Abstract
The role of statins in preventing prostate cancer is currently a controversial issue. The aim of this review is to investigate the effects of statins use on prostate cancer risk. Electronic databases (the Cochrane Library, PubMed, Medline, Embase, Web of Science, and ClinicalTrials.gov) were searched systematically up to April, 2015. Weighted averages were reported as relative risk (RR) with 95% confidence intervals (CIs). Statistic heterogeneity scores were assessed with the standard Cochran’s Q test and I2 statistic. The pooled estimates of randomized controlled trials (RCTs) and retrospective studies suggest that statins have a neutral effect on total prostate cancer (RR = 1·02, 95% CI: 0·90–1·14; and RR = 0·91, 95% CI: 0·79–1·02, respectively). This research provides no evidence to suggest that the use of statins for cholesterol lowering is beneficial for the prevention of low-grade or localized prostate cancer, although a plausible association between statins use and the reduction risk of advanced (RR = 0·87, 95% CI: 0·82–0·91) or high-grade prostate cancer (RR = 0·83, 95% CI: 0·66–0·99) is observed. Furthermore, it shows that prostate cancer risk does not statistically significant benefit from long-term statins use.
Prostate cancer (PCa) is the most commonly diagnosed cancer among men in the USA1. Although the data from the American Society showed that the estimated 5-year survival rate is 98·9%, PCa remains the second most common cause of cancer-related deaths in USA and the leading cause of death in older men1. Thus, there is an urgent need for a better understanding of the factors related to the development of PCa and its prognosis.
Statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) are the most widely used drugs for lowering cholesterol. Over the past 25 years, there has been increasingly great interest in the antitumour effects of statins, and laboratory research suggests that statins show an inhibitory potential on the growth of PCa, both in vitro and in vivo2,3,4,5. However, clinical studies have not yet shown a consensus as to whether statin use is associated with a decreased (or increased) risk of overall PCa.
Recently, two meta-analyses6,7 discussed the association of statins with PCa risk; however, they reached contradictory conclusions. However, because of considerable evidence implies that statin use may reduce the risk of PCa, it is both important and necessary to gain a better understanding of whether such therapy can influence disease outcomes. Therefore, we conducted a comprehensive review of all relevant published studies and provided a quantitative assessment of these issues by analysing factors causing inconsistent results.
Methods
Study selection
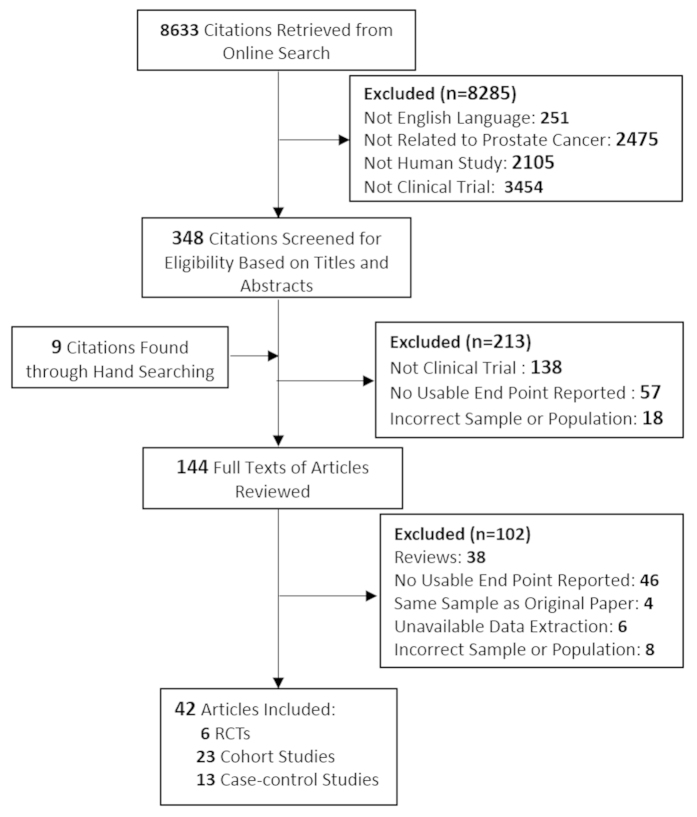
A literature search was performed without language restrictions using the databases of PubMed (Jan 1967–April 2015), MEDLINE (Jan 1967–April 2015), EMBASE (Jan 1990–April 2015), The Cochrane Library, Web of Science, and ClinicalTrials.gov. In addition, a manual search in published articles was conducted to identify additional relevant studies. After removing duplicate publications, two reviewers (Tan & Wei) independently assessed all remaining results by checking titles and abstracts. Studies investigating the association between statins and PCa were considered for further full-text assessment. All randomized controlled trials (RCTs), cohort studies, and case-control studies with both full-text articles and abstracts associated with the topic were considered to be eligible. Letters to the editor, comments, editorials, case reports, and animal studies were excluded. When studies reported outcomes from similar or overlapping databases or cohorts, only data from the most recent publication were included. We adapted a PRISMA (preferred reporting items for systematic reviews and meta-analyses) flow-chart to depict the study selection.
