Abstract
SDF-1/CXCL12 is a potent chemokine required for the homing and engraftment of hematopoietic stem and progenitor cells. Previous Data from our group has shown that in an SDF-1/CXCL12 transgenic mouse model, Lineage− Sca-1+ c-Kit+ (LSK) bone marrow cells have reduced mitochondrial membrane potential versus wild-type, These results suggested that SDF-1/CXCL12 may function to keep mitochondrial respiration low in immature blood cells in the bone marrow. Low mitochondrial metabolism helps to maintain low levels of reactive oxygen species (ROS), which can influence differentiation. To test whether SDF-1/CXCL12 regulates mitochondrial metabolism, we employed the human leukemia cell line HL-60, that expresses high levels of the SDF-1/CXCL12 receptor, CXCR4, as a model of hematopoietic progenitor cells in vitro. We treated HL-60 cells with SDF-1/CXCL12 for 2 and 24 hours. Oxygen consumption rates (OCR), mitochondrial-associated ATP production, mitochondrial mass, and mitochondrial membrane potential of HL-60 cells were significantly reduced at 2 hours and increased at 24 hours as compared to untreated control cells. These biphasic effects of SDF-1/CXCL12 were reproduced with lineage negative primary mouse bone marrow cells, suggesting a novel function of SDF-1/CXCL12 in modulating mitochondrial respiration by regulating mitochondrial oxidative phosphorylation, ATP production and mitochondrial content.
Keywords: SDF-1/CXCL12, Mitochondria, Oxygen Consumption, Blood Cells
Introduction
Stromal cell-derived factor 1α (SDF-1), also known as CXCL12, is an important member of the CXC family of chemokines. SDF-1/CXCL12 is expressed in a wide array of different tissues and cell types, including immune cells, endothelial cells, stromal cells, fibroblasts, and cancer cells [1]. The gene encoding SDF-1/CXCL12 is located at 10q11.1 and has 6 exons encoding 68 amino acids. It has a molecular weight of 8 kDa, and its promoter contains binding sites for transcription factors such as SP1 [2]. Signal transduction induced by SDF-1/CXCl12 is mediated through the chemokine receptor CXCR4 [3–6]. Knockout of SDF-1/CXCL12 is perinatal lethal and mice lacking SDF-1/CXCL12 have severe defects in gastrointestinal vascularization, cerebral development, and hematopoietic defects [7–9]. CXCR4 knockout studies reveal a strikingly similar phenotype to that of SDF-1/CXCL12 knockout mice, suggesting that the SDF-1/CXCL12 and CXCR4 signaling axis is non-promiscuous [10].
SDF-1/CXCL12 is a potent chemotactic factor for hematopoietic stem (HSCs) and progenitor (HPCs) cells [11,12]. It plays an essential role in the maintenance of HSCs, including homing, engraftment and repopulating activity, as well as HSC quiescence and retention in the bone marrow [13–17]. It has been shown to enhance the survival of HSCs and HPCs, an effect increased in synergy with other cytokines [5,18–20]. Treatment of mouse bone marrow cells and human cord blood HPCs with soluble SDF-1/CXCL12 enhanced their replating efficiency, and bone marrow cells from mice expressing a human SDF-1/CXCL12 transgene exhibited increased replating capacity of single macrophage-and multipotent progenitor- derived colonies [21].
SDF-1/CXCL12 appears to be a key regulator of HSCs in the bone marrow microenvironment [22]. The niche provides signals regulating HSC functions, such as self-renewal and long term repopulating capability, as well as the ability to undergo multiline age differentiation. Several groups have shown in genetic studies that mesenchymal progenitor, endothelial, and stromal cell populations play a critical role in the maintenance of HSCs in the niche and depending on which niche cells HSCs interact with, helps to define the specific “sub-niche” in which HSCs may reside [23–30]. Deletion of SDF-1/CXCL12 from different types of niche cells leads to the reduction in HSC numbers, competitive repopulation, and increases in splenic HSCs, all of which indicate an essential role for SDF-1/CXCL12 in HSC function in the bone marrow microenvironment [23,25,27,29,30].
