Abstract
Renal cell carcinoma (RCC), the most common type of kidney cancer in adult, rarely metastasizes to the ovary or fallopian tube, and most cases published in the literature were case reports. Herein, we describe the clinicopathologic features of 9 cases of RCC metastatic to the ovary (n=8) or the fallopian tube (n=1). The patients’ age at the onset of primary renal tumor was available in 8 patients, ranging from 37 to 73 years (mean, 51 years; median, 50 years). Ovarian metastasis was detected prior to or concurrently with the primary renal tumors in 3 patients, and after the diagnosis of renal tumors in 6 patients. The histotypes of the RCCs were clear cell (n=7), chromophobe (n=1), and unclassified (n=1). Immunohistochemical stainings were performed on the sections containing metastatic tumors in 4 cases. Interestingly, pagetoid intraepithelial spread in the tubal mucosa was observed in the case of RCC metastatic to the fallopian tube. Among the 8 patients with follow-up data, 5 patients died of disease and 3 were alive with disease with a follow-up period ranging from 3.7 months to 17 years (mean, 77 months; median, 53 months) after the diagnosis of primary kidney tumors. Diagnostically, metastatic RCC may mimic primary ovarian tumors clinically, morphologically or immunophenotypically. Pathologists should also keep in mind that both ovarian and kidney tumors express PAX8 and PAX2, the markers commonly used to diagnosis metastatic RCC. In addition, chromophobe RCC only rarely metastasizes, but it can be a diagnosis challenging when metastasizing to the ovary.
Keywords: Metastasis, Renal cell carcinoma, Secondary ovarian tumor
1. Introduction
Secondary ovarian tumors, extra-ovarian tumors that spread to the ovary, represent 15% to 20% of ovarian tumors [1, 2]. They may be diagnosed prior to, concurrently with, or after the diagnosis of the primary tumors [3]. Common extra-ovarian sites of origin of ovarian metastases include the gastrointestinal tract, breast, endocervix, and endometrium [1]. On the other hand, renal cell carcinoma (RCC) usually metastasizes to the lungs, lymph nodes, bones, brain and liver [4], and only very rarely to the ovary or fallopian tube. Most previous reports in the literature are single cases or small series of metastatic clear cell RCC [5–20], while chromophobe RCCs rarely metastasize to distant sites. In this study, we report the clinical and pathologic features of 9 cases of RCCs metastasizing to the ovary or the fallopian tube, including clear cell, chromophobe, and unclassified RCCs.
2. Materials and methods
After obtaining approval from Institutional Review Board, we searched the pathology records at The University of Texas MD Anderson Cancer Center from 1988 to 2014 and identified 9 cases of metastatic RCCs to the ovary (n = 8) or the fallopian tube (n = 1). Relevant clinical data were obtained via review of the patients’ electronic medical records, which included demographic, diagnosis, treatment, and follow-up information (updated through August 2015). Pathological reports, hematoxylin and eosin (H&E)-stained and immunohistochemical staining slides containing tissue sections of primary and metastatic RCCs were reviewed.
The following immunohistochemical stains were performed at outside institutions and were reviewed and recorded when the cases were sent to MD Anderson for consultation: actin, alpha-methylacyl CoA racemase (P504S), carbonic anhydrase, CD10, CD117, cytokeratin 5/6, cytokeratin 7 (CK7), cytokeratin 20 (CK20), E-cadherin, epithelial membrane antigen (EMA), high-molecular-weight keratin (HMWK), HMB-45, low-molecular-weight keratin (LMWK), Melan-A, pancytokeratin AE1/AE4, p53, p63, thrombomodulin, vimentin and WT-1. Immunohistochemical stains performed at our institution included AMACR (P504S) (Zeta Corporation.; clone13H4, 1:40 dilution), calretinin (Invitrogen; clone DC8, 1:120 dilution), CD10 (Novocastra; clone 56C6, 1:50 dilution), CK 5/6 (DAKO; clone D5/16B4, 1:50 dilution), CK7 (DAKO; clone OV-TL, 1:100 dilution), estrogen receptor (ER) (Leica Biosystems; clone 6F11, 1:35 dilution), inhibin (AbD Serotec; clone R1, 1:50 dilution), PAX8 (Biocare Medical; clone BC12, 1:100 dilution), PIN dual that includes HMWK and p63 (Biocare Medical; clone XM26, LL002, and BC4A4), RCC marker (DAKO; clone SPM314, 1:100 dilution), and vimentin (DAKO; clone V9, 1:900 dilution).