Data extraction
Data from each study were independently extracted by two reviewers (Tan & Wei) using a standardized data-extraction form. Any disagreements were resolved by consensus or by consultation with a third reviewer (Yang). The following information was checked for each article: first author’s last name, year of publication, location of study, study period, type of study design, mean follow-up time, drugs studied, duration of statin use, study population, number of male subjects, mean age of population, number of total cases of PCa, advanced (defined by the stage of the disease as ‘regional’ or ‘distant’ or the TNM stage within T3-4, N1-3 and M1) and localized PCa cases (defined by the stage of the disease as ‘localized’ or the TNM stage as T1-2, N0/x and M0/x.), high (Gleason sum ≥ 7) and low grade PCa cases (Gleason sum <7), PCa cases occurring during short- and long-term statins use (‘long-term’ was defined as ≥5 years of use; ‘short-term’ was defined as <5 years of use), risk estimates [including relative risk (RR), odds ratio (OR) and hazard ratio (HR)] adjusted for the maximum number of confounding variables with corresponding 95% confidence intervals (CIs). In addition, we also tried to contact authors via e-mail to obtain further information that had not been reported in their published articles.
Quality assessment
Two reviewers (Tan & Wei) independently used the Newcastle–Ottawa Scale (NOS) to assess the quality of the observational studies included (cohort and case-control studies). NOS comprises three parts (selection, comparability, and exposure for case-control studies or outcome for cohort studies) and scores of 4, 2 and 3 are assigned for these three parts, respectively. Studies with scores of 0–3, 4–6 and 7–9 were considered as low, moderate and high quality, respectively. The quality assessment of RCTs was conducted using the modified Jadad scale, which gives the following scores: generation of the allocation sequence (2), concealment of allocation (2), blinding (2), and incomplete outcome data (1). Scores of 1–3 indicate low quality and 4–7 indicate high quality.
Statistical analysis
RRs and their 95% CIs were used to assess the strength of association between statin use and the risk of PCa in RCTs and retrospective studies. Because HR was broadly equivalent to RR8,9, HRs were directly considered to be RRs. ORs were converted into RRs using the following formula: RR = OR/[(1 − P0) + (P0 × OR)], where P0 stands for the incidence of PCa in the non-statin use group10. We identified heterogeneity between studies using the standard Cochran’s Q test with a significance level of α = 0·10. We also examined heterogeneity with the I2 statistic, which quantifies inconsistency across studies to assess the impact of meta-analysis heterogeneity. An I2 statistic of 50% or more indicates a considerable level of heterogeneity. When heterogeneity was found, we attempted to determine potential sources of heterogeneity by examining individual study and subgroup characteristics. Fixed-effects models were used to pool risk estimates when heterogeneity among studies was considered statistically insignificant. Otherwise, random-effects model was applied to combine the results. We conducted subgroup analyses according to sample size, duration of statin use and stage or grade of PCa. Publication bias was detected using the Egger’s tests. Statistical significance was determined using the two-tailed test, where P < 0·05 was considered significant. STATA version 10 (Stata corporation, college station, TX) was employed to conduct all statistical analyses.
Results
