Abstract
Humans occupy by far the most complex foraging niche of all mammals, built around sophisticated technology, and at the same time exhibit unusually large brains. To examine the evolutionary processes underlying these features, we investigated how manipulation complexity is related to brain size, cognitive test performance, terrestriality, and diet quality in a sample of 36 non-human primate species. We categorized manipulation bouts in food-related contexts into unimanual and bimanual actions, and asynchronous or synchronous hand and finger use, and established levels of manipulative complexity using Guttman scaling. Manipulation categories followed a cumulative ranking. They were particularly high in species that use cognitively challenging food acquisition techniques, such as extractive foraging and tool use. Manipulation complexity was also consistently positively correlated with brain size and cognitive test performance. Terrestriality had a positive effect on this relationship, but diet quality did not affect it. Unlike a previous study on carnivores, we found that, among primates, brain size and complex manipulations to acquire food underwent correlated evolution, which may have been influenced by terrestriality. Accordingly, our results support the idea of an evolutionary feedback loop between manipulation complexity and cognition in the human lineage, which may have been enhanced by increasingly terrestrial habits.
Humans stand out among animals in having both a very complex foraging niche, built around sophisticated technology, and an unusually large brain. Is this combination just a coincidence, or instead the product of correlated evolution, as suggested by the relationship between use and manufacture of tools and brain size1,2? In carnivores, no correlation between brain size and forelimb dexterity during feeding was found3. Here, using a new method of assessing manipulation complexity in food-related contexts, we examine the relationship between foraging niche complexity and brain size for 36 non-human primate species from various taxonomic groups.
Manipulation complexity was previously defined according to a variety of contrasts: (i) the use of one hand rather than two hands in bimanual coordination; (ii) asymmetrical bimanual manipulation (i.e., both hands simultaneously performing different actions) versus symmetrical bimanual manipulation (i.e., both hands simultaneously performing the same action); (iii) uncoordinated two-handed patterns (i.e. both hands performing actions, independently) versus coordinated two-handed patterns (i.e. both hands performing actions dependent on each other in space and/or time); or (iv) any combination of these criteria4,5,6,7,8. However, in all these reports on how primates use their hands to perform object manipulation, no explicit evaluation of the level of complexity was undertaken. Therefore, our first aim was to test whether distinct food manipulation categories can be ranked cumulatively across species according to their difficulty. We can speak of “manipulation complexity” if there is a clear ranking pattern, that is if species which are able to perform a given type of manipulation are also able to perform all manipulations of a lower rank. Such an empirical evaluation of complexity, without a priori assumptions of which manipulations are more complex than others, has to our knowledge not yet been undertaken.
Second, we tested whether a species’ manipulation complexity is related to cognitively challenging food acquisition techniques, such as, extractive foraging or tool use2,9. Effective extractive foraging is likely to require complex manipulative skills, because finely tuned movements are an advantage for removing and holding food or manipulating it with different objects. Tool use, on the other hand, is considered cognitively and manipulatively difficult as it often involves bimanually coordinated actions causally relating two or more external objects10.
Third, an emerging consensus is that foraging skills have played an important role in cognitive evolution1,2,4,11,12. We therefore also tested whether the degree of food manipulation complexity is related to brain size or cognitive test performance.
We further tested the influence of other factors that may have affected the correlated evolution between brain size and manipulation complexity: diet quality and terrestriality13. High-quality diets may require more complex motor and cognitive skills than low-quality diets. For example, insectivorous and frugivorous primates may need to perform more complex manual food processing than folivorous primates, and are more likely to use tools12. Likewise, in a terrestrial habitat, in contrast to arboreal contexts, hands are less commonly needed for positional support, which may allow for the evolution of morphologies capable of more actions on objects and the use of complex actions that require the coordinated involvement of both hands14. In addition, discarded tools remain close to where they are used in terrestrial settings, while they tend to disappear from sight after being dropped in arboreal ones15,16.
If manipulation complexity and brain size coevolved among primates, and thus ecological requirements partly drove brain size evolution in this lineage, it is interesting to ask whether the human manipulation complexity score fits into the pattern based on the relationship between manipulation complexity and brain size in nonhuman primates. If humans fit the primate trend, this suggests that the process of correlated evolution between brain size and foraging ecology may also have played a major role in human brain evolution.
Material and Methods
Data collection
We assessed manipulations in food contexts in captive individuals of 36 primate species (Supplementary Table S1). All subjects were housed in their home enclosures in single-species groups of 2 to 27 individuals that included at least one adult male and one adult female, except for Saimiri sciureus (seven males), Propithecus verreauxi (two males, each housed together with a Eulemur mongoz) and Leontopithecus rosalia (one male). We sampled all available individuals, but immatures were excluded because their manipulation patterns are usually qualitatively different from those of adults14,15. Data on human manipulation complexity was collected in a similar way, observing everyday food consumption in a “natural” setting (in the cafeteria of the University of Zurich, Switzerland).
Data were collected by behavioural sampling between October 2011 and February 2014, for a total of 112 hours in various zoos (Supplementary Table S1). Manipulation was defined as making physical contact with a food item with the forelimbs, and thus did not include visual exploration or sniffing without contact. Behavioural sampling was conducted in bouts; a bout started as soon as an individual started to manipulate a food item and ended when contact was terminated or after a maximal duration of 5 minutes. After each individual bout, there was an interval of at least 2 minutes without sampling. At least 10 bouts per sex, and thus 20 bouts per species, were collected for most species (Supplementary Table S1). In total, 962 bouts were recorded.
