Abstract
Objective:
To examine whether occupational cognitive requirements, as a marker of adulthood cognitive activity, are associated with late-life cognition and cognitive decline.
Methods:
Main lifetime occupation information for 7,637 participants aged >65 years of the Chicago Health and Aging Project (CHAP) was linked with standardized data on worker attributes and job characteristics from the Occupational Information Network (O*NET). Ratings of cognitive processes required in 10 work-related tasks were used to create a summary measure of occupational cognitive requirements (possible range 0–7). Multivariable-adjusted linear mixed models were used to estimate the association of occupational cognitive requirements score (OCRS) with cognitive function and rate of cognitive decline.
Results:
Higher OCRS corresponded to significantly better late-life cognitive performance at baseline in 1993 (p < 0.001) and to slower decline in global cognitive function over time (p = 0.004). Within a genotyped subsample (n = 4,104), the associations of OCRS with rate of cognitive decline did not differ significantly by APOE ε4 carriership (p = 0.11).
Conclusions:
Findings suggest that occupational cognitive requirements are associated with better cognition and a slower rate of cognitive decline in older age. Adulthood cognitive activity may contribute to cognitive reserve in late life.
Alzheimer disease (AD) dementia affects a large number of older adults for whom there are few effective treatment options available.1 The long duration of the preclinical phase, which is thought to last from at least several years to over a decade2 and is characterized by a period of decline in cognitive function, has prompted increasing efforts to identify potentially modifiable risk factors from earlier stages in adulthood that affect disease onset and initial progression. A range of mid-adulthood lifestyle risk factors, including adherence to a healthy diet3 and regular participation in physical activity,4 are associated with reduced risk of late-onset AD dementia.5
There has been a growing interest in the role of cognitively stimulating activity during adulthood and its potentially protective effect on late-life cognitive decline and AD dementia risk. There is some evidence that engagement in leisure time cognitive activity is associated with higher levels of cognitive functioning in older age.6–8 More recent studies have explored occupation-related cognitive activity and complexity, but have produced inconsistent findings on whether higher occupation-related cognitive requirements are associated with late-life cognitive decline.9–14 A challenge central to these studies is the measurement of occupation-related cognitive activity, and most studies to date have relied on relatively crude measures, such as broad employment categories.
In this study, we used a detailed measure of occupation-related cognitive activity derived from the Department of Labor's Occupational Information Network (O*NET) database to test the degree to which occupation-related cognitive activity is associated with slower cognitive decline in later life. To that end, we linked the O*NET-derived measure of occupation-related cognitive activity with changes in cognitive function using data from a well-established longitudinal study of older adults: the Chicago Health and Aging Project (CHAP). We further tested this potential association for potential modification by carriership of an APOE ε4 allele, a known risk factor for AD dementia.15
METHODS
CHAP participants.
CHAP recruited persons aged 65+ years in 3 adjacent neighborhoods in Chicago, Illinois. Six data collection cycles were completed from 1993 to 2012 at approximately 3-year intervals. All cycles included in-home interviews with questions on sociodemographics, occupation history, psychosocial variables, medical history, and physical and cognitive functioning tests. Of the 10,516 CHAP participants, 7,765 (73.8%) had at least 2 assessments of cognitive function and thus were eligible for inclusion in this analysis.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Institutional Review Board of Rush University Medical Center. Written informed consent was received from all study participants.
Occupational cognitive requirements/O*Net linkage.
O*NET is a data collection project sponsored by the US Department of Labor to serve as the nation's primary source of standardized information on worker attributes and job characteristics for a broad range of occupations. Data collection occurs annually through surveys administered to a random sample of US workers. We used the first version of the O*NET database (O*Net 98) for our analyses instead of more recent versions so that job characteristics might better approximate the experiences of the mostly retired CHAP participants.