3. Results
The patients ranged in age from 37 to73 years (mean, 51 years; median, 50 years) at the time of diagnosis of primary kidney tumors. The clinicopathologic features of all 9 cases are summarized in Table 1.
Table 1.
Clinicopathologic features of 9 cases of renal cell carcinoma (RCC) metastatic to the ovary or fallopian tube.
| Pt | Age, y | Primary RCC
|
Ovarian or fallopian tube lesion
|
Time to metastases | Other metastases detected prior to or concurrently with ovarian metastasis | Follow-up | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | Size (cm) | FNG | Laterality | Local invasion | Margin | Location | Size (cm) | Laterality | |||||
| 1 | 73 | Clear cell RCC with sarcomatoid & rhabdoid features | 12.5 | 4 | Right | Invasion into the renal sinus, perinephric adipose tissue and renal vein | Negativea | Fallopian tube | 2.0 | Right | Synchronous | Retroperitoneal lymph nodes | DOD, 3.7 months |
| 2b | 60 | Clear cell RCC | 16.5 | 3 | Right | Invasion into the renal sinus and perinephric adipose tissue | Negative | Ovary | ~8.0 | Right | Synchronous | Bilateral adrenal glands | AWD, 40 months |
| 3 | NA | Chromophobe RCC | NA | Not applicablec | NA | NA | NA | Ovary | Right ovary 15.0; left ovary 5.5 | Bilateral | After resection of RCC | NA | NA |
| 4d | 48 | Clear cell RCC | 9.0 | 2 | Left | Invasion into renal vein | Negative | Ovary | 8.0 | Right | 14 months | None | AWD, 57 months |
| 5 | 37 | RCC, unclassified | NA | 4 | Left | NA | Negative | Ovary | NA | Bilateral | 8 months | Omentum, peritoneum & abdominal wall | AWD, 22 months |
| 6 | 45 | Clear cell RCC | 13.0 | 3 | Right | Invasion into perinephric adipose tissue and renal vein | Negative | Ovary | 7.0 | Left | 30 months | Liver | DOD, 48 months |
| 7e | 43 | Clear cell RCC | 9.0 | 3 | Right | Confined to renal parenchyma, no evidence of LVI | Negative | Ovary | 7.0 | Right | 20 months | Right psoas muscle | DOD, 109 months |
| 8 | 52 | Clear cell RCC | NA | NA | Left | NA | NA | Ovarian surface | 9.0 | Right | 10 months | Left adrenal | DOD, 11 years |
| 9 | 52 | Clear cell RCC | 7.2 | 3 | Right | NA | NA | Ovary | NA | Left | Prior to the diagnosis of RCC | None | DOD, 17 years |
Abbreviations: Pt indicates patient; y, year; FNG, Fuhrman nuclear grading; LVI, lymphovascular invasion; DOD, died of disease; AWD, alive with disease; NA, not available.
vascular margin is negative but tumor is present in lymphovascular space in the adjacent soft tissue.
Patient 2 also had a 2.8-cm mass in the renal hilum of the opposite site, which was considered to be a secondary primary tumor.
Fuhrman nuclear grade is not applicable to chromophobe RCC.
Patient 4 had a complex cystic adnexal mass with mural nodule, which was identified on both computed tomography (CT) scan and ultrasound.
Patient 7 had a cystic adnexal mass found on CT scan.
Metastases in the ovaries or fallopian tube were diagnosed prior to or concurrently with the primary kidney tumors in 3 patients (patients 1, 2, and 9). The clinical presentations of these 3 patients were described below. Patient 1 presented with fatigue, weight loss, and anemia. Computerized tomography (CT) showed a right kidney mass and retroperitoneal lymphadenopathy. During the surgery, right fallopian tube involvement was suspected. Pathologic examination confirmed that metastatic clear cell RCC involved the right fallopian tube, but no tumor was present in the right ovary. Patient 2 presented with weight loss and flank pain. The CT scan showed a large right kidney mass, a small left kidney mass, bilateral adrenal necrotic lesions, and a pelvic mass that was originally thought to be a fibroid. Pathological examination confirmed clear cell RCC metastatic to the ovary and bilateral adrenal glands. The mass in the opposite kidney was considered a second primary tumor. Patient 9 had a suspicious ovarian mass on pelvic examination during a work-up for abnormal Pap smear. Subsequently, ultrasound exam and CT scan demonstrated a left ovarian mass and a right renal mass, and clear cell RCC metastatic to the ovary was confirmed by pathological examination. In the remaining 6 patients, ovarian masses were found on restaging/follow-up CT scans after the resection of primary kidney tumors, and both primary ovarian tumors and metastatic tumors were suspected clinically. Subsequently, pathological examinations confirmed the diagnosis of metastatic RCC. The interval between detection of primary kidney tumor and ovarian metastasis ranged from 8 to 30 months (mean, 16 months; median, 14 months) for the 5 patients with available data. Several patients also had other metastases detected prior to or concurrently with ovarian metastasis, such as retroperitoneal lymph node, adrenal glands, omentum, peritoneal cavity, and liver, which was summarized in Table 1.