Despite work from several groups describing the role of SDF-1/CXCL12 in the maintenance of HSCs and HPCs in the various niches in the bone marrow [23,25,27,29,30], there is a paucity of information on the mechanism by which SDF-1/CXCL12 functions at the molecular level for immature blood cell function in the bone marrow. Regulation and restriction of mitochondrial metabolism has been shown to be critical in maintaining the quiescent state of HSCs in the bone marrow by preventing mitochondrial produced reactive oxygen species (ROS), which can promote differentiation and HSC attrition and potential dysfunction [31–36]. Recent work from our group has shown that SDF-1/CXCL12 can modulate mitochondrial activity and mitochondrial mass in murine bone marrow cells expressing a human SDF-1/CXCL12 transgene [37]. We therefore hypothesized that SDF-1/CXCL12 regulates mitochondrial respiration in early hematopoietic cells.
Materials and Methods
Oxygen Consumption Rates
Basal oxygen consumption rates (OCR) and mitochondrial-associated ATP production were obtained using the Seahorse Bioscience XF96 Extracellular Flux Analyzer from Seahorse Bioscience, and measurements were performed according to the manufacturer’s instructions and as described previously [37–39]. Mitochondrial-associated ATP production is the difference between the basal OCR and oligomycin-A repressed OCR [40].
Cell Culture and Lineage negative bone marrow cell isolation
HL-60 cells (ATCC CCL-240) were obtained from the American Type Culture Collection (Manassas, VA) and maintained in Iscove’s Modified Dulbecco’s Medium (IMDM) with 20% FBS. HL-60 cells were incubated in IMDM +20% FBS with and without 50 ng/ml SDF-1 (R&D, Minneapolis, MN) for two and 24 hours, respectively. This concentration of SDF-1 has been shown to elicit optimal responses in numerous of our assays [12,18,19,21,41]. C57Bl/6 strain mice were used to isolate lineage negative bone marrow cells. The Indiana University Committee on Use and Care of Animals approved the mouse studies. Mouse lineage negative cells were isolated using the Miltenyi Biotech (San Diego, CA) mouse Lineage Cell Depletion Kit. After lineage depletion, lineage negative cells were incubated in IMDM +10% FBS and stimulated with or without 50 ng/ml SDF-1 (R&D, Minneapolis, MN) for two and 24 hours, respectively.
Reagents and instruments
Anti-human CXCR4 APC conjugated antibody (Clone 12G5) and anti-human CXCR7 FITC conjugated antibody (Clone 358426) was from R&D, Minneapolis, Minnesota. Mitotracker Green FM and Mitotracker Red CMXRos were from Molecular Probes, Eugene, Oregon. Flow cytometry was performed with a FACS Calibre flow cytometer (BD Biosciences, Franklin Lakes, NJ). Flow cytometry data were analyzed using FlowJo (Ashland, Oregon). Oligomycin-A was purchased from Sigma-Aldrich (St. Louis, MO) and AMD3100 was a kind gift from AnorMed (Langley, BC, Canada).
Statistical analysis
Data were statistically analyzed and plotted using GraphPad Prism 6 (San Diego, CA). Differences were assessed with a 2-tailed Student t-test or one-way ANOVA with Tukey’s post-hoc correction. P values ≤0.05 were considered significant.
Results and Discussion
To investigate the potential role that SDF-1/CXCL12 plays in controlling mitochondrial respiration, we employed the human promyelocitic leukemia cell line, HL-60, as a beginning of modeling human immature subsets of hematopoietic cells and then proceeded to confirm the results in lineage− primary mouse bone marrow cells.
