Abstract
Objective:
To explore the effects of microglial activation on brain function and structure, and its relationship with peripheral inflammatory markers, in treated, HIV-positive individuals, using in vivo [11C]PBR28 PET (to measure the 18 kDa translocator protein [TSPO]).
Methods:
Cognitively healthy HIV-positive individuals on suppressive antiretroviral therapy and HIV-negative individuals (controls) underwent brain [11C]PBR28 PET and MRI. HIV-positive patients completed neuropsychological testing and CSF testing for chemokines. The concentration of bacterial ribosomal 16sDNA in plasma was measured as a marker of microbial translocation.
Results:
HIV-positive individuals showed global increases in TSPO expression compared to controls (corrected p < 0.01), with significant regional increases in the parietal (p = 0.001) and occipital (p = 0.046) lobes and in the globus pallidus (p = 0.035). TSPO binding in the hippocampus, amygdala, and thalamus were associated with poorer global cognitive performance in tasks assessing verbal and visual memory (p < 0.05). Increased TSPO binding was associated with increased brain white matter diffusion MRI mean diffusivity in HIV-positive individuals, a lower CD4/CD8 ratio, and both high pretreatment HIV RNA and plasma concentration ribosomal 16s DNA (p < 0.05).
Conclusions:
Cognitively healthy HIV-positive individuals show evidence for a chronically activated brain innate immune response and elevated blood markers of microbial translocation despite effective control of plasma viremia. Increased brain inflammation is associated with poorer cognitive performance and white matter microstructural pathology, suggesting a possible role in cognitive impairments found in some HIV-positive patients despite effective treatment.
Despite improvements in life expectancy for people living with HIV (PLWH) after the advent of combination antiretroviral therapy (cART),1 comorbidities including mild cognitive impairment remain common.2,3 There is evidence that the inflammatory process following HIV infection of the brain persists despite effective control of HIV RNA.4 Chronic activation of brain microglia therefore has been suggested to be a major contributing factor for HIV-associated brain disease.5,6
Translocator protein (TSPO) is highly expressed in the mitochondria of microglia and astrocytes.7 Following activation through host responses to cellular injury, microglia increase expression of TSPO.8 Increased binding of a TSPO radioligand therefore provides a proxy measure of brain microglial activation that can be assessed in vivo with PET.
Previous studies investigating microglial activation using TSPO PET in PLWH have employed [11C]PK11195, a first-generation TSPO radioligand. The findings have been contradictory, with some studies demonstrating differences in [11C]PK11195 binding between PLWH with and without cognitive impairment9,10 and others showing no significant differences between groups.11 Possible explanations for these discrepancies could be related to the difficulties in making accurate measures of brain binding with this ligand because of a lower proportion of the signal that arises from specifically bound radioligand.12 [11C]PBR28 is a second-generation TSPO radioligand with higher affinity for TSPO and a high displaceable binding fraction.13,14 Unlike [11C]PK11195, the affinity for the target protein is determined by the rs6971 single nucleotide polymorphism (SNP) in the TSPO gene,15 and therefore incorporation of genotypic data enables an accurate quantitative interpretation of TSPO PET data.16
The aim of this study was to test for increased microglial activation in cognitively healthy PLWH on effective cART. We also tested for correlations between TSPO expression measured by PBR28 and markers of peripheral immune activation, cognitive function, and brain structural MRI measures.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study was approved by the National Research Ethics Service (12/LO/1570). For the administration of [11C]PBR28, permission was obtained from the UK Administration of Radioactive Substances Advisory Committee (630/3764/29163). All participants provided informed consent prior to commencing any study procedures.
Participant selection.
HIV-positive cases.
Twelve participants were recruited from the HIV outpatient department at St. Mary's Hospital, Imperial College London, UK. Recruited PLWH were men who had been HIV-1-antibody positive for at least 2 years and were aged between 20 to 50 years and receiving stable cART with a plasma HIV RNA of <50 copies/mL at screening. Exclusion criteria included any active neurologic or psychiatric disease, current use of anti-inflammatories or benzodiazepines, use of recreational drugs, or alcohol abuse.
HIV-negative controls.
Historical data were available from 10 healthy HIV-negative participants between 20 to 60 years old who had undergone the same [11C]PBR28 PET scanning protocol and diffusion tensor imaging (DTI). Exclusion criteria were identical to those of the HIV-positive cases. All control participants provided written consent.
TSPO genotype.
Blood samples were obtained from HIV-positive participants and controls to determine the TSPO affinity genotype. All participants were genotyped for the rs6971 SNP. Only participants homozygous for the threonine rs6971 allele (low affinity binders) were excluded from the study.
Cognitive testing.
Cognitive testing was performed only in the HIV-positive group using CogState (CogState Ltd., Melbourne, Australia), a computerized battery previously validated in PLWH.17 A total of 8 cognitive domains were assessed (speed, visual attention, accuracy, visual learning, working memory, verbal learning, associate learning, and executive function). Overall scores from each task and composite Z scores were calculated for each participant and compared against age-matched normative population data (n = 879) provided by the manufacturer.
Biomarkers.
Ribosomal 16sDNA.
The concentration of bacterial DNA encoding the ribosomal 16s rRNA in plasma was measured by quantitative real-time PCR in all HIV-positive participants as previously described.18
CSF biomarkers.