The primary tumors were in the left kidney in 3 patients and in the right kidney in 5 patients; the laterality was unknown in 1 patient. The kidney tumors metastasized to the ipsilateral ovary or fallopian tube in 3 patients, to the contralateral ovary in 4 patients, and to both ovaries in 2 patients (Table 1). The sizes of the primary kidney tumors, available in 6 patients, ranged from 7.2 to 16.5 cm (mean, 11 cm; median, 11 cm). Nephrectomy specimens showed invasion into the renal sinus in 2 tumors, into perinephric adipose tissue in 3 tumors, and into the renal vein in 3 tumors (all considered pT3a in RCC). The margins were free of tumor in 6 of 6 cases.
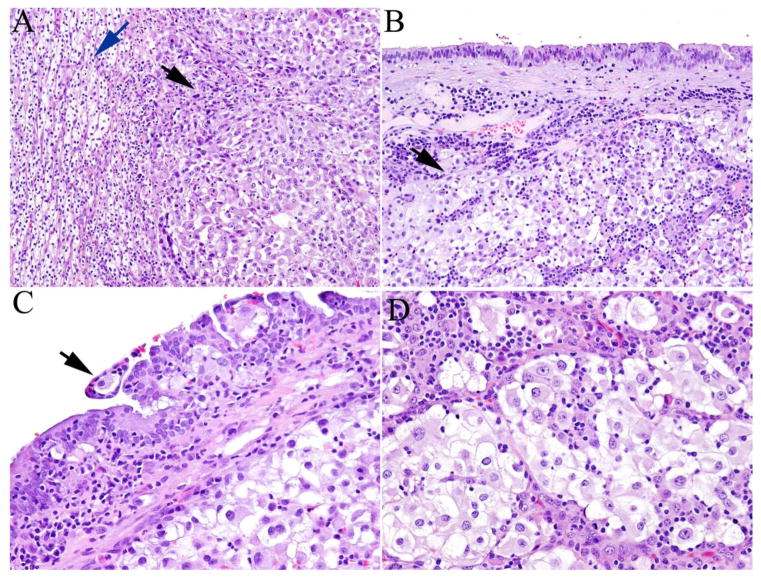
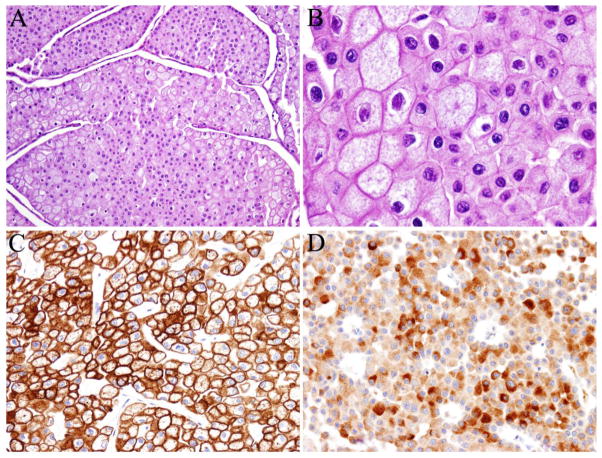
Among the 9 cases, the RCC histotypes were clear cell (n = 7), chromophobe (n = 1), and unclassified (n = 1). In general, microscopic examination of both primary and metastatic tumors showed similar morphologic features. Metastatic clear cell RCCs showed cells with clear cell cytoplasm and “chicken wire” vasculature, similar to primary RCCs. It is worth mentioning that in case 1, both primary and metastatic RCC to the fallopian tube showed clear cell RCC with sarcomatoid and rhabdoid features (Figure 1a–1d). Metastatic tumor cells grew predominantly beneath the fallopian tube mucosa, and rare foci of pagetoid spread were also identified in the tubal mucosa (Figure 1c). Case 3 showed polygonal tumor cells with “raisinoid” nuclei, perinuclear halo and distinct cell borders (Figure 2a, 2b). The tumor cells were positive for CK7 (Figure 2c), PAX8 and focally positive for RCC marker. Immunohistochemical staining for CD10 showed cytoplasmic positivity (Figure 2d). The tumor cells were negative for ER, calretinin, inhibin and vimentin. These findings were consistent with the diagnosis of metastatic chromophobe RCC. Case 5 showed a tumor with a papillary architecture, mimicking ovarian serous carcinoma. However, the tumor was immunopositive for PAX8, vimentin, P504S, P53, CK20 (focal), and negative for CK7, WT-1, PIN dual, cytokeratin 5/6, CD10 and ER, supporting the diagnosis of RCC, unclassified.
