Abstract
GLUT5, a fructose-transporting member of the facilitative glucose transporter (GLUT, SLC2) family, is a therapeutic target for diabetes and cancer but has no potent inhibitors. We virtually screened a library of 6 million chemicals onto a GLUT5 model and identified N-[4-(methylsulfonyl)-2-nitrophenyl]-1,3-benzodioxol-5-amine (MSNBA) as an inhibitor of GLUT5 fructose transport in proteoliposomes. MSNBA inhibition was specific to GLUT5; this inhibitor did not affect the fructose transport of human GLUT2 or the glucose transport of human GLUT1-4 or bacterial GlcPSe. In MCF7 cells, a human breast cancer cell line, MSNBA competitively inhibited GLUT5 fructose uptake with a KI of 3.2 ± 0.4 μM. Ligand docking, mutagenesis and functional studies indicate that MSNBA binds near the active site and inhibitor discrimination involves H387 of GLUT5. Thus, MSNBA is a selective and potent inhibitor of fructose transport via GLUT5, and the first chemical probe for this transporter. Our data indicate that active site differences in GLUT members could be exploited to further enhance ligand specificity.
Fructose is one of the most common dietary carbohydrates. During our evolution, humans have consumed relatively small amounts of fructose mostly from fruits. Recent studies indicate that fructose consumption increased by almost 50% among US adults in the last 40 years, with fructose accounting for at least 10% of daily calories on average1. Unlike glucose, fructose in serum is not regulated by insulin, and high levels of fructose consumption can cause dyslipidemia, impair glucose homeostasis and increase insulin resistance2. Some studies also link a fructose-rich diet with hypertension3,4.
Fructose transport across cell membranes is carried out by members of the facilitated glucose transporter (GLUT, SLC2) family. Among the 14 members of human GLUT protein family, only GLUT5 is fructose specific and lacks the ability to transport other carbohydrates such as glucose and galactose5,6,7. GLUT5 is expressed in intestinal epithelia, sperm, brain, fat, skeletal muscle and kidney cells8.
Cancer cells require more energy for their uncontrolled growth and usually exhibit increased rates of carbohydrates transport, compared to normal cells. GLUT5 is frequently overexpressed in cancer cells (~27% of analyzed tumors)9. For example, GLUT5 is not normally present in mammalian breast cells, but the breast carcinoma cell lines MCF7 and MDA-MB-231 exhibit elevated GLUT5 mRNA level and show high rates of fructose transport10. Additionally, in vitro studies linked fructose exposure to modification of the glycan structures on the cell surface that enhance cancer cell invasiveness and proliferation11. Also, abolishing GLUT5 expression in breast cancer cells inhibited tumor proliferation12. Pancreatic cancer cells prefer fructose in their nucleic acid synthesis, thus fructose promotes pancreatic cancer proliferation13. Consequently, GLUT5 activity is being explored as a marker for cancer, and development of fluorinated fructose analogs for use in positron emission tomography (PET) cancer diagnosis in GLUT5-overexpressing tumors is underway14.
In normal tissue, the expression of GLUT5 is upregulated by fructose15 and may be the underlying mechanism linking GLUT5 with metabolic disorders8. GLUT5 is upregulated in some diabetic patients and this expression is reversible with diabetes management treatment16.
Given the medical importance of GLUT5, its inhibitors could potentially serve as therapeutics for the treatment and management of cancer or diabetes. Nonetheless, selective and potent inhibitors for GLUT5 have not been described. Despite the high sequence similarity among GLUT members, known inhibitors of other GLUT proteins (for example cytochalasin B, phloretin, or forskolin) do not affect GLUT5, suggesting that subtle differences may be responsible for ligand specificity among GLUT family members (this work,17,18). Indeed, a recent study found two natural products that inhibit GLUT5; one of which, rubusoside, a natural sweetener from the Chinese sweet tea plant (Rubus suavissimus), inhibited both GLUT1 and GLUT5, the first example of a common inhibitor for these two GLUTs. The other molecule, astragalin-6-glucoside, a flavonoid compound from the American pokeweed (Phytolacca americana) did not affect GLUT1. These inhibitors, though not potent (IC50 values ~ 5 mM) allowed for the identification of active site differences between GLUT1 and GLUT5 responsible for ligand specificity17.
Viable drugs targeting the fructose transport of GLUT5 should not interfere with transport mediated by other GLUT proteins. For instance, disrupting glucose transport by the insulin-dependent GLUT4 can lead to diabetes19. GLUT1, another transporter of glucose, is expressed in most tissues, so its inhibition can cause serious side effects at the organism level. Ideally, GLUT5 specific inhibitors should only affect GLUT5 mediated fructose transport, without altering aspects of metabolism unrelated to fructose consumption.
In this work we describe a combination of in silico and in vitro experiments that determined a specific inhibitor of human GLUT5. We screened in silico a library of six million compounds for binding to a GLUT5 model. The top ranked 175 in silico hits were tested for inhibition of fructose transport by human GLUT5 in proteoliposomes, and we found that N-[4-(methylsulfonyl)-2-nitrophenyl]-1,3-benzodioxol-5-amine (MSNBA, SMILES: [S](=O)(=O)(C)c1cc(c(cc1)Nc2cc3c(cc2)OCO3)[N+](=O)[O−]) inhibited GLUT5. Additionally, MSNBA potently inhibited fructose uptake by GLUT5 in the human breast adenocarcinoma cell line MCF7.
MSNBA did not affect the transport activity of human GLUT1-4 or GlcPSe, appearing to be specific for GLUT5. Docking of MSNBA to the GLUT5 model, along with mutagenesis and functional studies on GLUT5, GLUT1 and GlcPSe, suggested that the inhibitor bound close to the active site and pinpointed a GLUT5-specific His residue as a key determinant of MSNBA recognition. MSNBA together with the active site differences between GLUT5 and GLUT1 highlighted by MSNBA inhibition can be exploited for the rational design of potent, specific inhibitors for GLUT5 that will aid in cancer or diabetes treatment. Additionally, MSNBA provides the first GLUT5-specific chemical probe that can be used to investigate the role of fructose uptake in mechanisms of these diseases.
