Abstract
Objective:
To examine sex differences in the relationship between clinical symptoms related to Alzheimer disease (AD) (verbal memory deficits) and neurodegeneration (hippocampal volume/intracranial volume ratio [HpVR]) across AD stages.
Methods:
The sample included 379 healthy participants, 694 participants with amnestic mild cognitive impairment (aMCI), and 235 participants with AD and dementia from the Alzheimer's Disease Neuroimaging Initiative who completed the Rey Auditory Verbal Learning Test (RAVLT). Cross-sectional analyses were conducted using linear regression to examine the interaction between sex and HpVR on RAVLT across and within diagnostic groups adjusting for age, education, and APOE ε4 status.
Results:
Across groups, there were significant sex × HpVR interactions for immediate and delayed recall (p < 0.01). Women outperformed men among individuals with moderate to larger HpVR, but not among individuals with smaller HpVR. In diagnosis-stratified analyses, the HpVR × sex interaction was significant in the aMCI group, but not in the control or AD dementia groups, for immediate and delayed recall (p < 0.01). Among controls, women outperformed men on both outcomes irrespective of HpVR (p < 0.001). In AD dementia, better RAVLT performance was independently associated with female sex (immediate, p = 0.04) and larger HpVR (delayed, p = 0.001).
Conclusion:
Women showed an advantage in verbal memory despite evidence of moderate hippocampal atrophy. This advantage may represent a sex-specific form of cognitive reserve delaying verbal memory decline until more advanced disease stages.
The cognitive reserve theory posits that favorable premorbid factors including higher education and IQ delay the onset of clinical deficits despite Alzheimer disease (AD)–related neurodegeneration because compensatory mechanisms are more readily engaged (e.g., alternate brain networks or cognitive strategies).1–3 The theory predicts that the onset of accelerated cognitive decline is closer in time to AD dementia diagnosis in individuals with greater cognitive reserve; however, after onset, their decline is more rapid because neurodegeneration is more advanced at that point.4–6
Throughout life, women outperform men on verbal memory tasks.7–9 We predict that this female advantage may reflect a sex-specific form of cognitive reserve resulting in a delay in the clinical manifestation of memory impairment until more advanced neurodegeneration overwhelms the female advantage and decline begins. Thereafter, we predict that women decline more rapidly than men due to greater pathologic burden. This sex difference is important clinically because verbal memory deficits are used to diagnose amnestic mild cognitive impairment (aMCI) and AD dementia and norms for clinical tests are currently not sex-adjusted. A true aMCI diagnosis may be delayed more often in women than men because the female advantage in verbal memory may mask underlying neurodegeneration, particularly in earlier disease stages.
The hippocampus mediates verbal memory10–12 and hippocampal volume is related to risk of aMCI13 and AD dementia.14 We tested the hypothesis that the female advantage in verbal memory reflects a form of cognitive reserve by examining sex differences in the relationship between AD-related clinical presentation (verbal memory performance) and neurodegeneration (hippocampal volume/intracranial volume ratio [HpVR]) across and within diagnostic categories (control, aMCI, AD dementia). We hypothesized that the magnitude of the female advantage in verbal memory will vary by HpVR; the female advantage will be evident among individuals with moderate-to-large HpVR but not among individuals with smaller HpVR.
METHODS
Participants and data source.
Cross-sectional data were extracted from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu) in June 2014. ADNI has been previously described in detail at www.adni-info.org. Since 2003, ADNI has recruited over 1,500 older adults over 3 phases (ADNI-1, ADNI-GO, ADNI-2) from over 50 sites in the United States and Canada. ADNI participants are aged 55–90 years and fall within the diagnostic categories of normal, early or late mild cognitive impairment (MCI), and early AD dementia, with MCI being the major target population. Study visits involve imaging, neuropsychological, and clinical assessments. Recruitment procedures for ADNI have been reported15 (www.loni.usc.edu/ADNI), and ADNI eligibility criteria are described at www.adni-info.org/Scientists/ADNIStudyProcedures.html.
Our sample included participants who had concurrent diagnostic, hippocampal volumetric, and verbal memory data available from one visit cycle (n = 1,384). Exclusionary criteria specific to our study included missing covariate data (62 missing APOE ε genotype), presence of a significant medical condition that could cause difficulty with protocol compliance (n = 2), evidence of brain infection, infarction, or other focal lesions at the participant's screening/baseline MRI (n = 9), and a MCI diagnosis that did not meet standard criteria for aMCI including objective memory impairment and a subjective memory complaint (n = 3).16
Standard protocol approvals, registrations, and patient consents.
ADNI was approved by the institutional review board at each site and was compliant with the Health Insurance Portability and Accountability Act. All participants provided written consent.
Neuropsychological outcomes.
Participants underwent clinical and cognitive evaluations at each ADNI visit. The cognitive evaluation included the following: (1) Mini-Mental State Examination (MMSE)17 to assess global cognitive function, (2) Clinical Dementia Rating (CDR)18 to assess dementia severity, and (3) American National Adult Reading Test19 to assess premorbid intelligence. The Rey Auditory Verbal Learning Test (RAVLT),20 a multitrial list learning and memory test that shows a female advantage,21 served as our measure of verbal episodic memory. On each of 5 successive trials, a list of 15 unrelated words was read aloud, and the participant was instructed to recall aloud as many words as possible (immediate recall score, range 0–75). A new list of 15 words (interference list) unrelated to the first list and to each other was then read aloud, and the participant was instructed to recall aloud as many words as possible. The participant was then, again, asked to recall the first word list. After a 30-minute delay in which other nonverbal tasks were administered, the participant was again instructed to recall as many words as possible from the first list (delayed recall score, range 0–15). The primary outcome measures were immediate and delayed recall scores.
Diagnostic criteria.