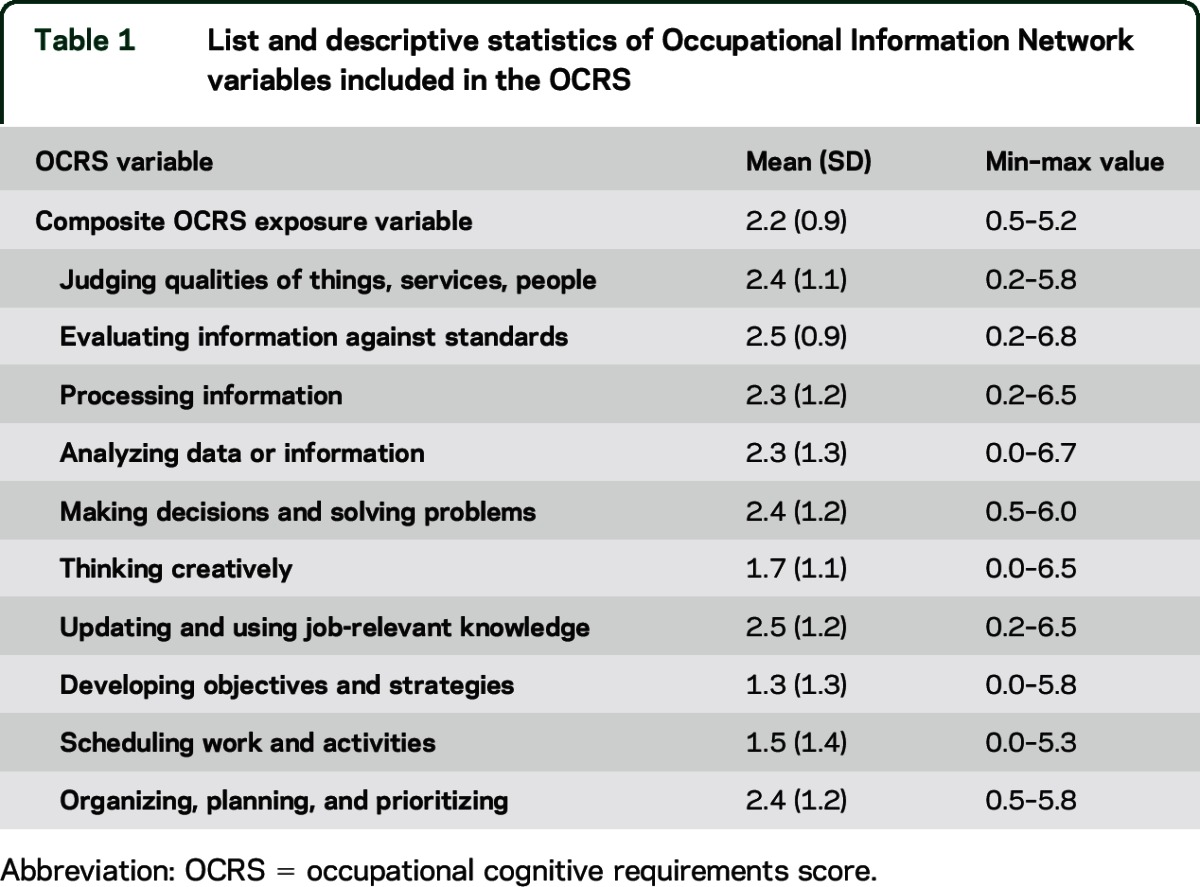
The O*NET data are compiled to provide standardized scores on the day-to-day needs of occupations and the qualifications and interests of the typical worker.16 We identified 10 cognitively related variables, listed in table 1. The scores for the 10 cognitively related constructs assess the level of activity needed to perform the job, ranging from 0 to 7, which were averaged across O*NET occupations to create a single occupational cognitive requirements score (OCRS) to be used in analysis (Cronbach α = 0.97).
Table 1.
