary of baseline characteristics
| Characteristics | RA patients (n = 24 859) | Non-RA patients (n = 87 304) | P-values | d |
|---|---|---|---|---|
| Number of patient-years, sum | 144 342 | 494 938 | ||
| Follow-up, mean (s.d.), years | 5.8 (4.4) | 5.7 (4.4) | <0.0001 | 0.023 |
| Agea, mean (s.d.), years | 60.0 (15.1) | 60.2 (15.9) | 0.071 | −0.013 |
| Charlson Co-morbidity Indexa, mean (s.d.) | 1.4 (0.9) | 0.3 (0.9) | <0.0001 | 1.222 |
| Females, n (%) | 17 202 (69.2) | 57 939 (66.4) | <0.0001 | 0.060 |
| Obesityb, n (%) | 2880 (11.6) | 9530 (10.9) | 0.003 | 0.022 |
| Current smokerb, n (%) | 6977 (28.1) | 24 122 (27.6) | 0.175 | 0.011 |
| Hypertensionc, n (%) | 9798 (39.4) | 33 382 (38.2) | 0.001 | 0.025 |
| Dyslipidaemiac, n (%) | 6761 (27.2) | 24 202 (27.7) | 0.103 | −0.011 |
| Diabetesc, n (%) | 1742 (7.0) | 7012 (8.0) | <0.0001 | −0.038 |
| Treatment status, n (%) | 10 393 (41.8) | 35 530 (40.7) | 0.027 | 0.022 |
| Cardiovascular risk category (NCEP algorithm), n (%) | <.0001 | 0.000 | ||
| Low | 4788 (19.3) | 18 169 (20.8) | ||
| Medium | 7683 (30.9) | 24 650 (28.2) | ||
| High | 7461 (30.0) | 25 322 (29.0) | ||
| Secondary prevention | 4927 (19.8) | 19 163 (21.9) |
aAge and Charlson Co-morbidity Index were evaluated at time of index date.
bObesity, current smoker: based on read codes of the 2 years preceding the index date.
cHypertension, dyslipidaemia and diabetes: based on diagnoses read codes, prescriptions and tests of the 2 years preceding index date.
d: standardized difference; NCEP: National Cholesterol Education Program.
The reporting of laboratory tests was similar in both groups overall, although CRP and ESR were reported more often in RA vs non-RA patients (33.9 vs 5.8% and 47.0 vs 12.2%, respectively, at index date). Overall, mean blood pressure, lipid and diabetes-related laboratory test results were stable and similar in the RA and non-RA cohorts over time since diagnosis, although CRP and ESR were higher in RA patients at diagnosis, decreasing over time [average (s.d.) from 24.6 mg/l (34.9) at index date to 14.8 mg/l (25.9) at 5 years for CRP, and from 31.9 mm/h (26.0) to 23.9 mm/h (20.9) for ESR; Table 2].
Table 2.
Summary of laboratory tests over time since index date, in RA and non-RA patients
| Blood test | At index date |
1 year after index |
3 years after index |
5 years after index |
||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Mean (s.d.) | n (%) | Mean (s.d.) | n (%) | Mean (s.d.) | n (%) | Mean (s.d.) | |
| RA patients (n = 24 859) | ||||||||
| HDL-C, mmol/l | 5709 (23.0) | 1.4 (0.5) | 5378 (21.6) | 1.4 (0.5) | 4474 (18.0) | 1.5 (0.5) | 3688 (14.8) | 1.5 (0.5) |