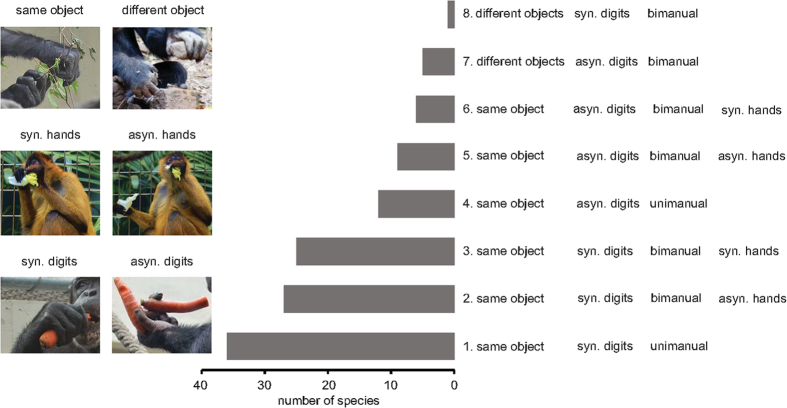
Observed manipulation bouts were divided into eight categories. These categories were based on all possible combination of the following: (i) use of the forelimbs, subdivided into unimanual and bimanual actions, (ii) asynchronous and synchronous use of hands, and (iii) asynchronous and synchronous use of digits. Furthermore, in bimanual actions we distinguished between the hands manipulating the same object or different objects, e.g. whether hands were both manipulating a fruit (same object) versus one hand is manipulating with a stick and the other hand is holding the fruit (different object) (Fig. 1). We only scored the presence of manipulation categories if the observed individuals of a primate species performed a manipulation category at least twice. Frequency or duration of manipulation categories were not assessed.
Figure 1. The eight manipulation complexity categories found through the Guttman scaling method (increasing complexity from category 1 to category 8), and the number of species able to perform actions in a particular manipulation complexity category.
Copyright S.A. Heldstab, Pan troglodytes nut cracking K. Koops.
Agreement between the two observers (inter-observer reliability) was assessed with the kappa statistic17, which corrects for agreement due to chance and was applied to the entire coding scheme. The acceptance criterion was set at 0.70, and all kappa statistics were substantially above this basal criterion (manipulation observed: K = 0.85, n = 20; level of manipulation: K = 0.72, n = 18; context of manipulation: K = 0.94, n = 18). Moreover, each species was observed by both observers together for at least 10 minutes to ensure reliability between different species.
Complexity levels
Using the deterministic Guttman scaling method based on the description of Green18, we assessed whether the manipulation categories followed a cumulative ranking. Using Guttman’s scaling method19 we can derive a rank order in a given set of skills, yielding a difficulty scale that is as cumulative as possible. The manipulation skills are ranked such that if an individual is able to perform a particular skill N, then that individual must also be able to perform all or most easier skills <N. Thus, in the ideal case of perfect nesting, if an individual’s score is known, this predicts the individual’s performances in all skills in the scale. For any empirical set of observed skills, the coefficient of reproducibility19 indicates to which extent the skills indeed do fit such a cumulative scale, or, in other words, whether there is a level of difficulty or complexity of the respective skills.
After ranking complexity levels of manipulations using the Guttman scale using all data on all species, mean manipulation complexities were calculated for each species as follows: For each manipulation bout the highest rank reached during the bout was determined. The mean complexity score for each species was then averaged over all observed bouts.
Additionally, we also performed all analyses using the highest manipulation complexity score reached over all observation bouts for each species. The results using this highest manipulation complexity score are largely identical to those obtained with mean complexity scores, but there is a ceiling effect and thus fewer distinctions between species (Supplementary Table S5).
Cognitive test performance and brain size
Two different meta-analyses of cognitive performance of a broad set of primate species provide quantitative estimates of cognitive test performance across primate genera11,20. Using the mean manipulation complexity for each genus (n = 15 genera for20/n = 19 genera for11), this allows for a direct test of a relationship between manipulation complexity and cognitive test performance on the genus level.
Endocranial volumes of mostly wild-derived female primates21 were used as a proxy of brain size on the species level (n = 36). To remove allometric effects of body size, female body mass was integrated as an independent variable in all multiple regression analyses22. Sources of data on endocranial volumes and body mass are given in Supplementary Table S1. Results showing that brain size is related to cognitive abilities in our primate sample are shown in Supplementary Table S4.
To test whether manipulative skills are particularly high in species known to perform cognitively challenging food acquisition techniques, tool use and extractive foraging were coded as binary variables (present = 1, absent = 0) for each species with data from the literature from wild primates23,24,25,26. In addition, we analysed correlations of manipulation complexity with neocortex and cerebellum size and with foraging group size as a measure of social complexity (results are reported and discussed in detail in the Supplementary material).
To test whether the human manipulation complexity score fits into the pattern between manipulation complexity and brain size in nonhuman primates, we conducted a bootstrapping analysis. The mean and width of the 95% confidence intervals were generated for sample estimates by bootstrapping (1000 iterations)27. At each iteration, a bootstrap sample of 25 and 30 nonhuman primate species was constructed by sampling at random without replacement. In a next step we tested whether the estimate of the phylogenetic generalized least-squares regression including the human manipulation complexity score was within the confidence limits calculated on the basis of nonhuman primates. If the data point for humans lies outside the confidence limits, then humans can be considered significantly different from the nonhuman primate trend with P < 0.05 (two-tailed). Furthermore, by using the “predict()” function in the “caper” package28 we calculated the human manipulation score which would be predicted for its brain size on the basis of the relationship between manipulation complexity and brain size in nonhuman primates.