HIV-positive patients underwent a single lumbar puncture. CSF HIV RNA concentration was measured using the COBAS AmpliPrep v2.1 (Roche, Basel, Switzerland) with a threshold of <50 copies/mL. CSF concentrations of eotaxin, macrophage inflammatory protein-1β (MIP-1β), interleukin-8 (IL-8), and interferon-γ-inducible protein 10 (IP-10) were investigated using the human chemokine cytokine assay (Meso Scale Discovery, Gaithersburg, MD). Chemokine concentrations were assessed in blood and CSF samples and were determined with Meso Scale Discovery Workbench software. The lower limit of detection in both plasma and CSF chemokines/cytokines (pg/mL) were eotaxin (8.3), MIP-1β (7.5), and IL-8 (0.6).
Brain imaging.
Volumetric imaging and DTI.
High-resolution T1 and T2 magnetic resonance images and DTI were obtained to determine the anatomical boundaries for volumetric analysis and to assess the effects of inflammation on white matter microstructure, respectively. Details of the MRI protocol are described in the e-Methods on the Neurology® Web site at Neurology.org.
[11C]PBR28 PET CT imaging.
Details on radioligand synthesis and PET protocol are described in the e-Methods. Each participant was injected with an IV bolus of 400 MBq of [11C]PBR28 over 20 seconds at the start of a 90-minute 3D mode dynamic PET acquisition. PET data were reconstructed using filtered back-projection with corrections for attenuation and scatter.
Imaging data analysis.
[11C]PBR28 PET CT.
Dynamic PET data were corrected for motion via frame-to-frame image registration and aligned with the individual's structural T1 MRI using SPM5 (Wellcome Trust Center for Neuroimaging; http://www.fil.ion.ucl.ac.uk/spm). The CIC neuroanatomical atlas19 was nonlinearly deformed into the individual's space, via T1 MRI data mapping, to obtain a personalized anatomical parcellation of regions of interest. Attention focused on 20 regions of moderate and high binding based on previous literature.20 Each region of interest (ROI) was then applied to the dynamic PET data to derive regional time-activity curves.
A 2-tissue compartment model, utilizing the metabolite-corrected plasma input function, was applied to the dynamic PET data using a fixed blood volume correction of 5%. For each ROI examined, the total volume of distribution (VT) was estimated from the rate constants as described previously by Gunn et al.21 The Logan graphical method employing a plasma input, 5% fixed blood volume, and a linear start time at 35 minutes was also used to estimate the absolute VT of each ROI and further applied at the voxel level to produce a parametric VT map for each participant. The main outcome measure is the total VT for each defined brain region and a parametric VT map for each participant. The VT for a radioligand is defined as the ratio of concentration in tissue to that in plasma at equilibrium (i.e., it is a partition coefficient) and is proportional to the level of specific radioligand binding to TSPO. The distribution volume ratio (DVR) in each ROI was also estimated by normalizing the VT to cortical gray matter in order to evaluate local regional differences. Normalization can control for unknown physiologic factors (unrelated to inflammation) that may affect radioligand brain uptake in TSPO PET imaging studies.22 The gray matter was selected as an area of control for normalization because of its relatively uniform pattern of low baseline binding in healthy participants. Model fitting and parameter estimation was performed using Matlab R2008b (The MathWorks Inc., Natick, MA).
DTI.
Standard DTI preprocessing methods were employed using the FMRIB software library.23 DTI parameter maps were generated using FMRIB's Diffusion Toolbox in FSL for fractional anisotropy (FA) and mean diffusivity (MD). The FA and MD output images were used as input for tract-based spatial statistics. A mean FA skeleton was created representing the centers of all tracts common to the group. Mean FA and MD in major white matter tracts for the HIV-positive participants were calculated by projecting each individual skeleton through masks for the major white matter tracts contained within the JHU white-matter tractography atlas, alongside additional cortico-thalamic and trans-collosal tracts defined by tractography.24
Statistical analysis.
Differences in [11C]PBR28 binding between HIV-positive participants and controls were studied both on ROI level using analysis of variance with TSPO affinity phenotype effect as a fixed factor using SPSS (SPSS, version 21, IBM, Armonk, NY) and parametrically using the FSL Randomise tool accounting for affinity status. Partial correlations were performed to investigate the relationships between PBR28 binding and clinical, cognitive, and MRI parameters including DTI adjusting for TSPO affinity phenotype. Multiple comparisons were corrected by bootstrapping for all measures.25 Associations between ribosomal 16sDNA and PBR28 binding were also calculated using partial correlation analysis adjusted for TSPO affinity status. Corrected p values below 0.05 were considered statistically significant. Independent t tests were used to contrast FA and MD between HIV-positive participants and controls.
RESULTS
Baseline characteristics and cognitive test results.
