Abstract
Exposure to estrogenic compounds has been shown to epigenetically reprogram the female reproductive tract and may contribute to ovarian cancer. The goal of this study was to compare the effect of estradiol or bisphenol A (BPA) on the expression of histone-modifying enzymes (HMEs) in ovarian cancer cells. Using 2 human ovarian cancer cell lines, we examined the expression of SET8, a histone methyltransferase, and SIRT1, a histone deacetylase, after exposure to estrogen or BPA. These experiments were carried out in complete media (fetal bovine serum) that contain natural hormones to understand the impact of additional exposure to estrogen or BPA on HME expression. We found differential expression of the HMEs in the different models examined and between the different compounds. Further, we determined that the changes in gene expression occurred via estrogen receptor signaling using the estrogen receptor antagonist, ICI 182,780 (fulvestrant).
Keywords: BPA, ovarian, SET8, SIRT1
Introduction
Ovarian cancer is the fifth leading cause of death in the United States, with approximately 200 000 new cases a year.1 Patients are typically diagnosed at a late stage due to a lack of symptoms and screening tools, making ovarian cancer the most lethal gynecological malignancy. The most common type of ovarian cancer, high-grade serious epithelial cancer, is typically treated with a combination of platinum drugs and taxols. The majority of patients respond initially, however, relapse occurs within approximately 2 years.2 Recurrent disease is no longer sensitive to platinum therapy,3 and only 30% to 35% of patients will survive more than 5 years.4
Environmental exposures have been demonstrated to contribute to disease development and progression.5 Specifically, bisphenol A (BPA) is a synthetic estrogen widely produced in the United States6 that has been shown to impact the development of the nervous7,8 and reproductive systems.9 Studies suggest that the ovary may be most vulnerable to estrogenic compounds during development. Follicle formation and growth are impacted by BPA exposure, resulting in delayed follicular assembly, multioocyte follicles, and reduced growth.10 Additionally, rodents neonatally exposed to BPA had altered ovarian morphology, an increase in cystic ovaries, cystic endometrial hyperplasia, and a decrease in primordial follicles.11 Furthermore, in the rhesus monkey, fetal exposure to BPA altered meiotic events, such as increasing synaptic defects, and with continuous exposure, the number of unenclosed oocytes increased.12 Taken together, these studies demonstrate that the ovary is negatively impacted by BPA exposure, particularly during development.
In rodents, the presence of BPA during organ development has also been shown to contribute to precancerous disease later in life. Animals exposed neonatally to BPA had an increased likelihood of prostatic intraepithelial neoplasia, a precursor to prostate cancer.13 The effects of BPA are not the result of mutations, but rather evidence indicates that BPA alters the epigenome.14,15 Tang et al16 studied the impact of estrogen and BPA exposure on the prostate methylome and found differential methylation patterns for multiple genes throughout the life of exposed animals. In addition, in both the developing prostate and oocytes, the expression of the DNA methyltransferase (DNMT) enzymes was shown to be altered following exposure to BPA.16,17 Furthermore, in ovarian tumors, the expression of DNMT1, 3A, and 3B was found to be increased compared to normal ovaries.18 These studies demonstrate that epigenetic changes occur in ovarian cancer and that BPA exposure can change the expression of epigenetic modifiers.
In addition to changes in DNA methylation, changes in histone modifications are also common in cancer. Since it is known that BPA changes the expression of DNMTs, the goal of the present study was to analyze the expression of histone-modifying enzymes (HMEs) that might be altered after exposure to estrogen or BPA in human ovarian cancer cell models. We analyzed the expression of HMEs that have previously been shown to be involved in estrogen signaling. SET8 is a histone monomethyltransferase that has been shown to be a coactivator of estrogen receptor (ER) α19 and plays a role in the epithelial to mesenchymal (EMT) transition in breast cancer.20 SIRT1 is a class III histone deacetylase that modifies both histone and nonhistone proteins, including p53 and ER-α.21 Furthermore, SIRT1 is increased in malignant ovarian cancer2 and also plays a role in EMT in prostate cancer.22 In addition, SIRT1 expression decreased after exposure to genistein, a phytoestrogen, in human prostate cancer cells23 and is involved in regulating ER-α transcription in breast cancer.24 Taken together, these studies indicate that SET8 and SIRT1 expression may be regulated by estrogenic compounds.