Results
In silico screening for potential GLUT5 inhibitors
The model of GLUT5 was initially generated on the basis of the inward-facing GlcPSe crystal structure (PDB ID 4LDS) with Coot20. GlcPSe, a glucose/H+ symporter from Staphyloccocus epidermidis, is a bacterial GLUT homologue that shares 30% amino acid sequence identity with human GLUT521. After the virtual screening studies were completed, the crystal structures of human GLUT122 and bovine GLUT523 were published and we generated new GLUT5 models on the basis of these new structures [PDB IDs: 4PYP (hGLUT1) and 4YB9 (bGLUT5)]. There is significant variation in the soluble loops depending upon the starting model, but the transmembrane substrate cavity in all GLUT5 homology models was invariable, consistent with the similarity of the crystal structures (Supplementary Fig. S1); the root-mean-square deviations for the superposition in the transmembrane helices was less than 1.5 Å for hGLUT5 models constructed from GlcPSe, hGLUT1 and bGLUT5 structures, as calculated with Superpose24. Our modeled GLUT5 was screened against Chemnavigator’s (http://www.chemnavigator.com) library of 6,273,384 small molecular compounds, and a list of 374 possible GLUT5 ligands was generated. Among these, considering commercial availability and cost, 175 were purchased and tested for inhibition of GLUT5 transport activity (Supplementary Table S1).
In vitro screening for specific inhibitors of GLUT5
Human GLUT5 was recombinantly expressed in insect cells, purified, and reconstituted in artificial lipids as previously reported17. The fructose entrance counter-flow transport with GLUT5 proteoliposomes was constant between 1 and 2 minutes, so the inhibition of transport by compounds was measured one minute after assay initiation (Fig. 1a). Consistent with previous reports, GLUT5 transport was not affected by classical inhibitors of GLUT1 such as phloretin25 or cytochalasin B10 (Fig. 1b). The 175 potential GLUT5 ligands (Supplementary Table S1) were grouped in batches of 5 chemicals (each at 1 mM concentration) and tested for inhibition of fructose entrance counter-flow transport by GLUT5 in proteoliposomes. For batches that caused decreased fructose transport, the individual compounds from the group were tested for inhibition.
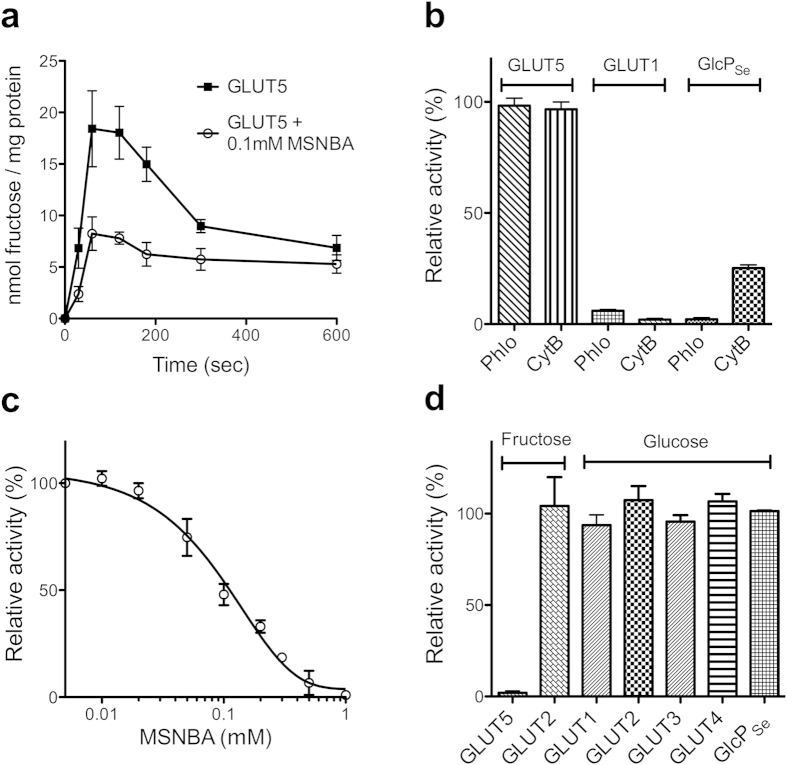
Figure 1. Effect of MSNBA on human GLUT1-5 and GlcPSe transport activities.
(a) Fructose entrance counter-flow transport in GLUT5 proteoliposomes, in the absence (filled squares) and presence (empty circles) of 0.1 mM MSNBA. Transport was initiated by the addition of GLUT5 proteoliposomes to the reaction solution containing 10 μM C14-fructose and stopped at the indicated time points. (b) Effect of common GLUT1 inhibitors on the relative transport activity of GLUT5, GLUT1 and GlcPSe. Glucose (for GLUT1) or fructose (for GLUT5) entrance counter-flow transport was measured one minute after initiation of transport by adding proteoliposomes to assay solution containing 10 μM C14-hexose and 2 mM phloretin (Phlo) or cytochalasin B (CytB). Glucose uptake for GlcPSe was measured one minute after initiation of transport with 30 μM C14-glucose in the presence of 2 mM phloretin or cytochalasin B in right-side-out vesicles. (c) Dose-dependent MSNBA inhibition of GLUT5 fructose transport in proteoliposomes. Each point was measured one minute after transport initiation, using the entrance counter-flow transport assay. IC50 of MSNBA inhibition was 0.10 ± 0.03 mM. Curve was calculated with Prism (GraphPad Software). (d) Effect of 2 mM MSNBA on the fructose transport by GLUT5 or GLUT2 or glucose transport by GLUT1-4 or GlcPSe. GLUT1-5 transport activity was measured in proteoliposomes, using the entrance counter-flow assay, as in (b). GlcPSe glucose transport was measured in right-side-out vesicles as in (b). Error bars represent standard deviations from 3 different experiments (a–d).