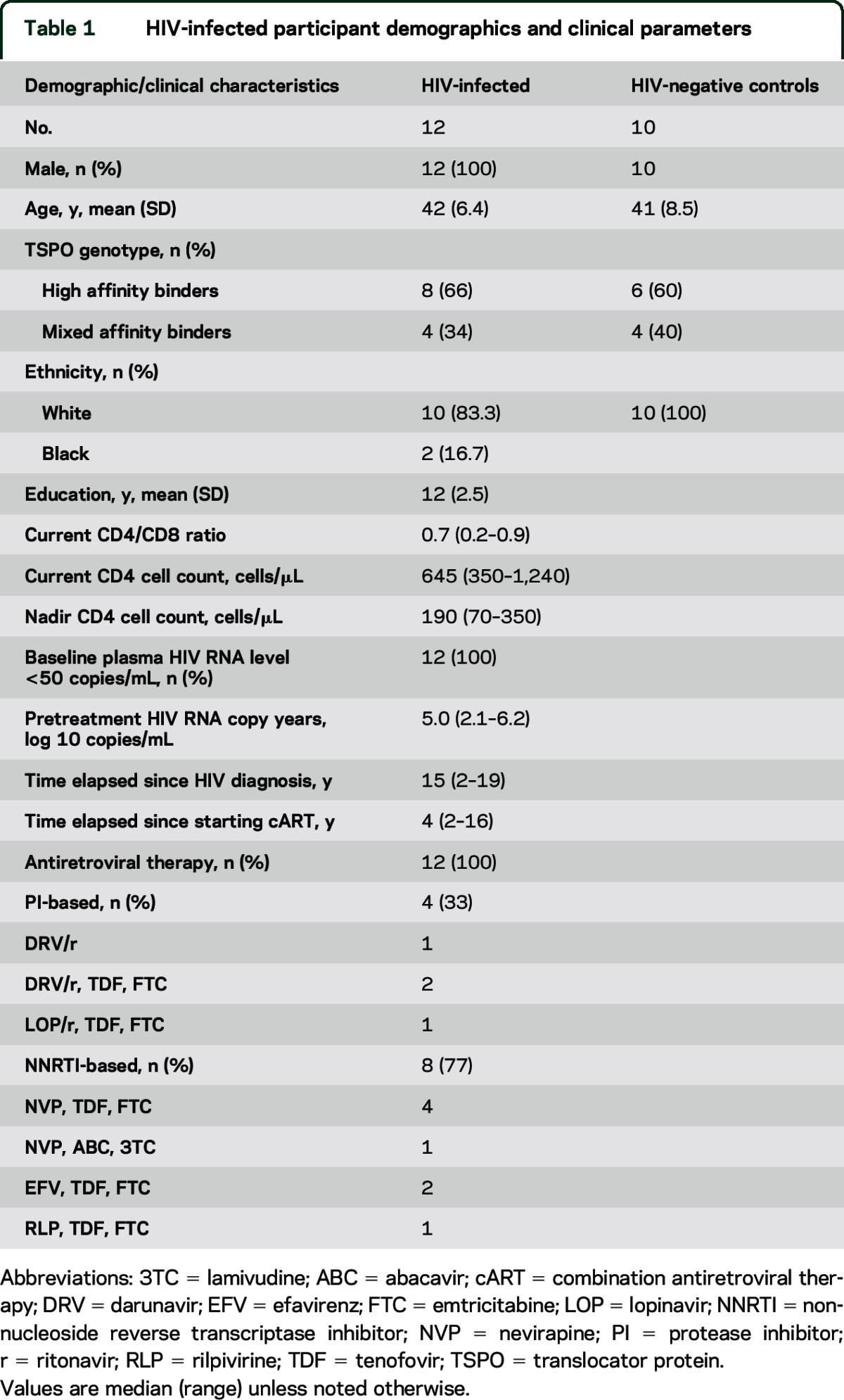
Patient demographics and clinical parameters for HIV-positive participants are presented in table 1. No significant differences were observed in overall composite Z scores in HIV-positive participants relative to the normative population provided by the CogState manufacturer (p > 0.05, all observations; see table e-1).
Table 1.
HIV-infected participant demographics and clinical parameters
TSPO binding in HIV-positive individuals compared to controls.
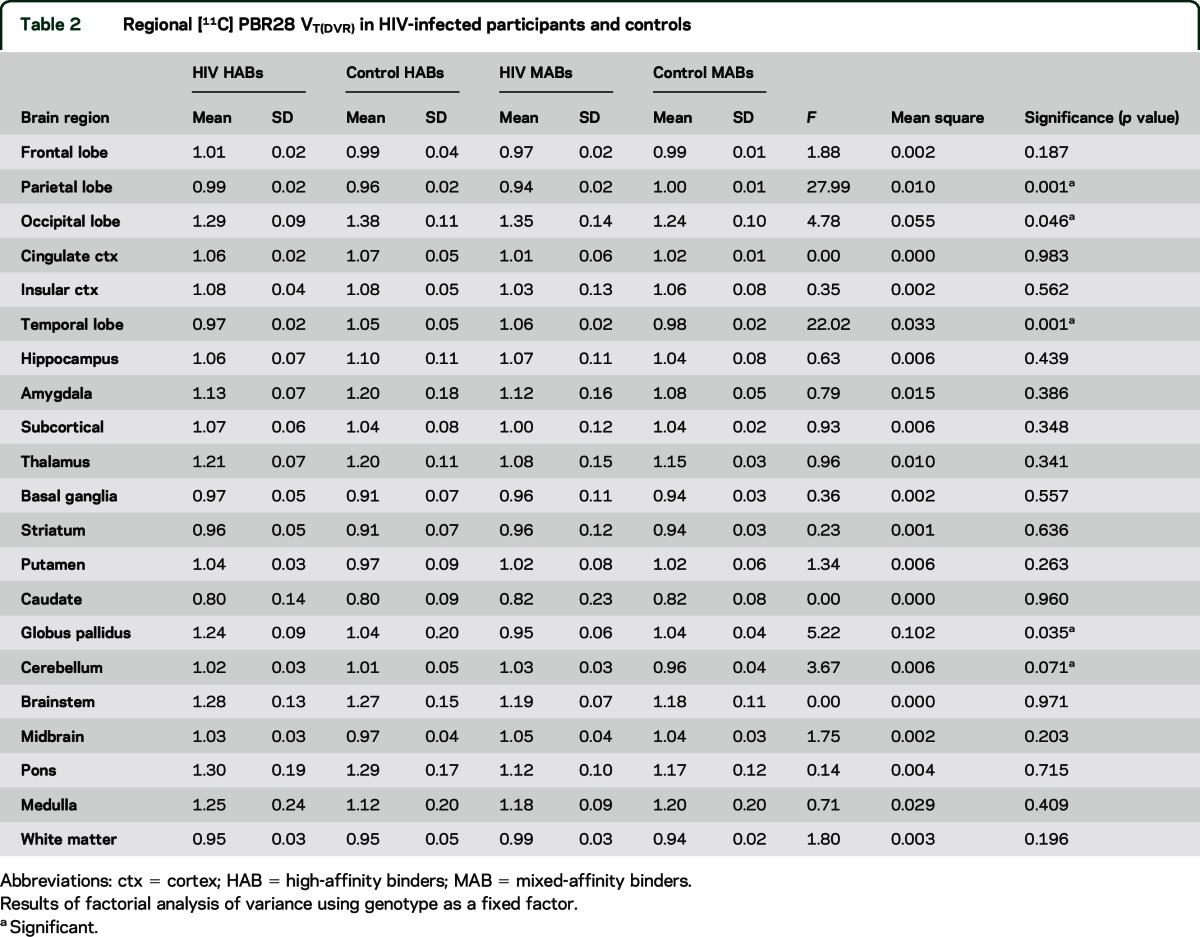
In HIV-positive individuals, a global increase in total [11C]PBR28 VT was observed when compared to HIV-negative controls in a parametric analysis (figure e-1). The global increase in [11C]PBR28 VT was observed in both high (C/C rs6971 alleles) and mixed (C/T rs6971 alleles) affinity binders. After normalization to cortical gray matter, these increases in [11C]PBR28 DVR in the HIV-positive participants (for both the high and mixed affinity genotypes) were found in the parietal (p = 0.001) and occipital (p = 0.046) lobes and in the globus pallidus (p = 0.035) (table 2) (figure e-2).
Table 2.
Regional [11C] PBR28 VT(DVR) in HIV-infected participants and controls
After adjusting for TSPO affinity genotype, a correlation between increased [11C]PBR28 DVR and lower CD4/CD8 ratio was found for the basal ganglia (p < 0.005, r = −0.555), amygdala (p < 0.005, r = −0.759), hippocampus (p < 0.005, r = −0.596), and thalamus (p ≤ 0.005, r = −0.525). Associations between