We had previously examined the effect of BPA exposure on SET8 and SIRT1 in prostate cancer cell models and found alterations in the expression of both HMEs that was distinct from estrogen regulation.25 Based on our previous findings, we hypothesized that exposure to BPA would alter the expression of SET8 and SIRT1 in ovarian cancer cells. We utilized 2 different cell models of human ovarian cancer and found that multiple doses of estrogen and BPA significantly altered gene expression. We also found that the changes in gene expression were dependent on ER signaling as they were reversed by an ER antagonist. Taken together, these data demonstrate that exposure to estrogenic compounds affects the expression of HMEs distinctly in different human ovarian cancer cell models.
Materials and Methods
Cell Culture and Treatment
The OVCAR3 and SKOV3 cell lines were a kind gift from Dr Kunle Odunsi (Roswell Park Cancer Institute, Buffalo, New York). The cells were maintained in RPMI/1640 medium supplemented with 100 U/mL of penicillin–streptomycin (Life Technologies, Carlsbad, California) and 10% heat-inactivated fetal bovine serum (FBS; Atlanta Biologicals, Norcross, Georgia). Cells were cultured at 37°C in a 5% CO2 heated incubator.
17β-Estradiol, BPA, and ICI 182,780 (fulvestrant) were purchased from Sigma-Aldrich (St Louis, Missouri). Cells were treated with 1, 5, or 10 nmol/L estradiol or 10, 25, or 50 nmol/L BPA for 24 hours. Where indicated, the ER inhibitor ICI was added at 10 nmol/L. All treatments were for 24 hours, and 95% ethanol (0.1%) was used as the vehicle control.
RNA Isolation and Polymerase Chain Reaction
OVCAR3 and SKOV3 cells were seeded in 6-well dishes. After 48 hours, the cells were treated with either 17β-estradiol or BPA with or without ICI. Total RNA was isolated via TRIzol reagent as recommended by the manufacturer (Life Technologies). Reverse transcription was done using the High-Capacity RNA-to-cDNA kit purchased from Life Technologies as recommended by the manufacturer. Polymerase chain reaction was performed to analyze the expression levels of SIRT1, SET8, and GAPDH. The expression level of each gene was normalized to the expression of GAPDH. GAPDH was chosen as the housekeeping gene because its expression did not change in response to estrogen or BPA. The relative expression was analyzed using ImageJ software. The experiments were repeated with 3 individual sets of samples.
Statistical Analysis
Data were expressed as mean ± standard deviation (SD). Bonferroni post hoc test (correction test) was performed following analysis of variance (Prism v4.0; GraphPad, California) for multiple comparisons to determine the statistical significance. A P value <.05 was considered statistically significant.
Results
Differential Expression of SET8 and SIRT1 in Response to Estrogen and BPA in SKOV3 Cells
Previous studies examining gene expression have been carried out under conditions of hormone deprivation. Typically in these studies, cells are maintained in full media while experiments are carried out after hormone deprivation.26–28 This approach is utilized since it eliminates confounding issues, such as the presence of other steroid hormones. One caveat to this approach is that it is not representative of physiological conditions, as there are still natural and synthetic estrogens present in the system. To understand what might be occurring under more physiologically relevant conditions, 2 experimental conditions were considered. First, we analyzed the expression of these genes in complete media (fetal bovine serum [FBS]) rather than after hormone deprivation (charcoal striped serum [CSS]). Second, given that individual exposures can vary, we used multiple doses of both estrogen and BPA to mimic human exposure. The concentrations of each compound were in the nmol/L range (estrogen: 1, 5, and 10; BPA: 10, 25, and 50), since these doses are thought to be comparable to human exposure.