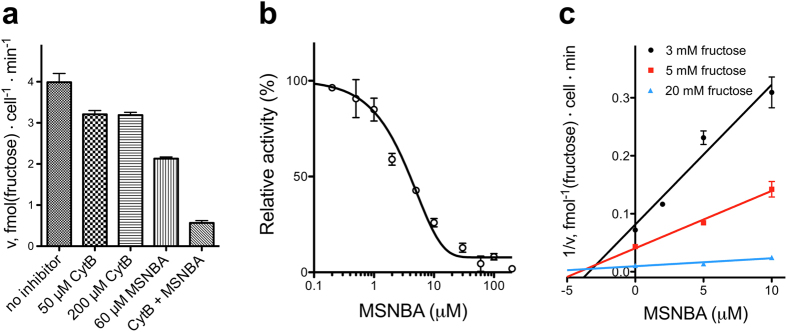
Figure 2. Inhibition of fructose uptake in MCF7 cells by MSNBA.
(a) Inhibitor effect on fructose uptake into MCF7 cells. Cytochalasin B (50 or 200 μM) and MSNBA (60 μM) individually or in combination (CytB + MSNBA is 50 μM cytochalasin B and 60 μM MSNBA), were pre-incubated with MCF7 cells for 5 minutes. The uptake assay was initiated by the addition of 10 mM fructose and the transport was stopped after 30 minutes. (b) Dose-dependent MSNBA inhibition of fructose uptake after 30 minutes in MCF7 cells in the presence of 10 mM fructose and 50 μM cytochalasin B. IC50 of MSNBA inhibition was 5.8 ± 0.5 μM. (c) Dixon plot of fructose uptake after 2 minutes into MCF7 cells with varying fructose and MSNBA concentrations, in the presence of 50 μM cytochalasin B. MSNBA displays competitive inhibition of fructose uptake with KI = 3.2 ± 0.4 μM. Kinetic parameters were determined with Prism (GraphPad Software).
We found one chemical with inhibitory activity against GLUT5: N-[4-(methylsulfonyl)-2-nitrophenyl]-1,3-benzodioxol-5-amine (MSNBA) (Fig. 1a,c,d; the chemical structure of MSNBA is shown in Fig. 3b). MSNBA inhibited GLUT5 mediated fructose transport with an IC50 of 0.10 ± 0.03 mM (Fig. 1c); this is 100-fold lower than the reported fructose KM for GLUT5 (~10 mM)10.
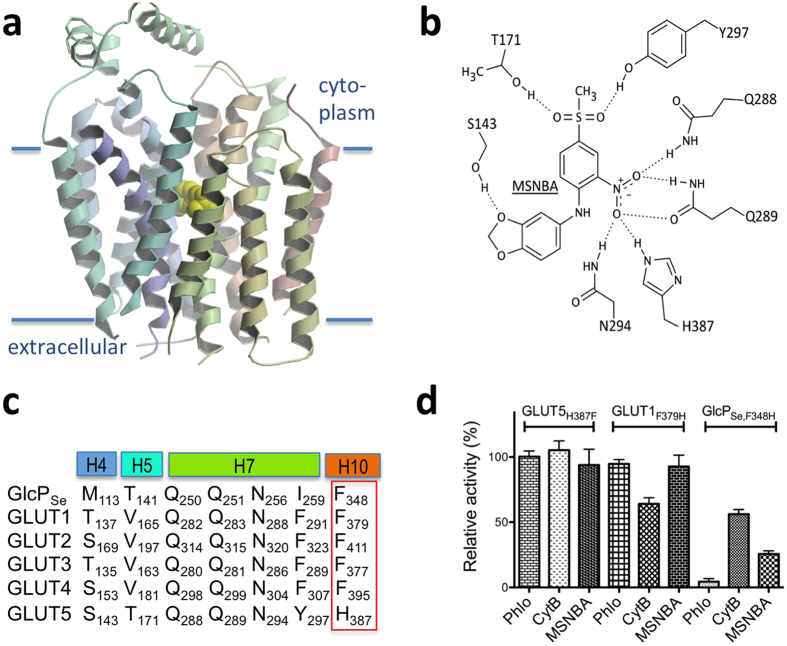
Figure 3. Modeled interaction of MSNBA with GLUT5.
(a) Binding of MSNBA (yellow CPK model) to the transmembrane site in GLUT5 as predicted by docking of the inhibitor to GLUT5 model with MOE. The figure was drawn with Molscript46 and raster3D47. (b) Interactions between MSNBA and side chains of GLUT5. Interacting residues come from helices 4 (S143), 5 (T171), 7 (Q288, Q289, N294 and Y297), and 10 (H387); see also Supplementary Fig. S4. (c) Multiple sequence alignment among GLUT homologues for the GLUT5 residues predicted to interact with MSNBA. H4, H5, H7 and H10 represent transmembrane helices 4, 5, 7 and 10, respectively, and are color-coded as in Fig. 3a. The red box highlights the residue explored by site-directed mutagenesis in GLUT1, GlcPSe and GLUT5. Alignment was generated with ClustalW48. (d) Effect of MSNBA and common GLUT1 inhibitors cytochalasin B (CytB) and phloretin (Phlo) on the relative transport activity of GLUT5H387F, GLUT1F379H or GlcPSe,F348H measured as in Fig. 1b except that for GlcPSe,F348H glucose concentration was 90 μM (corresponding to glucose KM for this mutant, Supplementary Fig. S5). All inhibitors were at 2 mM concentration.
Given the desirability of a selective inhibitor for GLUT5, we investigated whether MSNBA inhibits GLUT1-4 or GlcPSe. Among these GLUT1, GLUT3, GLUT4 and GlcPSe transport glucose, while GLUT2 transports both glucose and fructose6. Glucose transport of GLUT1, GLUT2, GLUT3 and GLUT4 or fructose transport of GLUT2 were assayed by the entrance counter-flow method in proteoliposomes loaded with the respective recombinant human GLUT. Glucose transport of GlcPSe was measured in right-side-out (RSO) vesicles from JM1100 E. coli cells (that lack endogenous glucose transport) expressing GlcPSe. High concentrations of MSNBA (2 mM) did not influence the glucose transport of GLUT1, GLUT2, GLUT3, GLUT4 or GlcPSe or the fructose transport of GLUT2 but completely abolished GLUT5 fructose transport (Fig. 1d).
The bioactivity profile of MSNBA was evaluated using three different online resources: i) PubChem (https://pubchem.ncbi.nlm.nih.gov), which has an entry for MSNBA (CID 4783927), does not show recorded bioactivities for this compound; ii) ChEMBL 21 (https://www.ebi.ac.uk/chembl/), and SureChEMBL (https://www.surechembl.org), have no entries for MSNBA. The on-line chemical portal, ChemSpider (https://www.chemspider.com), does not have additional information compared to PubChem. These searches indicate that there is no patented or published bioactivity profile for MSNBA, which further confirms our observations that this compound is highly specific for GLUT5.
