Abstract
Gnathodiaphyseal dysplasia (GDD) is a rare autosomal dominant disorder characterized by florid osseous dysplasia (FOD) in the jaws, bone fragility, and diaphyseal cortical thickening and bowing of long bones. We present a family with previously undiagnosed GDD. The disorder was identified by the characteristic gnathic and skeletal manifestations in the father. Clinical and radiological examination of the patient’s son also revealed the characteristic features of GDD. Gene sequencing revealed a novel mutation (c. 1067 G>A, p. Cys356Tyr) in the ANO5 gene, the gene that is causative for GDD. This mutation was predicted to be detrimental by computational analyses and by structural modeling of the protein. The implications for recognition and management of this disease are discussed.
INTRODUCTION
Gnathodiaphyseal dysplasia (GDD, OMIM#166260) is a rare autosomal dominant disorder characterized by florid osseous dysplasia (FOD) in the jaws, bone fragility, and diaphyseal cortical thickening and bowing of long bones.1 GDD was first recognized as a distinctive entity in 1969 by Akasaka et al,2 who described 21 affected individuals from a family spanning four generations. The characteristics that defined this entity were purulent osteomyelitis of the jaws and frequent bone fractures at a young age. In 2001, Riminucci et al. described the features of this disorder in a patient who had been previously misdiagnosed with polyostotic fibrous dysplasia, and proposed the term GDD.3 Notably, given its presentation of skeletal fragility and mixed radiodensity lesions in the jaws, GDD has been often misdiagnosed as fibrous dysplasia or osteogenesis imperfecta.
To date, there are four reported families with GDD.2, 4–6 The characteristic features of this disorder, as documented in all reports to date, include marked jaw involvement with FOD and a history of frequent fractures, often following minor trauma, and usually at a young age. In addition, there are isolated case reports describing findings similar to those seen in GDD. One report, published prior to the formal recognition of GDD7, described two patients with non-familial sclerotic lesions in the calvaria and a history of multiple long bone fractures. However, only one of these patients manifested jaw lesions characteristic of GDD, suggesting that only this latter individual represented a bona fide GDD case. Two case reports were published after the formal recognition of GDD.8, 9 Both reports described the characteristic jaw involvement and long bone features of GDD, but did not indicate the presence or absence of a family history. In one of these reports, there was no indication of any genetic analyses.8 The second case, in an infant, was reported as genetically confirmed, although the specific mutation was not described.9
Tsutmusi et al. performed linkage analysis of the large, original Japanese kindred and mapped the GDD locus to 11p14.3 – 15.1.10 The gene responsible for this disorder, anoctamin 5 (ANO5), was subsequently identified in 2004.6 The ANO5 gene encodes a 913 amino-acid protein and belongs to the newly discovered family of anoctamins, the functions of which are yet to be fully elucidated. The anoctamin proteins share a common structural feature of eight transmembrane domains, with a channel pore formed between the fifth and sixth loops.11, 12 ANO1 and ANO2 act as calcium-activated chloride channels, but the function of the reminder of the anoctamin genes remains poorly understood.11
To date, three discrete missense mutations of the ANO5 gene have been identified in GDD patients—two of these mutations alter the cysteine 356 residue (C356R and C356G)6, and another mutation alters the threonine 513 residue (T513I)4. However, the functional significance of these mutations remains unknown. Importantly, the function of ANO5 is poorly understood, and its functions specifically as they relate to the skeletal and jaw manifestations of GDD have not been investigated.
We present a new family with previously undiagnosed GDD. The diagnosis was initially made based on the clinical and radiographic manifestations in the father, and subsequently confirmed by sequencing of the ANO5 gene. Clinical and radiographic examination also confirmed the presence of the characteristic features in the patient’s son, and thus, contributes a new family of this disorder.