Diet quality and terrestriality
To test whether diet quality is related to manipulation complexity, we integrated diet quality data from wild primates24 (and references therein). Diet quality was determined by using temporal variation in the time spent feeding on diet components (and thus their estimated consumption) as in van Woerden29: Monthly mean intake of each food category, as estimated by feeding time, was multiplied by its relative energetic quality (8 for insects; 5 for fruits, seeds, and flowers; 3 for gum and young leaves; and 1 for mature leaves, as calculated from g crude fibre/kg dry matter by Langer30). Fibre content is commonly used as a measure of digestibility and thus energy gained per unit time31. An alternative, categorical scheme of main diet categories was defined as follows: Insectivorous and frugivorous primates are coded as 1 (diets requiring complex manipulation), and folivorous and gummivorous primates are coded as 0 (diets requiring less complex manipulation) (Supplementary Table S1). Results using this alternative, categorical scheme of main diet categories are largely identical to those obtained with diet quality, and diet categories are highly correlated because diets related to complex manipulations, such as fruits and insects, also have higher nutritional values (Supplementary Table S8).
As a proxy of terrestriality, primate species were placed in one of three categories based on their main travel habit, as follows: (1) terrestrial (more than 60% terrestrial), (0.5) partly terrestrial (more than 20% terrestrial) and (0) arboreal. Data on terrestriality were taken from the published literature23,24.
Statistical analyses
All statistical analyses were performed using JMPTM 10.032 and R2.13.133. The method of phylogenetic generalized least-squares regression (PGLS)34 with the “caper” package28 was used to control for phylogenetic non-independence. Phylogeny was based on a composite supertree including branch length estimations35 (Supplementary Fig. S2). Results using an alternative phylogenetic tree (phylogeny based on version 3 of 10K trees36) remained largely similar (Supplementary Table S9). The values of body mass, brain size and diet quality were loge transformed in order to reach residuals evenly distributed around zero.
Manipulation complexity of species that use tools or do not use tools as well as of species with or without extractive foraging was compared using PGLS. The correlation of cognitive test performance scores, brain size, diet quality, and terrestriality with manipulation complexity was tested for each variable separately. In a second step, a multiple regression model was run to include manipulation complexity as the response, brain size as effect, and diet quality, terrestriality and body mass as covariates. As diet quality data was not available for Ateles fusciceps and Saguinus imperator, those two species were excluded from the multiple regression models, yielding a sample size of n = 34 non-human primate species. To choose the best fitting from a set of models, the AIC values (Akaike Information Criterion37) of different models were compared. In these models, we also tested for interaction effects between the predictor variables. Bivariate plots of manipulation complexity against brain size, cognitive test performance, diet quality residuals (corrected for body mass), and terrestriality are shown for illustrative purpose only.
Ethical statement animals
All the observations were carried out in accordance with the Swiss legislation on animal experimentation and formally approved by the Kantonales Veterinäramt of Zurich.
Ethical statement humans
All the observation were carried out in accordance with the Swiss legislation on research involving human subjects. The subjects provided written informed consent, and the observations were approved by the Ethikkommission für psychologische und verwandte Forschung of the Philosophische Fakultät der Universität Zürich (step 1).
Results
We found that manipulation categories follow a cumulative ranking (Fig. 1). In total, 86% of species’ performances exactly fitted the resulting Guttman scale, and the coefficient of reproducibility was close to 1 (0.92), indicating that manipulation complexity is indeed cumulative across species. For example, species that are able to perform category 4 mostly also exhibit categories 1, 2, and 3, therefore category 4 can be seen as more complex than the latter.
The scale implies the following scale of manipulative complexity: First, manipulating two objects simultaneously is more complex than manipulating only one object with both hands. Second, the complexity of a manipulation increased with the capability to move digits asynchronously, and when using both hands instead of just one hand for the action. Third, manipulations with synchronous hand use tended to be more complex than manipulations with asynchronous hand use.
Manipulation complexity was significantly higher (P = 0.020) in primate species that regularly use tools and substantially higher (P = 0.056) in species that exhibit extractive foraging (Table 1, Supplementary Fig. S2). Manipulation complexity was also positively correlated with relative brain size (Table 2, Fig. 2) and with performance on cognitive tests (Table 1, Supplementary Fig. S3).
Table 1. PGLS models with manipulation complexity as response variable and tool use, extractive foraging or cognitive test performance as explanatory variables.
| Data set | n | λ | adj. r2 | Predictor variable | Estimate | Std. error | P-value |
|---|---|---|---|---|---|---|---|
| excluding Homo sapiens | 36 | 0.694 | 0.125 | tool use | 0.917 | 0.374 | 0.020 |
| including Homo sapiens | 37 | 0.731 | 0.172 | tool use | 1.052 | 0.361 | 0.006 |
| excluding Homo sapiens | 36 | 0.760 | 0.077 | extractive foraging | 0.520 | 0.263 | 0.056 |
| including Homo sapiens | 37 | 0.808 | 0.081 | extractive foraging | 0.554 | 0.271 | 0.048 |
| Deaner et al.20 | 15 | 0 | 0.552 | cog. performance | 0.983 | 0.230 | <0.001 |
| Reader et al.11 | 19 | 0.444 | 0.396 | cog. performance | 0.566 | 0.158 | 0.002 |
Significant effects are highlighted in bold face.