MSNBA inhibition of fructose uptake in MCF7 cells
To evaluate MSNBA inhibition of native human GLUT5, we measured the effect of MSNBA on the fructose uptake in cultured breast cancer cells MCF7, which express GLUT510. Besides GLUT5, MCF7 cells also express GLUT1 and GLUT29. Among these, only GLUT2 and GLUT5 are capable of fructose uptake. Cytochalasin B inhibits GLUT2 potently (KI of ~6 μM) but does not affect or bind GLUT518,26,27. Cytochalasin B at 50 or 200 μM had a moderate effect on fructose transport in MCF7 cells, while MSNBA by itself or in combination with cytochalasin B exhibited additional inhibition of fructose uptake (Fig. 2a). Therefore, to isolate the fructose uptake due to GLUT5, 50 μM of cytochalasin B was included in all uptake assays. We also measured glucose uptake into MCF7 cells (Supplementary Fig. S2) and found that MSNBA had no effect on glucose uptake while cytochalasin B reduced it by 20%.
In the presence of 10 mM fructose (reported KM for human GLUT510), MSNBA inhibited fructose uptake in MCF7 cells with an IC50 of 5.8 ± 0.5 μM (Fig. 2b). This is ~20 fold lower than the IC50 of MSNBA inhibition of the entrance counter-flow transport of fructose in GLUT5 proteoliposomes (Fig. 1c). The entrance counter-flow assay requires a high concentration of substrate (200 mM) and involves substrate exchange, not uptake. So it is likely that the counter-flow assay overestimates the inhibitor IC50.
To determine the mode of competition by MSNBA, initial velocity of uptake was measured by stopping the assay after two minutes (Supplementary Fig. S3). Analysis of inhibition of GLUT5 fructose uptake in MCF7 cells at different fructose and MSNBA concentrations shows that MSNBA is a competitive inhibitor of fructose transport with a KI of 3.2 ± 0.4 μM (Fig. 2c).
Determination of MSNBA binding site in GLUT5 model
GLUT proteins have 12 transmembrane (TM) helices organized into two domains (each of 6 helices, called the N- and C-domains), related by a two-fold pseudosymmetry axis. The substrate binding site is housed in the central cavity formed by the N- and C-domains21,22,28. The transport mechanism of GLUT proteins, similarly to other major facilitator superfamily (MFS) carriers, involves the alternating opening and closing of the substrate binding site to each side of the membrane. Consistent with this mechanism, available crystal structures of sugar porters capture the inward-facing (GlcPSe21, human GLUT122 and bovine GLUT523; PDB IDs 4LDS, 4PYP and 4BY9, respectively), outward-occluded (XylE28 and human GLUT329; PDB IDs 4GBY and 4ZWB, respectively) and outward-open (human GLUT329 and rat GLUT523; PDB IDs 4ZWC and 4YBQ, respectively) conformations. The GLUT5 model used for virtual screening was based on the GlcPSe crystal structure, which is in the inward-facing conformation.
The docked MSNBA binding site in the GLUT5 model is near the transmembrane active site (Fig. 3a). MSNBA putatively interacts with residues from the N-domain including S143 (helix 4) and T171 (helix 5), as well as the C-domain residues (Q288, Q289, N294, Y297) from helix 7, and H387 from helix 10 (Fig. 3b and Supplementary Fig. S4). These residues vary in their degree of conservation among human GLUT proteins or GlcPSe (Fig. 3c). Q288, Q289 and N294 are strictly conserved and essential for glucose transport as their mutations abolish transport activity in GLUT130 or GlcPSe21. S143 and T171 are not systematically conserved. There are two residues that are different in GLUT5 compared to glucose transporters: Y297 is a Phe in GLUT1-4 or Ile in GlcPSe, while H387 is Phe in GLUT1-4 and GlcPSe (Fig. 3c). GLUT1, GLUT3, GLUT4 and GlcPSe transport glucose but not fructose, GLUT2 transports both fructose and glucose, while GLUT5 transports only fructose7.
As MSNBA seemed to be specific for GLUT5, we focused on the residues in the MSNBA predicted binding site that were different in GLUT5 compared to GLUT1-4 and GlcPSe. Among these, H387 of GLUT5 potentially interacts with the nitro group of MSNBA (Fig. 3b and Supplementary Fig. S4), but the Phe present in GLUT1-4 as well as GlcPSe would have reduced interaction with MSNBA (Fig. 3c). To test if this residue is important for MSNBA inhibition, we constructed and expressed three mutant proteins: GlcPSe,F348H, GLUT1F379H and GLUT5H387F. As with the wild-type proteins, GLUT1F379H and GLUT5H387F were recombinantly produced in insect cells, purified, reconstituted in proteoliposomes, and then activity was assayed by the entrance counter-flow method in proteoliposomes. Glucose uptake by GlcPSe,F348H was measured in RSO vesicles of JM1100 E. coli cells. Unlike in wild-type transporters, 2 mM MSNBA inhibited 75% of the transport activity of GlcPSe,F348H (Fig. 3d), and left GLUT5H387F largely unaffected (Fig. 3d). Nevertheless, GLUT1F379H remained insensitive to MSNBA, similarly to wild-type GLUT1 (Figs 1b and 3d).
Interestingly, the susceptibility of GLUT1F379H to the well-known GLUT1 inhibitors cytochalasin B and phloretin (Fig. 3d) was different from that of the wild-type GLUT1 (Fig. 1b), with the mutant becoming completely resistant to phloretin and partially resistant to cytochalasin B. GlcPSe,F348H was also partially resistant to cytochalasin B inhibition but retained sensitivity to phloretin (Fig. 3d).