List and descriptive statistics of Occupational Information Network variables included in the OCRS
To link the OCRS to the main lifetime occupation in the CHAP data, open-ended questions in CHAP about occupational title, industry, and duties were first coded to the best-fitting 1990 Census Occupation Code (COC). These COC codes were linked to the O*NET database using a publicly available crosswalk developed by the National Crosswalk Service Center (http://www.xwalkcenter.org). Some COC codes link to more than one O*NET job code, and in this case, the OCRS was averaged across all O*NET occupations that linked to a CHAP participant. A small number of CHAP participants did not provide occupational data (n = 105) or worked in an occupation without available O*NET data (n = 23). These participants were excluded from this analysis.
Global cognitive measure.
At each in-home assessment, 4 brief cognitive function tests were administered, including the East Boston tests of immediate and delayed recall, the Mini-Mental State Examination, and the Symbol Digit Modalities Test. As previously described,17 raw scores for each test were converted to z scores, then averaged to create a global measure of cognitive function. Higher scores indicate higher cognitive function.
Covariates.
Educational attainment was reported by the participant as years of formal schooling completed. Race was assessed with the US Census questions. Income was assessed by asking participants to choose one of 10 levels of total family income from a show card. Time since baseline was calculated as days from baseline to cognitive function assessment date, rounded to nearest tenth of a year. At each cycle, a stratified random sample of participants was invited to participate in a more intensive clinical examination, which included testing for the presence of an APOE ε4 allele. About 43% of CHAP participants underwent APOE genotyping.
Statistical analysis.
Linear mixed models were used to estimate the association between the OCRS and the global measure of cognition. Diagnostic evaluation of the unadjusted association between OCRS and global cognition indicated the use of both a linear and quadratic term to account for a curvilinear relationship in baseline cognitive function; however, the quadratic term was not a significant predictor of cognitive function over time. Inclusion of the covariates did not change the functional form of the association. Using data from up to 6 cycles