A total of 8,633 articles were identified during the initial search (Fig. 1), and after employing exclusion criteria, a total of 42 studies were included, consisting of 23 cohort studies11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33, 13 case-control studies34,35,36,37,38,39,40,41,42,43,44,45,46 and 6 RCTs47,48,49,50,51,52, all of which involved more than 159,000 PCa cases. The characteristics of the cohort and case-control studies are presented in Supplementary Tables 1 and 2, respectively. Information regarding statins use and the diagnosis of PCa were mainly obtained from medical records and databases, the other sources were self-reported data. The 95% CI of 24 studies included 1·00, showed that no effect had been identified; 11 studies found a significant risk in the reduction of overall PCa in statin users; conversely, seven studies suggested an increased risk.
Figure 1. Trial Identification, Inclusion, and Exclusion.
Statins and risk of total PCa
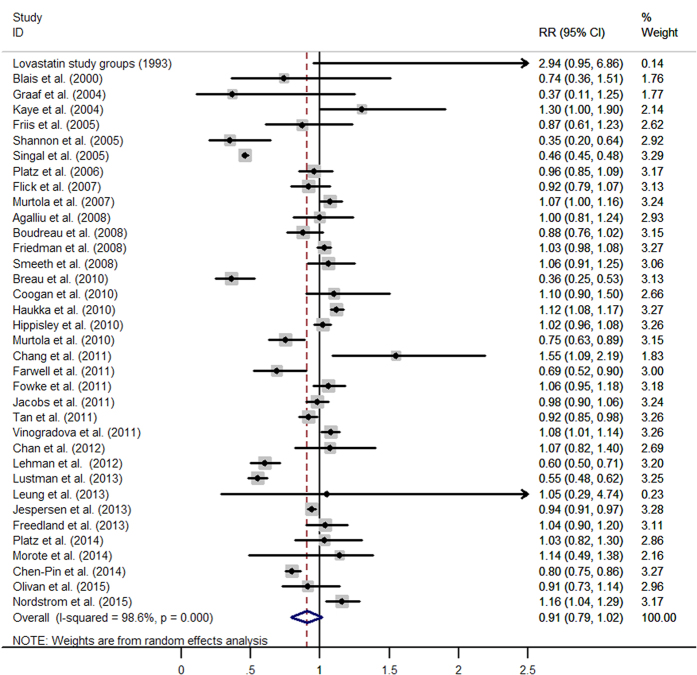
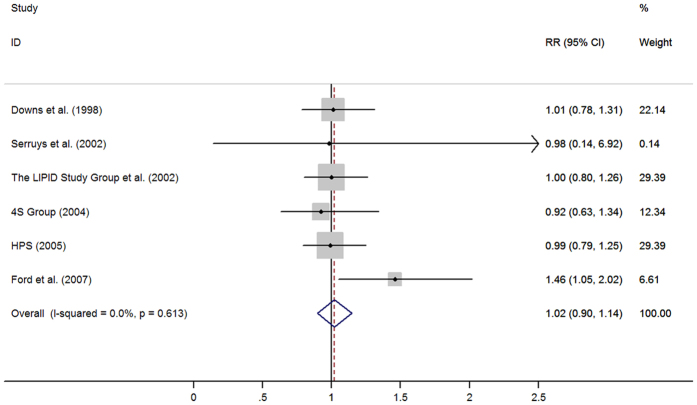
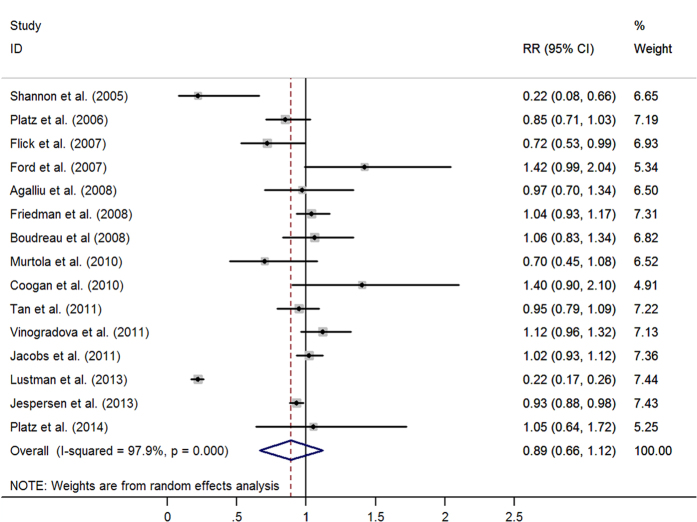
The pooled results from 36 retrospective studies (RR = 0·91, 95% CI: 0·79–1·02) (Fig. 2) and six RCTs (RR = 1·02, 95% CI: 0·90–1·14; I2 = 0·0%, p = 0·613) (Fig. 3) both suggested that statins have a neutral effect on total PCa. However, results of 23 cohort studies showed an inverse association (RR = 0·90, 95% CI: 0·82–0·99). Cumulative meta-analysis found there was no association between statins use and PCa risk since first studies in 1993 and remained stable after that, only a benefit was noted when Lustman et al.29 added in 2013. A summary of analyses results is shown in Table 1.
Figure 2. Statins use and risk of total prostate cancer in observational studies.
(From random-effects model, RR, relative risk; 95%CI, 95% confidence intervals).
Figure 3. Statins use and risk of total prostate cancer in 6 randomized controlled trials (From Fixed-effects model, RR, relative risk; 95%CI, 95% confidence intervals).
Table 1. The pooled estimates of meta-analysis in subgroups.
|
Pooled estimates |
|||||
|---|---|---|---|---|---|
| Outcome | No. of studies | RR | 95%CI | I2 statistic P-Value | |
| Total PCa | All studies | 42 | 0.92 | 0.82 to 1.03 | <0.001 |
| RCTs | 6 | 1.02 | 0.90 to 1.14 | 0.613 | |
| Without RCTs | 36 | 0.91 | 0.79 to 1.02 | <0.001 | |
| Cohort studies | 23 | 0.90 | 0.82 to 0.99 | <0.001 | |
| More than 10,000$ | 14 | 0.91 | 0.82 to 1.01 | <0.001 | |
| Case control studies | 13 | 0.90 | 0.68 to 1.12 | <0.001 | |
| More than 10,000 | 5 | 0.85 | 0.55 to 1.32 | <0.001 | |
| Advanced PCa | All studies | 11 | 0.87 | 0.82 to 0.91 | 0.082 |
| Cohort studies | 7 | 0.82 | 0.73 to 0.91 | 0.109 | |
| More than 10,000 | 6 | 0.81 | 0.72 to 0.90 | 0.161 | |
| Case control studies | 4 | 0.88 | 0.83 to 0.93 | 0.164 | |
| More than 10,000 | 2 | 0.84 | 0.71 to 1.00 | 0.076 | |
| Localized PCa | All studies | 8 | 0.98 | 0.91 to 1.06 | 0.001 |
| Cohort studies | 5 | 0.95 | 0.83 to 1.08 | <0.001 | |
| More than 10,000 | 4 | 0.95 | 0.83 to 1.08 | <0.001 | |
| Case control studies | 3 | 1.00 | 0.95 to 1.04 | 0.392 | |
| High-grade PCa | All studies | 15 | 0.83 | 0.66 to 0.99 | <0.001 |
| Cohort studies | 12 | 0.84 | 0.68 to 1.01 | <0.001 | |
| More than 10,000 | 6 | 0.83 | 0.57 to 1.08 | <0.001 | |
| Case control studies | 3 | 0.79 | 0.13 to 1.45 | <0.001 | |
| Low-grade PCa | All studies | 10 | 0.95 | 0.88 to 1.02 | 0.135 |
| Cohort studies | 7 | 0.96 | 0.85 to 1.07 | 0.091 | |
| More than 10,000 | 4 | 0.93 | 0.79 to 1.11 | 0.026 | |
| Case control studies | 3 | 0.92 | 0.75 to 1.10 | 0.261 | |
Abbreviations:PCa, prostate cancer; 95%CI, 95% confidence intervals; RR, relative risk.