Discussion
The investigation of GLUT5 involvement in diseases and its use as a therapeutic target could be greatly advanced by discovery of potent and specific inhibitors of its activity. Previously reported GLUT5 inhibitors are substrate analogs of low potency17. To determine novel ligands for GLUT5 we used a convergent virtual and in vitro screening approach that has previously allowed us to identify several “first-in-class” bioactive molecules: small molecule antagonists for the formyl peptide receptors; the first agonist31 for the G-protein estrogen receptor (GPER, or GPR30), “G1”, and the first GPER antagonist32, “G15”; a selective ABCG2 transporter inhibitor33; and two HIV integrase inhibitors, Raltegravir and Elvitegravir, that block metnase, a DNA-repair enzyme34.
We virtually screened a model of GLUT5 in an inward-facing conformation. Because of the conformational cycling undergone by MFS proteins, it is possible that inhibitors could bind or stabilize one conformation and lock the protein. Thus, virtual screens using one conformation may not identify all possible ligands. There are now crystal structures of GLUT5 homologs in outward-facing conformations23, and efforts to identify new ligands binding to these models are on-going in our labs.
Our virtual screening procedure follows a previously described workflow35, which centers on chemical structure standardization and preparation, followed by careful evaluation of the binding site. In this work, we virtually screened a chemical library of over 6 million compounds against a putative binding site model of GLUT5, and reduced the number of possible ligands to 374. Among these, 175 were tested for inhibition of GLUT5 activity in vitro, in proteoliposomes, with the entrance counter-flow transport method.
With this combination of virtual and in vitro screening we identified MSNBA as a selective inhibitor of fructose transport by GLUT5. MSNBA competitively inhibited fructose uptake by GLUT5 into MCF7 cells with a Ki of 3.2 ± 0.4 μM. In silico docking predicted that the MSNBA binding site is near the transmembrane substrate transit site; this prediction is consistent with the observed competitive inhibition. His 387 of GLUT5 may interact with the nitro group of MSNBA; this position aligns with Phe in glucose transporters and could be the molecular determinant of specificity for MSNBA. Indeed, mutants in this position in human GLUT5 (GLUT5H387F) and the bacterial glucose transporter GlcPSe (GlcPSe,F348H) showed changes in MSNBA inhibition consistent with a key role of His 387 in MSNBA recognition. GLUT1F379H remained insensitive to MSNBA inhibition, suggesting that other residues in the active site, besides His 387, are involved in binding MSNBA. However, the desensitization of GLUT1F379H to inhibition by cytochalasin B and phloretin strongly supports a significant role of this position in ligand recognition in different GLUT members. Among all human GLUTs only GLUT7 has a histidine residue aligned with GLUT5 His 387. We speculate that MSNBA may also inhibit GLUT7 and, thus it could be used to investigate the biological role of this less-studied GLUT member.
Taken together the data presented here and previous work on GLUT1 and GLUT5 inhibitors17 converge to suggest that, despite the overall similarity of GLUT members, small differences in their active sites can significantly impact ligand selectivity. This brings hope that while the molecular determinants of substrate specificity in GLUT1 and GLUT5 remain unclear, new small molecule chemical probes that selectively modulate the activity of each transporter can be discovered. Such probes could be important in the in vivo studies for various pathologies.
MSNBA is the first GLUT5-selective chemical probe, which completely blocks GLUT5-mediated fructose uptake. MSNBA may become an important starting point in the rational design of novel therapeutics against obesity, diabetes and cancer. Fructose is linked to obesity and diabetes, as fructose consumption is correlated with metabolic disorders, particularly impaired glucose homeostasis, insulin resistance and dyslipidemia2. Blocking fructose uptake from the gut into blood serum, could ameliorate the metabolic symptoms associated with our modern diets.
But the largest potential use of MSNBA is in understanding fundamental processes in cancer. Although we have shown that MSNBA can inhibit fructose uptake into an immortalized cancer cell line over a short time, the long-term effect on cells has yet to be evaluated. MSNBA could halt fructose-dependent proliferation or inhibit the metabolic changes seen in fructose-utilizing cancers.
Finally, MSNBA will allow experiments exploring fructose and its link to disease states. From epidemiological studies, the correlations between fructose and several diseases have been implied, but the molecular mechanisms are poorly understood. This specific GLUT5 inhibitor opens up new doors to understand the crucial relationship between our food and health.
Methods
Virtual screening
The 3D model for GLUT5 was built with the Coot software20, on the basis of GlcPSe crystal structure (PDB ID 4LDS), using amino acid replacement guided by sequence alignments. Homology models of human GLUT5 based upon either human GLUT1 (4PYP) or bovine GLUT5 (4YB9) were constructed upon publication of the structures; these models are virtually identical to the model based on GlcPSe (Supplementary Fig. S1). GLUT5 model was superimposed to the liganded XylE crystal structure (PDB ID 4GC0) to identify possible binding regions for GLUT5 ligands. All amino acid residues in GLUT5 model interacting with bromo-glucose were labeled and used to define the docking binding pocket through the following procedure: fructose molecule was placed in GLUT5 model using coordinates from bromo-glucose of XylE structure, and flexible docking was performed with Molecular Operating Environment (MOE, Chemical Computing Group Inc.), so that both ligand (fructose) and binding pocket residues were allowed to be flexible. Only the top 5 ligand-transporter scoring poses were saved and used with FRED docking36, given the time constraints of flexible docking on the ChemNavigator library (http://www.chemnavigator.com). The library was prepared for virtual screening as follows. Standard clean up procedure was employed where chemical structures were checked for valence errors, salt and solvent removal. A total of 6,273,384 chemicals were selected for further processing. Standardized chemical structures were then used to calculate 2D extended connectivity fingerprints (ECFP) using an in-house implementation of algorithm described in literature37. Conformer generation with Omega38 prepared input for 3D shape and docking applications. 3D conformations were generated using Merck Molecular Force Field 9439 with 400 maximum number of conformers for each chemical structure. Shape and pharmacophore screening were performed with ROCS40 based on lead molecules described in literature41. FRED program36 was used to dock conformers generated with Omega to the GLUT5 model. Ranking of hits was done using a combo score with 20%, 60% and 20% weights given to ECFP, ROCS and FRED scores, respectively. A total of 347 top scoring hits were selected based on the combo score described above, out of which 175 chemicals (Supplementary Table S1) were purchased and tested in GLUT5 proteoliposomes for inhibition of the fructose entrance counter-flow transport.