Fig. 1.
A case of clear cell renal cell carcinoma (RCC) metastatic to the fallopian tube. A, The primary renal tumor showed both areas of typical clear cell RCC (blue arrow) adjacent to areas with rhabdoid features (black arrow). B, Metastatic RCC predominantly grew beneath the fallopian tube mucosa (black arrow). C, Pagetoid intraepithelial spread of RCC in the tubal mucosa was seen (black arrow). D, High magnification showed areas with rhabdoid features resembling primary tumor (Hematoxylin and eosin stain, magnification ×100 in A and B, ×200 in C and D).
Fig. 2.
A case of chromophobe renal cell carcinoma (RCC) metastatic to the ovary. A and B, Ovarian metastasis showed polygonal cells with distinct cell borders and “raisinoid” nuclei (Hematoxylin and eosin stain, magnification ×100 in A, ×400 in B). The tumor cells were positive for cytokeratin 7 (membranous and cytoplasmic staining, C) and CD10 (cytoplasmic staining, D). (Immunohistochemical stain, magnification ×200 in C and D).
All 7 patients for whom a detailed clinical history was available underwent radical nephrectomy. Four patients also had chemotherapy. The follow-up information was available in 8 patients. Five patients died of disease and 3 were alive with disease. The follow-up period ranged from 3.7 months to 17 years (mean, 77 months; median, 53 months) after the diagnosis of primary kidney tumor. Of note, in patient 9, although ovarian metastasis was found prior to the diagnosis of RCC, the interval was very short.
4. Discussion
In the English literature, fewer than 30 cases of RCCs metastasizing to the ovary or fallopian tube have been previously reported, and most were case reports. Among the different histotypes of RCCs, clear cell carcinoma is the most common histotype metastasizing to the ovary. Other authors have reported papillary RCC spreading to the ovary [12], whereas chromophobe RCCs usually don’t metastasize. To our knowledge, our study of 9 cases is the largest case series so far, which included both clear cell, chromophobe and unclassified RCCs. Our study provides new insights into the clinicopathologic features of these tumors. Metastatic RCCs may mimic primary ovarian tumors clinically, morphologically or immunophenotypically and represent a diagnostic challenge. Several aspects of these metastatic tumors and primary tumors need to be considered in order to make a correct diagnosis:
Clinically, ovarian metastasis can be detected prior to, concurrently with, or after the resection of primary RCCs. Also, ovarian metastases can be very large in size. It may not be easy to differentiate primary ovarian neoplasms from metastatic carcinomas by clinical symptoms and radiological findings, especially when ovarian tumors present prior to the diagnosis of RCCs. Morphologically, although typically clear cell RCCs should be easily differentiated from primary ovarian clear cell carcinoma, clear cell RCCs with atypical features or other subtypes of RCCs may not be easily told apart from primary ovarian neoplasms. Ovarian clear cell carcinoma typically shows a variety of architectural patterns, including tubulocystic, solid and papillary patterns, prominent hyalinization of papillary cores, large atypical nuclei protruding into the lumen (hobnail cells), and intraluminal mucin [9]. In contrast, clear cell RCC lacks these features and shows prominent vasculature [6, 9]. However, clear cell RCCs may show rhabdoid or sarcomatoid features, as seen in one of our cases, which may cause confusion at metastatic sites, and the differential diagnosis of ovarian carcinosarcoma or even rhabdoid tumor may be entertained [9]. Chromophobe RCCs usually don’t metastasize. Therefore, metastatic chromophobe RCC to the ovary is extremely rare, and can mimic sex cord-stromal tumors (i.e., Sertoli-Leydig cell tumor, steroid cell tumor, or granulosa cell tumor). Moreover, papillary RCC can mimic ovarian serous carcinoma. Interestingly, pagetoid intra-epithelial spreading to the fallopian tube was identified in one of our cases. Previously, pagetoid spreading to the tubal mucosa has been reported in metastatic gastric cancer or neuroendocrine carcinoma [21, 22].