$Subgroups analyses in studies included more than 10,000 participants.
In sensitivity analyses in which one study at a time was excluded and the rest were analysed, the results remained stable and no evident variability was found (data not shown).
Statins and risk of advanced and localized PCa
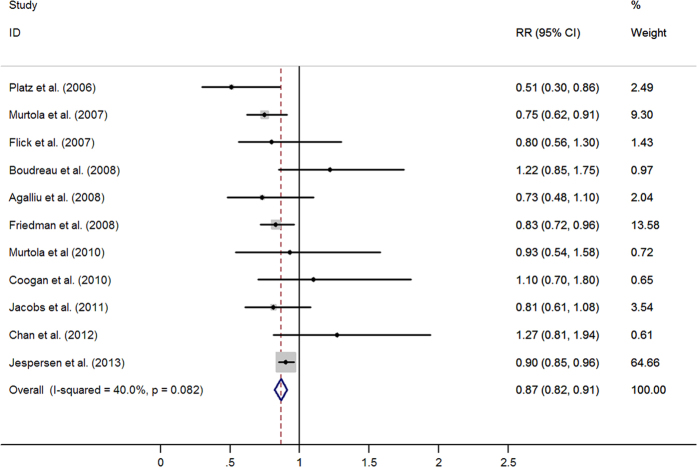
Eleven studies evaluated exposure to statins and the incidence of advanced PCa. The pooled estimates showed a statistically significant inverse association between statins use and the risk of advanced PCa (RR = 0·87, 95% CI: 0·82–0·91). No significant heterogeneity was observed (I2 = 40.0%, p = 0·082) (Fig. 4).
Figure 4. Statins use and risk of advanced prostate cancer (RR, relative risk; 95%CI, 95% confidence intervals).
Eight studies were available to evaluate the relationship between statins use and the incidence of localized PCa. However, the combined results showed that the association was neutral (RR = 0·98, 95% CI: 0·91–1·06; I2 = 71.6%, p = 0·001). (see Figure S1).
High-grade and low-grade PCa
Unexpectedly, the combined results of 15 retrospective studies found a significant association between statins use and the risk of high-grade PCa (RR = 0·83, 95% CI: 0·66–0·99; I2 = 90.3%, p < 0·001) (Figure S2). While this benefit was null among ten studies for the low-grade PCa (RR = 0·95, 95% CI: 0·88–1·02; I2 = 34·0%, p = 0·135) (Figure S3).
Long-term and short-term statin use
The combined RR of 15 trials suggested no statistically significant benefit from the use of long-term statins in relation to the risk of overall PCa (RR = 0·89, 95% CI: 0·66–1·12) (Fig. 5). In relation to long-term statin use, the pooled results showed an association with a decreased risk of advanced PCa (RR = 0·87, 95% CI: 0·79–0·95) and high-grade PCa (RR = 0·79, 95% CI: 0·65–0·92), but no association was observed with localized PCa and low-grade PCa. Synthesis of the available reports that had specifically examined statins use for more than 10 years in relation to total PCa (n = 3) indicated a protective association (RR = 0·92, 95% CI: 0·84–1·00) (Table 2).
Figure 5. Long-term statins use and risk of total prostate cancer (RR, relative risk; 95%CI, 95% confidence intervals).
Table 2. The analysis of relationship between the period of statins use and PCa risk.
| No. of studies | Pooled estimates | I2 statistic P-Value | |||
|---|---|---|---|---|---|
| Outcome | RR | 95%CI | |||
| Statins use less than 5 years | All studies | 14 | 0.88 | 0.78 to 0.98 | <0.001 |
| Total PCa | Cohort studies | 9 | 0.88 | 0.74 to 1.02 | <0.001 |
| More than 10,000 | 7 | 0.85 | 0.71 to 1.00 | <0.001 | |
| Case control studies | 5 | 0.88 | 0.70 to 1.06 | <0.001 | |
| More than 10,000 | 2 | 0.98 | 0.94 to 1.02 | 0.164 | |
| High-grade PCa | Cohort studies | 4 | 0.77 | 0.58 to 0.96 | 0.125 |
| Low-grade PCa | Cohort studies | 2 | 0.92 | 0.50 to 1.35 | 0.003 |
| Advanced PCa | All studies | 9 | 0.86 | 0.81 to 0.91 | 0.670 |
| Cohort studies | 6 | 0.81 | 0.71 to 0.91 | 0.755 | |
| Case-control studies | 3 | 0.88 | 0.82 to 0.95 | 0.447 | |
| Localized PCa | All studies | 7 | 1.02 | 0.95 to 1.09 | 0.051 |
| Cohort studies | 4 | 1.00 | 0.87 to 1.12 | 0.007 | |
| Case-control studies | 3 | 1.02 | 0.96 to 1.08 | 0.996 | |
| Statins use more than 5 years | All studies | 15 | 0.89 | 0.66 to 1.12 | <0.001 |
| Total PCa | Cohort studies | 9 | 0.84 | 0.52 to 1.16 | <0.001 |
| More than 10,000 | 7 | 0.73 | 0.50 to 1.06 | <0.001 | |
| Case control studies | 5 | 0.89 | 0.63 to 1.15 | <0.001 | |
| More than 10,000 | 2 | 1.01 | 0.84 to 1.20 | 0.03 | |
| High-grade PCa | Cohort studies | 5 | 0.79 | 0.65 to 0.92 | 0.669 |
| Low-grade PCa | Cohort studies | 3 | 0.94 | 0.71 to 1.16 | 0.072 |
| Advanced PCa | All studies | 9 | 0.87 | 0.79 to 0.95 | 0.049 |
| Cohort studies | 6 | 0.67 | 0.51 to 0.83 | 0.164 | |
| Case-control studies | 3 | 0.93 | 0.84 to 1.02 | 0.909 | |
| All studies | 7 | 0.97 | 0.88 to 1.05 | 0.059 | |
| Localized PCa | Cohort studies | 4 | 0.94 | 0.79 to 1.10 | 0.010 |
| Case-control studies | 3 | 0.96 | 0.88 to 1.03 | 0.859 | |
| Statins use more than 10 years | Case-control studies | 3 | 0.92 | 0.84 to 1.00 | 0.41 |
Abbreviations: PCa, prostate cancer; 95%CI, 95% confidence intervals; RR, relative risk.