of cognitive assessment and random effects accounting for the nested structure of these outcome data, the analytical model included main lifetime OCRS, OCRS-squared, time since baseline, and an interaction term between time and OCRS, while adjusting for race, sex, income decile, education, education-squared, age, age-squared, education × time, income × time, and age × time. Additionally, 3-way interactions with the OCRS and time were fitted to test differential associations by race or sex. We repeated the analytical model in the subset of participants with APOE genotyping to test the degree to which the association between OCRS and cognitive decline was modified by APOE. This model included an indicator for having at least one APOE ε4 allele and the 2-way interactions between APOE ε4 with OCRS and time and the 3-way interaction term APOE ε4 × OCRS × time. Models were validated graphically and analytically, and assumptions were judged to be adequately met. Analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC).
RESULTS
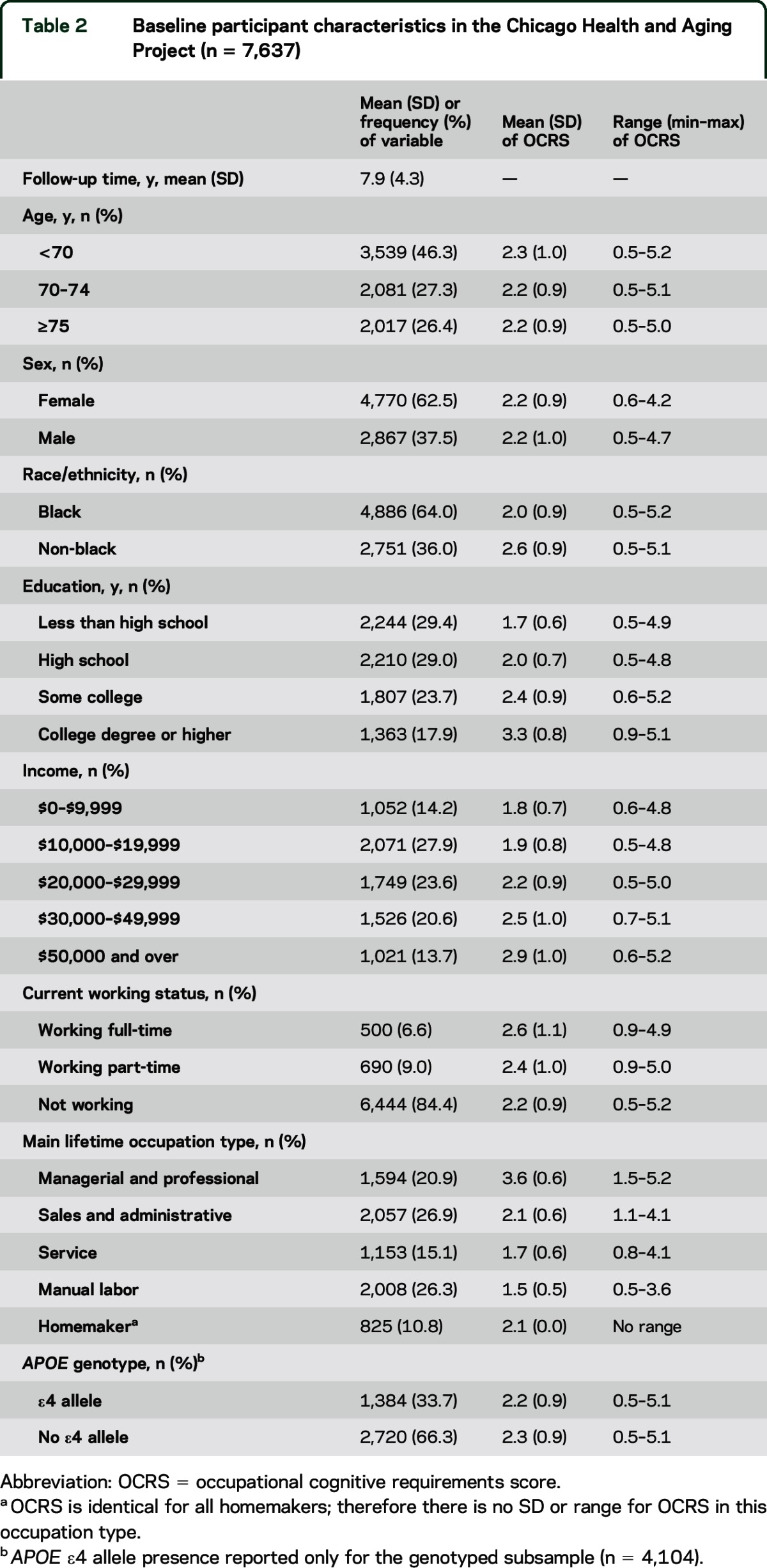
The baseline demographic and occupation variables of the participants included in this analysis are given in table 2. The CHAP sample is diverse in sex, race, socioeconomic status, and main lifetime occupation category. The OCRS mean was 2.2 (SD 0.9), with a range of 0.5–5.2, slightly compressed from the possible range of 0–7. OCRS differed across educational attainment, income, and occupation type categories, though a wide range of OCRS was represented within each category.
Table 2.
Baseline participant characteristics in the Chicago Health and Aging Project (n = 7,637)
At baseline, the mean global cognitive function score was 0.29 (SD 0.72). The results of the linear mixed model assessing the relationship between occupational cognitive requirements and overall cognitive function are presented in table 3. Cognitive function declined at a rate of 0.046 standard units per year after baseline (p < 0.001). At baseline, OCRS was associated with higher cognitive function, although this benefit receded at higher levels of OCRS. Higher OCRS was associated with slower decline in cognitive function over time (β = 0.003; p = 0.004). Further adjustment for cardiovascular disease and related risk factors, such as body mass index and smoking status, did not meaningfully alter the pattern of findings for baseline cognitive function (p < 0.001) or cognitive decline (p = 0.02).
Table 3.