Diagnostic criteria for an early AD dementia diagnosis in ADNI included a MMSE score between 20 and 26, a CDR of 0.5 or 1, and meeting the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association22 criteria for probable AD.15 Diagnostic criteria for an aMCI diagnosis included MMSE score between 24 and 30, CDR of 0.5, a subjective memory complaint, and objective memory loss as measured by education-adjusted scores on the Wechsler Memory Scale Logical Memory II, but without significant impairment in other cognitive domains or interference in daily life activities.15 The distinction of early vs late aMCI is based on a modest vs advanced impairment of the delayed recall portion of Logical Memory II.23 Classification as normal required a MMSE score between 24 and 30 and a CDR of 0.15
MRI acquisition.
Structural MRI scans were collected on a 1.5T scanner based on a standardized protocol that was validated across sites.24 High-resolution, T1-weighted volumetric magnetization-prepared rapid gradient echo sequences were collected in the sagittal plane, and T2-weighted fast-spin echo sequences were collected in the axial plane.24 Prior to data collection, customized imaging sequences were developed and validated on phantoms and on 137 participants. Additionally, a phantom scan was acquired for each participant and was centrally evaluated for an optimal signal-to-noise ratio. Validation procedures have been described24 (www.loni.usc.edu/ADNI).
Hippocampal volumetric MRI measures.
Hippocampal volume data were analyzed using FreeSurfer v4.3 (UCSF–FreeSurfer Methods, version 2012-12-11, ida.loni.usc.edu/pages/access/studyData.jsp). Semiautomated hippocampal volumetry was conducted using a previously validated high-dimensional brain mapping tool (Medtronic Surgical Navigation Technologies, Louisville, CO), which demonstrated similarity to manual hippocampal tracing.25 To measure hippocampal volume, 22 control points were placed manually on individual brain MRI to indicate hippocampal landmarks including the hippocampal head, tail, and at the superior, inferior, medial, and lateral boundaries on 5 equally spaced slices perpendicular to the long axis of the hippocampus.26 Individual brain scans were then fitted to a template brain using fluid image transformation.26 Hippocampal volume was then measured by counting the pixels that corresponded to the hippocampus. This hippocampal volumetry protocol has demonstrated reliability with an intraclass coefficient greater than 0.94.25 The right and left hippocampi were initially examined individually; however, because the predictive value of right vs left hippocampi was similar, we used the bilateral measure. In order to control for sex differences in head size, the HpVR was calculated using the formula hippocampal/intracranial volume × 103. Thus, HpVR represents a proportional, regional, gray matter volume.
Statistical analysis.
Participant characteristics (sociodemographic and clinical outcomes) and variables of interest (RAVLT scores and HpVR) were compared between sexes in the overall sample and within each diagnostic group using analyses of variance for continuous variables and χ2 tests for categorical variables. We ran a multivariable linear regression across diagnostic groups for each RAVLT outcome (immediate and delayed recall) in order to examine the independent and interactive associations of sex and HpVR on verbal memory performance. The first model examined the independent effects of sex and HpVR. The HpVR interaction term was added to the second model, but was eliminated if nonsignificant (p > 0.05). The covariates included age, education, APOE genotype, and diagnosis. APOE genotype was dichotomized by ε4 allele carrier status: APOE4 carriers and noncarriers. Secondary analyses examined the independent and interactive associations of sex and HpVR on RAVLT immediate and delayed recall within diagnostic group using the same approach.
RESULTS
Sample characteristics.
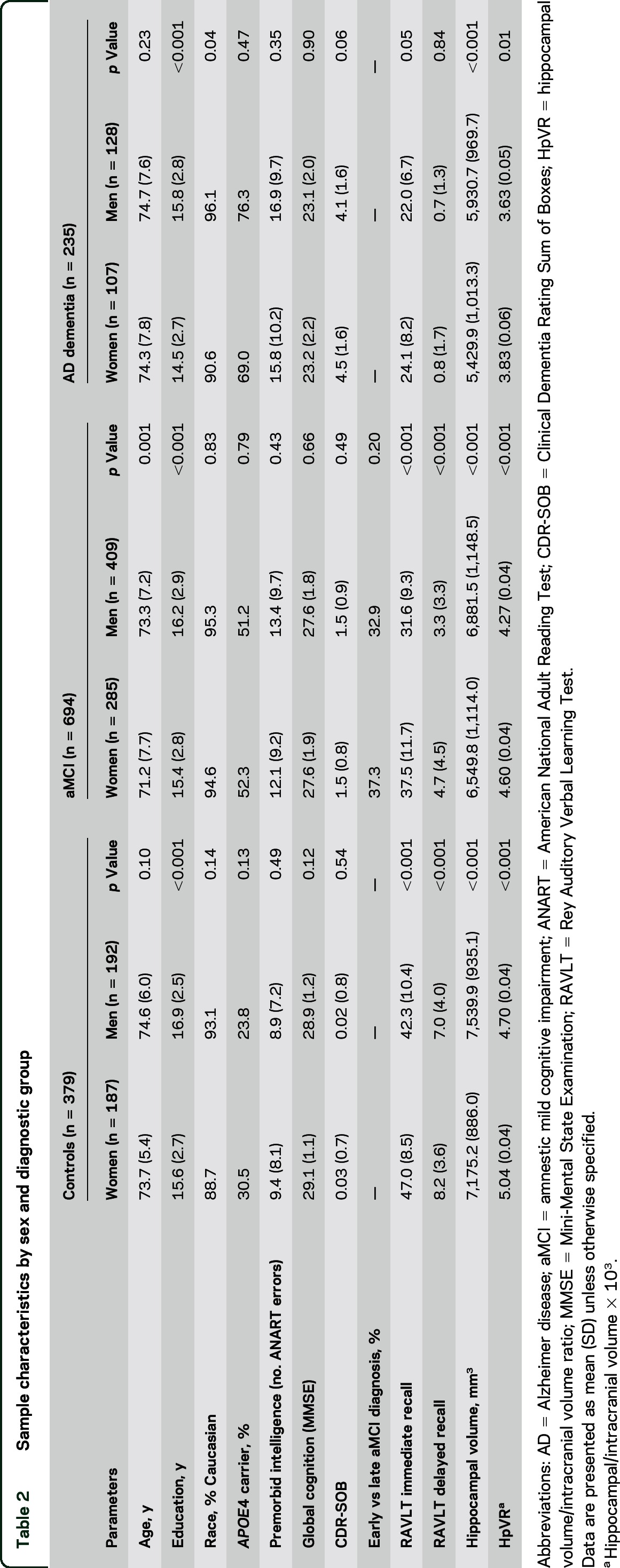
The sample comprised 1,308 participants including 379 controls, 694 individuals with aMCI (269 early MCI, 507 late MCI), and 235 individuals with AD dementia (tables 1 and 2). The majority of the participants were from ADNI 1 (n = 756) and 2 (n = 588) phases while 114 participants were from ADNI GO. Overall, across diagnostic groups, women were younger, less educated, and less likely to be white compared to men (p < 0.05). Race did not significantly relate to verbal memory scores (p > 0.05) and, therefore, was not included as a covariate. A comparison of men and women within diagnostic group separately demonstrated that women were less educated compared to men in each group (p < 0.001) and that women were significantly younger than men in the aMCI group only (p < 0.001). In the overall sample and within each diagnostic group, immediate recall scores were higher in women compared to men (p < 0.05). Delayed recall scores were higher in women compared to men in the overall sample and within control and aMCI subgroups (p < 0.001), but not the AD dementia subgroup. Although absolute hippocampal volumes were significantly larger in men compared to women in the overall sample and in each diagnostic group, the HpVR was significantly larger in women compared to men (p < 0.05).