Protein expression and purification of wild-type and mutant GLUTs
cDNAs of GLUT1, 3, 4 and 5 were purchased from Open Biosystems (GE Healthcare). cDNA of GLUT2 was a gift from Prof. Graeme Bell at University of Chicago. Full length DNA was subcloned into pFastBac1 vector (Life Technologies) with a N-terminal hexahistidine tag. Bacmids were generated in DH10Bac E. coli cells (Life Technologies). Baculoviruses were produced using Cellfectin II Reagent and amplified in Sf21 insect cells (Life Technologies). Cells were maintained at 26 °C, and P1 (106 pfu/mL) was collected from infected cells after 72 hours. Sf21 cells were propagated in HyClone SFX-Insect media (GE Healthcare), supplemented with 5% (v/v) fetal bovine serum (Biowest), 4.8 mM glutamine, antibiotics (100 units/mL Penicillin G and 100 μg/mL streptomycin sulfate) and amphotericin B (2.5 μg/mL). For recombinant protein expression, Sf21 cells in suspension culture at 2 × 106 cells/ml were infected with P3 viral stock (108 pfu/mL), at an MOI (multiplicity of infection) of 1.0 pfu/cell. Four days after viral infection, cells from 1 L culture were collected by centrifugation at 2,000 g and 25 °C. The cell pellet was resuspended in 120 mL of buffer A [50 mM sodium phosphate (NaPi) (pH 7.5), 5% (v/v) glycerol, 200 mM sodium chloride], with protease inhibitors (1 mM AEBSF, 10 μM E-64, 10 μM pepstatin A, 1 μM Aprotinin, 20 μM Bestatin, 20 μM Leupeptin), at 4 °C, and disrupted by sonication (Branson Ultrasonic). n-Dodecyl-β-D-maltopyranoside (DDM, EMD chemicals) was added to a final concentration of 1% (w/v) in the broken cell solution, and the mixture was incubated at 4 °C with stirring for 4 hours. The solubilized protein solution was clarified by ultracentrifugation at 200,000 g and 4 °C for 1 hour, and the supernatant was loaded onto the Ni-NTA resin (EMD Millipore). The column was washed with buffer containing 50 mM NaPi (pH 7.5), 500 mM NaCl, 5–20 mM imidazole, 5% (v/v) glycerol and 0.05% (w/v) DDM. GLUT1-5 were eluted with buffer A, containing 300 mM imidazole and 0.05% DDM (w/v). To generate DNA for mutant proteins, site-directed mutagenesis was performed on the pFastBac1 plasmid constructs of wild-type proteins and verified by DNA sequencing42. Mutant proteins were expressed and purified in the same manner as wild-type proteins with no modifications.
Proteoliposome preparation
Proteoliposomes were generated according to published protocols21,43 with minor modifications. Liposomes were made from a 95%/5% mix of soy phosphatidylcholine and cholesterol (Avanti Polar Lipids). Prepared liposomes were destabilized with 4 mM Triton X-100 and mixed with purified protein in a 100:1 (w/w) ratio in 100 mM KPi (pH 7.5), 20% (v/v) glycerol, 200 mM glucose for GLUT1-4 and GLUT1F379H or fructose for GLUT5 (wild-type and H387F mutant) and GLUT2. Detergent was removed by several additions of SM2 BioBeads (BioRad), followed by incubation overnight at 4 °C. After filtering out BioBeads, proteoliposomes were diluted with 100 mM KPi (pH 7.5), 200 mM glucose for GLUT1 (wild-type and F379H mutant) and GLUT2-4 or fructose for GLUT5 (wild-type and H387F mutant) and GLUT2, and then collected by ultracentrifugation at 200,000 g, for 1 h, at 4 °C. Proteoliposomes were resuspended in 100 mM KPi (pH 7.5), to an OD600nm ~30.
Entrance counter-flow transport assay for human GLUTs
For the entrance counter-flow transport in proteoliposomes, the assay was started by the addition of 5 μL proteoliposomes solution (OD600nm ~30) to 200 μL assay solution (100 mM KPi buffer at pH 7.5), containing 10 μM 14C-radiolabeled glucose (for GLUT1 wild-type or F379H mutant and GLUT2-4) or fructose (for GLUT5 wild-type or H387F mutant and GLUT2) (Moravek Biochemicals). After one minute (or different time points as specified), the transport was stopped with ice-chilled quench buffer [0.1 M KPi (pH 5.5) and 0.1 M lithium chloride]. The solution was filtered onto a cellulose nitrate membrane filter (Whatman; 0.4 μm pore size), and the filter was washed three times with quench buffer. The membrane filter was placed into a vial filled with BioSafe II scintillation liquid (Research Products International Corp.), and radioactivity was quantified with LS 6500 scintillation counter (Beckman). Compounds tested for the inhibition study were purchased from Sigma Chemicals (Supplementary Table S1). Stocks of 100 mg/ml for each compound were made in either water or dimethyl sulfoxide (DMSO). Chemicals at 1 mM final concentration in the assay volume were screened for inhibition of GLUT5 fructose transport in proteoliposomes. Tested inhibitors were added 1 min prior to addition of proteoliposomes solution. Cytochalasin B (Enzo Life Sciences) and phloretin (Alfa Aesar) were dissolved in DMSO at stock concentrations of 100 mM. Kinetic parameters were determined by nonlinear algorithm plots supplied by Prism (GraphPad Software). DMSO up to 5% (v/v) concentration in the transport assay did not affect activity. Data is presented as relative activity normalized to radioactivity of no inhibitor added as 100% and empty liposomes as 0%.
Hexose uptake in MCF7 cells
MCF7 cells were acquired from the American Type Culture Collection (ATCC HTB-22) and cultured in DMEM/F12 media (HyClone) supplemented with 10% (v/v) fetal bovine serum (Biowest) and 1% Pen/Strep (Lonza), and grown in a 37 °C/CO2 incubator. Cells were seeded into 12-well culture plates with 1 ml media/well and assayed 4 days after seeding (~200,000 cells per well). Before assays, media was removed and replaced with 300 μL phosphate-buffered saline (PBS). MSNBA and/or cytochalasin B in DMSO or DMSO control were added to MCF7 in PBS and pre-incubated 5 minutes at 37 °C. Then 14C-fructose (total concentration of cold and radioactive fructose of 3, 5, 10 or 20 mM, as indicated) or 14C-glucose (total concentration of cold and radioactive glucose of 10 mM) was added to each well and incubated at 22 °C for times indicated. Reaction was terminated by addition of 1 ml of ice-cold PBS. Cells were washed again with PBS and then lysed with 1% (w/v) Triton X-100 and 10 mM NaOH. Lysates were transferred to scintillation vial, diluted with 10 ml scintillation cocktail and measured with Becker LS 6500 Multi-purpose Scintillation Counter. Each measurement was the result of averaging 3 wells.