A) Effects on HL-60 cells
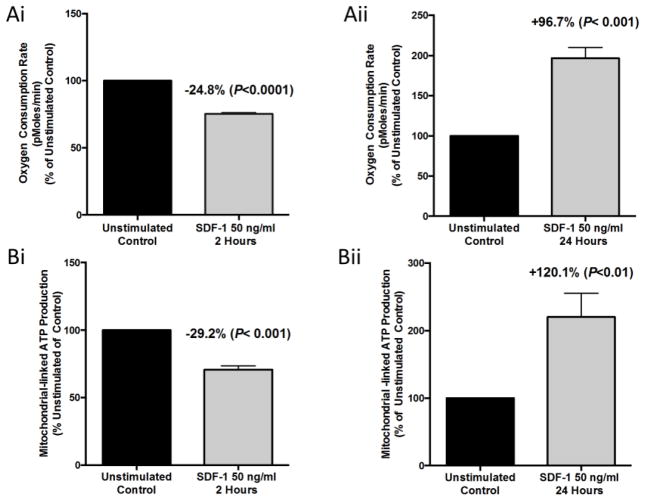
We maintained HL-60 cells in culture with IMDM +20% fetal bovine serum under standard cell culture conditions, then cultured them for two and 24 hours, respectively, with or without 50 ng/ml of SDF-1/CXCL12 to assess rapid and more delayed effects. We assessed oxygen consumption rates (OCR), a measure of mitochondrial respiration, for each group on a Seahorse Bioscience XF96 Extracellular Flux Analyzer (Seahorse XF96). Cells treated for two hours with SDF-1/CXCL12 had a significant reduction in OCR of 24.8±1.0% (mean ± SEM) as compared to two-hour unstimulated control cells (Figure 1Ai). In contrast, HL-60 cells treated for 24 hours with SDF-1/CXCL12 had a significantly increased OCR of 96.7±13.3% over unstimulated 24-hour control cells (Figure 1Aii). This suggests that SDF-1/CXCL12 plays a potential role modulating mitochondrial respiration in a bi-phasic manner as indicated by changes in mitochondrial OCR.
Figure 1. SDF-1/CXCL12 Regulates mitochondrial respiration of HL-60 cells in a biphasic manner.
The human leukemia cell line, HL-60, was treated with 50 ng/ml of SDF-1 for 2 and 24 hours respectively. After each time point, cells were collected and their oxygen consumption rates (OCR) was measured (A) on the Seahorse Bioscience Extracellular Flux Analyzer. (B) Mitochondrial-linked ATP production of SDF-1 treated HL-60 cells was also measured using the Seahorse Bioscience Extracellular Flux Analyzer. Results are the mean ± SEM of four independent experiments with significant differences as compared to unstimulated control shown in the figure.
We next analyzed mitochondrial-associated ATP production by using the Seahorse XF96 (Figure 1Bi and 1Bii). Mitochondrial-associated ATP production is the difference between the OCR and oligomycin-A (0.6 μM) repressed OCR [34]. Mitochondrial-associated ATP production was significantly reduced in the two-hour SDF-1/CXCL12 treated group compared to the unstimulated control cells (−29.2 ± 2.9%), while the 24-hour treated group was significantly increased versus control (+120.1 ± 20.3%). These results show that as OCR is reciprocally decreased or increased over time by SDF-1/CXCL12 treatment of HL-60 cells, there are closely related changes in ATP production suggesting that these processes are tightly coupled and regulated in a biphasic manner in vitro.
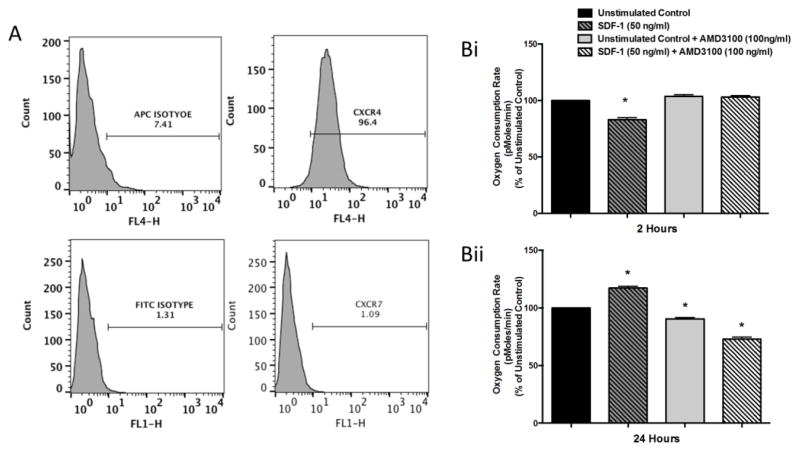
Next we sought to confirm that the changes in mitochondrial respiration and ATP production in HL-60 cells are mediated through the SDF-1/CXCL12-CXCR4 axis. We first measured surface expression of human CXCR4 and CXCR7, both receptors for SDF-1/CXCL12, using fluorochrome conjugated antibodies. (Figure 2A). Once we determined that the HL-60 cells expressed CXCR4 with minimal or no expression of CXCR7, we used the CXCR4 antagonist AMD3100 [42] to attempt to block the SDF-1/CXCL12-CXCR4 mediated changes in mitochondrial respiration. HL-60 cells were pretreated for 30 minutes with 100 ng/ml of AMD3100 and then stimulated for 2 and 24 hours, respectively, with 50 ng/ml of SDF-1/CXCL12. HL-60 cells treated with SDF-1/CXCL12 for two hours have significantly reduced OCR as compared to untreated control cells, while both the AMD3100 alone group and the SDF-1/CXCL12 + AMD3100 group were not significantly different from untreated control (Figure 2Bi). Cells treated with SDF-1/CXCL12 for 24 hours had significantly increased OCR as compared to untreated control and cells pretreated with AMD3100 alone or pretreated with AMD3100 and then stimulated with SDF-1/CXCL12 for 24 hours had significantly reduced OCR as compared to untreated control cells (Figure 2Bii). These results show that blocking CXCR4 with AMD3100 oblates the SDF-1/CXCL12 mediated biphasic changes in OCR, suggesting that these metabolic effects of SDF-1/CXCL12 are mediated through the CXCR4 receptor.