Table 1.
Overall sample characteristics by sex
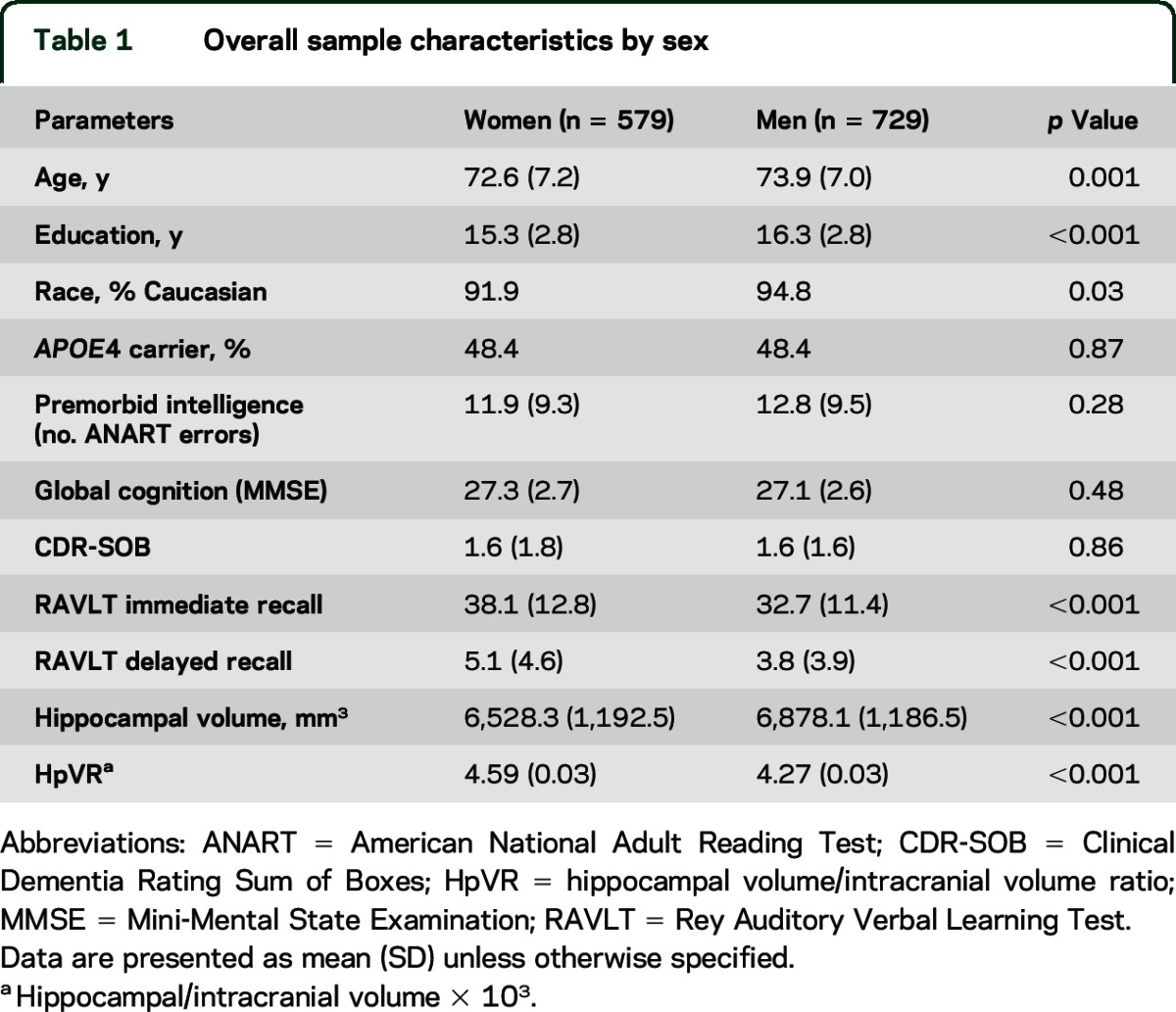
Table 2.
Sample characteristics by sex and diagnostic group
Linear regression results.