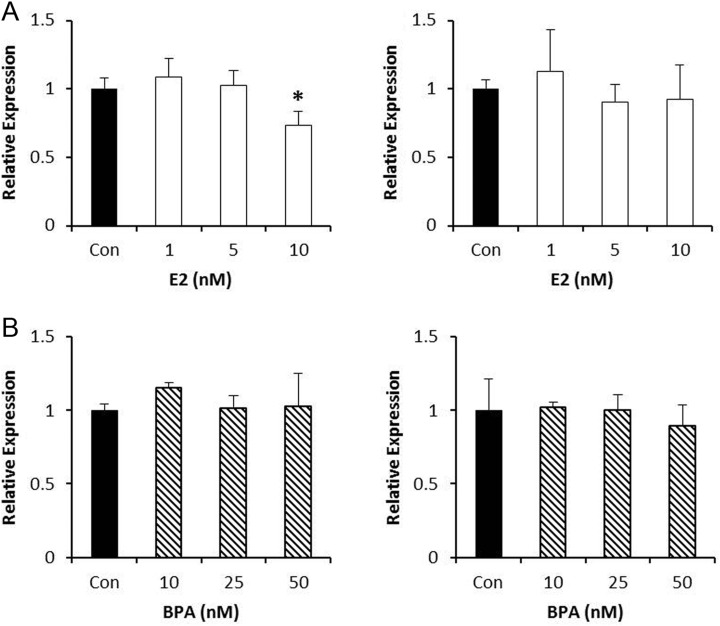
To understand the effects of estrogen and BPA on the expression of HME genes, we first utilized the SKOV3 ovarian serous adenocarcinoma cell model. These cells are chemosensitive and express both ERs. The samples were collected 24 hours after exposure, since this time point has been previously shown to allow sufficient time for transcription following hormone treatment.29 We found that the level of SET8 messenger RNA (mRNA) was decreased by 25% with 10 nmol/L estrogen, however, there was no change in the level of SIRT1 mRNA at any of the doses examined (Figure 1A). By contrast, none of the doses of BPA examined affected SET8 mRNA levels. Similarly, BPA exposure did not significantly change the level of SIRT1 mRNA (Figure 1B). These data imply the expression of HMEs in SKOV3 cells is not significantly impacted by additional hormone exposure.
Figure 1.
Effect of estrogen or bisphenol A (BPA) on gene expression of histone-modifying enzymes (HMEs) in SKOV3 cells. A, SKOV3 cells were treated with the indicated doses of estrogen for 24 hours, and the expression of SET8 (left panel) or SIRT1 (right panel) was analyzed. B, SKOV3 cells were treated with the indicated doses of BPA for 24 hours, and the expression of SET8 (left panel) or SIRT1 (right panel) was analyzed. ImageJ was used to quantitate the results, and the expression of each gene was normalized to GAPDH from the same sample. Results are from 3 independent experiments (*P < .05).
Differential Expression of SET8 and SIRT1 in Response to Estrogen and BPA in OVCAR3 Cells
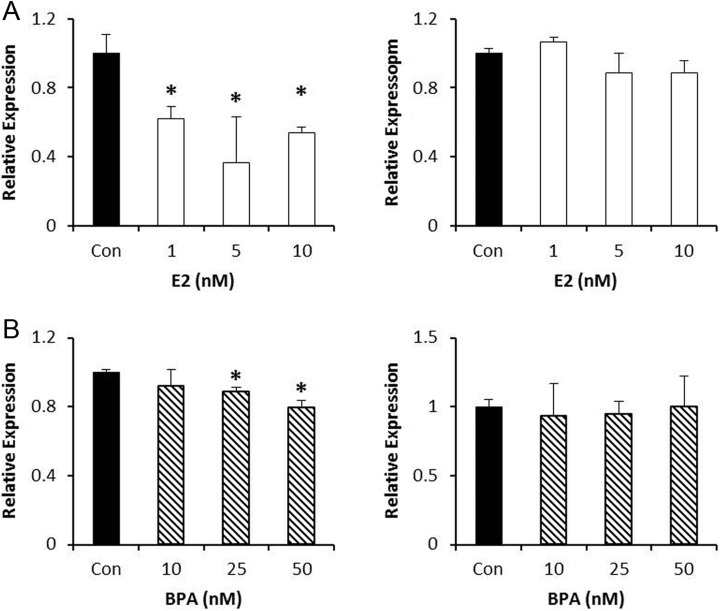
Ovarian cancer is considered a heterogeneous disease with different molecular signatures. To aid our understanding of the differences, and the impact of estrogenic compounds, we analyzed the expression of SET8 and SIRT1 in response to estrogen and BPA in another chemosensitive epithelial ovarian cancer model, OVCAR3 cells. OVCAR3 cells also express the ERs. SET8 mRNA was decreased by 38%, 64%, and 46% following exposure to 1, 5, and 10 nmol/L estrogen. There was, however, no effect on SIRT1 mRNA levels (Figure 2A). Similarly, both 25 and 50 nmol/L BPA decreased SET8 mRNA levels significantly by 10% and 20%, respectively. Similar to estrogen, BPA had no effect on SIRT1 mRNA in OVCAR3 cells (Figure 2B).