Figure 2. SDF-1/CXCL12 mediated effects on HL-60 cells are CXCR4 specific.
(A) HL-60 cell were stained with anti-hCXCR4-APC and anti-hCXCR7-FITC conjugated antibodies and the level of surface expression of each receptor was determined by flow cytometry. (B) HL-60 cells were pre-treated with 100ng/ml of the CXCR4 antagonist, AMD3100, for 30 minutes. After pre-treatment, cells were treated with 50ng/ml of SDF-1 for 2 and 24 hours respectively. After each time point, cells were collected and their oxygen consumption rates (OCR) (B) were measured on the Seahorse Bioscience Extracellular Flux Analyzer. Results are the mean ± SD of one experiment with triplicates of each group. *, p<0.05 comparing each group to unstimulated control.
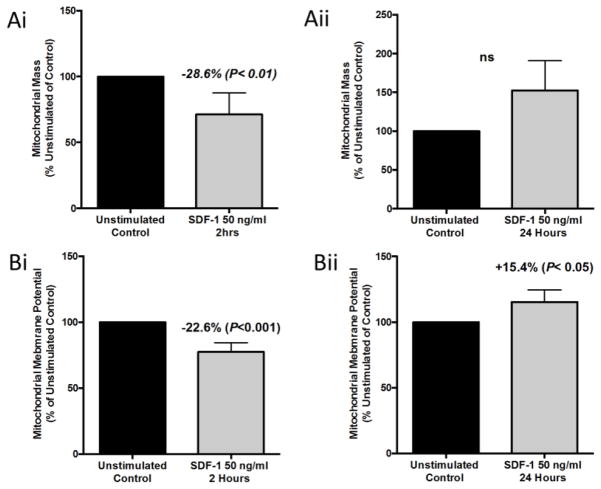
To further investigate the mechanism by which SDF-1/CXCL12 mediates changes in mitochondrial respiration, we investigated changes in mitochondrial mass and mitochondrial membrane potential (Δψm). We hypothesized that changes in mitochondrial respiration and ATP production should be accompanied by concomitant changes in mitochondrial mass and Δψm. To assess these changes we used the mitochondrial specific dyes MitoTracker Green FM (50nM) and MitoTracker Red CMXROS (50 nM). MitoTracker Green FM is a mitochondrial specific dye that is mitochondrial membrane potential independent and is an indicator of mitochondrial mass, while Mitotracker Red CMXRos is membrane potential dependent and used as an indicator of Δψm (a measure of mitochondrial electron transport chain function). HL-60 cells were cultured for two and 24 hour, respectively, with 50 ng/ml of SDF-1/CXCL12. After each time point, cells were harvested and stained with MitoTracker Green FM or MitoTracker Red CMXRos and mitochondrial mass and Δψm was measured by flow cytometry. Both the mitochondrial mass and Δψm for the two-hour SDF-1/CXCL12 stimulated group was significantly decreased versus unstimulated control (Figure 3Ai and 3Bi). There was a trend toward increased mitochondrial mass at 24 hours (Figure 3Aii). The Δψm of the 24-hour SDF-1/CXCL12 stimulated group was significantly increased versus control (Figure 3Bii). Much like the OCR and ATP production, SDF-1/CXCL12 is also producing biphasic changes in mitochondrial mass and Δψm, suggesting that it may be regulating several different aspect of mitochondrial respiration through regulation of electron transport, oxidative phosphorylation, and mitochondrial biogenesis.