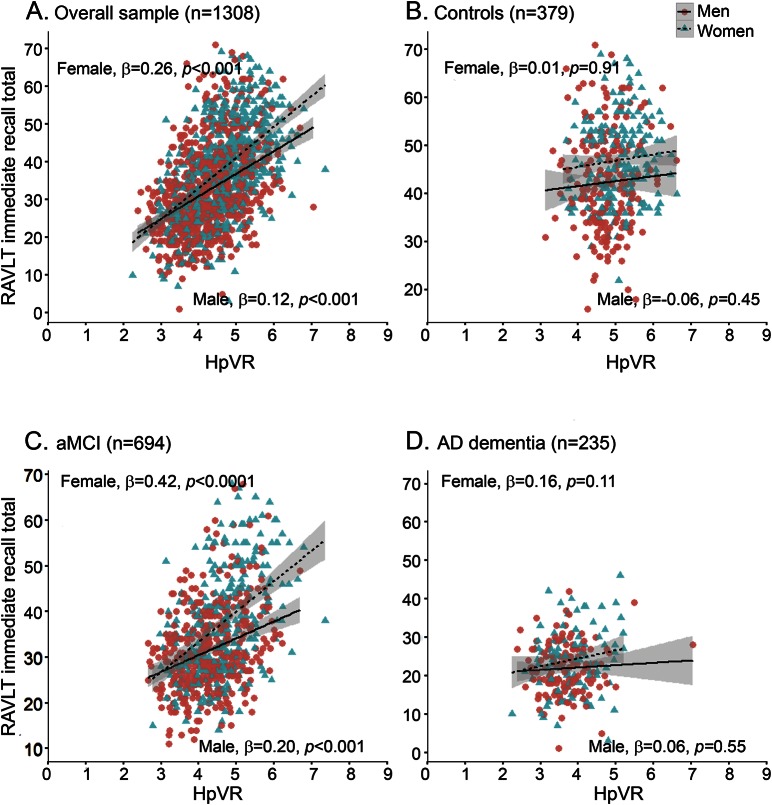
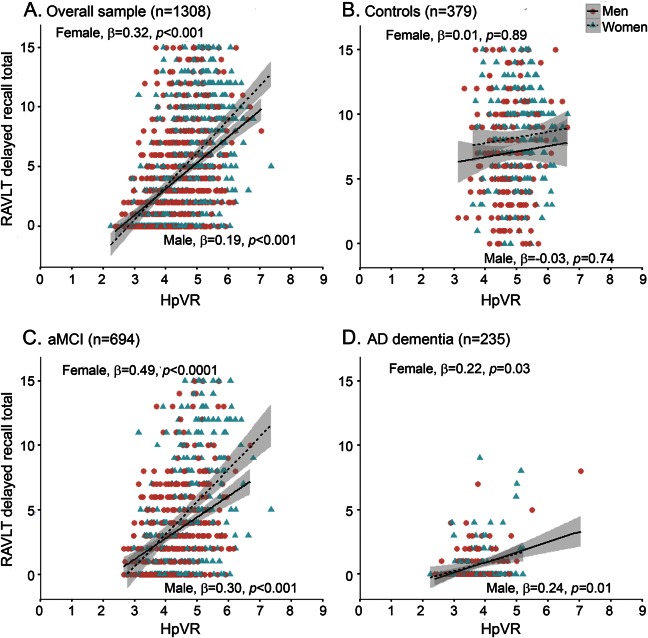
The hypothesis that the magnitude of the female advantage in verbal memory would vary by HpVR was supported by the finding of a significant sex × HpVR interaction for both immediate (p = 0.001) and delayed recall on the RAVLT (p = 0.008; table 3). The magnitude of the positive association between HpVR and RAVLT performance was stronger in women compared to men for immediate (B [unstandardized coefficient] = 3.89, β [standardized coefficient] = 0.26, SE = 0.63, p < 0.001 for women vs B = 1.84, β = 0.12, SE = 0.49, p < 0.001 for men) and delayed (B = 1.64, β = 0.32, SE = 0.23, p < 0.001 for women vs B = 1.03, β = 0.19, SE = 0.18, p < 0.001 for men) recall. Figures 1A and 2A show this relationship demonstrating that women with larger HpVR (higher end of HpVR linear spectrum) significantly outperformed men with larger HpVR on immediate (figure 1A) and delayed (figure 2A) recall, but this sex difference was absent among individuals with smaller HpVR.
Table 3.
Results of multivariable linear regression analyses modeling the independent and interactive effects of sex and HpVR on verbal memory performance
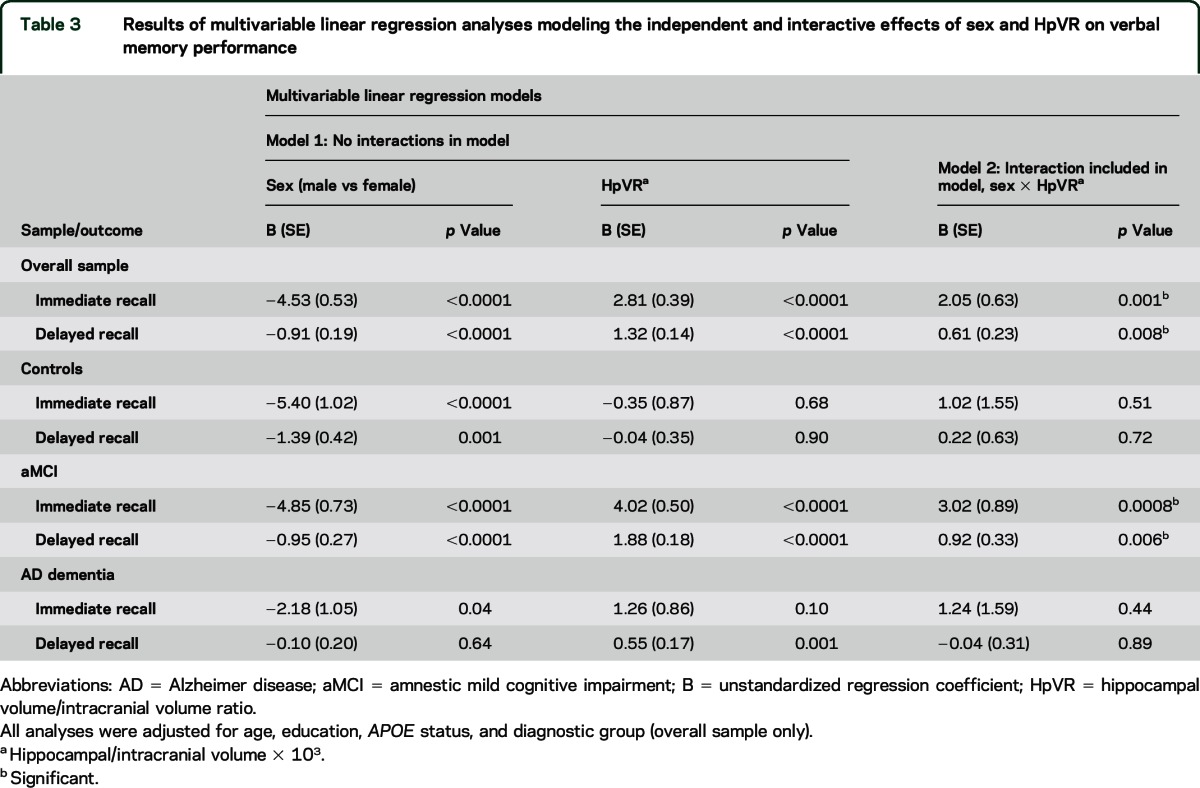
Figure 1. Relationship between hippocampal volume/intracranial volume ratio (HpVR) and Rey Auditory Verbal Learning Test (RAVLT) immediate recall scores in men and women.