Table 2. PGLS models with manipulation complexity as response variable and brain size as explanatory variables, terrestriality and diet quality as covariates singly and as combined models (n = 34, Homo sapiens excluded).
| Model | P-value model | λ | adj. r2 | AIC | ∆AIC | Predictor variables | Estimate | Std. error | P-value |
|---|---|---|---|---|---|---|---|---|---|
| model 1 | <0.001 | 0 | 0.728 | 60.773 | – | log brain | 1.286 | 0.387 | 0.002 |
| log body | −0.551 | 0.312 | 0.087 | ||||||
| terrestriality | 0.948 | 0.328 | 0.007 | ||||||
| model 2 | <0.001 | 0 | 0.721 | 62.463 | 1.690 | log brain | 1.376 | 0.429 | 0.003 |
| log body | −0.618 | 0.346 | 0.081 | ||||||
| terrestriality | 0.927 | 0.335 | 0.010 | ||||||
| log diet quality | −0.313 | 0.607 | 0.610 | ||||||
| model 3 | <0.001 | 0 | 0.663 | 67.105 | 6.332 | log brain | 1.274 | 0.430 | 0.006 |
| log body | −0.416 | 0.343 | 0.234 | ||||||
| model 4 | <0.001 | 0 | 0.659 | 68.438 | 7.665 | log brain | 1.422 | 0.474 | 0.005 |
| log body | −0.531 | 0.376 | 0.165 | ||||||
| log diet quality | −0.513 | 0.666 | 0.447 | ||||||
| model 5 | <0.001 | 0.147 | 0.567 | 68.498 | 7.725 | terrestriality | 0.863 | 0.374 | 0.028 |
| log body | 0.439 | 0.098 | <0.001 | ||||||
| model 6 | <0.001 | 0.211 | 0.461 | 73.783 | 13.010 | log diet quality | −0.069 | 0.659 | 0.918 |
| log body | 0.531 | 0.101 | <0.001 |
Body mass is always included as covariate.
Significant effects and best-fitting models are highlighted in bold face.
Figure 2. Relationship between manipulation complexity and ln (brain size), for various types of substrate use (raw species values, blue = terrestrial, green = partly terrestrial, red = arboreal) shown for visualisation purpose.
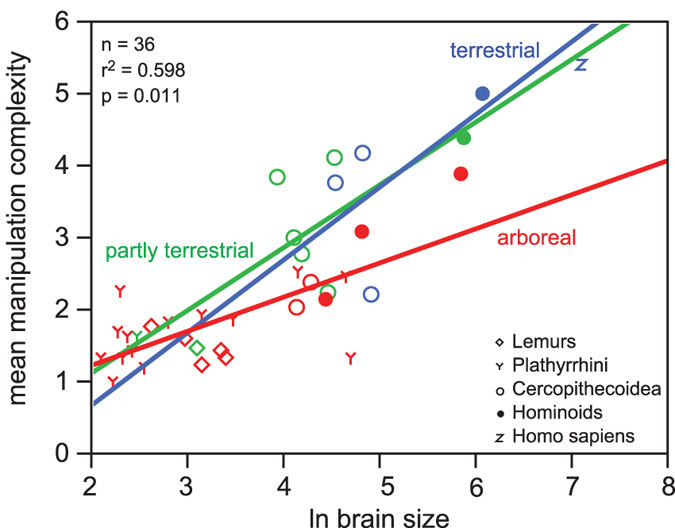
Species values are listed in Supplementary Table S1. Homo sapiens is not included in the calculation of the correlation and is only shown for illustrative purposes. Statistics see Table 2.
From visual inspection, the relationship between manipulation complexity and brain size appeared to be steeper in terrestrial species, but the effect of the interaction was not statistically significant (Table 2 and Supplementary Table S2, Fig. 2 and Supplementary Fig. S3). Diet quality did not affect the relationship between manipulation complexity and brain size (Table 2 and Supplementary Fig. S3).
Humans reached by far the highest manipulation complexity score of all tested species (Supplementary Table S1). Including Homo sapiens in the analyses increased the magnitude and significance of the difference in manipulation complexity between species that use tools (P = 0.006) and perform extractive foraging (P = 0.048), compared to species that do not (Table 2 and Supplementary Table S3). Furthermore, the human data point always lies above the upper confidence limit calculated using the relationship between manipulation complexity and brain size of nonhuman primates. This indicates that the human manipulation complexity score and brain size were higher compared to nonhuman primates (Table 3). The human manipulation complexity score calculated using the bootstrapping approach, on the basis of nonhuman primates, was 4.98, which is below the actual measured score of 5.40. Therefore, the human manipulation complexity score was higher than predicted for our brain size, and additional factors (e.g. bipedality, see discussion) may have enhanced human manipulative skills.
Table 3. Mean and width of the 95% confidence intervals obtained through bootstrapping.
| Data set | 95% CI mean | 95% CI width | |
|---|---|---|---|
| 25 species | nonhuman primates | 1.205 | 1.186–1.225 |
| Homo sapiens included | 1.294 | above the upper CI | |
| 30 species | nonhuman primates | 1.236 | 1.224–1.249 |
| Homo sapiens included | 1.294 | above the upper CI |
Discussion
If we aim to understand the evolutionary link between primate manipulative skills and the flourishing of complex technology among humans, we need explicit and consistent operational definitions of manipulative skills as well as a thorough evaluation of the levels of difficulty of the various manipulative actions across a broad variety of primate species38. Here, we provide manipulation categories that can be easily distinguished, and show that they can be ranked cumulatively according to their complexity across a broad range of primate species, and that this complexity measure is correlated with brain size and relevant ecological aspects (extractive foraging and tool use).
Consistent with previous studies, non-human primate taxa considered to be the most dexterous–specifically chimpanzees16,39,40, gorillas4,41, orangutans4,16,42, geladas4 and macaques4,7–also showed high manipulative complexity in our study. These findings support the accuracy of our rankings of the manipulation categories according to difficulty.