greater pretreatment HIV RNA and [11C]PBR28 DVR in the occipital lobe (p < 0.005, r = 0.684), parietal lobe (p < 0.005, r = 0.662), caudate (p < 0.005, r = 0.600), and striatum (p < 0.005, r = 0.604) were observed. Significant associations between other HIV clinical parameters and [11C]PBR28 DVR including nadir CD4 count, age, type of cART, years of known duration of HIV infection, and years since starting cART were not found (p > 0.1, all observations).
TSPO binding and neurocognitive performance.
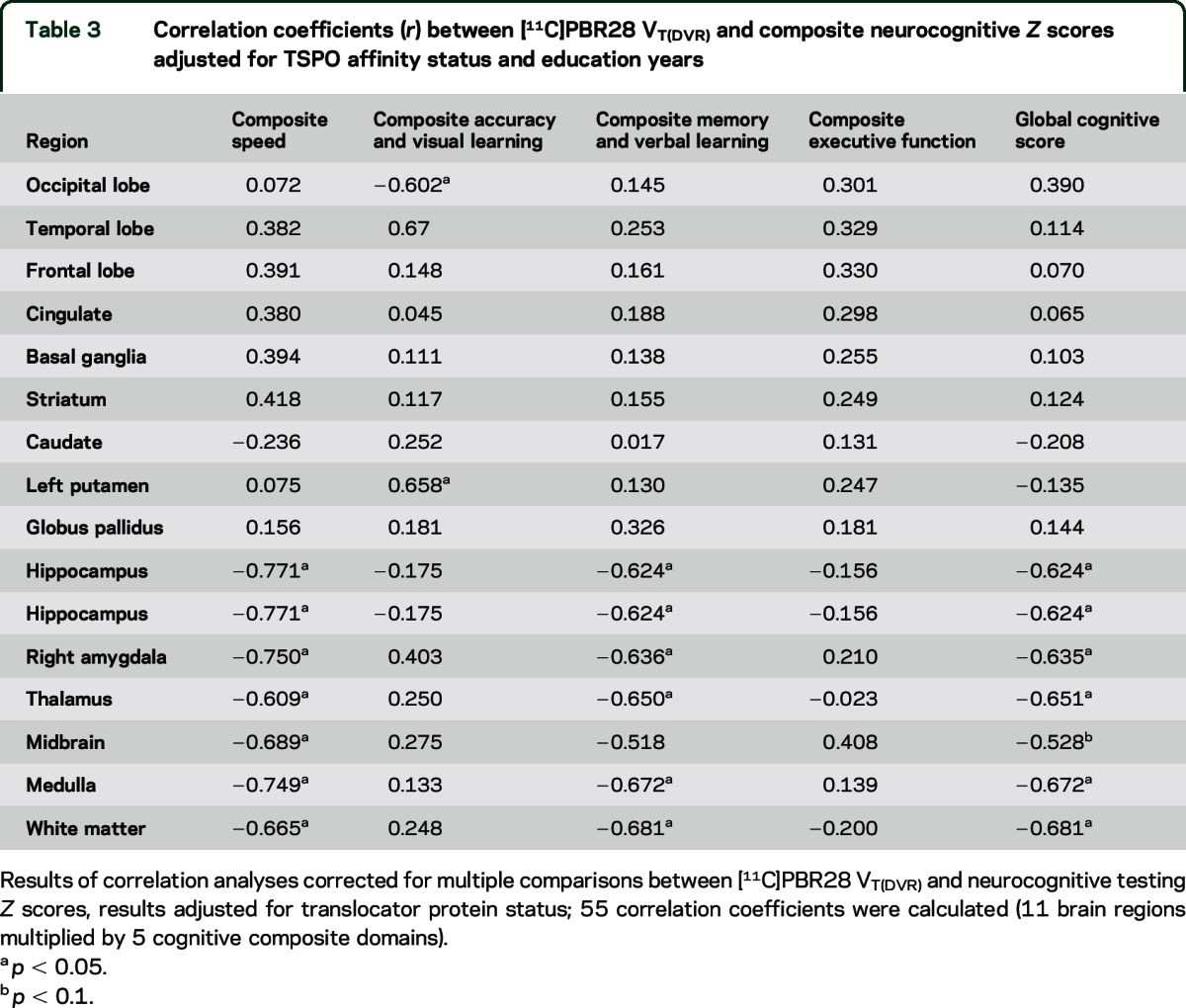
After adjusting for TSPO affinity genotype and education, greater [11C]PBR28 DVR in the hippocampus, amygdala, and thalamus all were associated with poorer cognitive global performance in HIV-positive individuals (table 3). The strongest relationships were observed between [11C]PBR28 DVR in the hippocampus and thalamus and tests of memory (r = −0.624, p < 0.05) and verbal learning (r = −0.650, p < 0.05).
Table 3.
Correlation coefficients (r) between [11C]PBR28 VT(DVR) and composite neurocognitive Z scores adjusted for TSPO affinity status and education years
TSPO binding and white matter structure.
Significantly reduced brain white matter FA and increased MD were observed in HIV-positive when compared with HIV-negative controls in the splenius of the corpus callosum, forceps major, corticospinal, and cingulum hippocampus white matter tracts (tables e-2 and e-3). After adjusting for TSPO genotype, significant correlations between increased [11C]PBR28 