Transport assay for GlcPSe
Glucose transport assay for GlcPSe, wild-type and F348H mutant, was performed as described previously21. GlcPSe was cloned into the pBAD vector (Invitrogen) with C-terminal 6xHis tag. F348H mutation was done with the site-directed mutagenesis method42 and verified by DNA sequencing. GlcPSe proteins were expressed in the glucose transport deficient Escherichia coli strain JM1100 (the Coli Genetic Stock Center). Cells were grown at 37 °C, in Luria Broth medium, with 100 μg/mL ampicillin. Protein expression was induced with 0.3 mM L-arabinose at O.D.600nm 0.6. After 3 hours, cells were harvested by centrifugation at 2,500 g for 5 min. The right-side-out (RSO) membrane vesicles of JM1100 E. coli cells were prepared as described previously21,44,45. Transport assay was initiated by the addition of 14C-radiolabeled glucose (Moravek Biochemicals) to 50 μL RSO vesicles in 100 mM KPi buffer pH 7.5, at O.D.600nm of 2.0. After one minute, the transport was stopped and the radioactivity was measured as described above (see ‘Entrance counter-flow transport assay for human GLUTs’). When inhibitors were used, they were incubated with RSO vesicles 1 minute before transport initiation.
Additional Information
How to cite this article: George Thompson, A. M. et al. Discovery of a specific inhibitor of human GLUT5 by virtual screening and in vitro transport evaluation. Sci. Rep. 6, 24240; doi: 10.1038/srep24240 (2016).
Supplementary Material
Acknowledgments
We thank Professor Graeme Bell (University of Chicago) for providing GLUT2 cDNA. This work was supported by NIH Grants R01-DK091754 (to J.C.), P30-CA118100 (to the University of New Mexico Cancer Center), and U54-CA189205 (to T.I.O., Illuminating the Druggable Genome Knowledge Management Center). We thank Dr. Ajit Jadhav (NIH/NCATS) for confirming and analyzing the purity of MSNBA. We thank Openeye Scientific Software (Santa Fe, NM) for allowing us to use their software.
Footnotes
A patent application has been filed describing the potential use of N-[4-(methylsulfonyl)-2-nitrophenyl]-1,3-benzodioxol-5-amine (MSNBA).
Author Contributions T.I.O. and J.C. designed experiments. A.M.G.T., O.U., P.B., C.V.I., A.W. and J.C. performed experiments. All authors, except A.W. analyzed data and wrote the manuscript.
References
- Vos M. B., Kimmons J. E., Gillespie C., Welsh J. & Blanck H. M. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J. Med. 10, 160 (2008). [PMC free article] [PubMed] [Google Scholar]
- Tappy L. & Lê K.-A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 90, 23–46 (2010). [DOI] [PubMed] [Google Scholar]
- Hwang I. S., Ho H., Hoffman B. B. & Reaven G. M. Fructose-induced insulin resistance and hypertension in rats. Hypertension 10, 512–516 (1987). [DOI] [PubMed] [Google Scholar]
- Bremer A. A., Mietus-Snyder M. & Lustig R. H. Toward a unifying hypothesis of metabolic syndrome. Pediatrics 129, 557–570 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burant C. F., Takeda J., Brot-Laroche E., Bell G. I. & Davidson N. O. Fructose transporter in human spermatozoa and small intestine is GLUT5. J. Biol. Chem. 267, 14523–14526 (1992). [PubMed] [Google Scholar]
- Thorens B. & Mueckler M. Glucose transporters in the 21st Century. Am. J. Physiol. Endocrinol. Metab. 298, E141–145 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldry M. & Thorens B. The SLC2 family of facilitated hexose and polyol transporters. Pflüg. Arch. Eur. J. Physiol. 447, 480–489 (2004). [DOI] [PubMed] [Google Scholar]
- Douard V. & Ferraris R. P. Regulation of the fructose transporter GLUT5 in health and disease. Am. J. Physiol. - Endocrinol. Metab. 295, E227–E237 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy A. et al. Differential subcellular distribution of glucose transporters GLUT1–6 and GLUT9 in human cancer: Ultrastructural localization of GLUT1 and GLUT5 in breast tumor tissues. J. Cell. Physiol. 207, 614–627 (2006). [DOI] [PubMed] [Google Scholar]
- Zamora-León S. P. et al. Expression of the fructose transporter GLUT5 in human breast cancer. Proc. Natl. Acad. Sci. 93, 1847–1852 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzavi-Karbassi B. et al. Fructose as a carbon source induces an aggressive phenotype in MDA-MB-468 breast tumor cells. Int. J. Oncol. 37, 615–622 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K. K., Chan J. Y. W., Chung K. K. W. & Fung K.-P. Inhibition of cell proliferation in human breast tumor cells by antisense oligonucleotides against facilitative glucose transporter 5. J. Cell. Biochem. 93, 1134–1142 (2004). [DOI] [PubMed] [Google Scholar]
- Liu H. et al. Fructose induces transketolase flux to promote pancreatic cancer growth. Cancer Res. 70, 6368–6376 (2010). [DOI] [PubMed] [Google Scholar]
- Soueidan O.-M. et al. New fluorinated fructose analogs as selective probes of the hexose transporter protein GLUT5. Org. Biomol. Chem. 13, 6511–6521 (2015). [DOI] [PubMed] [Google Scholar]
- David E. S., Cingari D. S. & Ferraris R. P. Dietary Induction of Intestinal Fructose Absorption in Weaning Rats. Pediatr. Res. 37, 777–782 (1995). [DOI] [PubMed] [Google Scholar]
- Stuart C. A., Howell M. E. A. & Yin D. Overexpression of GLUT5 in diabetic muscle is reversed by pioglitazone. Diabetes Care 30, 925–931 (2007). [DOI] [PubMed] [Google Scholar]