$Subgroups analyses in studies included more than 10,000 participants.
Intriguingly, a benefit was noted among short-term statins users (n = 14) (RR = 0·88, 95% CI: 0·78–0·98) (Figure S4). In subgroup analyses, a statistically significant inverse association was identified with advanced PCa, but not with localized PCa.
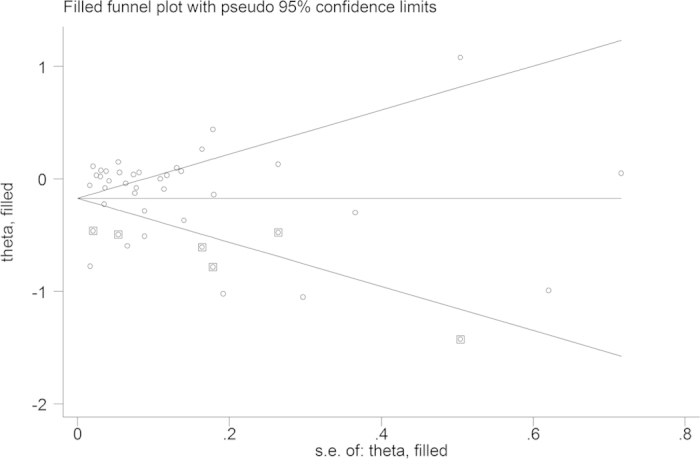
Publication bias
A potential publication bias was observed (Begg’s test, p = 0·002; Egger’s test, p = 0·380). Therefore, we performed a sensitivity analysis using the trim and fill method (Fig. 6.). The Filled estimate showed a reverse association (RR = 0·825, 95% CI: 0·737–0·924), means a possibly potential publication bias might exist.
Figure 6. Funnel plot for publication bias.
Discussion
There is no evidence provided by this research to suggest that the use of statins at low doses for managing hypercholesterolemia is beneficial for the prevention of total, low-grade, or localized PCa. This is generally consistent with a previous meta-analysis6 included 13 observational studies and six RCTs. Meanwhile, in three other meta-analyses of randomized controlled trials, they also found statins had a neutral effect on cancer and cancer death risk, and no type of cancer was affected by statins use53,54,55. However, a recent meta-analysis7 by Bansal et al. in 2012 included 27 studies and found approximately 7% reduction risk of total PCa in statins users compared with non-users. This inconsistency is likely to be associated with the inclusion of 16 new studies published after 2011, which suggested that statins lowered the incidence of total PCa. As expected, no association was found between the long-term statins use and the incidence of total PCa in their study. While the results of Bonovas et al.6 and Bansal et al.7 both showed an inverse association between statins use and the risk of advanced PCa, which was consistent with the result of our trial. In addition, we found that a benefit was noted in high-grade PCa, to our knowledge, which was found for the first time. While, this result should be approached with caution, as there was significant heterogeneity and upper CI was very close to 1.00.
As described above, the use of statins is both significantly inversely related to the risk of clinically advanced PCa and high-grade PCa. Given the known effects of statins on the PCa cell cycle and apoptosis, especially its ability to decrease the development of existing cancer rather than initiation of cancer, this finding may be plausibly explained. While, this finding may also be explained by a detection bias32. Because of the difference in social and economic statuses between statins users and non-users, patients who use statins may have better access to health care and receive greater preventive care, such as PSA screening or prostate biopsies, contributing to the early detection of PCa, thus lowering the risk of advanced/high-grade PCa. Another possible source of detection bias is the influence of statins on serum PSA. Hamilton et al.56 found that statins users have lower PSA than non-users and that levels of PSA decline after commencing statins use. However, Mondul et al.57 found that detection bias was unlikely to explain this potential inverse association. Hence, we cannot definitively declare that the observed association between statins and advanced PCa/high-grade PCa is causal or that it should be attributed to varying uptakes of PSA testing between statins users and non-users.
Intriguingly, we found no statistically significant benefit from the long-term use of statins, but a benefit was noted from short-term statins use. Whether this finding is attributed to either residual confounding or type I error of studies is unknown. One possible explanation is various definitions of duration of exposure in each trial and the irregular use of statins in many participants, with months of non-use between periods of use. Hence, the cumulative amount of statin defined daily doses (DDDs) could be small despite the long duration use. It should be noted that the inverse association between the risk of PCa and statins use was dose-dependent with a cumulative amount of statins use21. Thus, future studies should take fully into account of influence of cumulative amount of DDDs on the overall statins exposure.