Furthermore, the different manipulation categories found in this study correspond quite well to the food processing behaviours observed in wild living primates. For instance, the 72 functionally distinct manipulative actions in wild living mountain gorillas recorded by Byrne and colleagues41 would be classified as levels 1 to 6 in our manipulation complexity scheme. But we also observed tool use of level 7 in the captive Western lowland gorillas. Chimpanzees of the Taï National Park (Côte d’Ivoire)43 and of the Mahale Mountains National Park (Tanzania)44 are able to perform all manipulation categories 1 to 7 found in our study.
Free ranging Japanese macaques7 exhibit asynchronous and synchronous hand and digit use according to complexity levels 1–6 in our scheme, matching our findings for Tonkean macaque and Barbary macaque. The long-tailed macaques, on the other hand, for which we also found level 7 manipulations, are renowned for high manipulation complexity, tool use and extractive foraging in the wild4.
Complexity of manipulations
Most previous studies on object manipulation and tool-use in primates were either experimentally induced or directed towards experimentally provided standardized objects (e.g.5,39,40). In contrast, in our study, manipulations were unconstrained and undertaken in a non-experimental setting, without introducing new objects or changes to the daily routine of the animals. Animals were free to select food items and any additional objects available within the enclosure. Our data demonstrate that the types of food (sometimes, including chopped fruits or pellets) available to the primates under study did not limit their capacity for manipulations. First, several species exhibited manipulations of high complexity classified in category 7. Second, even species with generally low manipulation complexity showed a few manipulations of higher complexity. Third, species differed in manipulation complexity even when the food items were identical. For example, all primates in the Parc Zoologique et Botanique de Mulhouse (France) were fed with similar-sized chopped fruits. Yet the highest manipulation complexity category ever reached, by different species held in this specific zoo, varied between 1 and 6. In summary, manipulations were not constrained by the availability of a particular food item or object, and all species had the opportunity to show the full range of manipulations.
In our study, the complexity of a manipulation increased when using both hands instead of just one hand for the action. Other studies have indeed found that patterns performed unimanually are more straightforward for the brain to program than patterns performed bimanually5,6,8. For instance, reaching, grasping or holding a food item arise very early in infancy, suggesting a low level of manipulative complexity45.
Second, we found that manipulating two objects is more complex than manipulating the same object with both forelimbs. This corresponds to earlier studies on ontogeny of nut cracking in wild chimpanzees. Biro and colleagues46 found that in infants in early stages of development interactions with nuts or stones were restricted to the manipulation of single objects on their own, such as holding a stone or rolling a nut. This stage was then followed at later ages by performing actions on multiple objects, indicating that manipulating one object is less complex than manipulating two objects.
Third, the complexity of a manipulation increased with the capability to move digits asynchronously. Again, this pattern also occurs during ontogeny. During human development, individuated movements of the fingers become superimposed on more fundamental grasping movements involving synchronous digits. Reflexive closure of the entire hand, which is present at birth, is followed by voluntary grasping at 2–3 months of age. Thumb opposition and finger individuation start to appear at 10–12 months indicating that manipulations with asynchronous digits are more complex than with synchronous digits45. Furthermore, a study in capuchins showed that they performed manipulation involving synchronous digits much quicker than manipulations involving asynchronous digit use suggesting that using the digits asynchronously seems to be more complex than synchronous digit use47.
Fourth, manipulations with synchronous hand use tended to be more complex than manipulations with asynchronous hand use. This finding is surprising as previous studies suggested that patterns of asynchronous hand use are more complex than those of synchronous hand use within species5,6,8. In future studies we will revisit this unexpected result by assessing whether the order of emergence of these manipulation categories during ontogeny matches the order of the complexity scale found in this study.
Ecology
Primate species engaging in extractive foraging and tool use tended to have higher manipulation complexity than those that do not. Previous studies have shown that exactly these two food acquisition modes are mastered relatively late in development, and attributed their late appearance to them being cognitively demanding and involving complex manipulative patterns15,42. Both suggestions are supported by our results.
Cognitive abilities
Contrary to a study on forelimb dexterity in carnivores3, we found that in primates brain size and cognitive test performance exhibit correlated evolution with manipulation complexity. This relationship between brain size and manipulation complexity persists even after controlling for foraging group size (Supplementary Table S7). The difference between primates and carnivores may be due to the underlying adaptation for grasping in the primate forelimb. Correlated evolution of brain size and manipulation complexity may be hindered by a phylogenetic constraint of paw morphology in carnivores, a group in which arboreality is only a secondary adaptation. To explain patterns of correlated evolution that shaped brain size variation across mammals, such discrepancies between orders should be studied in more detail.
Additional results on the relationship between the size of specific brain regions (neocortex or cerebellum size) and manipulation complexity showed that relative cerebellum size was not correlated with manipulation complexity (Supplementary Table S6). Relative neocortex size on the other hand was positively correlated with manipulation complexity (Supplementary Table S6). This may indicate a closer link between manipulation complexity and cognitive rather than motor skills. However, the cerebellum is involved not only in sensory-motor control and automatized learning of motor skills, but may also play a role in understanding and producing complex behavioural sequences including tool use1,48. Because our results on brain parts depend on a relatively small sample, they must be regarded with caution.