Adjusted associations between occupational cognitive requirements and cognitive aging
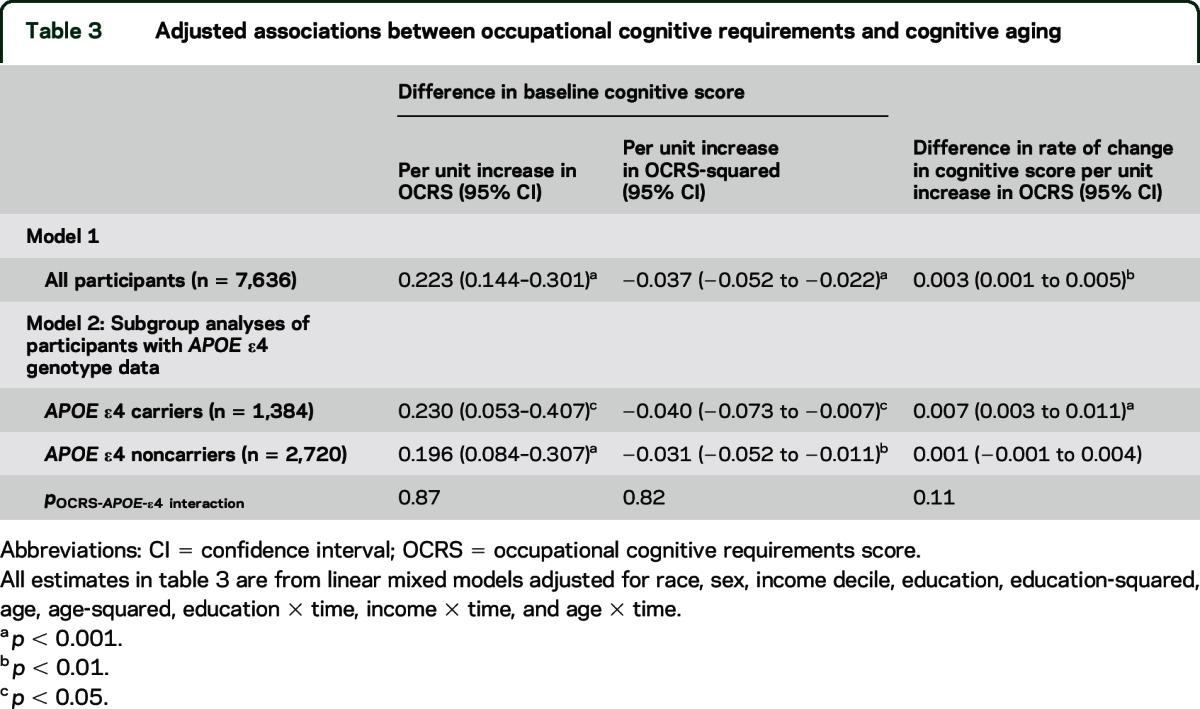
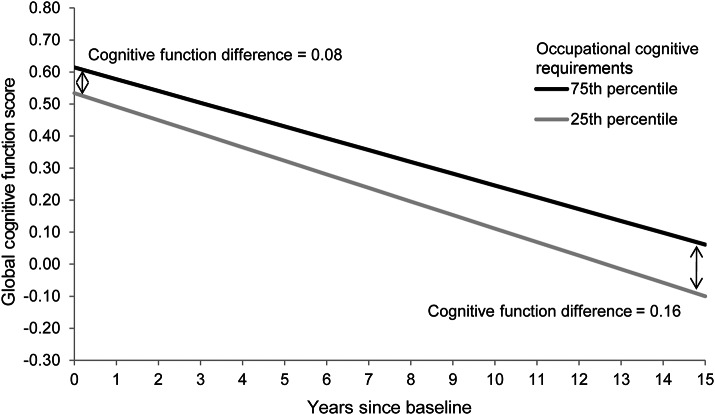
Figure 1 illustrates the differences in predicted cognitive trajectories of main lifetime OCRS in the 75th percentile (OCRS = 3.0) and 25th percentile (OCRS = 1.5), after adjustment for covariates. The late-life cognitive function difference between OCRS in the 75th percentile and 25th percentile is 0.08 at baseline, but doubles to 0.16 after 15 years of follow-up.