binding and MD values across most brain white matter tracks were observed in the PLWH group (table e-4). No significant correlations were found between [11C]PBR28 binding and FA values in any of the white matter tracts investigated (p > 0.10, all observations).
TSPO binding and biomarkers.
CSF biomarkers.
CSF was obtained from 10/12 HIV-positive participants recruited (the other 2 participants declined the procedure). All individuals had CSF HIV RNA <50 copies/mL. Mean eotaxin, MIP-1β, and IL-8 CSF concentrations were 12.9 pg/mL (SD = 11), 146.2 pg/mL (SD = 18), and 2.8 pg/mL (SD = 4.6), respectively. CSF concentrations of IP-10 were below the limit of detection for the assay. No associations between TSPO binding and any of the CSF chemokines were observed (p > 0.1, all observations).
Plasma r16S DNA.
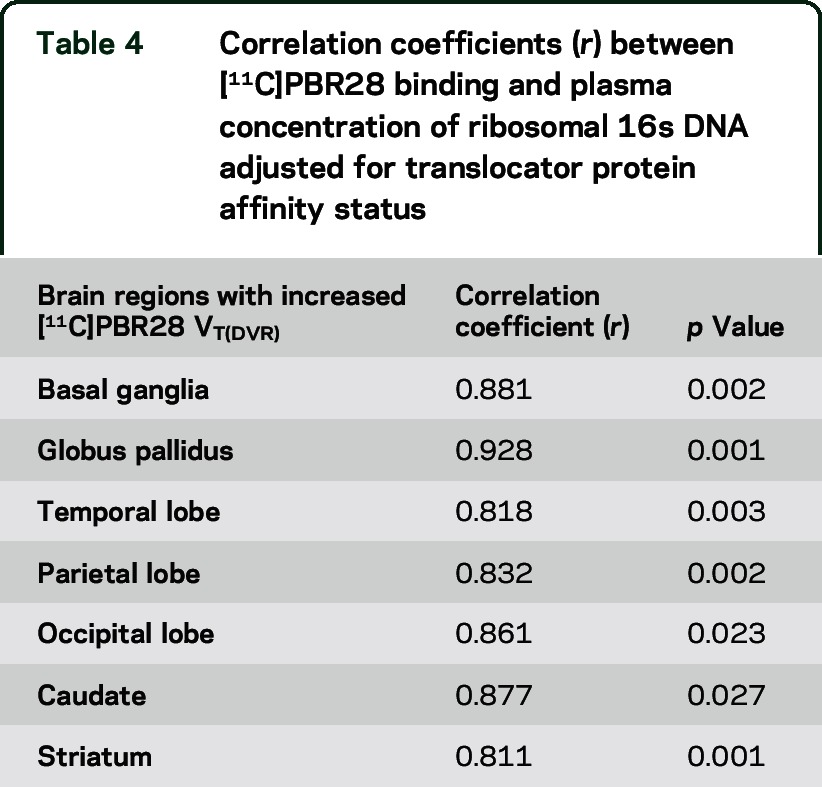
Plasma mean r16S DNA was 10.9 copies/mL in the HIV-positive group range (SD = 14.2). An association between greater concentration of ribosomal 16s DNA in plasma and increased [11C]PBR28 DVR was observed in brain regions with significant microglial activation such as the basal ganglia and the globus pallidus (table 4). We observed a significant association between plasma concentration of ribosomal 16s DNA and greater MD values in the right inferior longitudinal fasciculus (r = 0.601; p = 0.07), and an association trend in the forceps major (r = 0.532; p = 0.07) and right inferior fronto-occipital fasciculus (r = 0.509; p = 0.09) (figure e-3).