Figure 2.
Effect of estrogen or bisphenol A (BPA) on gene expression of histone-modifying enzymes (HMEs) in OVCAR3 cells. A, OVCAR3 cells were treated with the indicated doses of estrogen for 24 hours, and the expression of SET8 (left panel) or SIRT1 (right panel) was analyzed. B, OVCAR3 cells were treated with the indicated doses of BPA for 24 hours, and the expression of SET8 (left panel) or SIRT1 (right panel) was analyzed. ImageJ was used to quantitate the results, and the expression of each gene was normalized to GAPDH from the same sample. Results are from 3 independent experiments (*P < .05).
Differential Expression of SET8 and SIRT1 by Estrogen and BPA Is Dependent on ER Signaling
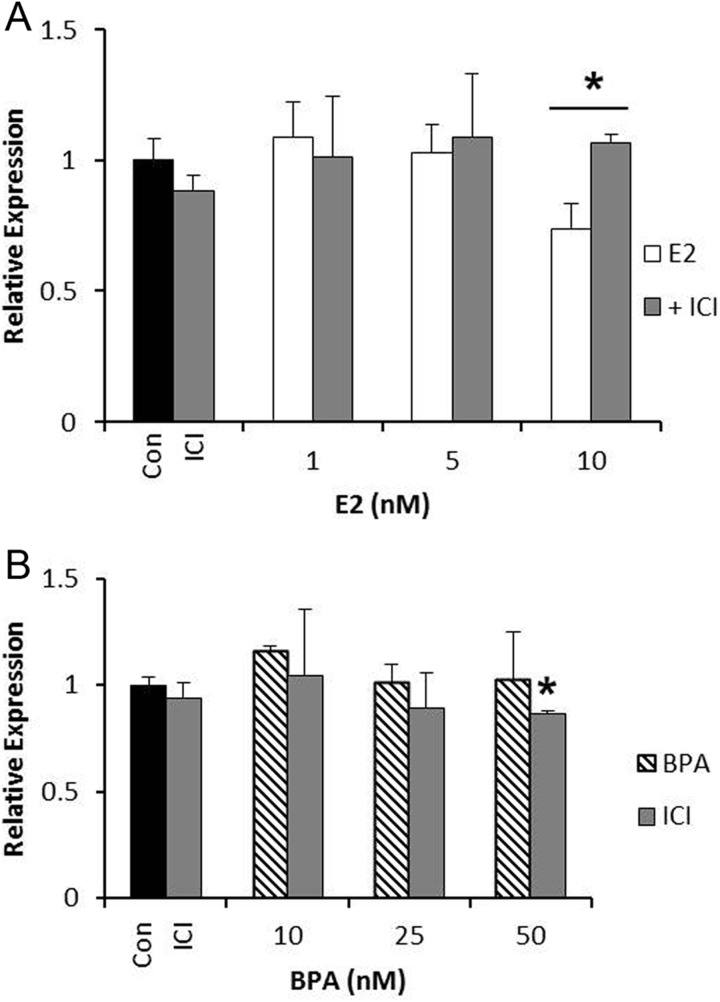
To determine whether estrogen and BPA are altering the expression of SET8 and SIRT1 through ER signaling, we utilized the ER antagonist, ICI 182,780 (fulvestrant). The treatments were carried out as above in the presence of estrogen or BPA with ICI. In SKOV3 cells, as expected, the expression of SET8 was reversed in the presence of ICI (Figure 3A). Interestingly, even though BPA alone did not affect the expression of SET8, we found a 15% decrease in SET8 mRNA at the 50 nmol/L dose when BPA was combined with ICI (Figure 3B). These data show that the combination of an ER antagonist and BPA alters the expression of SET8 even when BPA alone had no effect.