CASE REPORTS
Case 1
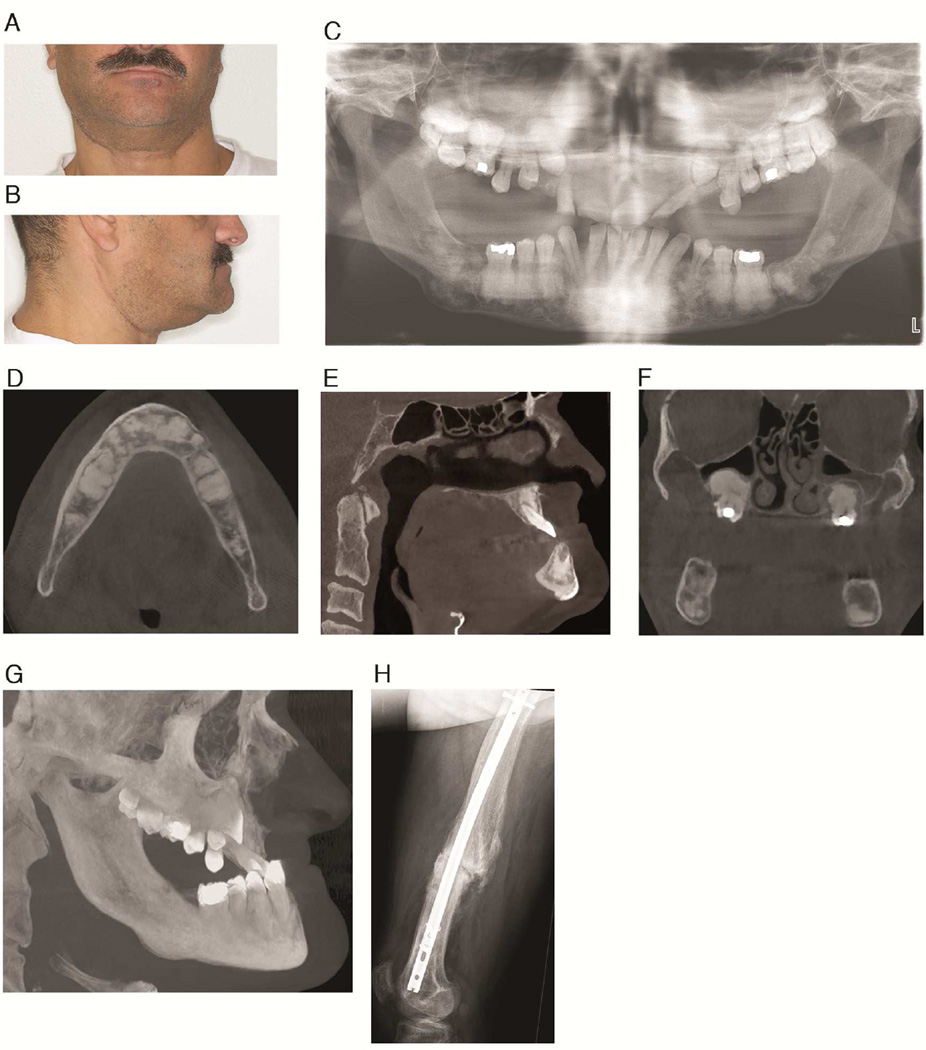
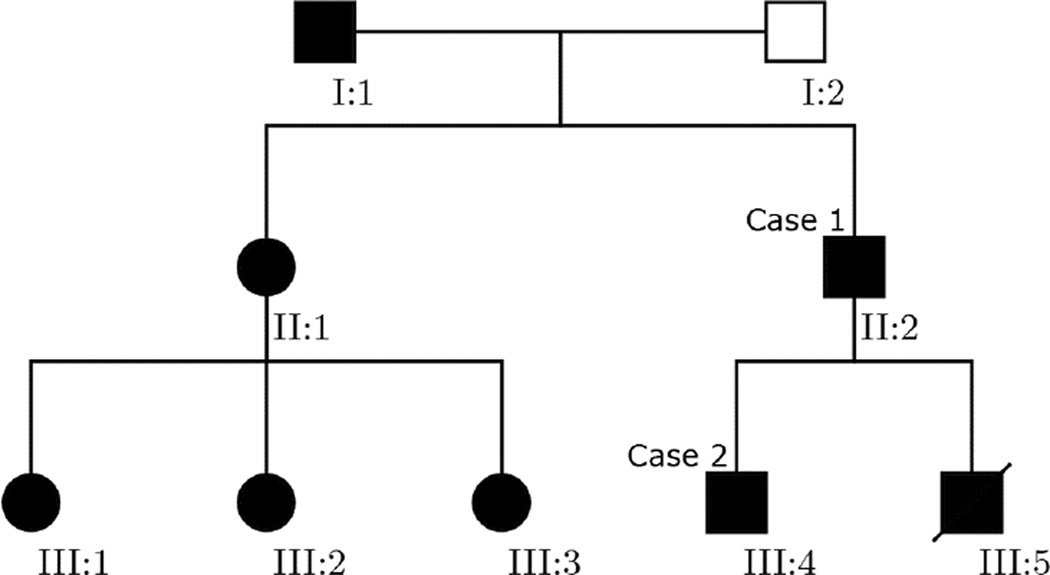
The index patient is a 41-year-old man of German and Italian descent (Patient 1), who presented to the Veterans Affairs Greater Los Angeles Healthcare System with Ludwig’s angina and osteomyelitis in the right posterior mandible. His radiographs (Figure. 1), which consisted of a panoramic radiograph and a cone beam computed tomography (CBCT) scan, were evaluated at the UCLA Oral and Maxillofacial Radiology clinic. The patient was partially edentulous with multiple impacted teeth (Figure 1C). Multiple well-defined, amorphous mixed radiodensity lesions were present predominantly in the tooth-bearing areas of the maxilla and mandible, with expansion and loss of normal trabecular architecture (Figures 1D–F). The lesions consisted of a central radiodense area, with the radiodensity of bone/cementum, surrounded by a radiolucent halo. In the maxilla, the lesions protruded into the maxillary sinus lumen, causing considerable hypoplasia of the sinuses (Figure 1F). The overall radiographic features were characteristic of FOD. Further review of his history revealed a left femoral fracture due to minor trauma when the patient was a child (Figure. 