Table 4.
Correlation coefficients (r) between [11C]PBR28 binding and plasma concentration of ribosomal 16s DNA adjusted for translocator protein affinity status
DISCUSSION
The main goal of this study was to assess the possible role of microglial activation in PLWH on cART. We observed increased brain TSPO radioligand uptake in vivo in HIV-positive individuals on effective ART using [11C]PBR28 PET, suggesting microglia (and, possibly, astroglial activation).12 In contrast to some previous reports on TSPO radioligand uptake9,11 in cognitively healthy PLWH, we found evidence for widespread increases in brain TSPO radioligand uptake. Regions of greatest TSPO uptake, and, by inference, greatest neuroinflammation, were found in subcortical brain gray matter, particularly in the basal ganglia (globus pallidus, caudate, and striatum). Markers of increased glial activation and neuronal injury in subcortical brain regions have been demonstrated previously by us using proton magnetic resonance spectroscopy, which has shown pathologically elevated myo-inositol and choline and reduced NAA brain metabolites in the basal ganglia in treated HIV-positive participants26,27 in conjunction with elevated markers of immune activation.28
Increased TSPO binding was associated with poorer cognitive performance on cognitive tasks evaluating verbal learning and memory. Subcortical-frontal white matter microstructural pathology has been associated previously with impaired performance on verbal learning29 and memory30 tests and symptoms of poor attention, problem-solving difficulties, and information retention and recall31 in PLWH. With our evidence for substantial innate immune activation, we speculate that the relationships we observed between regions with TSPO radioligand uptake and increased MD in white matter may reflect increased inflammation.32
Although the cohort of PLWH we recruited did not have cognitive symptoms, our results showing associations with cognitive test performance suggest a possible link between neuroinflammation and their subsequent development. However, the clinical meaningfulness of the association between increases in TSPO radioligand uptake in subcortical brain regions and poorer cognitive performance is uncertain, as the HIV-positive cases by definition were within normal limits of cognitive function. Further studies are needed to assess whether these are markers of risk for future cognitive impairment. The anatomical distribution implied by the correlations also needs further work to confirm; we have not tested directly for differences between regions in participants and the independence of relationships between cognitive performance measures. As an exploratory characterization, the study also may lack power to adequately test correlations such as between basal ganglia TSPO radioligand uptake and performance in cognitive domains such as composite speed and executive function.
We found increased TSPO radioligand uptake was associated with a lower CD4/CD8 ratio and increased pretreatment HIV RNA. In virally suppressed PLWH, a lower current CD4/CD8 ratio has been associated with immune activation33 and cognitive impairments.34 We hypothesize that the association between lower CD4/CD8 ratio and greater TSPO expression in the brain that we have observed reflects a causal relationship among peripheral immune activation, breakdown of the blood–brain barrier, and subsequent brain immune activation, possibly by facilitating HIV entry into the CNS early during infection.
Finally, we observed a significant association between plasma concentration of ribosomal 16SDNA, a marker of microbial translocation, and increased TSPO expression. The plasma concentration of ribosomal 16S DNA also correlated with increased MD of white matter tracts. Microbial translocation from the gastrointestinal tract has been strongly associated with persistent immune activation in PLWH35 and HAD.36 An increase in PET [11C]PBR28 uptake and a marker of microbial translocation in a nonhuman primate brain model was observed after IV administration of Escherichia coli lipopolysaccharide (LPS), a microbial product with potent immune stimulatory activity. The underlying microglial activation was correlated with increased inflammatory cytokine levels, suggesting mediation by LPS-induced inflammatory cytokines such as IL-6 and IL-1β.37 More recently, [11C]PBR28 PET evidence for microglial activation was reported for humans following administration of LPS.38 We found a significant association between plasma ribosomal 16SDNA and levels of the inflammatory chemokine IL-8, which is consistent with the hypothesis that the effects of microbial translocation on microglial activation are mediated by circulating inflammatory chemokines.
This study describes a direct association between in vivo microglial activation and microbial translocation in cognitively healthy PLWH stable on cART. These results suggest a model whereby microglial activation in HIV-positive individuals is driven in part by systemic inflammatory responses, which are, in turn, triggered by the translocation of microbial products across the impaired gut–blood barrier. This model is consistent with immune activation–mediated injury via nonspecific activation39 rather than direct damage of HIV infection per se in the CNS compartment.