Figure 3.
Estrogen and bisphenol A (BPA) signal through the estrogen receptor to alter SET8 expression in SKOV3 cells. A, SKOV3 cells were treated with estrogen alone or in the presence of ICI (10 nmol/L) for 24 hours, and SET8 expression was analyzed. B, SKOV3 cells were treated with BPA alone or in the presence of ICI (10 nmol/L) for 24 hours, and SET8 expression was analyzed. ImageJ was used to quantitate the results, and the expression was normalized to GAPDH from the same sample. Results are from 3 independent experiments (*P < .05).
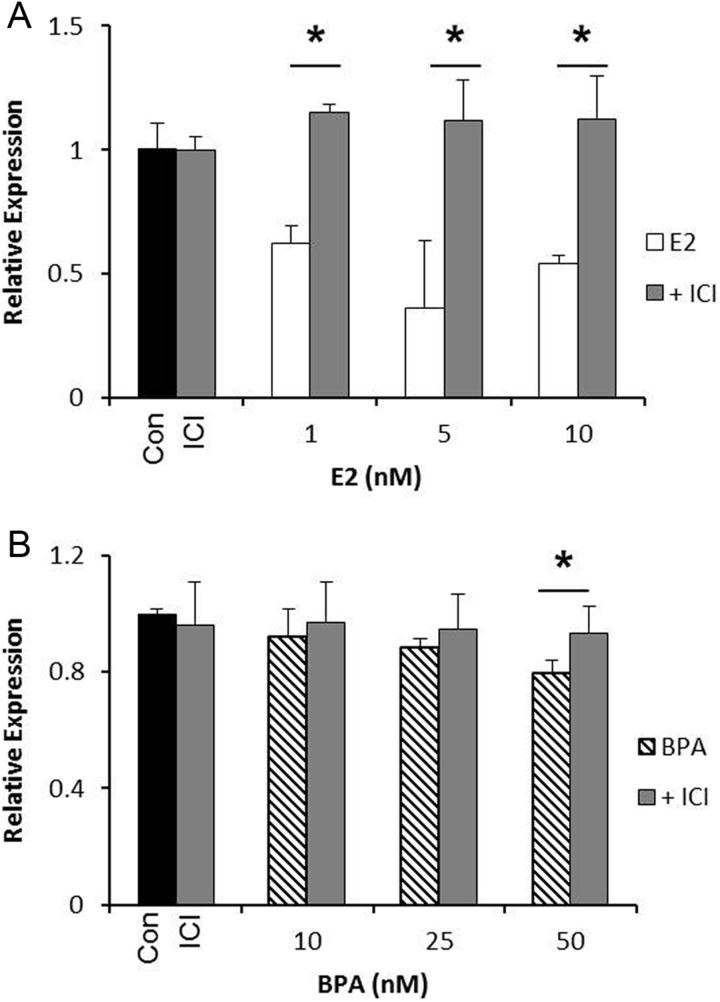
The same experiments were carried out in OVCAR3 cells to determine whether the changes in gene expression were mediated by ER signaling. Treatment with ICI reversed the decrease in SET8 mRNA by estrogen alone, demonstrating that ER signaling is the mechanism by which estrogen regulates SET8 expression (Figure 4A). Bisphenol A alone induced a decrease in SET8 mRNA at 25 and 50 nmol/L. Treatment with ICI reversed this effect at both doses, although it did not reach statistical significance for the 25 nmol/L dose (average expression was 0.95; Figure 4B). Taken together, these data indicate that both estrogen and BPA are regulating gene expression in ovarian cancer via ER signaling.
Figure 4.
Estrogen and bisphenol A (BPA) signal through the estrogen receptor to alter SET8 expression in OVCAR3 cells. A, OVCAR3 cells were treated with estrogen alone or in the presence of ICI (10 nmol/L) for 24 hours, and SET8 expression was analyzed. B, OVCAR3 cells were treated with BPA alone or in the presence of ICI (10 nmol/L) for 24 hours, and SET8 expression was analyzed. ImageJ was used to quantitate the results, and the expression was normalized to GAPDH from the same sample. Results are from 3 independent experiments (*P < .05).