RAVLT immediate recall scores as a function of HpVR (hippocampal/intracranial volume × 103) and sex in the (A) overall group, (B) controls, (C) amnestic mild cognitive impairment (aMCI), and (D) Alzheimer disease (AD) dementia. β = sex-specific standardized regression coefficient of the relationship between RAVLT scores and HpVR controlling for age, education, APOE4, and diagnosis (overall sample only); HpVR = hippocampal/intracranial volume × 103.
Figure 2. Relationship between hippocampal volume/intracranial volume ratio (HpVR) and Rey Auditory Verbal Learning Test (RAVLT) delayed recall scores in men and women.
RAVLT delayed recall scores as a function of HpVR (hippocampal/intracranial volume × 103) and sex in the (A) overall group, (B) controls, (C) amnestic mild cognitive impairment (aMCI), and (D) Alzheimer disease (AD) dementia. β = sex-specific standardized regression coefficient of the relationship between RAVLT scores and HpVR controlling for age, education, APOE4, and diagnosis (overall sample only); HpVR = hippocampal/intracranial volume × 103.
In diagnosis-stratified analyses, the HpVR × sex interaction was significant in the aMCI group, but not the AD dementia or control group. Specifically, there was a HpVR × sex interaction for immediate (p = 0.0008) and delayed (p = 0.006) recall in the aMCI group. Similar to the results in the overall sample, the association between HpVR and RAVLT performance was stronger in women compared to men for immediate (B = 5.71, β = 0.42, SE = 0.70, p < 0.0001 for women vs B = 2.69, β = 0.20, SE = 0.63, p < 0.001 for men) and delayed (B = 2.40, β = 0.26, SE = 0.26, p < 0.0001 for women vs B = 1.48, β = 0.30, SE = 0.23, p < 0.001 for men) recall. Among individuals with aMCI with larger HpVR, women outperformed men on immediate (figure 1C) and delayed (figure 2C) recall, but this sex difference was not evident among individuals with aMCI with smaller HpVR (lower end of HpVR linear spectrum). Conversely, the HpVR × sex interaction was not significant in control or AD dementia groups (p > 0.05). Among controls, women significantly outperformed men in immediate and delayed recall irrespective of HpVR (p = 0.01) and HpVR was not significantly associated with immediate (p = 0.53) or delayed recall (p = 0.95) (figures 1B and 2B). Among patients with AD dementia, women significantly outperformed men in immediate (p = 0.04) but not delayed recall (p > 0.05). Regardless of sex, smaller HpVR in AD dementia were significantly associated with poorer delayed recall performance (p = 0.001) but did not reach statistical significance for immediate recall (p = 0.10).
DISCUSSION
We examined whether sex modifies the relationship between verbal memory and HpVR. Consistent with the broader scientific literature, we found that, compared to men, women performed better on immediate and delayed measures of verbal memory7–9 and had larger HpVR.27 In the first study to examine how sex modifies the relationship between verbal memory and hippocampal volume, we found overall that the female advantage in verbal memory was apparent among individuals with moderate to large HpVR but the advantage was absent among individuals with smaller HpVR. Results were driven by the interaction between sex and HpVR in the aMCI group, where females outperformed men when volumes were moderate to large but not when HpVR were small.
We hypothesized that in the AD dementia group, there would be no female advantage in verbal memory and that there would be no association between sex and HpVR on memory because of the greater loss of hippocampal volume. As hypothesized, delayed recall scores did not significantly differ between male and female patients with AD dementia; however, a floor effect limits interpretation. Counter to hypotheses, female patients with AD dementia outperformed male patients with AD dementia in immediate recall (p = 0.04); however, the sex difference was smaller compared to controls and aMCI groups (mean difference = 2.1, 4.7, and 5.9, respectively). These results suggest a diminution of the female advantage but not an elimination. Others have also reported an elimination28 or even a reversal29 of the female advantage in verbal memory in AD dementia. Smaller HpVR were associated with poorer delayed recall performance and trended towards an association with immediate recall (p = 0.10) among patients with AD dementia.
Among controls, women showed an advantage in verbal memory over men and had larger HpVR, but memory performance was unrelated to HpVR. The lack of a relationship between hippocampal volume and memory performance in healthy older adults has been demonstrated previously,30 and may be due to a limited range of variability among controls compared to the aMCI or AD dementia group (SD = 928, 1,146, and 1,019, respectively) or because of the low prevalence in controls of AD-specific mechanisms underlying both hippocampal atrophy and memory deficits. In controls, it is presumed that there is a smaller degree of neurodegeneration and less reliance on reserves to maintain normal performance. Therefore, differences in cognitive reserve among controls are likely latent; however, an effect might be detectable in a larger control group. One interpretation of these findings is that the reliable female advantage in verbal memory may represent a greater cognitive reserve in women in the domain of verbal memory. The cognitive reserve theory posits that high levels of certain premorbid factors such as IQ, education, and occupational attainment confer an advantage in the ability to compensate for neuropathologic changes by, for example, engaging alternative brain networks or cognitive strategies.1–3 The theory was originally proposed to explain why individuals vary in clinical presentation of AD yet have a similar degree of neurodegeneration.1 In this way, women's high premorbid performance on verbal memory tests might confer an advantage in the ability to maintain verbal memory performance despite loss of hippocampal volume. In support of the theory that the female advantage in verbal memory may reflect a domain-specific cognitive reserve, a study found that, among healthy adults, men show an earlier decline in verbal memory compared to women.31 The current study more directly demonstrates that the female advantage is maintained despite moderate levels of neurodegeneration. Furthermore, our results suggest that the clinical manifestation of verbal memory impairment is delayed until a more advanced level of neurodegeneration in women vs men. Indeed, using a standardized cutoff for impairment on the RAVLT,32,33 women in the current study reached the cutoff of impairment at a smaller HpVR than men for both immediate (5 vs 6, respectively; figure 1A) and delayed recall (7.5 and ∼8.5, respectively). Thus, impairment in verbal memory was evident at a smaller HpVR in women compared to men.