Figure 1. Global cognitive function score over time by levels of occupational cognitive requirements.
The predicted values are derived from linear mixed models adjusted for race, sex, income decile, education, education-squared, age, age-squared, education × time, income × time, and age × time.
In analyses testing differential associations by race and sex, the protective effects of OCRS on cognitive decline were somewhat weaker among black than among white participants (p = 0.02). Female sex was not associated with differential decline in cognitive function nor did it modify the association between OCRS and cognitive decline (p = 0.84).
Within the genotyped subsample, higher OCRS remained significantly associated with both baseline postretirement cognitive function and slower decline in cognitive function over time (table 3). APOE ε4 allele was not significantly associated with baseline cognitive function (β = −0.016; p = 0.75), but was associated with faster decline in cognitive function over time (β = −0.030; p < 0.001). A 3-way interaction term between APOE ε4 allele, OCRS, and time indicated that the association of higher OCRS with slower decline was more pronounced among APOE ε4 allele carriers, but this difference in slopes was not statistically significant (p = 0.11). Figure 2 illustrates the differences in predicted cognitive trajectories of main lifetime OCRS in the 75th percentile (OCRS = 3.0) and 25th percentile (OCRS = 1.5), separately by APOE ε4 allele carriership.
Figure 2. Global cognition score over time by APOE ε4 allele and levels of occupational cognitive requirements.
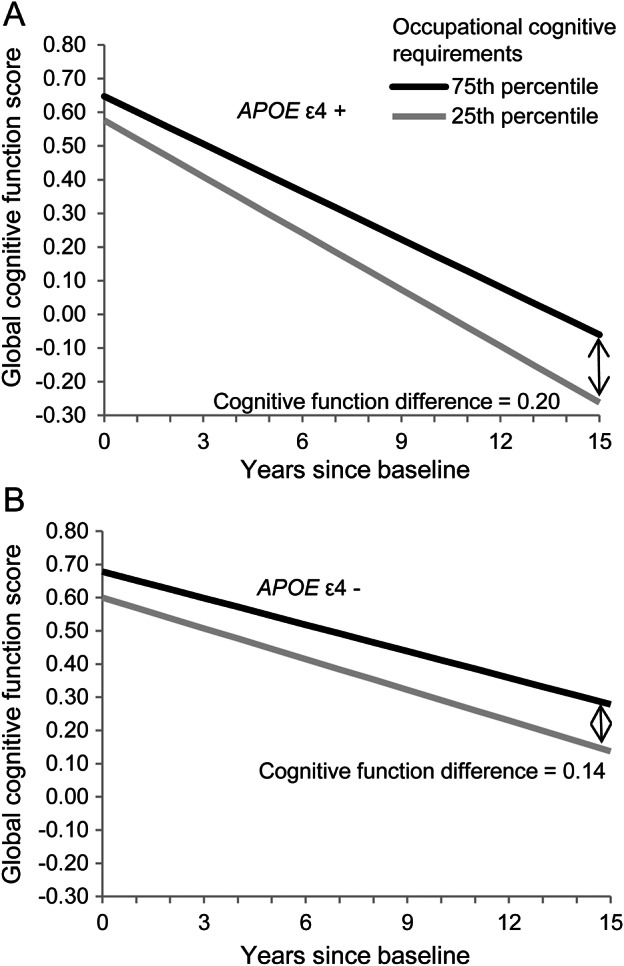
(A) APOE ε4+: Participants with an APOE ε4 allele. (B) APOE ε4−: Participants without an APOE ε4 allele. The predicted values are derived from linear mixed models adjusted for race, sex, income decile, education, education-squared, age, age-squared, education × time, income × time, and age × time.