Metastatic clear cell RCC can be diagnosed on H&E alone if the patient has a known history of clear cell RCC. However, immunohistochemical stainings may be helpful if the clinical history is not available or the patient has a non-clear cell RCC. Pathologists should keep in mind that both ovarian and kidney neoplasms are typically positive for PAX8, PAX2. Although ovarian neoplasms are frequently positive for CK7, a subset of kidney neoplasms are also positive for CK7 (i.e., papillary RCC and chromophobe RCCs). Therefore, the above markers cannot be used to differentiate metastatic RCCs from primary ovarian tumors. The markers that may be helpful are listed below. Ovarian clear cell carcinoma is typically positive for HMWK and negative for CD10, although a small subset of ovarian clear cell carcinomas may show CD10 positivity at apical borders [23]. In contrast, clear cell RCC is typically positive for CD10 and negative for HMWK. Immunohistochemical staining for RCC marker is typically positive in clear cell RCC, but is not very specific and can also be positive in a small percentage of ovarian neoplasms [24]. Hepatocyte nuclear factor-1beta (HNF-1beta), Napsin-A, AE1/AE3, EMA, ARID1A (BAF-250a), CD15 and vimentin can be expressed in both ovarian and renal clear cell carcinoma and therefore cannot be used to tell them apart. A subset of papillary and chromophobe RCCs also express CD10 [25–27]. However, chromophobe RCC may show a cytoplasmic staining pattern for CD10, instead of membranous staining pattern typically seen in RCC [27]. Typical high grade serous carcinoma is positive for WT-1, and negative staining for WT-1 favors metastatic carcinoma (as in case 5). Immunohistochemical staining for calretinin and inhibin is also helpful to differentiate metastatic RCC from ovarian sex cord-stromal tumor. The latter is typically positive for calretinin and inhibin [28].
The renal-ovarian axis has been proposed by some authors as one of the mechanisms of RCC metastasizing to the ovary [5]. The renal-ovarian axis refers to the finding that the left ovarian vein frequently drains into the left renal vein, instead of the inferior vena cava. However, earlier studies [5–20] and our study demonstrated that RCC can metastasize to ipsilateral, contralateral, or bilateral ovaries. RCCs may spread to the ovarian or fallopian tube by hematogenous dissemination or direct invasion from primary tumor or metastatic retroperitoneal lymph nodes, or as part of peritoneal carcinomatosis.
In conclusion, pathologists should be aware that RCC can metastasize to the ovary and the fallopian tube. Even though rare, it can be a potential diagnostic pitfall, and these secondary tumors may be mistaken for primary ovarian tumors. Clinical correlation and ancillary studies described above may aid the correct diagnosis.
Table 2.
Immunohistochemical staining performed in 4 cases to confirm the diagnosis of metastatic renal cell carcinoma (RCC).
| Pt | Diagnosis | Positive markers | Negative markers |
|---|---|---|---|
| 3 | Chromophobe RCC | CK7, PAX8, CD10 (cytoplasmic), RCC (rare cells) | ER, calretinin, inhibin, vimentin |
| 4 | Clear cell RCC | EMA | CK7, CK20 |
| 5a | RCC, unclassified | PAX8, vimentin, P504S, p53, CK20 (focal) | CK7, WT-1, PIN dual (HMWK and p63), cytokeratin 5/6, CD10, ER |
| 8 | Clear cell RCC | Vimentin, LMWK | HMWK |
Abbreviations: CK7 indicates cytokeratin 7; ER, estrogen receptor; EMA, epithelial membrane antigen; CK20, cytokeratin 20; P504S, alpha-methylacyl CoA racemase; HMWK, high-molecular-weight keratin; LMWK, low-molecular-weight keratin.