Our results might help to explain the paradoxical sex differences in the incidence of aMCI vs AD dementia. Some,34,35 but not all studies,36,37 reported that incidence of AD dementia is higher in women, whereas incidence of MCI has been reported to be higher in men.38,39 In the present cross-sectional study, there was a higher proportion of aMCI diagnoses in men vs women (56.8% vs 48.5%, p < 0.002), but a nonsignificant higher proportion of AD dementia diagnoses in women vs men (19.7% vs 17.8%, p = 0.08%). One possible explanation for this apparent paradox is that verbal memory deficits are central in diagnosing aMCI, and cognitive reserve in that domain may mask a true aMCI diagnosis in women. Furthermore, a delayed onset of verbal memory impairment in women and accelerated decline thereafter would lead to a shorter window of time for an aMCI diagnosis in women that may not be captured in longitudinal assessments given at 12- to 24-month intervals.
Our study has limitations. With a cross-sectional design, the temporal relationship between verbal memory and HpVR cannot be determined. However, hippocampal volumes are a biomarker of imminent cognitive decline and progression from MCI to AD dementia in longitudinal studies.12,13 This design also limits our test of the cognitive reserve theory because we were unable to measure rates of decline in men vs women. Population-based, longitudinal analyses are needed to more definitively test the theory that the female advantage in verbal memory may serve as a form of cognitive reserve. The control and AD dementia groups were smaller than the MCI group, thereby limiting statistical power to detect a sex by HpVR interaction within these groups; however, no trend for an interaction was evident in the control and AD dementia groups. The ADNI cohort represents a convenience sample of volunteers and is, therefore, susceptible to selection bias. Past use of hormone therapy was not assessed in ADNI and, therefore, not adjusted for because of the difficulty in acquiring self-reported medication history from participants with memory problems.
Our findings replicate previous findings that women perform better on a verbal memory task and have larger HpVR compared to men. The relationship between verbal memory and HpVR varies by sex; women show an advantage in verbal memory despite minimal to moderate levels of hippocampal atrophy. Findings suggest that women might show a sex-specific cognitive reserve in the domain of verbal memory. If replicated, our findings suggest the need to evaluate whether diagnosis of aMCI is made at a later disease stage in women compared to men because this sex-specific advantage in verbal memory masks underlying neurodegeneration. If so, then sex-based norms in clinical memory tests might improve diagnostic accuracy in women.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the ADNI study subjects and investigators for their participation.
GLOSSARY
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- aMCI
amnestic mild cognitive impairment
- CDR
Clinical Dementia Rating
- HpVR
hippocampal volume/intracranial volume ratio
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- RAVLT
Rey Auditory Verbal Learning Test
Footnotes
Editorial, page 1364
Supplemental data at Neurology.org
Contributor Information
Collaborators: Alzheimer's Disease Neuroimaging Initiative, Michael Weiner, Paul Aisen, Michael Weiner, Paul Aisen, Ronald Petersen, Clifford R. Jack, Jr., William Jagust, John Q. Trojanowki, Arthur W. Toga, Laurel Beckett, Robert C. Green, Andrew J. Saykin, John Morris, Enchi Liu, Robert C. Green, Tom Montine, Ronald Petersen, Paul Aisen, Anthony Gamst, Ronald G. Thomas, Michael Donohue, Sarah Walter, Devon Gessert, Tamie Sather, Laurel Beckett, Danielle Harvey, Anthony Gamst, Michael Donohue, John Kornak, Clifford R. Jack, Jr., Anders Dale, Matthew Bernstein, Joel Felmlee, Nick Fox, Paul Thompson, Norbert Schuff, Gene Alexander, Charles DeCarli, William Jagust, Dan Bandy, Robert A. Koeppe, Norm Foster, Eric M. Reiman, Kewei Chen, Chet Mathis, John Morris, Nigel J. Cairns, Lisa Taylor-Reinwald, J.Q. Trojanowki, Les Shaw, Virginia M.Y. Lee, Magdalena Korecka, Arthur W. Toga, Karen Crawford, Scott Neu, Andrew J. Saykin, Tatiana M. Foroud, Steven Potkin, Li Shen, Zaven Kachaturian, Richard Frank, Peter J. Snyder, Susan Molchan, Jeffrey Kaye, Joseph Quinn, Betty Lind, Sara Dolen, Lon S. Schneider, Sonia Pawluczyk, Bryan M. Spann, James Brewer, Helen Vanderswag, Judith L. Heidebrink, Joanne L. Lord, Ronald Petersen, Kris Johnson, Rachelle