| LDL-C, mmol/l | 4621 (18.6) | 3.0 (1.0) | 4386 (17.6) | 3.0 (1.0) | 3597 (14.5) | 3.0 (1.0) | 3030 (12.2) | 2.9 (1.0) |
| Total cholesterol, mmol/l | 8018 (32.3) | 5.2 (1.2) | 7485 (30.1) | 5.2 (1.2) | 6025 (24.2) | 5.1 (1.1) | 4815 (19.4) | 5.1 (1.1) |
| Triglyceride level, mmol/l | 5847 (23.5) | 1.6 (1.0) | 5458 (22.0) | 1.6 (1.0) | 4437 (17.8) | 1.6 (1.0) | 3661 (14.7) | 1.6 (0.9) |
| Diastolic BP, mmHg | 17 779 (71.5) | 79.0 (10.1) | 16 021 (64.4) | 79.0 (10.0) | 12 173 (49.0) | 79.0 (10.0) | 9157 (36.8) | 78.5 (10.0) |
| Systolic BP, mmHg | 17 779 (71.5) | 136.2 (19.2) | 16 021 (64.4) | 136.5 (18.9) | 12 173 (49.0) | 136.5 (18.9) | 9157 (36.8) | 136.3 (18.1) |
| CRP, mg/l | 8434 (33.9) | 24.6 (34.9) | 8607 (34.6) | 18.8 (29.8) | 4967 (20.0) | 18.8 (29.8) | 3647 (14.7) | 14.8 (25.9) |
| ESR, mm/h | 11 694 (47.0) | 31.9 (26.0) | 11 440 (46.0) | 27.0 (23.5) | 6912 (27.8) | 27.0 (23.5) | 5165 (20.8) | 23.9 (20.9) |
| HbA1c, % | 1448 (5.8) | 7.1 (1.5) | 1475 (5.9) | 6.9 (1.5) | 1222 (4.9) | 6.9 (1.5) | 1009 (4.1) | 7.0 (1.6) |
| FG, mmol/l | 1645 (6.6) | 5.5 (1.5) | 1515 (6.1) | 5.5 (1.7) | 1215 (4.9) | 5.5 (1.7) | 1029 (4.1) | 5.4 (1.4) |
| Non-RA patients (n = 87 304) | ||||||||
| HDL-C, mmol/l | 19 754 (22.6) | 1.4 (0.5) | 18 791 (21.5) | 1.4 (0.5) | 16 092 (18.4) | 1.5 (0.5) | 13 113 (15.0) | 1.5 (0.5) |
| LDL-C, mmol/l | 16 340 (18.7) | 3.0 (1.0) | 15 558 (17.8) | 3.0 (1.0) | 13 282 (15.2) | 2.9 (1.1) | 10 953 (12.5) | 2.9 (1.0) |
| Total cholesterol, mmol/l | 27 177 (31.1) | 5.2 (1.2) | 25 524 (29.2) | 5.2 (1.2) | 21 041 (24.1) | 5.1 (1.2) | 16 786 (19.2) | 5.1 (1.2) |
| Triglyceride level, mmol/l | 20 462 (23.4) | 1.6 (1.0) | 19 361 (22.2) | 1.6 (1.0) | 16 251 (18.6) | 1.6 (0.9) | 13 123 (15) | 1.6 (0.9) |
| Diastolic BP, mmHg | 60 889 (69.7) | 79.3 (10.7) | 54 339 (62.2) | 78.9 (10.1) | 40 515 (46.4) | 78.9 (10.1) | 30 049 (34.4) | 78.5 (9.7) |
| Systolic BP, mmHg | 60 889 (69.7) | 136.4 (19.6) | 54 339 (62.2) | 136.4 (58.9) | 40 515 (46.4) | 136.4 (58.9) | 30 049 (34.4) | 136.4 (17.7) |
| CRP, mg/l | 5095 (5.8) | 10.6 (24.6) | 4915 (5.6) | 10.8 (25.7) | 4229 (4.8) | 10.8 (25.7) | 3482 (4) | 10.7 (25.9) |
| ESR, mm/h | 10 630 (12.2) | 17.2 (19.1) | 9986 (11.4) | 16.8 (17.2) | 7876 (9.0) | 16.8 (17.2) | 6382 (7.3) | 17.2 (17.0) |
| HbA1c, % | 5365 (6.1) | 7.2 (1.5) | 5116 (5.9) | 7.2 (1.6) | 4264 (4.9) | 7.2 (1.5) | 3479 (4.0) | 7.1 (1.5) |
| FG, mmol/l | 5730 (6.6) | 5.7 (1.8) | 5529 (6.3) | 5.7 (1.7) | 4751 (5.4) | 5.7 (1.6) | 3967 (4.5) | 5.6 (1.6) |
BP: blood pressure; FG: fasting blood glucose; HbA1c: haemoglobin A1c; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol.