In patient 5, immunohistochemical staining was performed on both primary kidney tumor (positive for pancytokeratin AE1/AE3, E-cadherin, vimentin, CK20, CD10, CD117, P504S, and carbonic anhydrase. The tumor cells were negative for CK7, thrombomodulin, actin, HMB-45, cytokeratin 5/6, WT-1, P63, and Melan-A), as well as abdominal wall masses (as shown in the table). The primary and metastatic tumors (including the ovarian tumor) showed similar morphology, and this case was diagnosed as RCC, unclassified.
Acknowledgments
We thank Sunita Patterson from the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center for editing the manuscript.
Footnotes
Conflicts of interest and funding disclosures: The authors have no conflicts of interest to declare. This work was supported in part by grant from the Cancer Prevention and Research Institute of Texas, the MD Anderson Cancer Center SPORE in Ovarian Cancer (National Institutes of Health grant), Multi-investigator Grant of Cancer Prevention and Research Institute of Texas (CPRIT), and a Sister Institution Grant from MD Anderson Cancer Center Global Academic Programs. Drs. Li Liang and Vipulkumar Dadhania are supported by the training grant T32CA163185 from NIH/NCI.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Waal YR, Thomas CM, Oei AL, Sweep FC, Massuger LF. Secondary ovarian malignancies: frequency, origin, and characteristics. Int J Gynecol Cancer. 2009;19:1160–1165. doi: 10.1111/IGC.0b013e3181b33cce. [DOI] [PubMed] [Google Scholar]
- 2.Yada-Hashimoto N, Yamamoto T, Kamiura S, et al. Metastatic ovarian tumors: a review of 64 cases. Gynecol Oncol. 2003;89:314–317. doi: 10.1016/s0090-8258(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 3.Petru E, Pickel H, Heydarfadai M, et al. Nongenital cancers metastatic to the ovary. Gynecol Oncol. 1992;44:83–86. doi: 10.1016/0090-8258(92)90017-d. [DOI] [PubMed] [Google Scholar]
- 4.Saitoh H. Distant metastasis of renal adenocarcinoma. Cancer. 1981;48:1487–1491. doi: 10.1002/1097-0142(19810915)48:6<1487::aid-cncr2820480635>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Kostrzewa M, Zyla M, Wladzinski J, Stetkiewicz T, Stachowiak G, Wilczynski JR. Metastases of renal clear cell carcinoma to ovary--case report and review of the literature. Eur J Gynaecol Oncol. 2015;36:219–222. [PubMed] [Google Scholar]
- 6.Kato Y, Numata A, Wada N, et al. A case of metastatic renal cell carcinoma to the ovary. Hinyokika Kiyo. 2006;52:923–927. [PubMed] [Google Scholar]
- 7.Bauerova L, Dundr P, Fischerova D, Pesl M, Zikan M, Burgetova A. Ovarian metastasis of clear cell renal cell carcinoma: A case report. Can Urol Assoc J. 2014;8:E188–192. doi: 10.5489/cuaj.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolanbay M, Kutuk MS, Uludag S, Ozgun MT, Ozturk F, Ozcelik B. A case of renal cell carcinoma with solitary metastasis to the ovary. J Obstet Gynaecol. 2015;35:218–219. doi: 10.3109/01443615.2014.948814. [DOI] [PubMed] [Google Scholar]
- 9.Young RH, Hart WR. Renal cell carcinoma metastatic to the ovary: a report of three cases emphasizing possible confusion with ovarian clear cell adenocarcinoma. Int J Gynecol Pathol. 1992;11:96–104. [PubMed] [Google Scholar]
- 10.Insabato L, De Rosa G, Franco R, D’Onofrio V, Di Vizio D. Ovarian metastasis from renal cell carcinoma: a report of three cases. Int J Surg Pathol. 2003;11:309–312. doi: 10.1177/106689690301100408. [DOI] [PubMed] [Google Scholar]
- 11.Guney S, Guney N, Ozcan D, Sayilgan T, Ozakin E. Ovarian metastasis of a primary renal cell carcinoma: case report and review of literature. Eur J Gynaecol Oncol. 2010;31:339–341. [PubMed] [Google Scholar]