Our study has several limitations. First, the small sample size and the stringent inclusion criteria limit the generalizability of the results. However, the robustness and convergence of independent observations (using PET and magnetic resonance) strongly demonstrate the associations under at least some conditions. Our study also is not longitudinal, so we cannot test the causal relationships proposed. Second, because of the small number of participants in each group by genotype, we decided to adjust statistically by genotype status using factorial analysis of variance rather than comparing the groups directly. The lack of significant associations between TSPO expression and CSF biomarkers may be due to the selection of chemokines measured in this study and limitations of study power rather than absence of any relationship between parenchymal and CSF inflammatory profiles. Nonetheless, the lack of correlation suggests that independent information is provided by the PET and CSF cytokine measures, possibly consistent with data showing an influence of the inflammatory phenotype of the choroid plexus, as well as parenchyma, on soluble CSF inflammatory markers.40
Microglial activation has been proposed as a pathogenic mechanism in HIV-associated brain disease. Our data suggest that in vivo microglial activation is present in individuals with chronic HIV infection on effective cART, without any cognitive or neurologic deficit, and that microglial activation is associated with white microstructural changes and poor performance on some cognitive tests. We also identified a correlation between biomarkers of microbial translocation and microglial activation, which we hypothesize may be mediated by the expression of inflammatory chemokines. Therefore, our findings suggest a link between peripheral inflammation and microglial activation in treated HIV-positive patients. Longitudinal studies are needed to assess whether increased [11C]PBR28 PET signal (or other markers of neuroinflammation) are markers of risk for progression from asymptomatic, mild impairments of cognitive performance to symptomatic cognitive impairment.
Supplementary Material
GLOSSARY
- cART
combination antiretroviral therapy
- DTI
diffusion tensor imaging
- DVR
distribution volume ratio
- FA
fractional anisotropy
- IL-8
interleukin-8
- IP-10
interferon-γ-inducible protein 10
- LPS
lipopolysaccharide
- MD
mean diffusivity
- MIP-1β
macrophage inflammatory protein-1β
- PLWH
people living with HIV
- ROI
region of interest
- SNP
single nucleotide polymorphism
- TSPO
translocator protein
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Jaime H. Vera: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, statistical analysis, study supervision, obtaining funding. Qi Guo: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, statistical analysis. James H. Cole: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, statistical analysis. Adriano Boasso: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data. Louise Greathead: analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data. Peter Kelleher: study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, contribution of vital reagents/tools/patients, study supervision, obtaining funding. Eugenii A. Rabiner: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, study supervision. Nicola Kalk: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and final approval, acquisition of data, study supervision. Courtney Bishop: analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Roger N. Gunn: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Paul Matthews: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, contribution of vital reagents/tools/patients, study supervision, obtaining funding. Alan Winston: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, study supervision, obtaining funding.
STUDY FUNDING
Supported by Wellcome Trust and GlaxoSmithKline through a Wellcome Trust Translational Medicine and Therapeutics Fellowship, of which J.H.V. is a recipient. P.M.M. received support from the Edmond J. Safra Foundation. He is an NIHR Senior Investigator.
DISCLOSURE
J. Vera has received honoraria from Merck and Janssen Cilag and sponsorship to attend scientific conferences from Janssen Cilag, Gilead Sciences, and AbbVie. Q. Guo, J. Cole, A. Boasso, L. Greathead, P. Kelleher, E.A. Rabiner, N. Kalk, C. Bishop, and R. Gunn report no disclosures relevant to the manuscript. P. Mathews has received honoraria or speakers fees from GlaxoSmithKline, Biogen, Novartis, IXICO, Transparency Life Sciences, and Adelphi Communications. A. Winston has received honoraria and research grants and been a consultant or investigator in clinical trials sponsored by Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen Cilag, Roche, Pfizer, and ViiV Healthcare. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Dore GJ, Correll PK, Li Y, Kaldor JM, Cooper DA, Brew BJ. Changes to AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS 1999;13:1249–1253. [DOI] [PubMed] [Google Scholar]
- 2.Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol 2002;8(suppl 2):115–121. [DOI] [PubMed] [Google Scholar]
- 3.Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 2010;24:1243–1250. [DOI] [PubMed] [Google Scholar]
- 4.Peluso MJ, Meyerhoff DJ, Price RW, et al. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis 2013;207:1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol 2005;64:529–536. [DOI] [PubMed] [Google Scholar]
- 6.Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci 2002;202:13–23. [DOI] [PubMed] [Google Scholar]
- 7.Papadopoulos V, Baraldi M, Guilarte TR, et al. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 2006;27:402–409. [DOI] [PubMed] [Google Scholar]
- 8.Stephenson DT, Schober DA, Smalstig EB, Mincy RE, Gehlert DR, Clemens JA. Peripheral benzodiazepine receptors are colocalized with activated microglia following transient global forebrain ischemia in the rat. J Neurosci 1995;15:5263–5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammoud DA, Endres CJ, Chander AR, et al. Imaging glial cell activation with [11C]-R-PK11195 in patients with AIDS. J Neurovirol 2005;11:346–355. [DOI] [PubMed] [Google Scholar]