1H), and subsequent multiple fractures of the appendicular skeleton and cranium. The patient also provided a history of frequent fractures and jaw lesions in other family members (Figure 2) including his father, sister and all three of her children, and his 21-year-old son. His other son reportedly died of neonatal sepsis and also had multiple bone fractures. Based on this history, coupled with the classical radiographic features of FOD, a diagnosis of GDD was made.
Fig. 1. GDD Patient 1.
A and B: Frontal and lateral views of the patient. C: Panoramic radiograph showing extensive mixed density lesions and multiple impacted teeth. D: Axial CT slice demonstrating sclerotic lesions in the mandible. E. Sagittal CT slice showing maxillary hypoplasia and expansile mixed radiodensity lesions in the maxilla and mandible. F. Coronal CT slice showing displacement of the sinus floor causing maxillary sinus hypoplasia. G. 3D maximum intensity projection reconstruction of the maxillofacial CT scan demonstrating maxillary hypoplasia. H. Lateral radiograph of the femur showing a previous fracture.
Fig. 2.
Pedigree demonstrating the pattern of inheritance of the disease manifestations. Case 1 and case 2 are denoted by individuals II:2 and III:4, respectively.
Sequencing of the ANO5 gene was performed in the germline DNA of this patient (Prevention Genetics Laboratory, Marshfield, WI) and revealed a novel heterozygous mutation—c.1067 G>A (p.Cys356Tyr, Table 1). This amino acid change was assessed to be detrimental by three independent computational prediction programs—PolyPhen-2, Mutation Taster2, and SIFT.13–15 No other mutations were present in the ANO5 gene.
Table 1.