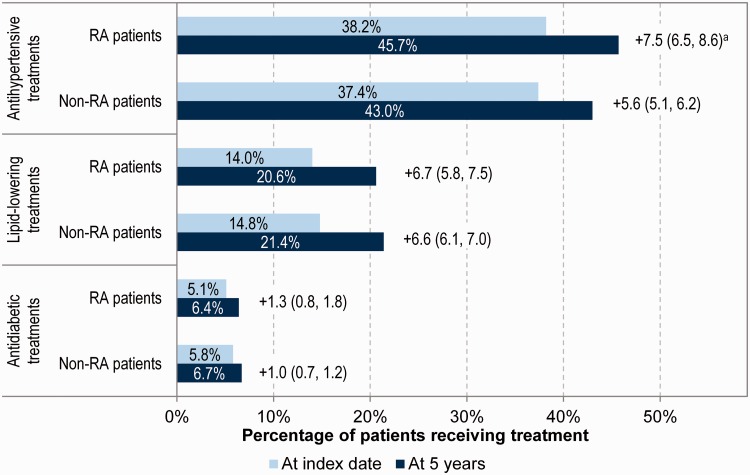
The percentage of RA patients who were prescribed treatments for hypertension, lipid-lowering therapies and diabetes at RA diagnosis was 38.2, 14.0 and 5.1%, respectively, and these values were comparable to those in the non-RA cohort (37.4, 14.8 and 5.8%). When comparing these treatment percentages between baseline and 5 years, there was a general trend towards an increased proportion of patients being managed for CV risk factors in RA and non-RA patients at 5 years (Fig. 1). The trend was slightly higher in RA patients (+7.5%, 95% CI 6.5, 8.6) than in non-RA patients (+5.6%, 95% CI 5.1, 6.2) for antihypertensive use. There was no difference in the proportion of RA patients treated for CV risk factors compared with matched non-RA patients treated for CV risk factors over the 5 year analysis based on the comparison of CIs (Fig. 1).
Fig. 1.
Summary of treatments prescribed at index date
All data are percentages. aChange in percentage of patients receiving treatment (95% CI).
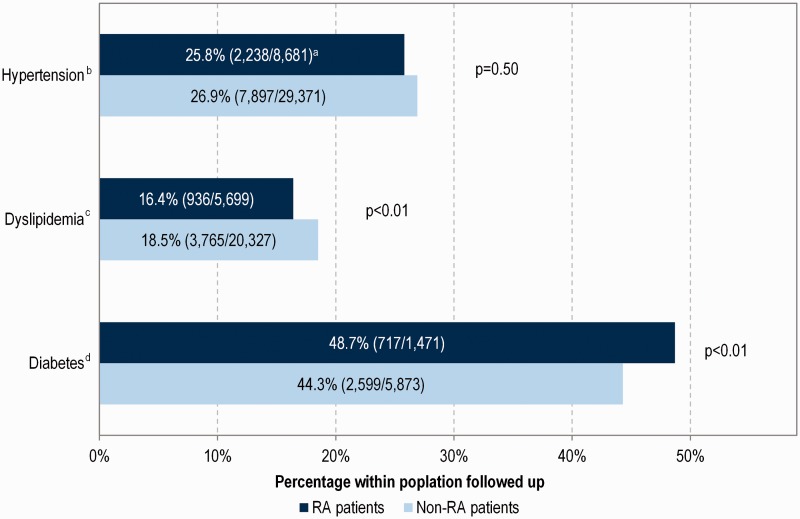
Within patients with the same risk factors at the index date, there was no difference between RA and non-RA patients reaching goals for hypertension at 1 year (Fig. 2; P = 0.50) although small but significant differences were found for dyslipidaemia and diabetes (16.4 vs 18.5%, P < 0.01 for lipid goals in RA and non-RA patients, respectively, and 48.7 vs 44.3%, P < 0.01 for HbA1c goals).
Fig. 2.
Summary of patients reaching blood pressure, lipids and haemoglobin A1c goals at 1 year post index date
aPercentage (number of patients reaching target level/number of patients followed up). bHypertension target levels: blood pressure <140/90 mmHg. cDyslipidaemia target levels: low-density lipoprotein cholesterol <2 mmol/l or total cholesterol <4 mmol/l. dDiabetes target levels: haemoglobin A1c <7.5%.