Terrestriality
The primate pattern suggests that not only full terrestriality, but already a partly terrestrial habit may have positively affected the correlated evolution between manipulation complexity and brain size. The secondary adoption of a partly terrestrial habit may therefore have facilitated innovation by allowing more frequent and repeated actions on objects, as well as the use of complex actions requiring the coordinated involvement of both hands14,16. Furthermore, food manipulation complexity in mainly terrestrial species might also be higher as terrestriality affects the availability of food and raw materials to be used as tools49,50. Terrestrial habitats present a wider range of possible substrates and materials, such as stones and grass stems, in addition to twigs and leaves that can be used as tools49,50. Similarly, in birds, complex manipulations and tool-use behaviour have been observed in free-ranging species foraging a high proportion of time on the ground, such as ravens and several crow species including New Caledonian crows51,52.
That terrestrial species showed higher manipulation complexity compared to arboreal ones might also be due to the fact that manipulation and locomotion pose different and frequently opposed selection pressures on primate hand morphology. The forelimb-dominated climbing and suspensory behaviours of arboreal species such as e.g. in gibbons favours a long hand functioning as a grasping hook during suspension and/or climbing that is thus less well suited for manipulative functions53,54. In contrast, terrestrial quadrupedalism favours a short hand, which is far more compatible with an enhanced thumb/hand relationship, which in turn enables more complex manipulations as in geladas and baboons55. However, hand morphology alone does not explain manipulation complexity. The aye-aye (Daubentonia madagascerensis), an arboreal species with long digits, especially the thin middle one (D3), would be presumed to have a rather limited capacity for complex manipulations based on its hand morphology. Conversely, in a comparative study, aye-ayes were able to perform more complex manipulations than other lemurs by using the thumbs to secure the food in a sophisticated way56. This finding demonstrates that fine motor control in the brain can sometimes override motor limitations imposed by body morphology. It is also consistent with our finding that brain size is related to manipulation complexity, since aye-ayes are very large-brained lemurs.
The human case
We also found that humans reach by far the highest manipulation complexity, even higher than predicted for our brain size. Human foragers occupy the most complex foraging niche of all mammals57, and forager diet requires intensive processing and relies heavily on enhanced manipulative skills58. There is ample fossil evidence that, over the course of human evolution, increasingly bipedal habits freed the hand from the constraints of locomotion and hands could evolve primarily for manipulation, including tool use and eventually tool production59,60. The occurrence of human-like hand proportions and features linked to precision grip in very early hominins even hint at the possibility that manipulative skills were an early autapomorphy of the human lineage that co-evolved with habitual bipedalism and was not necessarily related to stone tool production61,62,63. Admittedly, primate species engaging in tool use also showed higher manipulation complexity than those that do not, suggesting that tool use is also involved in enhancing manipulation complexity. However, as the amount of variation in manipulation complexity explained by tool use or extractive foraging is rather small, brain size and terrestriality may be more important factors.
Our comparative evidence also suggests that terrestriality alone already improves manipulative skills. Thus, in human evolutionary history, the combination of terrestriality with bipedality may have boosted a positive feedback loop with manipulation complexity, far beyond the range of other primates, combined with an unusually large brain and the corresponding cognitive abilities. Together with previous findings that terrestriality is crucial for acquiring and maintaining complex tool variants in primates16, our study lends support to the notion that the combination of intelligence and terrestriality may have been a major pacemaker of hominin technological evolution64.
That this simple categorization of manipulation complexity yields a consistent pattern of correlation between processing food and brain size obviously does not rule out that finding food may also play an important role in the evolution of cognitive abilities. Although our results add manipulation complexity to a suite of emerging evidence linking cognition with ecological rather than with social factors1,2,12,13, the outcomes of the present study are also consistent with a role for social factors, as among primates, the developmental acquisition of all complex manipulative skills has a major social-learning component65. However, if social challenges alone (independent of social learning of skills) were responsible for the evolution of the unusually large human brain (e.g.66), we would expect human manipulation complexity to be lower than expected for our brain size. The fact that the opposite was actually found, is not favourable to the idea that only purely social challenges were involved.
Additional Information
How to cite this article: Heldstab, S. A. et al. Manipulation complexity in primates coevolved with brain size and terrestriality. Sci. Rep. 6, 24528; doi: 10.1038/srep24528 (2016).
Supplementary Material
Acknowledgments
We sincerely thank Janneke van Woerden, Erik Willems and Kathelijne Koops for contributing data to our compilation. We are grateful to Rob Barton and Ellen Meulman for their constructive comments. We thank the following zoos for allowing us to collect behavioural data: Bioparco di Roma (Italy), Parc Zoologique & Botanique de Mulhouse (France), and Toni’s Zoo, Zoo Basel and Zoo Zurich (Switzerland). Financial support was provided through Swiss National Science Foundation (31003A-144210) and the University of Zurich.
Footnotes
Author Contributions S.A.H. and Z.K.K. performed behavioural observations and statistical analyses. All authors (S.A.H., Z.K.K., S.E.K., J.M.B., C.P.v.S. and K.I.) contributed to the design of the study. S.A.H., C.P.v.S. and K.I. wrote the paper, to which all authors contributed.