ANO5 mutation in GDD patient 1. For comparison, the wild-type sequence and previously documented ANO5 mutations in GDD are listed. The codon altered by the mutation is indicated in bold font. The predicted amino acids are denoted below the DNA sequence.
| Exon 11 | Exon 15 | |
|---|---|---|
| Wild type | …CCA CTC TGT GAT CAA… Pro Leu Cys Asp Gln |
…CAG ATA ACC ACA TCA… Gln Ile Thr Thr Ser |
| GDD patient 1 | …CCA CTC TAT GAT CAA… Pro Leu Tyr Asp Gln |
_ |
| Tsutsumi et al. (2003)10 | …CCA CTC CGT GAT CAA… Pro Leu Arg Asp Gln |
_ |
| Tsutsumi et al. (2004)6 | …CCA CTC GGT GAT CAA… Pro Leu Gly Asp Gln |
_ |
| Marconi et al. (2013)4 | _ | …CAG ATA ATC ACA TCA… Gln Ile Ile Thr Ser |
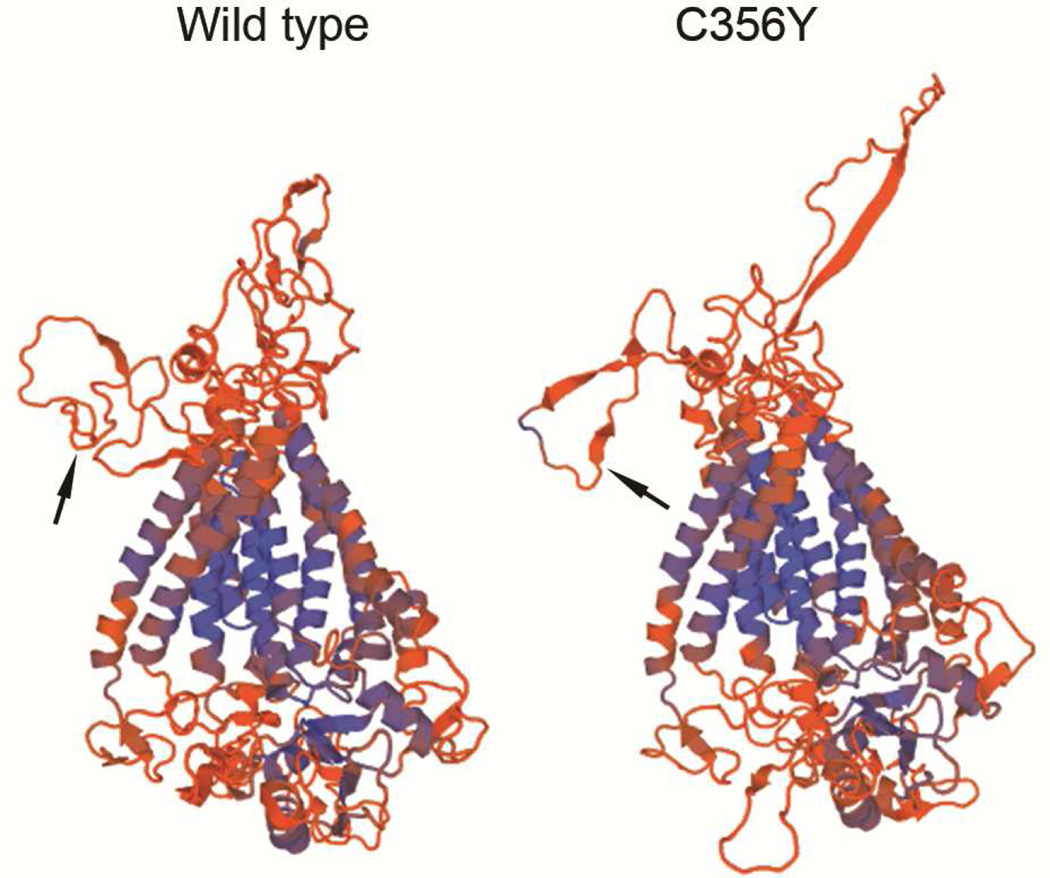
To further characterize the effect of the mutation on ANO5 protein structure, we constructed molecular models of the wild type and C356Y mutant ANO5 protein using the SWISS-MODEL server.16 The predicted structure of wild type ANO5 (Figure 3) was consistent with the putative structure of the anoctamin family members—eight transmembrane domains, the N– and C– terminals within the cytoplasm, and extracellular loops.12 Interestingly, the structure of the extracellular domains in the ANO5 C356Y mutant deviated markedly from the wild-type structure (Figure 3). These deviations were particularly evident in the region around the location of the mutated residue.
Fig. 3. Modeled structure of the ANO5 wild type and C356Y mutant proteins.