Several sensitivity analyses were performed. First, we removed 11% of the sample who reported homemaking as their main lifetime occupation. The baseline linear and quadratic cognitive function estimates increased slightly (β = 0.272; p < 0.001 and β = −0.046; p < 0.001, respectively), as did the cognitive decline estimate (β = 0.004; p < 0.001). Second, we removed the highest and lowest 5% of cognitive function scores. The baseline linear and quadratic cognitive function estimates attenuated slightly but remain significant (β = 0.182; p < 0.001 and β = −0.030; p < 0.001, respectively), but there was no change in the cognitive decline estimate (β = 0.003; p = 0.002). Finally, an analysis examining the separate tests of cognitive function indicated that higher OCRS was associated with slower decline in episodic memory (p = 0.04), but not executive function (p = 0.17).
DISCUSSION
In this study, we found evidence for a relationship between the cognitive requirements of an individual's main lifetime occupation and both cognitive function at baseline and rate of cognitive decline in older age. Greater occupational cognitive requirements were associated with higher levels of cognitive function at baseline, with smaller benefit at the upper end of occupational cognitive requirements. These findings are consistent with previous cross-sectional research on late-life cognitive function, in which occupation-related cognitive stimulation has been shown to be associated with higher levels of cognitive function.18–20 They are also consistent with the cognitive reserve hypothesis, in which a cognitively stimulating environment is thought to increase neuronal capacity, which helps the brain compensate for neuronal damage that might manifest clinically as AD dementia.21
Previous research examining the cognitive reserve hypothesis has focused primarily on the association between early-life or late-life cognitive activity in preventing or delaying cognitive decline in older age.6,8 In contrast, we focus on mid-adulthood, using a specific measure of occupation-related cognitive demands and complexity, providing evidence that higher levels of occupation-related cognitive requirements are not only associated with higher level of cognitive function, but also with a slower rate of decline. Our findings are consistent with another cohort study of American older adults, the Health and Retirement Study, which used a similar O*NET-derived measure of mental demands at work,13 as well as a study of German older adults that used O*NET-derived measures of enriched work environment.14
The exact mechanisms by which work-related cognitive demands and complexity may lead to slower cognitive decline later in life remain mostly unknown. It is possible that participation in cognitive stimulating activities throughout a large part of a person's adult life may contribute to cognitive reserve, and buffer or conceal the effects of neurodegeneration on cognitive function at more advanced ages.21 In terms of potential neurologic pathways, environmental stimulation may increase the level of neurotrophins available in brain tissue, which may protect or repair existing neurons as well as actively promote neurogenesis. This pathway, which is also referred to as differential preservation,22 may enhance neural reserve that protects against the adverse effects of brain deterioration due to aging, stress, and neurodegenerative disease.23
A major strength of our research is the use of the O*NET data, which provide validated assessments of occupational exposure. O*NET measures the actual experience of workers in real time, which minimizes recall bias that may affect self-reported information on specific work functions that occurred 20–30 years earlier in life. The specific O*NET constructs included in the occupational cognitive requirements index include job tasks that recruit different cognitive domains, which is ideal for comparison with a global cognitive function measure, as is available in CHAP. The OCRS used in this article is distinct from socioeconomic status: the score is correlated with education, income, and broad occupational category, but the full range of the OCRS was represented across all levels of each of these covariates.
By combining the O*NET data with observations from CHAP, we were able to produce these results in a large racially and socioeconomic diverse US-based sample. With few exceptions,13,20 the majority of studies examining detailed occupational complexity and cognitive aging have used data from European studies. Because of international differences in work environments and population heterogeneity, analysis in a US-based sample is important for generalizability to older Americans. Although findings similar to ours have been observed previously in a US-based cohort of older adults,13 our findings are derived from a diverse cohort with a wide range of educational and occupational exposures, and using a well-validated, comprehensive assessment of cognitive function involving several tests administered in-person at regular intervals with high rates of follow-up participation.
The effect of having at least one APOE ε4 allele is associated with higher risk of AD15 as well as faster late-life cognitive decline.24 Our results were consistent; we found faster annual decline among those with APOE ε4 genotype. We also found some evidence that occupational cognitive requirements may attenuate some of the risk of APOE ε4 genotype on decline; however, this finding did not meet conventional criteria of statistical significance (p = 0.11).