- George Thompson A. M., Iancu C. V., Nguyen T. T. H., Kim D. & Choe J. Inhibition of human GLUT1 and GLUT5 by plant carbohydrate products; insights into transport specificity. Sci. Rep. 5, 12804 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inukai K. et al. Characterization of rat GLUT5 and functional analysis of chimeric proteins of GLUT1 glucose transporter and GLUT5 fructose transporter. Endocrinology 136, 4850–4857 (1995). [DOI] [PubMed] [Google Scholar]
- Gaster M., Staehr P., Beck-Nielsen H., Schrøder H. D. & Handberg A. GLUT4 Is Reduced in Slow Muscle Fibers of Type 2 Diabetic Patients Is Insulin Resistance in Type 2 Diabetes a Slow, Type 1 Fiber Disease? Diabetes 50, 1324–1329 (2001). [DOI] [PubMed] [Google Scholar]
- Emsley P., Lohkamp B., Scott W. G. & Cowtan K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iancu C. V., Zamoon J., Woo S., Aleshin A. & Choe J. Crystal structure of a glucose/H+ symporter and its mechanism of action. Proc. Natl. Acad. Sci. USA 110, 17862–17867 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng D. et al. Crystal structure of the human glucose transporter GLUT1. Nature 510, 121–125 (2014). [DOI] [PubMed] [Google Scholar]
- Nomura N. et al. Structure and mechanism of the mammalian fructose transporter GLUT5. Nature 526, 397–401 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissinel E. & Henrick K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 60, 2256–2268 (2004). [DOI] [PubMed] [Google Scholar]
- Corpe C. P. et al. The regulation of GLUT5 and GLUT2 activity in the adaptation of intestinal brush-border fructose transport in diabetes. Pflüg. Arch. Eur. J. Physiol. 432, 192–201 (1996). [DOI] [PubMed] [Google Scholar]
- Concha I. I. et al. Human Erythrocytes Express GLUT5 and Transport Fructose. Blood 89, 7 (1997). [PubMed] [Google Scholar]
- Colville C. A., Seatter M. J., Jess T. J., Gould G. W. & Thomas H. M. Kinetic analysis of the liver-type (GLUT2) and brain-type (GLUT3) glucose transporters in Xenopus oocytes: substrate specificities and effects of transport inhibitors. Biochem. J. 290 (Pt 3), 701–706 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L. et al. Crystal structure of a bacterial homologue of glucose transporters GLUT1-4. Nature 490, 361–366 (2012). [DOI] [PubMed] [Google Scholar]
- Deng D. et al. Molecular basis of ligand recognition and transport by glucose transporters. Nature, doi: 10.1038/nature14655 (2015). [DOI] [PubMed] [Google Scholar]
- Hruz P. W. & Mueckler M. M. Cysteine-scanning mutagenesis of transmembrane segment 7 of the GLUT1 glucose transporter. J. Biol. Chem. 274, 36176–36180 (1999). [DOI] [PubMed] [Google Scholar]
- Bologa C. G. et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat. Chem. Biol. 2, 207–212 (2006). [DOI] [PubMed] [Google Scholar]
- Dennis M. K. et al. In vivo effects of a GPR30 antagonist. Nat. Chem. Biol. 5, 421–427 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strouse J. J. et al. A selective ATP-binding cassette subfamily G member 2 efflux inhibitor revealed via high-throughput flow cytometry. J. Biomol. Screen. 18, 26–38 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E. A. et al. Targeting the transposase domain of the DNA repair component Metnase to enhance chemotherapy. Cancer Res. 72, 6200–6208 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologa C. G. & Oprea T. I. Compound collection preparation for virtual screening. Methods Mol. Biol. Clifton NJ 910, 125–143 (2012). [DOI] [PubMed] [Google Scholar]
- McGann M. FRED pose prediction and virtual screening accuracy. J. Chem. Inf. Model. 51, 578–596 (2011). [DOI] [PubMed] [Google Scholar]
- Rogers D. & Hahn M. Extended-Connectivity Fingerprints. J. Chem. Inf. Model. 50, 742–754 (2010). [DOI] [PubMed] [Google Scholar]
- Hawkins P. C. D., Skillman A. G., Warren G. L., Ellingson B. A. & Stahl M. T. Conformer Generation with OMEGA: Algorithm and Validation Using High Quality Structures from the Protein Databank and Cambridge Structural Database. J. Chem. Inf. Model. 50, 572–584 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren T. A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 17, 490–519 (1996). [Google Scholar]
- Hawkins P. C. D., Skillman A. G. & Nicholls A. Comparison of shape-matching and docking as virtual screening tools. J. Med. Chem. 50, 74–82 (2007). [DOI] [PubMed] [Google Scholar]
- McQuade D. T., Plutschack M. B. & Seeberger P. H. Passive fructose transporters in disease: a molecular overview of their structural specificity. Org. Biomol. Chem. 11, 4909–4920 (2013). [DOI] [PubMed] [Google Scholar]
- Braman J., Papworth C. & Greener A. Site-directed mutagenesis using double-stranded plasmid DNA templates. Methods Mol. Biol. Clifton NJ 57, 31–44 (1996). [DOI] [PubMed] [Google Scholar]
- Geertsma E. R., Nik Mahmood N. a. B., Schuurman-Wolters G. K. & Poolman B. Membrane reconstitution of ABC transporters and assays of translocator function. Nat. Protoc. 3, 256–266 (2008). [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Bacterial membranes. Methods Enzymol. 22, 99–120 (1971). [Google Scholar]
- Short S. A., Kaback H. R. & Kohn L. D. Localization of D-lactate dehydrogenase in native and reconstituted Escherichia coli membrane vesicles. J. Biol. Chem. 250, 4291–4296 (1975). [PubMed] [Google Scholar]
- Kraulis P. J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991). [Google Scholar]
- Merritt E. A. & Bacon D. J. Raster3D: photorealistic molecular graphics. Methods Enzymol. 277, 505–524 (1997). [DOI] [PubMed] [Google Scholar]
- Larkin M. A. et al. Clustal W and Clustal X version 2.0. Bioinforma. Oxf. Engl. 23, 2947–2948 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.