This study has several limitations. First, the combined estimates in this study are inconsistent between cohort and case-control studies in some subgroups. These inconsistencies are likely to be attributed to inherent limitations, notably bias and unmeasured confounding factors existed in observational studies. At this stage, more RCTs would be required to evaluate these relationships. Second, significant heterogeneities were observed in some analyses that we conducted. Fortunately, the heterogeneities lowered down in planned subgroups, reflecting that stage or grade of PCa and period of statins use all contributed to heterogeneities. Furthermore, the number and content of adjusted confounders were varied among studies. Provided it is known that 5α-reductase inhibitors, aspirin and antidiabetic can affect the risk of PCa, which could have produced inaccuracy in the effect estimates. However, these information was unavailable in several studies18,25,27,29. To minimize these confounding biases, multivariable adjusted-effect estimates were selected. At last, a potential publication bias was noted among 42 studies, which might be attributed to the lower quality of some literature and the data of some meeting abstracts were unavailable. Thus, the part of our results should be explained with caution.
Conclusions
Statins have a neutral effect on PCa risk. However, a plausible link was found between a decreased risk of advanced PCa/high-grade PCa and statins use. It is considered that further studies are required to address the risk of overall PCa and clinically important advanced PCa/high-grade PCa among statin users with potential sources that may cause detection bias being well controlled.
Additional Information
How to cite this article: Tan, P. et al. LDL-lowering therapy and the risk of prostate cancer: a meta-analysis of 6 randomized controlled trials and 36 observational studies. Sci. Rep. 6, 24521; doi: 10.1038/srep24521 (2016).
Supplementary Material
Acknowledgments
WQ received a grant from the National Natural Science Foundation of China (Grant no. 81370855, 81200551), and has received a grant from Foundation of Science & Technology Department of Sichuan Province (Grant no. 2013SZ0006). YL has received a research grant from the Prostate Cancer Foundation Young Investigator Award 2013, the National Natural Science Foundation of China (Grant no. 81300627), and a grant from Foundation of Science & Technology Department of Sichuan Province (Grant no. 2015SZ0230). The funders of this research had no role in study selection, data extraction, analysis or interpretation, writing of this article, or the decision to publish. TP had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Author Contributions W.Q., Y.L. and T.P. had the idea for and designed this review. T.P., W.S.Y., Z.C., T.Z. and G.L. identified reports of trials and extracted data. Y.L. provided statistical advice and W.S.Y. and N.P. did all statistical analyses. Z.C. checked for statistical inconsistency and interpreted data. Y.L., T.P. and W.S.Y. contributed to data interpretation. T.P. drafted the report and all other authors (W.Q., Y.L., W.S.Y., Z.C., T.Z., N.P. and G.L.) critically reviewed the article. W.Q. is guarantor.
References
- Siegel R., Ma J., Zou Z. & Jemal A. Cancer statistics. CA-Cancer J. Clin. 64, 9–29 (2014). [DOI] [PubMed] [Google Scholar]
- Carlberg M. et al. Mevalonic acid is limiting for N-linked glycosylation and translocation of the insulin-like growth factor-1 receptor to the cell surface. Evidence for a new link between 3-hydroxy-3-methylglutaryl-coenzyme a reductase and cell growth. J. Biol. Chem. 271, 17453–17462 (1996). [DOI] [PubMed] [Google Scholar]
- Wong W. W., Dimitroulakos J., Minden M. D. & Penn L. Z. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 16, 508–519, 10.1038/sj.leu.2402476 (2002). [DOI] [PubMed] [Google Scholar]
- Jain M. K. & Ridker P. M. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat. Rev. Drug. Discov. 4, 977–987 (2005). [DOI] [PubMed] [Google Scholar]
- Dulak J. & Jozkowicz A. Anti-angiogenic and anti-inflammatory effects of statins: relevance to anti-cancer therapy. Curr. Cancer Drug Tar. 5, 579–594 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonovas S., Filioussi K. & Sitaras N. M. Statin use and the risk of prostate cancer: A metaanalysis of 6 randomized clinical trials and 13 observational studies. Int. J. Cancer 123, 899–904, 10.1002/ijc.23550 (2008). [DOI] [PubMed] [Google Scholar]
- Bansal D., Undela K., D’Cruz S. & Schifano F. Statin use and risk of prostate cancer: a meta-analysis of observational studies. PLoS One 7, e46691, 10.1371/journal.pone.0046691 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruance S. L., Reid J. E., Grace M. & Samore M. Hazard ratio in clinical trials. Antimicrob. Agents Ch. 48, 2787–2792, 10.1128/aac.48.8.2787-2792.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lemos M. L. How to survive the survival plots. Lancet. 360, 954, 10.1016/s0140-6736(02)11063-4 (2002). [DOI] [PubMed] [Google Scholar]