S. Doody, Javier Villanueva-Meyer, Munir Chowdhury, Yaakov Stern, Lawrence S. Honig, Karen L. Bell, John C. Morris, Beau Ances, Maria Carroll, Sue Leon, Mark A. Mintun, Stacy Schneider, Daniel Marson, Randall Griffith, David Clark, Hillel Grossman, Effie Mitsis, Aliza Romirowsky, Leyla deToledo-Morrell, Raj C. Shah, Ranjan Duara, Daniel Varon, Peggy Roberts, Marilyn Albert, Chiadi Onyike, Stephanie Kielb, Henry Rusinek, Mony J de Leon, Lidia Glodzik, Susan De Santi, P. Murali Doraiswamy, Jeffrey R. Petrella, R. Edward Coleman, Steven E. Arnold, Jason H. Karlawish, David Wolk, Charles D. Smith, Greg Jicha, Peter Hardy, Oscar L. Lopez, MaryAnn Oakley, Donna M. Simpson, Anton P. Porsteinsson, Bonnie S. Goldstein, Kim Martin, Kelly M. Makino, M. Saleem Ismail, Connie Brand, Ruth A. Mulnard, Gaby Thai, Catherine Mc-Adams-Ortiz, Kyle Womack, Dana Mathews, Mary Quiceno, Ramon Diaz-Arrastia, Richard King, Myron Weiner, Kristen Martin-Cook, Michael DeVous, Allan I. Levey, James J. Lah, Janet S. Cellar, Jeffrey M. Burns, Heather S. Anderson, Russell H. Swerdlow, Liana Apostolova, Po H. Lu, George Bartzokis, Daniel H.S. Silverman, Neill R Graff-Radford, Francine Parfitt, Heather Johnson, Martin R. Farlow, Ann Marie Hake, Brandy R. Matthews, Scott Herring, Christopher H. van Dyck, Richard E. Carson, Martha G. MacAvoy, Howard Chertkow, Howard Bergman, Chris Hosein, Sandra Black, Bojana Stefanovic, Curtis Caldwell, Ging-Yuek Robin Hsiung, Howard Feldman, Benita Mudge, Michele Assaly, Andrew Kertesz, John Rogers, Dick Trost, Charles Bernick, Donna Munic, Diana Kerwin, Marek-Marsel Mesulam, Kristina Lipowski, Chuang-Kuo Wu, Nancy Johnson, Carl Sadowsky, Walter Martinez, Teresa Villena, Raymond Scott Turner, Kathleen Johnson, Brigid Reynolds, Reisa A. Sperling, Keith A. Johnson, Gad Marshall, Meghan Frey, Jerome Yesavage, Joy L. Taylor, Barton Lane, Allyson Rosen, Jared Tinklenberg, Marwan Sabbagh, Christine Belden, Sandra Jacobson, Neil Kowall, Ronald Killiany, Andrew E. Budson, Alexander Norbash, Patricia Lynn Johnson, Thomas O. Obisesan, Saba Wolday, Salome K. Bwayo, Alan Lerner, Leon Hudson, Paula Ogrocki, Evan Fletcher, Owen Carmichael, John Olichney, Charles DeCarli, Smita Kittur, Michael Borrie, T-Y Lee, Rob Bartha, Sterling Johnson, Sanjay Asthana, Cynthia M. Carlsson, Steven G. Potkin, Adrian Preda, Dana Nguyen, Pierre Tariot, Adam Fleisher, Stephanie Reeder, Vernice Bates, Horacio Capote, Michelle Rainka, Douglas W. Scharre, Maria Kataki, Earl A. Zimmerman, Dzintra Celmins, Alice D. Brown, Godfrey D. Pearlson, Karen Blank, Karen Anderson, Andrew J. Saykin, Robert B. Santulli, Eben S. Schwartz, Kaycee M. Sink, Jeff D. Williamson, Pradeep Garg, Franklin Watkins, Brian R. Ott, Henry Querfurth, Geoffrey Tremont, Stephen Salloway, Paul Malloy, Stephen Correia, Howard J. Rosen, Bruce L. Miller, Jacobo Mintzer, Crystal Flynn Longmire, Kenneth Spicer, Elizabether Finger, Irina Rachinsky, John Rogers, Andrew Kertesz, Dick Drost, Nunzio Pomara, Raymundo Hernando, Antero Sarrael, Susan K. Schultz, Laura L. Boles Ponto, Hyungsub Shim, Karen Elizabeth Smith, Norman Relkin, Gloria Chaing, Lisa Raudin, Amanda Smith, Kristin Fargher, and Balebail Ashok Raj
AUTHOR CONTRIBUTIONS
E.E.S., A.B., P.M.M.: study concept. E.E.S., A.B., P.M.M., R.B.L., L.H.R., W.M., S.L.: study design. E.E.S., S.L.: data acquisition. L.H.R., E.E.S.: statistical analysis. E.E.S., A.B., P.M.M., R.B.L., S.L.: data interpretation. E.E.S.: initial manuscript preparation. All authors provided a critical review of manuscript for important intellectual content and contributed to and approved the final manuscript.
STUDY FUNDING
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (NIH grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through contributions from nonprofit partners the Alzheimer's Association and Alzheimer's Drug Discovery Foundation, with participation from the US Food and Drug Administration and from the following: Abbott; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Amorfix Life Sciences Ltd.; AstraZeneca; Bayer HealthCare; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research and Development, LLC; Johnson and Johnson Pharmaceutical Research and Development LLC; Medpace, Inc.; Merck and Co., Inc.; Meso Scale Diagnostics, LLC; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. Drs. Sundermann and Lipton's time was supported by funding for the Einstein Aging Study: National Institute on Aging Grant AG003949, AG026728, TL1RR000087, T32-GM007288, the Leonard and Sylvia Marx Foundation, and the Czap Foundation.