Figure 3. SDF-1/CXCL12 Regulates mitochondrial mass and mitochondrial membrane potential of HL-60 cells in a biphasic manner.
(A & B) HL-60 cells were treated with 50ng/ml of SDF-1 for 2 and 24 hours respectively. After each time point, cells were collected and stained with 50 nM MitoTracker Green (A) to measure mitochondrial mass or 50 nM MitoTracker Red CMXRos (B) to measure mitochondrial membrane potential. Mitochondrial mass and membrane potential were analyzed by flow cytometry. Results are the mean ± SEM of five independent experiments with significant differences as compared to unstimulated control shown in the figure.
B) Effect on Mouse Primary Bone Marrow Lineage− Cells
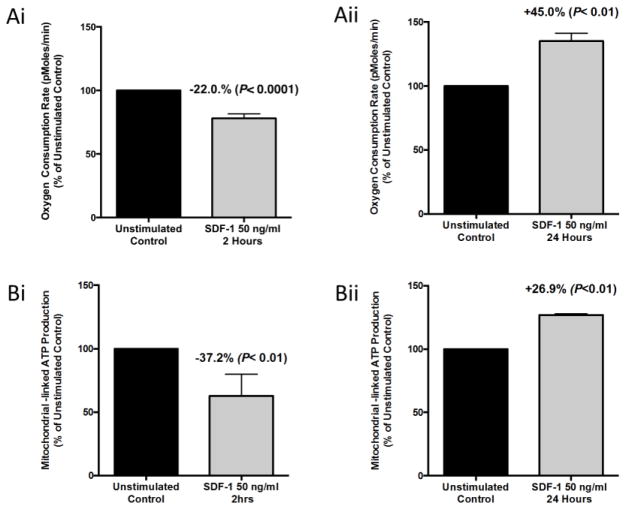
To determine if SDF-1/CXCL12 effects on HL-60 cells could be mimicked in primary cells, we isolated lineage negative (Lin−) bone marrow cells (enriched for immature blood cells) from C57Bl/6 mice and cultured them with or without 50 ng/ml SDF-1/CXCL12 for two and 24 hours, respectively. Cells were then collected after each time point and OCR rates of each group were measured on the Seahorse Bioscience XF96 Extracellular Flux Analyzer. As already noted for the human HL-60 cells (Figure 1), the OCR for the two-hour SDF-1/CXCL12 stimulated group was significantly reduced versus unstimulated two-hour control (Figure 4Ai). The OCR of the 24-hour SDF-1/CXCL12 stimulated group, however, was significantly increased (Figure 4Aii). These results are similar to the changes in OCR of the SDF-1/CXCL12 treated HL-60 cells. Next we analyzed mitochondrial-associated ATP production using the Seahorse Bioscience XF96 Extracellular Flux Analyzer (Figure 4Bi and 4Bii). Treatment of Lin− cells with SDF-1/CXCL12 produced a significant decrease in the mitochondrial-associated ATP production versus two-hour unstimulated control and treatment of Lin− cells with SDF-1/CXCL12 for 24 hours produced a significant increase in the mitochondrial-associated ATP production as compared to control cells. These results demonstrate that ex vivo treatment of primary mouse immature blood cells in Lin- bone marrow cells with SDF-1/CXCL12 regulates mitochondrial respiration in a biphasic manner similar to that seen in HL-60 cells.
Figure 4. SDF-1/CXCL12 Regulates mitochondrial respiration of lineage negative bone marrow cells in a biphasic manner.
Lineage negative bone marrow cells were isolated from C57Bl/6 mice and treated with 50ng/ml of SDF-1 for 2 and 24 hours respectively. After each time point the the OCR (A) and mitochondrial-linked ATP production (B) were measured on the Seahorse Bioscience Extracellular Flux Analyzer. Results are the mean ± SEM of three independent experiments with significant differences as compared to unstimulated control shown in the figure.