- 10.Garvey LJ, Pavese N, Politis M, et al. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART; an 11C-PK11195 PET study. AIDS 2014;28:67–72. [DOI] [PubMed] [Google Scholar]
- 11.Wiley CA, Lopresti BJ, Becker JT, et al. Positron emission tomography imaging of peripheral benzodiazepine receptor binding in human immunodeficiency virus-infected subjects with and without cognitive impairment. J Neurovirol 2006;12:262–271. [DOI] [PubMed] [Google Scholar]
- 12.Matthews PM, Datta G. Positron-emission tomography molecular imaging of glia and myelin in drug discovery for multiple sclerosis. Expert Opin Drug Discov 2015;10:557–570. [DOI] [PubMed] [Google Scholar]
- 13.Chauveau F, Boutin H, Van Camp N, Dolle F, Tavitian B. Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med Mol Imaging 2008;35:2304–2319. [DOI] [PubMed] [Google Scholar]
- 14.Owen DR, Guo Q, Kalk NJ, et al. Determination of [(11)C]PBR28 binding potential in vivo: a first human TSPO blocking study. J Cereb Blood Flow Metab 2014;34:989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owen DR, Yeo AJ, Gunn RN, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab 2012;32:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreisl WC, Jenko KJ, Hines CS, et al. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab 2013;33:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Overton ET, Kauwe JS, Paul R, et al. Performances on the CogState and standard neuropsychological batteries among HIV patients without dementia. AIDS Behav 2011;15:1902–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page EE, Greathead L, Metcalf R, et al. Loss of Th22 cells is associated with increased immune activation and IDO-1 activity in HIV-1 infection. J Acquir Immune Defic Syndr 2014;67:227–235. [DOI] [PubMed] [Google Scholar]
- 19.Tziortzi AC, Searle GE, Tzimopoulou S, et al. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage 2011;54:264–277. [DOI] [PubMed] [Google Scholar]
- 20.Fujita M, Imaizumi M, Zoghbi SS, et al. Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. Neuroimage 2008;40:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunn RN, Lammertsma AA, Grasby PM. Quantitative analysis of [carbonyl-(11)C]WAY-100635 PET studies. Nucl Med Biol 2000;27:477–482. [DOI] [PubMed] [Google Scholar]
- 22.Jucaite A, Cselenyi Z, Arvidsson A, et al. Kinetic analysis and test-retest variability of the radioligand [11C](R)-PK11195 binding to TSPO in the human brain: a PET study in control subjects. EJNMMI Res 2012;2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 24.Squarcina L, Bertoldo A, Ham TE, Heckemann R, Sharp DJ. A robust method for investigating thalamic white matter tracts after traumatic brain injury. Neuroimage 2012;63:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westfall PH. On using the bootstrap for multiple comparisons. J Biopharm Stat 2011;21:1187–1205. [DOI] [PubMed] [Google Scholar]
- 26.Chang L, Ernst T, Leonido-Yee M, Walot I, Singer E. Cerebral metabolite abnormalities correlate with clinical severity of HIV-1 cognitive motor complex. Neurology 1999;52:100–108. [DOI] [PubMed] [Google Scholar]
- 27.Schuettfort G, Hattingen E, Pilatus U, et al. Proton 1H- and phosphorus 31P-MR spectroscopy (MRS) in asymptomatic HIV-positive patients. J Int AIDS Soc 2014;17(4 suppl 3):19577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vera JH, Garvey LJ, Allsop JM, et al. Alterations in cerebrospinal fluid chemokines are associated with maraviroc exposure and in vivo metabolites measurable by magnetic resonance spectroscopy. HIV Clin Trials 2012;13:222–227. [DOI] [PubMed] [Google Scholar]
- 29.Jiang ZG, Piggee C, Heyes MP, et al. Glutamate is a mediator of neurotoxicity in secretions of activated HIV-1-infected macrophages. J Neuroimmunol 2001;117:97–107. [DOI] [PubMed] [Google Scholar]
- 30.Kuhlmann AC, Guilarte TR. The peripheral benzodiazepine receptor is a sensitive indicator of domoic acid neurotoxicity. Brain Res 1997;751:281–288. [DOI] [PubMed] [Google Scholar]
- 31.Hinkin CH, Hardy DJ, Mason KI, et al. Verbal and spatial working memory performance among HIV-infected adults. J Int Neuropsychol Soc 2002;8:532–538. [DOI] [PubMed] [Google Scholar]
- 32.DeBoy CA, Zhang J, Dike S, et al. High resolution diffusion tensor imaging of axonal damage in focal inflammatory and demyelinating lesions in rat spinal cord. Brain 2007;130:2199–2210. [DOI] [PubMed] [Google Scholar]
- 33.Serrano-Villar S, Gutierrez C, Vallejo A, et al. The CD4/CD8 ratio in HIV-infected subjects is independently associated with T-cell activation despite long-term viral suppression. J Infect 2013;66:57–66. [DOI] [PubMed] [Google Scholar]
- 34.Serrano-Villar S, Perez-Elias MJ, Dronda F, et al. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS One 2014;9:e85798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12:1365–1371. [DOI] [PubMed] [Google Scholar]
- 36.Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One 2008;3:e2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hannestad J, Gallezot JD, Schafbauer T, et al. Endotoxin-induced systemic inflammation activates microglia: [(1)(1)C]PBR28 positron emission tomography in nonhuman primates. Neuroimage 2012;63:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandiego CGJ, Lim K, Lin S, et al. Systemic endotoxin induces a robust increase in microglial activation measured with [11C]PBR28 and PET in humans. J Nucl Med 2015;56(suppl 3):468. [Google Scholar]
- 39.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev 2007;7:161–167. [DOI] [PubMed] [Google Scholar]
- 40.Gordon LB, Nolan SC, Ksander BR, Knopf PM, Harling-Berg CJ. Normal cerebrospinal fluid suppresses the in vitro development of cytotoxic T cells: role of the brain microenvironment in CNS immune regulation. J Neuroimmunol 1998;88:77–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