DISCLOSURE
E. Sundermann, A. Biegon, and L. Rubin report no disclosures relevant to the manuscript. R. Lipton reports research support from the NIH: PO1 AG003949 (Program Director), PO1AG027734 (Project Leader), RO1AG025119 (Investigator), RO1AG022374-06A2 (Investigator), RO1AG034119 (Investigator), RO1AG12101 (Investigator), K23AG030857 (Mentor), K23NS0514090μ1A1 (Mentor), and K23NS47256 (Mentor), the National Headache Foundation, and the Migraine Research Fund; serves on the editorial boards of Neurology® and Cephalalgia and as senior advisor to Headache; has reviewed for the NIA and NINDS; holds stock options in eNeura Therapeutics (a company without commercial products); and serves as consultant, advisory board member, or has received honoraria from Alder, Allergan, American Headache Society, Autonomic Technologies, Avanir, Boston Scientific, Bristol Myers Squibb, Colucid, Dr. Reddy's, Electrocore, Eli Lilly, Endo, eNeura Therapeutics, Informa, Labrys, Merck, Novartis, Teva, and Vedanta. W. Mowrey reports no disclosures relevant to the manuscript. S. Landau has served as a paid consultant for Genentech, Synarc, Biogen, and Janssen. P. Maki reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA 1994;271:1004–1010. [PubMed] [Google Scholar]
- 2.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 2002;8:448–460. [PubMed] [Google Scholar]
- 3.Stern Y, Zarahn E, Hilton HJ, Flynn J, DeLaPaz R, Rakitin B. Exploring the neural basis of cognitive reserve. J Clin Exp Neuropsychol 2003;25:691–701. [DOI] [PubMed] [Google Scholar]
- 4.Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology 1999;53:1942–1947. [DOI] [PubMed] [Google Scholar]
- 5.Le Carret N, Auriacombe S, Letenneur L, Bergua V, Dartigues JF, Fabrigoule C. Influence of education on the pattern of cognitive deterioration in AD patients: the cognitive reserve hypothesis. Brain Cogn 2005;57:120–126. [DOI] [PubMed] [Google Scholar]
- 6.Hall CB, Derby C, LeValley A, Katz MJ, Verghese J, Lipton RB. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology 2007;69:1657–1664. [DOI] [PubMed] [Google Scholar]
- 7.Kramer JH, Delis DC, Daniel MH. Sex differences in verbal learning. J Clin Psychol 1988;44, 907–915. [Google Scholar]
- 8.Aartsen MJ, Martin M, Zimprich D. Gender differences in level and change in cognitive functioning: results from the Longitudinal Aging Study Amsterdam. Gerontology 2004;50:35–38. [DOI] [PubMed] [Google Scholar]
- 9.Barrett-Connor E, Kritz-Silverstein D. Gender differences in cognitive function with age: the Rancho Bernardo study. J Am Geriatr Soc 1999;47:159–164. [DOI] [PubMed] [Google Scholar]
- 10.Kilpatrick C, Murrie V, Cook M, Andrewes D, Desmond P, Hopper J. Degree of left hippocampal atrophy correlates with severity of neuropsychological deficits. Seizure 1997;6:213–218. [DOI] [PubMed] [Google Scholar]
- 11.Ystad MA, Lundervold AJ, Wehling E, et al. Hippocampal volumes are important predictors for memory function in elderly women. BMC Med Imaging 2009;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci 2000;3:1149–1152. [DOI] [PubMed] [Google Scholar]
- 13.Kantarci K, Weigand SD, Przybelski SA, et al. MRI and MRS predictors of mild cognitive impairment in a population-based sample. Neurology 2013;81:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landau SM, Harvey D, Madison CM, et al. ; Alzheimer's Disease Neuroimaging Initiative. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 2010;75:230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 2010;74:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 19.Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol 1991;13:933–949. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt M. Rey Auditory Verbal Learning Test: A Handbook. Los Angeles: Psychological Services; 1996. [Google Scholar]
- 21.Gale SD, Baxter L, Connor DJ, Herring A, Comer J. Sex differences on the Rey Auditory Verbal Learning Test and the brief visuospatial memory test-revised in the elderly: normative data in 172 participants. J Clin Exp Neuropsychol 2007;29:561–567. [DOI] [PubMed] [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 23.Aisen PS, Petersen RC, Donohue MC, et al. Clinical core of the Alzheimer's disease neuroimaging initiative: progress and plans. Alzheimers Dement 2010;6:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 2008;27:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu YY, Schuff N, Du AT, et al. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J Magn Reson Imaging 2002;16:305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen GE, Joshi SC, Miller MI. Volumetric transformation of brain anatomy. IEEE Trans Med Imaging 1997;16:864–877. [DOI] [PubMed] [Google Scholar]
- 27.Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex 1994;4:344–360. [DOI] [PubMed] [Google Scholar]
- 28.Beinhoff U, Tumani H, Brettschneider J, Bittner D, Riepe MW. Gender-specificities in Alzheimer's disease and mild cognitive impairment. J Neurol 2008;255:117–122. [DOI] [PubMed] [Google Scholar]
- 29.Chapman RM, Mapstone M, Gardner MN, et al. Women have farther to fall: gender differences between normal elderly and Alzheimer's disease in verbal memory engender better detection of Alzheimer's disease in women. J Int Neuropsychol Soc 2011;17:654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wirth M, Villeneuve S, Haase CM, et al. Associations between Alzheimer disease biomarkers, neurodegeneration, and cognition in cognitively normal older people. JAMA Neurol 2013;70:1512–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramer JH, Yaffe K, Lengenfelder J, Delis DC. Age and gender interactions on verbal memory performance. J Int Neuropsychol Soc 2003;9:97–102. [DOI] [PubMed] [Google Scholar]
- 32.Stark SM, Yassa MA, Lacy JW, Stark CE. A task to assess behavioral pattern separation (BPS) in humans: data from healthy aging and mild cognitive impairment. Neuropsychologia 2013;51:2442–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lines CR, McCarroll KA, Lipton RB, Block GA; Prevention of Alzheimer's in Society's Elderly Study Group. Telephone screening for amnestic mild cognitive impairment. Neurology 2003;60:261–266. [DOI] [PubMed] [Google Scholar]
- 34.Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry 1998;55:809–815. [DOI] [PubMed] [Google Scholar]
- 35.Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand 1987;76:465–479. [DOI] [PubMed] [Google Scholar]
- 36.Katz MJ, Lipton RB, Hall CB, et al. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord 2012;26:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc 2004;52:195–204. [DOI] [PubMed] [Google Scholar]
- 38.Roberts RO, Geda YE, Knopman DS, et al. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology 2012;78:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brodaty H, Heffernan M, Kochan NA, et al. Mild cognitive impairment in a community sample: the Sydney Memory and Ageing Study. Alzheimers Dement 2013;9:310–317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.