- 12.Stolnicu S, Borda A, Radulescu D, Puscasiu L, Berger N, Nogales FF. Metastasis from papillary renal cell carcinoma masquerading as primary ovarian clear cell tumor. Pathol Res Pract. 2007;203:819–822. doi: 10.1016/j.prp.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Valappil SV, Toon PG, Anandaram PS. Ovarian metastasis from primary renal cell carcinoma: report of a case and review of literature. Gynecol Oncol. 2004;94:846–849. doi: 10.1016/j.ygyno.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 14.Hammock L, Ghorab Z, Gomez-Fernandez CR. Metastatic renal cell carcinoma to the ovary: a case report and discussion of differential diagnoses. Arch Pathol Lab Med. 2003;127:e123–126. doi: 10.5858/2003-127-e123-MRCCTT. [DOI] [PubMed] [Google Scholar]
- 15.Buller RE, Braga CA, Tanagho EA, Miller T. Renal-cell carcinoma metastatic to the ovary. A case report. J Reprod Med. 1983;28:217–220. [PubMed] [Google Scholar]
- 16.Anagnostou VK, Tiniakos DG, Chorti M, et al. Right sited renal cell carcinoma metastasizing to the contralateral ovary: case report and review of the literature. Pathol Oncol Res. 2009;15:123–127. doi: 10.1007/s12253-008-9039-7. [DOI] [PubMed] [Google Scholar]
- 17.Toquero L, Aboumarzouk OM, Abbasi Z. Renal cell carcinoma metastasis to the ovary: a case report. Cases J. 2009;2:7472. doi: 10.4076/1757-1626-2-7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Udoji E, Herts BR. Renal cell carcinoma metastatic to the ovary. J Urol. 2012;188:603–604. doi: 10.1016/j.juro.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 19.Fields S, Libson E, Lavie O, Beller U. Renal cell carcinoma metastatic to the ovary. Ultrasound and CT appearance Clin Imaging. 1996;20:42–44. doi: 10.1016/0899-7071(94)00075-1. [DOI] [PubMed] [Google Scholar]
- 20.Liu FS, Ho ES, Lu F, Chang CY. Solitary metastasis of renal cell carcinoma to the ovaries: a case report. Zhonghua Yi Xue Za Zhi (Taipei) 1992;50:165–168. [PubMed] [Google Scholar]
- 21.Roma AA. Metastatic gastric adenocarcinoma primarily presenting in the fallopian tube. Ann Diagn Pathol. 2012;16:63–66. doi: 10.1016/j.anndiagpath.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Stewart CJ, Leung YC, Whitehouse A. Fallopian tube metastases of non-gynaecological origin: a series of 20 cases emphasizing patterns of involvement including intra-epithelial spread. Histopathology. 2012;60:E106–114. doi: 10.1111/j.1365-2559.2012.04194.x. [DOI] [PubMed] [Google Scholar]
- 23.Leroy X, Farine MO, Buob D, Wacrenier A, Copin MC. Diagnostic value of cytokeratin 7, CD10 and mesothelin in distinguishing ovarian clear cell carcinoma from metastasis of renal clear cell carcinoma. Histopathology. 2007;51:874–6. doi: 10.1111/j.1365-2559.2007.02874.x. [DOI] [PubMed] [Google Scholar]
- 24.Cameron RI, Ashe P, O’Rourke DM, Foster H, McCluggage WG. A panel of immunohistochemical stains assists in the distinction between ovarian and renal clear cell carcinoma. Int J Gynecol Pathol. 2003;22:272–276. doi: 10.1097/01.PGP.0000071044.12278.43. [DOI] [PubMed] [Google Scholar]
- 25.Reuter VE, Argani P, Zhou M, Delahunt B. Best practices recommendations in the application of immunohistochemistry in the kidney tumors: report from the International Society of Urologic Pathology consensus conference. Am J Surg Pathol. 2014;38:e35–49. doi: 10.1097/PAS.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 26.Walter B, Hartmann A, Hofstadter F, et al. Immunohistochemical marker panel differentiates between the three most common subtypes of renal cell carcinoma independent from histomorphologic criteria. Virchows Arch. 2012;460:343–352. doi: 10.1007/s00428-011-1187-6. [DOI] [PubMed] [Google Scholar]
- 27.Martignoni G, Pea M, Brunelli M, et al. CD10 is expressed in a subset of chromophobe renal cell carcinomas. Mod Pathol. 2004;17:1455–1463. doi: 10.1038/modpathol.3800236. [DOI] [PubMed] [Google Scholar]
- 28.Rabban JT, Zaloudek CJ. A practical approach to immunohistochemical diagnosis of ovarian germ cell tumours and sex cord-stromal tumours. Histopathology. 2013;62:71–88. doi: 10.1111/his.12052. [DOI] [PubMed] [Google Scholar]