The black arrows point to the location of amino acid residue 356, located in the predicted extracellular domain. Note marked deviation in the structure of the extracellular loops of the mutant ANO5, compared with the wild-type structure.
The patient was referred to the Endocrine service at the Veterans Affairs Greater Los Angeles Healthcare System. He did not have a history of other endocrine disorders. Dual x-ray absorptiometry was normal, and demonstrated good preservation of bone density. Other markers of bone and calcium metabolism, including bone-specific serum alkaline phosphatase, serum osteocalcin, urine N-telopeptide, serum total calcium, serum phosphate, and serum 25-hydroxyvitamin D, were within normal ranges (Table 2).
Table 2.
Laboratory investigations in GDD patient 1
| Analyte | Result | Reference Range |
|---|---|---|
| Serum bone-specific alkaline phosphatase | 36 | 16–56 U/L |
| Serum total alkaline phosphataste | 103 | 40–115 U/L |
| Serum calcium | 9.1 | 8.4–10.2 mg/dL |
| Serum phosphorus | 4.2 | 2.5–4.9 mg/dL |
| Serum 25-hydroxyvitamin D | 41 | 30–80 ng/mL |
| Serum intact PTH | 17 | 14–72 pg/mL |
| Serum TSH | 1.28 | 0.55–4.78 uIU/mL |
| Serum osteocalcin | 32 | 9–38 ng/mL |
| Urine N-telopeptide/creatinine ratio | 34 | 9–36 nmol BCE/nmol creat |
| Bone densitometry, DXA (Z-score)* | ||
| Right femoral neck | − 0.7 | |
| L1-L4 region | + 0.7 | |
| Right hip | – 0.3 | |
Evaluation of the left femoral neck and hip was not possible due to orthopedic hardware.
Case 2
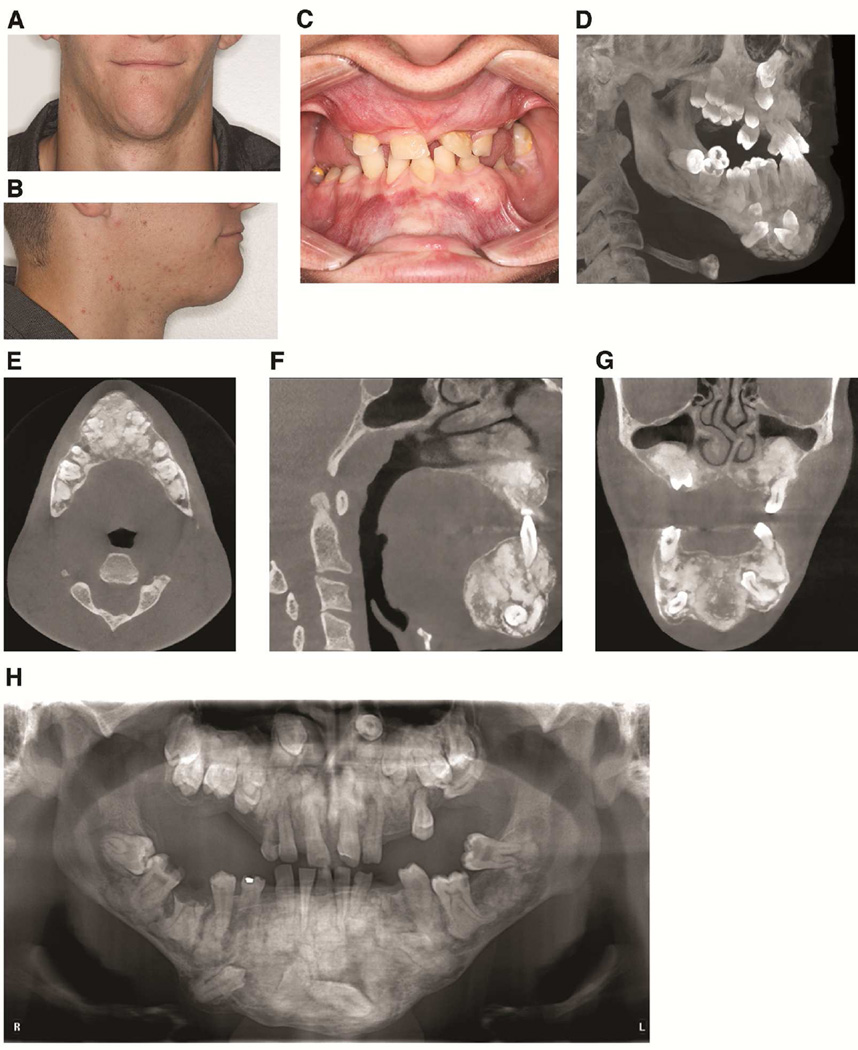
The patient’s 21-year-old son was examined at the UCLA Dental Clinic. Clinical examination revealed expansion of the anterior mandible (Figure 4A, 4B, and 4C). Clinical intraoral examination revealed multiple missing teeth, malocclusion, poor periodontal health, and mobility of several teeth (Figure 4C). Panoramic and CBCT depicted multiple impacted teeth (Figure 4D–H). Similar to his father, cementum/bone-like masses were present in the tooth-bearing areas of the jaws (Figure 4D–H). The buccal and lingual cortical plates in the anterior mandible were expanded and thinned. The lesions protruded into the maxillary sinuses, which were hypoplastic. Overall, the radiographic features were consistent with FOD. Notably, the FOD lesions were considerably more extensive and expansile compared with those in his father.
Fig. 4. GDD Patient 2.