Conclusions
Regulation of mitochondrial respiration is essential to HSC and HPC maintenance and proper function [31–36]. Disregulation of mitochondrial function can lead to various disorders including leukemia or bone marrow failure. Many groups have shown an essential role for SDF-1/CXCL12 in HSC maintenance, but its role in mitochondrial regulation had yet to be established. Our findings in HL-60 and primary Lin− bone marrow cells suggest that SDF-1/CXCL12 regulates mitochondrial OCR, ATP production as well as Δψm and mitochondrial mass in a biphasic fashion and that these effects are mediated through CXCR4. These results suggest that SDF-1/CXCL12 plays a potential role in regulating mitochondrial respiration in immature blood cell types. Mitochondrial regulation in a biphasic manner also suggests that time-dependent changes in SDF-1/CXCL12, as well as changes in the expression of SDF-1CXCL12 by various niche cells, may differentially effect hematopoietic cells in the bone marrow and potentially influence HSC maintenance and differentiation. It is clear that mitochondrial metabolism of HSCs and HPCs is important [33–37] and these results may have important implications for the regulation of mitochondrial metabolism of HSCs and HPCs in the bone marrow microenvironment. By continuing to study the role of SDF-1/CXCL12 in mitochondrial regulation in more defined HSC and HPC populations, studies may elucidate the role that it plays in regulating HSC and HPC function by various SDF-1/CXCL12 expressing niche cells.
Acknowledgments
These Studies were supported by: U.S. Public Health Service Grants from the National Institutes of Health: R01 HL67384, R01 HL56416, R01 HL112669, P01 DK90948, and U54 DK106846 to H.E.B. S.M-G was supported as a Pre-Doctoral Fellow by NIH T32 DK07519 to H.E.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Montresor A, Toffali L, Mirenda M, et al. JAK2 tyrosine kinase mediates integrin activation induced by CXCL12 in B-cell chronic lymphocytic leukemia. Oncotarget. 2015;6:34245–34257. doi: 10.18632/oncotarget.5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shirozu M, Nakano T, Inazawa J, et al. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 3.Bleul CC, Farzan M, Choe H, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 4.Oberlin E, Amara A, Bachelerie F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y, Gotoh A, Kwon HJ, et al. Enhancement of intracellular signaling associated with hematopoietic progenitor cell survival in response to SDF-1/CXCL12 in synergy with other cytokines. Blood. 2002;99:4307–4317. doi: 10.1182/blood.v99.12.4307. [DOI] [PubMed] [Google Scholar]
- 6.Joo EK, Broxmeyer HE, Kwon HJ, et al. Enhancement of cell survival by stromal cell-derived factor-1/CXCL12 involves activation of CREB and induction of Mcl-1 and c-Fos in factor-dependent human cell line MO7e. Stem Cells Dev. 2004;13:563–570. doi: 10.1089/scd.2004.13.563. [DOI] [PubMed] [Google Scholar]
- 7.Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 8.Zou YR, Kottmann AH, Kuroda M, et al. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 9.Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 10.Ma Q, Jones D, Borghesani PR, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in C. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aiuti A, Webb IJ, Bleul C, et al. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim CH, Broxmeyer HE. In vitro behavior of hematopoietic progenitor cells under the influence of chemoattractants: stromal cell-derived factor-1, steel factor, and the bone marrow environment. Blood. 1998;91:100–110. [PubMed] [Google Scholar]
- 13.Kawabata K, Ujikawa M, Egawa T, et al. A cell-autonomous requirement for CXCR4 in long-term lymphoid and myeloid reconstitution. Proc Natl Acad Sci USA. 1999;96:5663–5667. doi: 10.1073/pnas.96.10.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 15.Christopherson KW, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 16.Bonig H, Priestley GV, Nilsson LM, et al. PTX-sensitive signals in bone marrow homing of fetal and adult hematopoietic progenitor cells. Blood. 2004;104:2299–2306. doi: 10.1182/blood-2004-04-1605. [DOI] [PubMed] [Google Scholar]
- 17.Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205:777–783. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broxmeyer HE, Cooper S, Kohli L, et al. Transgenic expression of stromal cell-derived factor-1/CXC chemokine ligand 12 enhances myeloid progenitor cell survival/antiapoptosis in vitro in response to growth factor withdrawal and enhances myelopoiesis in vivo. J Immunol. 2003;170:421–429. doi: 10.4049/jimmunol.170.1.421. [DOI] [PubMed] [Google Scholar]