Discussion
We set out to determine whether estrogen and the endocrine disruptor, BPA, affect the expression of HMEs in human ovarian cancer cells in the presence of natural hormones. We found that both estrogen and BPA regulate the expression of SET8, but there was very little effect on SIRT1. Furthermore, we found that the effects exerted by these compounds differ between the 2 models of human ovarian cancer analyzed, with the OVCAR3 cells having greater sensitivity to estrogenic compounds. Additionally, we showed that the regulation of gene expression was via ER signaling, as the effects were reversed in all cases by the ER antagonist ICI.
Although many studies have demonstrated the impact of estrogenic compounds on ovarian development and function, the effects of BPA on ovarian cancer are not completely understood. Bisphenol A was found to increase the growth of ovarian cancer via ER signaling.30 This was due to an increase in the expression of cell cycle proteins, such as cyclin D, E2F1, and PCNA, with a subsequent decrease in the cell cycle inhibitor p21.30,31 Additionally, BPA exposure has been shown to alter the expression of apoptotic genes, decreasing caspases, and proapoptotic genes.31 These studies provide insight into the mechanism by which BPA may impact ovarian cancer. We sought to contribute to our understanding by examining the effect of BPA on epigenetic reprogramming. Since it has already been shown that BPA impacts the expression of DNMTs, we examined the effect of BPA on HME expression.
We studied 2 HMEs, SIRT1 and SET8, both of which have been implicated in estrogen signaling. SIRT1 has been shown to be involved in numerous cellular functions including the regulation of gene expression, cell survival, proliferation, differentiation, metabolism, and immune response. Of particular interest to cancer, SIRT1 activation has been positively correlated with drug resistance and EMT.21 In breast cancer with mutated BRCA1, however, SIRT1 is decreased and this was correlated with increased expression of the antiapoptotic protein, survivin.32 In malignant epithelial ovarian cancer, SIRT1 expression is increased in a subset of patients and this correlated with increased survival.33 Taken together, these studies suggest that SIRT1 may be protective in a subset of breast and ovarian cancer.
Given its prominent role in cancer, we were surprised to find that SIRT1 expression was not impacted by BPA exposure and was only decreased by 10 nmol/L estrogen in SKOV3 cells. SIRT1 is elevated in ovarian cancer, and it may be that in both OVCAR3 and SKOV3 cells, SIRT1 is insensitive to estrogenic compounds. This is supported by the fact that ICI alone had no effect on SIRT1 expression in either cell line.
Compared to SIRT1, little is known about the role of SET8 in ovarian cancer. We found that SET8 regulation was very sensitive to estrogen, but not BPA, in OVCAR3 cells. SET8 expression did not change in SKOV3 cells, implying that there is an underlying difference between these 2 cell models of ovarian cancer. Ovarian cancer is a heterogeneous disease, with many molecular signatures. The 2 model systems used in this study may reflect this and explain the different outcomes with regard to estrogenic exposures.
The results of the current study mimic the results found in our previous study analyzing prostate cancer.25 We found few changes in SIRT1, but SET8 expression was sensitive to estrogenic exposures. Similar to that study, estrogen decreased the expression of SET8, indicating that in 2 distinct cancer models, SET8 expression is sensitive to estrogen signaling. In our prostate cancer models, however, BPA increased the expression of SET8, whereas in the present study, BPA caused a slight reduction in SET8 expression. In both the prostate and ovarian cells, ERs are expressed and involved in regulating SET8, however, the presence and relative amounts of ERs may be important. Interestingly, SET8 expression was only greatly impacted in prostate cancer cells when both ERs were present. If only ER-β was present, SET8 expression was minimally impacted. This may be the case in ovarian cancer cells as well. Both ERs are present in the ovarian cancer cells, however, the relative amounts of ER-α to ER-β may explain the differences in response to estrogenic compounds.