A and B: Frontal and lateral views showing expansion of the anterior mandible. C: Intraoral view showing multiple areas of expansion in the maxilla and mandible. D: 3D maximum intensity projection reconstruction of the maxillofacial CT scan showing maxillary hypoplasia, multiple impacted teeth, and expansile mixed radiodensity lesions. E. Axial slice showing expansile sclerotic lesions in the mandible. F. Sagittal CT slice showing expansile lesions in both jaws. G. Displacement of the sinus floor with resultant maxillary sinus hypoplasia. H. Panoramic radiograph showing extensive mixed radiodensity lesions and multiple impacted teeth.
DISCUSSION
GDD is a rare autosomal dominant disorder characterized by FOD of the jaws, bone fragility and diaphyseal sclerosis of tubular bones, and is due to an underlying mutation in the ANO5 gene. FOD is one of the categories of fibro-osseous lesions—a group of clinically and radiographically diverse lesions which share a similar histological appearance where normal bone is replaced by fibrous tissue containing varying amounts or combinations of calcified material.17 The three broad categories that comprise jaw fibro-osseous lesions include fibrous dysplasia (FD), osseous dysplasia (subcategorized as periapical, florid, and focal, depending on the extent of jaw involvement), and cemento-ossifying fibromas (COF).17 Notably, some reports of GDD incorrectly identified the jaw lesions as COF, although the radiographic features presented were consistent with FOD. This distinction is clinically significant—COF is a true neoplasm that requires surgical removal, whereas FOD is a localized skeletal dysplasia and does not require surgical intervention except when necessary for cosmetic or functional purposes. Clearly separating these two entities is important to prevent unnecessary radical excision of FOD lesions.
This manuscript highlights three clinically important issues. First, given its rarity and relatively recent characterization, GDD is undiagnosed or often misdiagnosed as polyostotic fibrous dysplasia, based on the fibro-osseous nature of the jaw lesions, or as osteogenesis imperfecta considering the manifestation of multiple bone fractures. However, unlike FD, which can affect any skeletal site, FOD occurs exclusively in the jaws, and is limited to the tooth-bearing areas. The presence of FOD lesions was the first indication of GDD in our patient, underscoring the importance of radiographic recognition of these lesions as distinct from FD and other fibro-osseous lesions. Importantly, the molecular pathogenesis of FD and FOD are distinct—FD is caused by somatic activating mutations of GNAS1.18 In contrast, FOD lesions do not harbor GNAS1 mutations19.