Discussion
This study is the first to describe the management of CV risk factors of RA patients in a UK clinical practice setting and compare it with matched non-RA patients. Given that the risk of a CV event is dependent on multiple factors, its management is dictated by the evaluation of these risk factors and the determination of overall CV risk. Thus, it is important to control for the baseline CV risk when evaluating the management of CV risk factors. To our knowledge, this is the only study to have matched RA patients to non-RA patients on baseline CV risks when evaluating the management of CV risks factors in RA patients. Given the large number of patients included in the study and the average follow-up of >5 years, we believe that the study population and its management of CV risk factors is representative of general RA patients seen in a clinical practice setting.
No meaningful differences were found between RA and non-RA patients in terms of the frequency of CV risk factor evaluation and treatments at baseline. However, there were more patients managed for hypertension in the RA-cohort vs the non-RA cohort at 5 years, and there was a modest difference of 2% fewer patients in the RA cohort reaching lipid goals. The evidence for hypertension being more prevalent in RA patients is mixed, because reports are contradictory and dependent on the definition of hypertension used in the analysis [19]. However, results from a recent study indicate a significantly increased prevalence of hypertension in RA patients vs controls and would support our findings [20]. Our study also found that RA patients experienced a modest absolute 2% lower achievement in lipid goals. Studies of lipid levels in RA are inconclusive and seem to be influenced by the duration of RA and treatment for RA. Studies conducted prior to RA diagnosis seem to indicate that RA patients have low HDL vs matched controls [21]. However, other studies have shown that RA patients have lower LDL and total cholesterol levels and, in spite of these lower levels, there is a paradoxical increase in CV risk [22, 23]. Our study was not designed to compare the lipid levels between RA and non-RA patients. Given that we matched patients on baseline CV risk factors, we could not observe a difference in baseline lipid levels in our cohort. However, in spite of the similarity in testing and treatment for lipid lowering between RA and non-RA patients, we found that attainment of lipid goals was modestly lower in RA patients. Further studies would need to be conducted to confirm our observation and its relevance. Studying the patterns of lipid management in RA patients is important because new therapies introduced to manage RA have been associated with increasing lipid levels [17].
Some of our findings are contrary to those reported in the literature on management of CV risk factors in RA patients. A recent study concluded that RA patients were less likely to be diagnosed with hypertension than patients without RA [24]. Another study found that one-third of eligible patients lacked appropriate lipid testing despite the presence of traditional CV risk factors [25], whereas others concluded that the health-care quality in RA appears to be suboptimal for co-morbid disease [26]. We postulate that the difference between our findings and those from other studies can be explained partly by the difference in methodology and the settings of the studies. Firstly, as stated previously, we controlled for baseline CV risk in our study via matching, which was not done in the other studies. Secondly, our study was conducted in the general practice setting vs rheumatologist setting, because there is evidence to support that rheumatologists identify and manage CV risk factors significantly less frequently in RA patients when compared with primary care providers [26]. Thirdly, it is the only study based on data from the UK, whereas the other studies were based on US data.
Our general finding that there is no substantial difference in the evaluation, treatment and attainment of CV risk factor goals indicates that the higher incidence of CV events among RA patients observed in our data (CV event incidence rate in our data set of 4.29, 95% CI 4.15, 4.44 in RA patients vs 3.11, 95% CI 3.04, 3.17 in non-RA patients per 100 patient-years; 8.97% of RA patients and 6.97% of non-RA primary prevention patients had a CV event over a 5 year follow-up) may not be driven by poor management of traditional CV risk factors alone. This finding indirectly supports the literature indicating that there might be other factors contributing to increased CV events in RA patients. Although traditional risk factors are known to play an important role in the general population, their relative contributions to CV risk in RA is less clear [19]. Moreover, there is evidence that the increased CV risk in RA patients might not be explained by traditional risk factors alone [27]. The mechanisms underlying increased CV events in RA have yet to be elucidated fully. However, there is emerging evidence to suggest that the immune dysregulation, chronic high-grade inflammation and metabolic disturbances found in RA patients could contribute to the increased CV events in RA patients [6–9]. The increase in CV risk in RA patients is acknowledge by the consensus guidelines for the management of RA, which recommend the evaluation for CV risk at baseline using a traditional CV risk algorithm, such as Framingham Heart Study-based algorithm or the MONIC Study-based SCORE algorithm. The Dutch guidelines recommend the application of CV risk management (CVRM) in RA patients, because RA is considered as an independent risk factor for CV disease. A recent study showed that CVRM guidelines performed poorly in RA patients, with an overall increase in 10 year CV risk despite implemention of CVRM [28]. Given that these algorithms were not developed in the RA-specific population, and to account for the increased CV risk in RA patients vs the general population, the EULAR guidelines recommend that the risk calculated, using these algorithms, should be multiplied by a factor of 1.5.