Interestingly, we found that the combination of estrogen or BPA with ICI decreased the expression of SET8 in SKOV3 cells, even though BPA alone had no effect. It is important to note that ICI alone had no effect on SET8 expression but only reduced SET8 expression when in combination with BPA. Although it has been shown that antiestrogens inhibit ovarian cancer proliferation in vitro and in vivo, few studies have been conducted examining the effect of ER antagonists on ovarian cancer.34 Given this, it is hard to speculate on the impact of our finding that 50 nmol/L BPA combined with ICI decreased SET8 expression.
Many studies have been performed to show that HMEs play a role in estrogen signaling. We set out not only to determine whether estrogen and BPA regulate the expression of HMEs but also to determine whether they do so in the presence of physiological hormones. This is an important distinction as it more closely resembles the disease state. It would be of interest to examine what happens to the expression of these enzymes with prolonged exposure to estrogen or BPA. Additionally, determining how current therapies are impacted in the presence of endocrine disruptors like BPA may be important for understanding disease progression.
Conclusion
It is clear from our study that with a very short exposure time (24 hours), changes in HME gene expression were observed in response to estrogen and BPA. Perhaps most interestingly, we found that the OVCAR3 model was sensitive to estrogenic exposures, whereas the SKOV3 model was insensitive. This may be a reflection of different molecular signatures of ovarian cancer and be relevant to designing new therapeutic strategies for ovarian cancer.
Acknowledgments
The authors would like to thank the Canisius Earned Excellence Program for funding part of this work.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Mei L, Chen H, Wei DM, et al. Maintenance chemotherapy for ovarian cancer. Cochrane Database Syst Rev. 2014;3:CD007414 doi:10.1002/14651858. [DOI] [PubMed] [Google Scholar]
- 2. Marsh DJ, Shah JS, Cole AJ. Histones and their modification in ovarian cancer—drivers of disease and therapeutic targets. Front Oncol. 2014;4:144 doi:10.3389/fonc.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suh DH, Kim MK, Kim HS, Chung HH, Song YS. Epigenetic therapies as a promising strategy for overcoming chemoresistance in epithelial ovarian cancer. J Can Prev. 2013;18(3):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chene G, Lamblin G, Le Bail-Carval K, Chabert P, Bakrin N, Mellier G. Early preinvasive lesions in ovarian cancer. Biomed Res Int. 2014;2014:639252 doi:10.1155/2014/639252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alwis ID, Maroni DM, Hendry IR, et al. Neonatal diethylstilbestrol exposure disrupts female reproductive tract structure/function via both direct and indirect mechanisms in the hamster. Reprod Toxicol. 2011;32(4):472–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol. 2013;42:132–155. [DOI] [PubMed] [Google Scholar]
- 7. Mathisen GH, Yazdani M, Rakkestad KE, et al. Prenatal exposure to bisphenol A interferes with the development of cerebellar granule neurons in mice and chicken. Int J of Dev Neuro. 2013;31(8):762–769. [DOI] [PubMed] [Google Scholar]
- 8. Itoh K, Yaoi T, Fushiki S. Bisphenol A, an endocrine disrupting chemical, and brain development. Neuropathology. 2012;32(4):447–457. [DOI] [PubMed] [Google Scholar]
- 9. Peretz J, Vrooman L, Ricke WA, et al. Bisphenol A and reproductive health: update of experimental and human evidence, 2007-2013. Environl Health Perspect. 2014;122(8):775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cruz G, Foster W, Paredest A, Yi KD, Uzumcu M. Long-term effects of early-life exposure to environmental oestrogens on ovarian function: role of epigenetics. J Neuroendocrinol. 2014;26(9):613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gau H, Yang BJ, Li N, et al. Bisphenol A and hormone-associated cancers: current progress and perspectives. Medicine. 2015;94(1):e211 doi:10.1097/MD.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hunt PA, Lawson C, Gieske M, et al. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc Natl Acad Sci U S A. 2012;109(43):17525–17530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;166(11):5624–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prins GS, Tang WY, Belmonte J, Ho SM. Perinatal exposure to oestradiol and bisphenol A alters the prostate epigenome and increases susceptibility to carcinogenesis. Basic Clin Pharmacol Toxicol. 