- Zhang J. & Yu K. F. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 280, 1690–1691 (1998). [DOI] [PubMed] [Google Scholar]
- Lovastatin 5-year safety and efficacy study. Lovastatin Study Groups I through IV. Arch. Intern. Med. 153, 1079–1087 (1993). [PubMed] [Google Scholar]
- Friis S. et al. Cancer risk among statin users: a population-based cohort study. Int. J. Cancer 114, 643–647, 10.1002/ijc.20758 (2005). [DOI] [PubMed] [Google Scholar]
- Platz E. A. et al. Statin drugs and risk of advanced prostate cancer. J. Natl. Cancer Inst. 98, 1819–1825, 10.1093/jnci/djj499 (2006). [DOI] [PubMed] [Google Scholar]
- Flick E. D. et al. Statin use and risk of prostate cancer in the California Men’s Health Study cohort. Cancer Epidem. Biomar. 16, 2218–2225, 10.1158/1055-9965.epi-07-0197 (2007). [DOI] [PubMed] [Google Scholar]
- Boudreau D. M., Yu O., Buist D. S. & Miglioretti D. L. Statin use and prostate cancer risk in a large population-based setting. Cancer Cause. Control. 19, 767–774, 10.1007/s10552-008-9139-4 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman G. D. et al. Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361,859 recipients. Pharmacoepidem. Dr. S. 17, 27–36, 10.1002/pds.1507 (2008). [DOI] [PubMed] [Google Scholar]
- Smeeth L., Douglas I., Hall A. J., Hubbard R. & Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br. J. Clin. Pharmaco. 67, 99–109, 10.1111/j.1365-2125.2008.03308.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breau R. H. et al. The association between statin use and the diagnosis of prostate cancer in a population based cohort. J. Urol. 184, 494–499, 10.1016/j.juro.2010.03.149 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukka J. et al. Incidence of cancer and statin usage–record linkage study. Int. J. Cancer 126, 279–284, 10.1002/ijc.24536 (2010). [DOI] [PubMed] [Google Scholar]
- Hippisley-Cox J. & Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 340, c2197, 10.1136/bmj.c2197 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtola T. J. et al. Prostate cancer and PSA among statin users in the Finnish prostate cancer screening trial. Int. J. Cancer 127, 1650–1659, 10.1002/ijc.25165 (2010). [DOI] [PubMed] [Google Scholar]
- Farwell W. R., D’Avolio L. W., Scranton R. E., Lawler E. V. & Gaziano J. M. Statins and prostate cancer diagnosis and grade in a veterans population. J. Natl. Cancer Inst. 103, 885–892, 10.1093/jnci/djr108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowke J. H. et al. The associations between statin use and prostate cancer screening, prostate size, high-grade prostatic intraepithelial neoplasia (PIN), and prostate cancer. Cancer Cause. Control. 22, 417–426, 10.1007/s10552-010-9713-4 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E. J., Newton C. C., Thun M. J. & Gapstur S. M. Long-term use of cholesterol-lowering drugs and cancer incidence in a large United States cohort. Cancer Res. 71, 1763–1771, 10.1158/0008-5472.can-10-2953 (2011). [DOI] [PubMed] [Google Scholar]
- Tan N., Klein E. A., Li J., Moussa A. S. & Jones J. S. Statin use and risk of prostate cancer in a population of men who underwent biopsy. J. Urol. 186, 86–90, 10.1016/j.juro.2011.03.004 (2011). [DOI] [PubMed] [Google Scholar]
- Chan J. M. et al. Statin use and risk of prostate cancer in the prospective Osteoporotic Fractures in Men (MrOS) Study. Cancer Epidem. Biomar. 21, 1886–1888, 10.1158/1055-9965.epi-12-0816 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman D. M., Lorenzo C., Hernandez J. & Wang C. P. Statin use as a moderator of metformin effect on risk for prostate cancer among type 2 diabetic patients. Diabetes. Care. 35, 1002–1007, 10.2337/dc11-1829 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedland S. J. et al. Statin use and risk of prostate cancer and high-grade prostate cancer: results from the REDUCE study. Prostate Cancer P. D. 16, 254–259, 10.1038/pcan.2013.10 (2013). [DOI] [PubMed] [Google Scholar]
- Lustman A., Nakar S., Cohen A. D. & Vinker S. Statin use and incident prostate cancer risk: does the statin brand matter? A population-based cohort study. Prostate Cancer P. D. 17, 6–9, 10.1038/pcan.2013.34 (2013). [DOI] [PubMed] [Google Scholar]
- Chen-Pin W. et al. Statins and Finasteride Use Differentially Modify the Impact of Metformin on Prostate Cancer Incidence in Men with Type 2 Diabetes. Ann. Transl. Med. Epidemiol. 1 (2014). [PMC free article] [PubMed] [Google Scholar]
- Morote J. et al. Role of serum cholesterol and statin use in the risk of prostate cancer detection and tumor aggressiveness. Int. J. Mol. Sci. 15, 13615–13623, 10.3390/ijms150813615 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz E. A. et al. Statin drug use is not associated with prostate cancer risk in men who are regularly screened. J. Urol. 192, 379–384, 10.1016/j.juro.2014.01.095 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom T., Clements M., Karlsson R., Adolfsson J. & Gronberg H. The risk of prostate cancer for men on aspirin, statin or antidiabetic medications. Eur. J. Cancer 51, 725–733, 10.1016/j.ejca.2015.02.003 (2015). [DOI] [PubMed] [Google Scholar]