There were several limitations to our analysis. Our study was retrospective, implying the potential presence of some sources of bias due to confounding factors. However, this was partly accounted for by propensity score matching of patients on both their RA and CV risk profiles. Only data from a general practice setting were available, and no data from rheumatologists were available. This could impact the identification of RA patients into our study, which is based on read codes in groups 1 and 2 of Thomas et al. [17]. This list of codes did not include seronegative for RF RA codes and had a high sensitivity (93%) but a relative low specificity (49%); thus, there could be some false positives in our case cohort [15]. In addition, the present analysis used the NCEP guidelines, which were cited by the British Society of Cardiology and in effect during the time when these patients were managed for their CV risk, that is, before 2011. The NCEP guidelines were used to categorize the RA into different CV risk categories, which were later used to match patients. The results could be different if the new ACC/AHA guidelines were used to match patients. However, as the new guidelines were not in place when these patients were evaluated and managed for CV risk, it would not be appropriate to use the new guidelines in this analysis. There were also a large number of missing values for laboratory test data. The method of last observation carried forward was used to handle missing data after the index date. Other imputation methods were also investigated, such as the simple mean imputation approach, producing similar results. Finally, only prescription data were available, which are known to differ from dispensed or taken medications.
Despite limitations, the study was based on data with both a large sample size and a good follow-up period. The results are generalizable to the UK population and are representative of its clinical practice, because the study was based on the CPRD general practice database, which covers a large proportion of the UK population.
Conclusion
There were no differences observed between RA and non-RA patients in terms of CV risk management and testing, although RA patients had experienced a modest 2% lower achievement of lipid goals, which may not be clinically meaningful. Based on this analysis, it seems that the higher CV risk in patients with RA is unlikely to be driven by differences in traditional CV risk factor management alone. A focus should be placed on CV risk stratification of RA patients and studies to determine how best to tailor management of CV risk to the different risk groups.
Acknowledgements
This study was supported by Bristol-Myers Squibb. Bristol-Myers Squibb contracted MAPI for this study. Editorial assistance was provided by MAPI. K.P.L. and D.H.S. received no funding from Bristol-Myers Squibb for their efforts on this paper. K.P.L. is supported by the NIH K08 AR 060257. E.A. is a PhD student at Erasmus University Rotterdam, supervised by M.P.M.H.R.M. and M.A.
Funding: This study and the present communication were financially supported by Bristol-Myers Squibb Company (Princeton, NJ, USA).
Disclosure statement: F.B. and H.C. are employees of MAPI. D.H.S. is supported by research grants to Brigham and Women’s Hospital by Amgen, Lilly and Pfizer. He serves in unpaid capacities on a Pfizer-sponsored trial on an unrelated topic. He receives royalties from UpToDate on unrelated topics. E.A. is a shareholder and employee of Bristol-Myers Squibb Company and a PhD student at Erasmus University Rotterdam, supervised by M.P.M.H.R.M and M.A. All other authors have declared no conflicts of interest.