Second, as with other FOD lesions, GDD patients are predisposed to developing osteomyelitis, initiated by infections from the teeth or the periodontium. These infections often have life-threatening consequences, as was observed in our index patient. Maintenance of optimal dental and periodontal health is paramount to reducing the risk of such consequences. Notably, given the presence of the extensive jaw sclerosis, implant therapy may not be feasible due to reduced vascularity in the areas of FOD.
Third, there are no established guidelines to manage fracture risk in these patients. The pathogenesis of bone fragility in GDD patients is not known. Indeed, laboratory investigations of markers of bone turnover, calcium homeostasis, and vitamin D status were within normal limits. Potential approaches to manage fracture risk may include bone anabolic agents such as parathyroid hormone, and this therapy has been initiated for patient 1. We certainly recognize that anabolic therapy could potentially exacerbate mineralization of the FOD lesions. Continued oral health monitoring in this patient will allow us to minimize the risk of FOD–associated osteomyelitis, as we develop approaches to reduce fracture risk. In one prior report, pamidronate was used to manage the fracture risk.9 Anti-resorptives, such as bisphosphonates and Denosomab increase the risk of osteonecrosis of the jaw.20–22 It is important to note that the hypovascularity associated with the extensive sclerotic FOD lesions increases the propensity to develop osteomyelitis, and the use of anti-resorptive therapy may heighten the risk of developing osteonecrosis.
Our report also highlights issues of biological significance. GDD is caused by inherited germline mutations of the ANO5 gene. The anoctamin family of genes comprises 10 genes that share several regions of homology. They are involved in ion transport and lipid scrambling.11 ANO1 and ANO2 are known to function as calcium-activated chloride channels.12 It is likely that ANO5 may also have similar functions. However, the function of ANO5 in bone pathophysiology is unknown. In this manuscript we report a novel mutation of the ANO5 gene, at the C356 residue (C356Y). Previous GDD mutations (Table 1) described at this residue have resulted in different amino acid substitutions, C356R and C356G mutations.6 The mutation in our patient underscores the importance of this cysteine residue in ANO5 function, at least in the pathogenesis of skeletal fragility and FOD development. The C356 residue is predicted to be in the first extracellular loop. Interestingly, our molecular modeling analyses show that the C356Y mutation changes the three-dimensional structure of this extracellular loop, suggesting that this domain of the protein has an important function that may be important in GDD development. Further molecular studies on the role of ANO5 in bone pathophysiology are important to better understand the role of this gene in causing GDD, and perhaps to sporadic FOD in general.
CONCLUSION
We present a new family with previously undiagnosed GDD. Detailed clinical and radiographic features of the father and son are presented. Genetic analyses of the father demonstrated a novel mutation C356Y, emphasizing the functional importance of this residue in ANO5 function, at least in bone physiology.
Acknowledgments
Funding Sources
AML was supported in part by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under award number K23HD068552. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: NONE
Portions of this manuscript were presented at the Annual Meetings of the Endocrine Society (2014, Dr. Karen T. Le, primary presenter,) and the American Academy of Oral and Maxillofacial Radiology (2014, Dr. Hannah Duong, primary presenter). Dr. Hannah Duong received the Dentsply Rinn Clinical Award for her presentation.