References
- Barton R. A. Embodied cognitive evolution and the cerebellum. Phil. Trans. R. Soc. B 367, 2097–2107 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S. T. & Gibson K. R. Object manipulation, tool use and sensorimotor intelligence as feeding adaptations in Cebus monkeys and great apes. J. Hum. Evol. 6, 623–641 (1977). [Google Scholar]
- Iwaniuk A. N., Pellis S. M. & Whishaw I. Q. Brain size is not correlated with forelimb dexterity in fissiped carnivores (Carnivora): a comparative test of the principle of proper mass. Brain Behav. Evol. 54, 167–180 (1999). [DOI] [PubMed] [Google Scholar]
- van Schaik C. P., Deaner R. O. & Merrill M. Y. The conditions for tool use in primates: implications for the evolution of material culture. J. Hum. Evol. 36, 719–741 (1999). [DOI] [PubMed] [Google Scholar]
- Hopkins W. D. & de Waal F. B. Behavioral laterality in captive bonobos (Pan paniscus): Replication and extension. Int. J. Primatol. 16, 261–276 (1995). [Google Scholar]
- Byrne R. W. In Stone Knapping: The Necessary Conditions for a Uniquely Hominid Behaviour (eds Roux V. & Bril B.) 159–169 (University of Cambridge: McDonald Institute for Archeological Research, 2005). [Google Scholar]
- Leca J.-B., Gunst N. & Huffman M. Complexity in object manipulation by Japanese macaques (Macaca fuscata): a cross-sectional analysis of manual coordination in stone handling patterns. J. Comp. Psychol. 125, 61 (2011). [DOI] [PubMed] [Google Scholar]
- Elliott J. M. & Connolly K. A classification of manipulative hand movements. Dev. Med. Child Neurol. 26, 283–296 (1984). [DOI] [PubMed] [Google Scholar]
- Biro D. et al. Cultural innovation and transmission of tool use in wild chimpanzees: evidence from field experiments. Anim. Cog. 6, 213–223 (2003). [DOI] [PubMed] [Google Scholar]
- Tomasello M. & Call J. Primate Cognition. (Oxford University Press, 1997). [Google Scholar]
- Reader S. M., Hager Y. & Laland K. N. The evolution of primate general and cultural intelligence. Phil. Trans. R. Soc. B 366, 1017–1027 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin A. D., Young H. C., Mosdossy K. N. & Fedigan L. M. Seasonality, extractive foraging and the evolution of primate sensorimotor intelligence. J. Hum. Evol. 71, 77–86 (2014). [DOI] [PubMed] [Google Scholar]
- Byrne R. W. In Machiavellian Intelligence II: Extensions and Evaluations Vol. 2 (eds Whiten A. & Byrne Richard W) 289–311 (Cambridge University Press, 1997). [Google Scholar]
- Fragaszy D. & Visalberghi E. Socially biased learning in monkeys. Anim. Learn. Behav. 32, 24–35 (2004). [DOI] [PubMed] [Google Scholar]
- Gunst N., Boinski S. & Fragaszy D. M. Development of skilled detection and extraction of embedded prey by wild brown capuchin monkeys (Cebus apella apella). J. Comp. Psychol. 124, 194–204 (2010). [DOI] [PubMed] [Google Scholar]
- Meulman E. J., Sanz C. M., Visalberghi E. & van Schaik C. P. The role of terrestriality in promoting primate technology. Evol. Anthropol. 21, 58–68 (2012). [DOI] [PubMed] [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 20, 37–46 (1960). [Google Scholar]
- Green B. F. A method of scalogram analysis using summary statistics. Psychometrika 21, 79–88 (1956). [Google Scholar]
- Guttman L. A basis for scaling qualitative data. Am. Sociol. Rev 9, 139–150 (1944). [Google Scholar]
- Deaner R. O., Isler K., Burkart J. & van Schaik C. Overall brain size, and not encephalization quotient, best predicts cognitive ability across non-human primates. Brain Behav. Evol. 70, 115–124 (2007). [DOI] [PubMed] [Google Scholar]
- Isler K. et al. Endocranial volumes of primate species: scaling analyses using a comprehensive and reliable data set. J. Hum. Evol. 55, 967–978 (2008). [DOI] [PubMed] [Google Scholar]
- Freckleton R. P. On the misuse of residuals in ecology: regression of residuals vs. multiple regression. Anim. Ecol. 71, 542–545 (2002). [Google Scholar]
- Myers P. et al. The Animal Diversity Web, (2006). Available at: < http://animaldiversity.org/accounts/Mammalia/>, (Date of access: 05/09/2014).
- Rowe N. & Myers M. All the World’s Primates, (2011). Available at: < http://www.alltheworldsprimates.org>, (Date of access: 08/09/2014).
- Gibson K. Cognition, brain size and the extraction of embedded food resources. Primate ontogeny, cognition and social behaviour 3, 93–104 (1986). [Google Scholar]
- Jaeggi A. V. & Van Schaik C. P. The evolution of food sharing in primates. Behav. Ecol. Sociobiol. 65, 2125–2140 (2011). [Google Scholar]
- Efron B. & Tibshirani R. J. An Introduction to the Bootstrap. (Chapman and Hall, 1993). [Google Scholar]
- Orme D. The caper package: comparative analysis of phylogenetics and evolution in R. R package version 5 (2013). [Google Scholar]
- van Woerden J. T., van Schaik C. P. & Isler K. Effects of seasonality on brain size evolution: Evidence from strepsirrhine primates. Am. Naturalist 176, 758–767 (2010). [DOI] [PubMed] [Google Scholar]
- Langer P. & Yoder A. Lactation, weaning period, food quality, and digestive tract differentiations in eutheria. Evolution 57, 1196–1215 (2003). [DOI] [PubMed] [Google Scholar]
- McNab B. K. The Physiological Ecology of Vertebrates: A View from Energetics. (Cornell University Press, 2002). [Google Scholar]
- JMP version 10.0 (1989-2016). SAS Institute Inc., Cary, North Carolina, USA.
- R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/.