References
- 1.Aviña-Zubieta JA, Choi HK, Sadatsafavi M. et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 2008;59:1690–7 [DOI] [PubMed] [Google Scholar]
- 2.Kaplan MJ. Cardiovascular complications of rheumatoid arthritis: assessment, prevention, and treatment. Rheum Dis Clin North Am 2010;36:405–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meune C, Touzé E, Trinquart L, Allanore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology 2009;48:1309–13 [DOI] [PubMed] [Google Scholar]
- 4.Goodson N, Symmons D. Rheumatoid arthritis in women: still associated with an increased mortality. Ann Rheum Dis 2002;61:955–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riise T, Jacobsen BK, Gran JT, Haga HJ, Arnesen E. Total mortality is increased in rheumatoid arthritis. A 17-year prospective study. Clin Rheumatol 2001;20:123–7 [DOI] [PubMed] [Google Scholar]
- 6.Solomon DH, Goodson NJ, Katz JN. et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis 2006;65:1608–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.del Rincón ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 2001;44:2737–45 [DOI] [PubMed] [Google Scholar]
- 8.Aubry MC, Maradit-Kremers H, Reinalda MS. et al. Differences in atherosclerotic coronary heart disease between subjects with and without rheumatoid arthritis. J Rheumatol 2007;34:937–42 [PubMed] [Google Scholar]
- 9.Aviña-Zubieta JA, Abrahamowicz M, De Vera MA. et al. Immediate and past cumulative effects of oral glucocorticoids on the risk of acute myocardial infarction in rheumatoid arthritis: a population-based study. Rheumatology 2013;52:68–75 [DOI] [PubMed] [Google Scholar]
- 10.Kremers HM, Crowson CS, Therneau TM, Roger VL, Gabriel SE. High ten-year risk of cardiovascular disease in newly diagnosed rheumatoid arthritis patients: a population-based cohort study. Arthritis Rheum 2008;58:2268–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naranjo A, Sokka T, Descalzo MA. et al. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008;10:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnabe C, Martin BJ, Ghali WA. Systematic review and meta-analysis: anti-tumor necrosis factor α therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res 2011;63:522–9 [DOI] [PubMed] [Google Scholar]
- 13.Westlake SL, Colebatch AN, Baird J. et al. Tumour necrosis factor antagonists and the risk of cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology 2011;50:518–31 [DOI] [PubMed] [Google Scholar]
- 14.Bartels CM, Kind AJ, Everett C. et al. Low frequency of primary lipid screening among Medicare patients with rheumatoid arthritis. Arthritis Rheum 2011;63:1221–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toms TE, Panoulas VF, Kitas GD. Dyslipidaemia in rheumatological autoimmune diseases. Open Cardiovasc Med J 2011;5:64–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Souto A, Salgado E, Maneiro JR. et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: a systematic review and meta-analysis. Arthritis Rheumatol 2015;67:117–27 [DOI] [PubMed] [Google Scholar]
- 17.Thomas SL, Edwards CJ, Smeeth L, Cooper C, Hall AJ. How accurate are diagnoses for rheumatoid arthritis and juvenile idiopathic arthritis in the general practice research database? Arthritis Rheum 2008;59:1314–21. [DOI] [PubMed] [Google Scholar]
- 18.Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421 [PubMed] [Google Scholar]
- 19.Liao KP, Solomon DH. Traditional cardiovascular risk factors, inflammation and cardiovascular risk in rheumatoid arthritis. Rheumatology 2013;52:45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung CP, Giles JT, Petri M. et al. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum 2012;41:535–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi HK, Seeger JD. Lipid profiles among US elderly with untreated rheumatoid arthritis–the Third National Health and Nutrition Examination Survey. J Rheumatol 2005;32:2311–6 [PubMed] [Google Scholar]
- 22.Gabriel SE, Crowson CS. Risk factors for cardiovascular disease in rheumatoid arthritis. Curr Opin Rheumatol 2012;24:171–6 [DOI] [PubMed] [Google Scholar]
- 23.Liao KP, Cai T, Gainer VS. et al. Lipid and lipoprotein levels and trend in rheumatoid arthritis compared to the general population. Arthritis Care Res 2013;65:2046–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartels CM, Johnson H, Voelker K. et al. Impact of rheumatoid arthritis on receiving a diagnosis of hypertension among patients with regular primary care. Arthritis Care Res 2014;66:1281–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartels CM, Kind AJ, Thorpe CT. et al. Lipid testing in patients with rheumatoid arthritis and key cardiovascular-related comorbidities: a medicare analysis. Semin Arthritis Rheum 2012;42:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLean CH, Louie R, Leake B. et al. Quality of care for patients with rheumatoid arthritis. JAMA 2000;284:984–92 [DOI] [PubMed] [Google Scholar]
- 27.Peters MJ, Symmons DP, McCarey D. et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 2010;69:325–31 [DOI] [PubMed] [Google Scholar]
- 28.Heslinga M. et al. Is cardiovascular risk management in patients with rheumatoid arthritis effective? Two year follow up reveals unexpected rise in cardiovascular risk!. Ann Rheum Dis 2015;74(Suppl 2):192 [Google Scholar]