- Blais L., Desgagne A. & LeLorier J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch. Intern. Med. 160, 2363–2368 (2000). [DOI] [PubMed] [Google Scholar]
- Graaf M. R., Beiderbeck A. B., Egberts A. C., Richel D. J. & Guchelaar H. J. The risk of cancer in users of statins. J. Clin. Oncol. 22, 2388–2394, 10.1200/jco.2004.02.027 (2004). [DOI] [PubMed] [Google Scholar]
- Kaye J. A. & Jick H. Statin use and cancer risk in the General Practice Research Database. Brit. J. Cancer 90, 635–637, 10.1038/sj.bjc.6601566 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon J. et al. Statins and prostate cancer risk: a case-control study. Am J. Epidemiol. 162, 318–325, 10.1093/aje/kwi203 (2005). [DOI] [PubMed] [Google Scholar]
- Singal R., Khurana V., Caldito G. & Fort C. Statins and prostate cancer risk: a large case-control study in veterans. J. Clin. Oncol. 23, 1004 (2005). [Google Scholar]
- Murtola T. J., Tammela T. L., Lahtela J. & Auvinen A. Cholesterol-lowering drugs and prostate cancer risk: a population-based case-control study. Cancer Epidem. Biomar. 16, 2226–2232, 10.1158/1055-9965.epi-07-0599 (2007). [DOI] [PubMed] [Google Scholar]
- Agalliu I., Salinas C. A., Hansten P. D., Ostrander E. A. & Stanford J. L. Statin use and risk of prostate cancer: results from a population-based epidemiologic study. Am J. Epidemiol. 168, 250–260, 10.1093/aje/kwn141 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan P. F., Kelly J. P., Strom B. L. & Rosenberg L. Statin and NSAID use and prostate cancer risk. Pharmacoepidem. Dr. S. 19, 752–755, 10.1002/pds.1970 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Ho S. C., Chiu H. F. & Yang C. Y. Statins increase the risk of prostate cancer: a population-based case-control study. Prostate 71, 1818–1824, 10.1002/pros.21401 (2011). [DOI] [PubMed] [Google Scholar]
- Vinogradova Y., Coupland C. & Hippisley-Cox J. Exposure to statins and risk of common cancers: a series of nested case-control studies. BMC Cancer. 11, 409, 10.1186/1471-2407-11-409 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen C. G., Norgaard M., Friis S., Skriver C. & Borre M. Statin use and risk of prostate cancer: a Danish population-based case-control study, 1997–2010. Cancer Epidemiol. 38, 42–47, 10.1016/j.canep.2013.10.010 (2013). [DOI] [PubMed] [Google Scholar]
- Leung H. W., Chan A. L., Lo D., Leung J. H. & Chen H. L. Common cancer risk and statins: a population-based case-control study in a Chinese population. Expert Opin. Drug Saf. 12, 19–27, 10.1517/14740338.2013.744392 (2013). [DOI] [PubMed] [Google Scholar]
- Olivan M. et al. Simultaneous treatment with statins and aspirin reduces the risk of prostate cancer detection and tumorigenic properties in prostate cancer cell lines. Biomed Res. Int. 2015, 762178, 10.1155/2015/762178 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs J. R. et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 279, 1615–1622 (1998). [DOI] [PubMed] [Google Scholar]
- Serruys P. W. et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA. 287, 3215–3222 (2002). [DOI] [PubMed] [Google Scholar]
- Disease)., L. S. G. L.-t. I. w. P. i. I. Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: the LIPID trial follow-up. Lancet. 359, 1379–1387, 10.1016/s0140-6736(02)08351-4 (2002). [DOI] [PubMed] [Google Scholar]
- Strandberg T. E. et al. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S). Lancet. 364, 771–777, 10.1016/s0140-6736(04)16936-5 (2004). [DOI] [PubMed] [Google Scholar]
- Group. H. P. S. C. The effects of cholesterol lowering with simvastatin on cause-specific mortality and on cancer incidence in 20,536 high-risk people: a randomised placebo-controlled trial [ISRCTN48489393]. BMC Med. 3, 6, 10.1186/1741-7015-3-6 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford I. et al. Long-term follow-up of the West of Scotland Coronary Prevention Study. N. Engl. J. Med. 357, 1477–1486, 10.1056/NEJMoa065994 (2007). [DOI] [PubMed] [Google Scholar]
- Dale K. M., Coleman C. I., Henyan N. N., Kluger J. & White C. M. Statins and cancer risk: a meta-analysis. JAMA. 295, 74–80, 10.1001/jama.295.1.74 (2006). [DOI] [PubMed] [Google Scholar]
- Kuoppala J., Lamminpaa A. & Pukkala E. Statins and cancer: A systematic review and meta-analysis. Eur. J. Cancer 44, 2122–2132, 10.1016/j.ejca.2008.06.025 (2008). [DOI] [PubMed] [Google Scholar]
- Browning D. R. & Martin R. M. Statins and risk of cancer: a systematic review and metaanalysis. Int. J. Cancer 120, 833–843, 10.1002/ijc.22366 (2007). [DOI] [PubMed] [Google Scholar]
- Hamilton R. J., Goldberg K. C., Platz E. A. & Freedland S. J. The influence of statin medications on prostate-specific antigen levels. J. Natl. Cancer Inst. 100, 1511–1518, 10.1093/jnci/djn362 (2008). [DOI] [PubMed] [Google Scholar]
- Mondul A. M., Caffo B. & Platz E. A. Minimal detection bias in the inverse association between statin drug use and advanced prostate cancer risk: a simulation study. Cancer Epidemiol. 35, e6–11, 10.1016/j.canep.2010.11.005 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.