- Martins E. P. & Hansen T. F. Phylogenies and the comparative method: A general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Naturalist 149, 646–667 (1997). [Google Scholar]
- Perelman P. et al. A molecular phylogeny of living primates. PLos Gen. 7, e1001342 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold C., Matthews L. J. & Nunn C. L. The 10 kTrees website: a new online resource for primate phylogeny. Evol. Anthropol. 19, 114–118 (2010). [Google Scholar]
- Akaike H. A new look at the statistical model identification. Trans. Aut. Control 19, 716–723 (1974). [Google Scholar]
- McGrew W. & Marchant L. On the other hand: current issues in and meta-analysis of the behavioral laterality of hand function in nonhuman primates. Yearb. Phys. Anthropol. 40, 201–232 (1997). [Google Scholar]
- Torigoe T. Comparison of object manipulation among 74 species of non-human primates. Primates 26, 182–194 (1985). [Google Scholar]
- Hayashi M. Stacking of blocks by chimpanzees: developmental processes and physical understanding. Anim. Cog. 10, 89–103 (2007). [DOI] [PubMed] [Google Scholar]
- Byrne R. W. & Byrne J. M. Manual dexterity in the gorilla: bimanual and digit role differentiation in a natural task. Anim. Cog. 4, 347–361 (2001). [DOI] [PubMed] [Google Scholar]
- Meulman E., Seed A. & Mann J. If at first you don’t succeed… Studies of ontogeny shed light on the cognitive demands of habitual tool use. Phil. Trans. R. Soc. B 368, 20130050 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesch C. & Boesch H. In Hands of Primates 31–43 (Springer, 1993). [Google Scholar]
- Corp N. & Byrne R. W. Leaf processing by wild chimpanzees: Physically defended leaves reveal complex manual skills. Ethology 108, 673–696 (2002). [Google Scholar]
- Gordon A. In Handbook of Brain and Behaviour in Human Development. (eds Kalverboer A. F. & Gramsbergen A.) 513–537 (Kluwer Academic Publishers, 2001). [Google Scholar]
- Biro D., Sousa C. & Matsuzawa T. In Cognitive Development in Chimpanzees (eds Matsuzawa Tetsuro, Tomonaga M. & Tanaka M.) 476–508 (Springer, 2006). [Google Scholar]
- Christel M. I. & Fragaszy D. Manual function in Cebus apella. Digital mobility, preshaping, and endurance in repetitive grasping. Int. J. Primatol. 21, 697–719 (2000). [Google Scholar]
- Barton R. A. & Venditti C. Rapid evolution of the cerebellum in humans and other great apes. Curr. Biol. 24, 2440–2444 (2014). [DOI] [PubMed] [Google Scholar]
- Visalberghi E., Fragaszy D. M., Izar P. & Ottoni E. B. Terrestriality and tool use. Science 308, 951–952, author reply 951-952 (2005). [DOI] [PubMed] [Google Scholar]
- McGrew W. C. The Cultured Chimpanzee: Reflections on Cultural Primatology. (Cambridge University Press, 2004). [Google Scholar]
- Emery N. J. & Clayton N. S. Tool use and physical cognition in birds and mammals. Curr. Opin. Neurobiol. 19, 27–33 (2009). [DOI] [PubMed] [Google Scholar]
- Rutz C., Bluff L. A., Weir A. A. & Kacelnik A. Video cameras on wild birds. Science 318, 765–765 (2007). [DOI] [PubMed] [Google Scholar]
- Susman R. L. Comparative and functional morphology of hominoid fingers. Am. J. Phys. Anthropol. 50, 215–236 (1979). [DOI] [PubMed] [Google Scholar]
- Inouye S. E. Ontogeny and allometry of African ape manual rays. J. Hum. Evol 23, 107–138 (1992). [Google Scholar]
- Maier W. In Hands of Primates 191–198 (Springer, 1993). [Google Scholar]
- Pellis S. M. & Pellis V. C. Anatomy is important, but need not be destiny: Novel uses of the thumb in aye-ayes compared to other lemurs. Behav. Brain Res. 231, 378–385 (2012). [DOI] [PubMed] [Google Scholar]
- Schuppli C., Isler K. & van Schaik C. P. How to explain the unusually late age at skill competence among humans. J. Hum. Evol. 63, 843–850 (2012). [DOI] [PubMed] [Google Scholar]
- Kaplan H., Hill K., Lancaster J. & Hurtado A. M. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185 (2000). [Google Scholar]
- Marzke M. W. Precision grips, hand morphology, and tools. Am. J. Phys. Anthropol. 102, 91–110 (1997). [DOI] [PubMed] [Google Scholar]
- Skinner M. M. et al. Human-like hand use in Australopithecus africanus. Science 347, 395–399 (2015). [DOI] [PubMed] [Google Scholar]
- Almécija S., Moyà-Solà S. & Alba D. M. Early origin for human-like precision grasping: a comparative study of pollical distal phalanges in fossil hominins. PLos One 5, e11727 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almécija S., Smaers J. B. & Jungers W. L. The evolution of human and ape hand proportions. Nature communications 6, doi: 10.1038/ncomms87 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panger M. A., Brooks A. S., Richmond B. G. & Wood B. Older than the Oldowan? Rethinking the emergence of hominin tool use. Evolutionary Anthropology: Issues, News, and Reviews 11, 235–245 (2002). [Google Scholar]
- Schick K. D. & Toth N. Making Silent Stones Speak: Human Evolution and the Dawn of Technology. (Simon and Schuster, 1994). [Google Scholar]
- van Schaik C. P. & Pradhan G. R. A model for tool-use traditions in primates: implications for the coevolution of culture and cognition. J. Hum. Evol. 44, 645–664 (2003). [DOI] [PubMed] [Google Scholar]
- Dunbar R. I. M. & Shultz S. Evolution in the social brain. Science 317, 1344–1347 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.