- 19.Broxmeyer HE, Kohli L, Kim CH, et al. Stromal cell-derived factor-1/CXCL12 directly enhances survival/antiapoptosis of myeloid progenitor cells through CXCR4 and G(alpha)i proteins and enhances engraftment of competitive, repopulating stem cells. J Leukoc Biol. 2003;73:630–638. doi: 10.1189/jlb.1002495. [DOI] [PubMed] [Google Scholar]
- 20.Tzeng YS, Li H, Kang YL, et al. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood. 2011;117:429–439. doi: 10.1182/blood-2010-01-266833. [DOI] [PubMed] [Google Scholar]
- 21.Broxmeyer HE, Mejia JA, Hangoc G, et al. SDF-1/CXCL12 enhances in vitro replating capacity of murine and human multipotential and macrophage progenitor cells. Stem Cells Dev. 2007;16:589–596. doi: 10.1089/scd.2007.0044. [DOI] [PubMed] [Google Scholar]
- 22.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omatsu Y, Sugiyama T, Kohara H, et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ugarte F, Forsberg EC. Haematopoietic stem cell niches: new insights inspire new questions. EMBO J. 2013;32:2535–2547. doi: 10.1038/emboj.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nombela-Arrieta C, Pivarnik G, Winkel B, et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol. 2013;15:533–543. doi: 10.1038/ncb2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anthony BA, Link DC. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol. 2014;35:32–37. doi: 10.1016/j.it.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu WM, Liu X, Shen J, et al. Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell. 2013;12:62–74. doi: 10.1016/j.stem.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian P, He XC, Paulson A, et al. The Dlk1-Gtl2 locus preserves LT-HSC function by inhibiting the PI3K-mTOR pathway to restrict mitochondrial metabolism. Cell Stem Cell. 2015 Nov 23; doi: 10.1016/j.stem.2015.11.001. pii: S1934-5909(15)00499-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mantel C, Messina-Graham S, Moh A, et al. Mouse hematopoietic cell-targeted STAT3 deletion: stem/progenitor cell defects, mitochondrial dysfunction, ROS overproduction, and a rapid aging-like phenotype. Blood. 2012;120:2589–2599. doi: 10.1182/blood-2012-01-404004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yalcin S, Marinkovic D, Mungamuri SK, et al. ROS-mediated amplification of AKT/mTOR signalling pathway leads to myeloproliferative syndrome in Foxo3(−/−) mice. EMBO J. 2010;29:4118–4131. doi: 10.1038/emboj.2010.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantel CR, O’Leary HA, Chitteti BR, et al. Enhancing hematopoietic stem cell transplantation efficacy by mitigating oxygen shock. Cell. 2015;161:1553–1565. doi: 10.1016/j.cell.2015.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broxmeyer HE, O’Leary HA, Huang X, Mantel C. The importance of hypoxia and extra physiologic oxygen shock/stress for collection and processing of stem and progenitor cells to understand true physiology/pathology of these cells ex vivo. Curr Opin Hematol. 2015;22:273–278. doi: 10.1097/MOH.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantel C, Messina-Graham S, Broxmeyer HE. Upregulation of nascent mitochondrial biogenesis in mouse hematopoietic stem cells parallels upregulation of CD34 and loss of pluripotency: a potential strategy for reducing oxidative risk in stem cells. Cell Cycle. 2010;9:2008–2017. doi: 10.4161/cc.9.10.11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerencser AA, Neilson A, Choi SW, et al. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem. 2009;81:6868–6878. doi: 10.1021/ac900881z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernier M, Paul RK, Martin-Montalvo A, et al. Negative regulation of STAT3 protein-mediated cellular respiration by SIRT1 protein. J Biol Chem. 2011;286:19270–19279. doi: 10.1074/jbc.M110.200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Nuebel E, Wisidagama DR, et al. Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat Protoc. 2012;7:1068–1085. doi: 10.1038/nprot.2012.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capitano ML, Hangoc G, Cooper S, Broxmeyer HE. Mild heat treatment primes human CD34(+) cord blood cells for migration toward SDF-1alpha and enhances engraftment in an NSG mouse model. Stem Cells. 2015;33:1975–1984. doi: 10.1002/stem.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]