REFERENCES
- 1.Online Mendelian Inheritance in Man, OMIM®. McKusick-Nathans Institute of Genetic Medicine. Baltimore, MD: Johns Hopkins University; [Google Scholar]
- 2.Akasaka Y, Nakajima T, Koyama K, Furuya K, Mitsuka Y. Familial cases of a new systemic bone disease, hereditary gnatho-diaphyseal sclerosis. Nihon Seikeigeka Gakkai zasshi. 1969;43:381–394. [PubMed] [Google Scholar]
- 3.Riminucci M, Collins MT, Corsi A, et al. Gnathodiaphyseal dysplasia: a syndrome of fibro-osseous lesions of jawbones, bone fragility, and long bone bowing. J Bone Miner Res. 2001;16:1710–1718. doi: 10.1359/jbmr.2001.16.9.1710. [DOI] [PubMed] [Google Scholar]
- 4.Marconi C, Brunamonti Binello P, Badiali G, et al. A novel missense mutation in ANO5/TMEM16E is causative for gnathodiaphyseal dyplasia in a large Italian pedigree. European journal of human genetics : EJHG. 2013;21:613–619. doi: 10.1038/ejhg.2012.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roginsky VV, Ivanov AL, Khonsari RH. Recurring gnathodiaphyseal dysplasia in two Russian brothers. International journal of oral and maxillofacial surgery. 2010;39:397–401. doi: 10.1016/j.ijom.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Tsutsumi S, Kamata N, Vokes TJ, et al. The novel gene encoding a putative transmembrane protein is mutated in gnathodiaphyseal dysplasia (GDD) Am J Hum Genet. 2004;74:1255–1261. doi: 10.1086/421527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimura G, Haga N, Ikeuchi S, Yamaguchi T, Aoki K, Yamato M. Fragile bone syndrome associated with craniognathic fibro-osseous lesions and abnormal modeling of the tubular bones: report of two cases and review of the literature. Skeletal radiology. 1996;25:717–722. doi: 10.1007/s002560050167. [DOI] [PubMed] [Google Scholar]
- 8.Ahluwalia J, Ly JQ, Norman E, Costello RF, Jr, Beall DP. Gnathodiaphyseal dysplasia. Clinical imaging. 2007;31:67–69. doi: 10.1016/j.clinimag.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Herman TE, Siegel MJ, Sargar K. Gnathodiaphyseal dysplasia. Journal of perinatology : official journal of the California Perinatal Association. 2014;34:412–414. doi: 10.1038/jp.2013.178. [DOI] [PubMed] [Google Scholar]
- 10.Tsutsumi S, Kamata N, Maruoka Y, et al. Autosomal dominant gnathodiaphyseal dysplasia maps to chromosome 11p14.3-15.1. J Bone Miner Res. 2003;18:413–418. doi: 10.1359/jbmr.2003.18.3.413. [DOI] [PubMed] [Google Scholar]
- 11.Picollo A, Malvezzi M, Accardi A. TMEM16 Proteins: Unknown Structure and Confusing Functions. Journal of Molecular Biology. 2015;427:94–105. doi: 10.1016/j.jmb.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedemonte N, Galietta LJ. Structure and function of TMEM16 proteins (anoctamins) Physiological reviews. 2014;94:419–459. doi: 10.1152/physrev.00039.2011. [DOI] [PubMed] [Google Scholar]
- 13.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nature methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001;11:863–874. doi: 10.1101/gr.176601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nature methods. 2014;11:361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 16.Bordoli L, Kiefer F, Arnold K, Benkert P, Battey J, Schwede T. Protein structure homology modeling using SWISS-MODEL workspace. Nat Protoc. 2009;4:1–13. doi: 10.1038/nprot.2008.197. [DOI] [PubMed] [Google Scholar]
- 17.Waldron CA. Fibro-osseous lesions of the jaws. J Oral Maxillofac Surg. 1993;51:828–835. doi: 10.1016/s0278-2391(10)80097-7. [DOI] [PubMed] [Google Scholar]
- 18.Bianco P, Riminucci M, Majolagbe A, et al. Mutations of the GNAS1 gene, stromal cell dysfunction, and osteomalacic changes in non-McCune-Albright fibrous dysplasia of bone. J Bone Miner Res. 2000;15:120–128. doi: 10.1359/jbmr.2000.15.1.120. [DOI] [PubMed] [Google Scholar]
- 19.Patel MM, Wilkey JF, Abdelsayed R, D'Silva NJ, Malchoff C, Mallya SM. Analysis of GNAS mutations in cemento-ossifying fibromas and cemento-osseous dysplasias of the jaws. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontology. 2010;109:739–743. doi: 10.1016/j.tripleo.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 21.Aghaloo TL, Dry SM, Mallya S, Tetradis S. Stage 0 osteonecrosis of the jaw in a patient on denosumab. J Oral Maxillofac Surg. 2014;72:702–716. doi: 10.1016/j.joms.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aghaloo TL, Felsenfeld AL, Tetradis S. Osteonecrosis of the jaw in a patient on Denosumab. J Oral Maxillofac Surg. 2010;68:959–963. doi: 10.1016/j.joms.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]