2008;102(2):134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dhimolea E, Wadia P, Murray T, et al. Prenatal exposure to BPA alters the epigenome of the rat mammary gland and increases the propensity to neoplastic development. PloS One. 2014;9(7):e99800 doi:10.1371/journal.pone.0099800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang WY, Morey LM, Cheung YY, Birch L, Prins GS, Ho SM. Neonatal exposure to estradiol/bisphenol A alters promoter methylation and expression of Nsbp1 and Hpcal1 genes and transcriptional programs Dnmt3a/b and Mbd2/4 in the rat prostate gland throughout life. Endocrinology. 2012;153(1):42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chao HH, Zhang XF, Chen B, et al. Bisphenol A exposure modifies methylation of imprinted genes in mouse oocytes via the estrogen receptor signaling pathway. Histochem Cell Biol. 2012;137(2):249–259. [DOI] [PubMed] [Google Scholar]
- 18. Lee J-Y, Jeong W, Lim W, et al. Hypermethylation and post-transcriptional regulation of DNA methyltransferases in the ovarian carcinomas of the laying hen. PloS One. 2013;8(4):e61658 doi:10.1371/journal.pone.0061658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Y, Sun L, Zhang Y, et al. The histone modifications governing TFF1 transcription mediated by estrogen receptor. J Biol Chem. 2011;286(16):13925–13936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang F, Sun L, Li Q, et al. SET8 promotes epithelial-mesenchymal transition and confers TWIST dual transcriptional activities. EMBO J. 2012;31(1):110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Z, Chen WY. Emerging roles of SIRT1 in cancer drug resistance. Genes Cancer. 2013;4(3-4):82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Byles V, Zhu L, Lovaas JD, et al. SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis. Oncogene. 2012;31(43):4619–4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kikuno N, Shiina H, Urakami S, et al. Genistein mediated histone acetylation and demethylation activates tumor suppressor genes in prostate cancer cells. Int J Cancer. 2008;123(3):552–560. [DOI] [PubMed] [Google Scholar]
- 24. Moore R, Faller D. Sirt1 represses estrogen signaling, ligand independent ERa-mediated transcription, and cell proliferation in estrogen-responsive breast cells. J Endocrinol. 2013;216(3):273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burton K, Shaw L, Morey LM. Differential effect of estradiol and bisphenol A on Set8 and Sirt1 expression in prostate cancer. Toxicol Rep. 2015;2:817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balasubramaniam S, Comstock CES, Ertel A, et al. Aberrant BAF57 signaling facilitates prometastaic phenotypes. Clin Cancer Res. 2013;19(10):2657–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schiewer MJ, Goodwin JF, Han S, et al. Dual Roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012;2(12):1134–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chou YW, Zhang L, Muniyan S, et al. Androgens upregulate Cdc25C protein by inhibiting its proteasomal and lysosomal degradation pathways. PloS One. 2013;8(4):e61934 doi:10.1371/journal.pone.0061934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ngan S, Stronach EA, Photiou A, et al. Microarray coupled to quantitative RT-PCR analysis of androgen-regulated genes in human LNCaP prostate cancer cells. Oncogene. 2009;28(19):2051–2063. [DOI] [PubMed] [Google Scholar]
- 30. Hwang KA, Kang NH, Yi BR, Lee HR, Park MA, Choi KC. Genistein, a soy phytoestrogen, prevents the growth of BG-1 ovarian cancer cells induced by 17b-estradiol or bisphenol A via the inhibition of cell cycle progression. In J Oncol. 2013;42(2):733–740. [DOI] [PubMed] [Google Scholar]
- 31. Ptak A, Wrobel A, Gregoraszczuk EL. Effect of bisphenol-A on the expression of selected genes involved in cell cycle and apoptosis in the OVCAR-3 cell line. Toxicol Lett. 2011;202(1):30–35. [DOI] [PubMed] [Google Scholar]
- 32. Wang RH, Zheng Y, Kim HS, et al. Interplay among BRAC1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol Cell. 2008;32(1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jang KY, Kim KS, Hwang SH, et al. Expression and prognostic significance of SIRT1 in ovarian epithelial tumours. Pathology. 2009;41(4):366–371. [DOI] [PubMed] [Google Scholar]
- 34. Simpkins F, Garcia-Soto A, Slingerland J. New insights on the role of hormonal therapy in ovarian cancer. Steroids. 2013;78(6):530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]