About 11% of our sample identified the homemaker role as their main lifetime occupation. Nearly all of these participants were women, and had lower levels of income and educational attainment as compared to those who worked outside of the home. Much of the existing research on occupational complexity excludes homemakers, which may result in a sample of women that is not representative of the general population, especially among studies of older adults. O*NET provides data on the occupational cognitive requirements of homemakers, and thus we were able to include homemakers in our analysis. We conducted a sensitivity analysis in which we removed homemakers from the sample, which produced little change in the overall results.
This study is subject to several limitations. Although O*NET measures minimize potential for recall bias, they do not capture variations due to personal experience. The assumption is that all persons who had a specific occupation had identical occupational cognitive requirements, which is probably not true. Moreover, we assume that the main lifetime occupation was the predominant job throughout the life course; persons who had more than one occupation throughout their lifetime may be subject to incomplete measurement. Additionally, the cognitive requirements of an occupation at the time of O*NET data collection may differ from the occupational requirements in the years when our sample was working, primarily the 1960s, 1970s, and 1980s. We attempted to minimize this difference by using the first release of the O*NET from 1998, instead of more recent versions, but differences may still persist.
Because CHAP did not collect data on midlife risk factors, we were unable to adjust for the potentially confounding health and psychosocial functioning factors of participants in mid-adulthood. Some residual confounding may therefore be present and the true magnitude of the OCRS effect may be somewhat overestimated. Cognition at labor force entry may influence the cognitive requirements of the selected occupation,25,26 as may other health-related covariates. Likewise, physical and psychosocial occupational attributes, such as job strain27 or lead exposure,28 may correspond with OCRS but affect late-life cognition through separate mechanisms. Adjustment for a variety of late-life health and psychosocial functioning status factors did not affect significant independent effects of OCRS on baseline cognitive function or decline in cognitive function over time.
Our results are based primarily on a measure of global cognitive function, with some evidence that our measures of occupational cognitive requirements are related to decline in episodic memory and general cognition, but not executive function. Few studies have attempted to link specific cognitive domains of stimulation in lifetime occupation to decline in specific cognitive domains in older age.10,14 More research is needed to understand the pathways by which different forms of lifetime cognitive stimulation differentially affect decline in specific cognitive domains in late life.
Data on American time use show that employed adults spend over one-third of their weekday hours at work,29 and thus the duration of exposure to occupational risk factors can be considerable. This study represents a systematic evaluation of midlife occupation-related cognitive activity in relation to cognitive aging, and provides evidence in support of the hypothesis that adulthood cognitive activity may enhance cognitive reserve in late life.
ACKNOWLEDGMENT
The authors thank the residents of Morgan Park, Washington Heights, and Beverly who participated in the study; Ann Marie Lane for community development and oversight of project coordination; Michelle Bos, Holly Hadden, Flavio LaMorticella, and Jennifer Tarpey for coordination of the study; Todd Beck, MS, for analytic programming; and the staff of the Rush Institute for Healthy Aging.
GLOSSARY
- AD
Alzheimer disease
- CHAP
Chicago Health and Aging Project
- COC
Census Occupation Code
- O*NET
Occupational Information Network
- OCRS
occupational cognitive requirements score
AUTHOR CONTRIBUTIONS
L. Pool contributed to study concept and design, data analysis and interpretation, and drafting and revising the manuscript. J. Weuve contributed to data analysis and interpretation and revising the manuscript. R. Wilson and U. Bültmann contributed to revising the manuscript. D. Evans supervised the study and contributed to revising the manuscript. C. Mendes de Leon contributed to study concept and design, data analysis and interpretation, revising the manuscript, and supervising the study.
STUDY FUNDING
Supported by National Institute on Aging grants R01AG11101, R01AG032247, and T32AG027708.
DISCLOSURE
L. Pool receives support from the NIH (T32AG027708). J. Weuve is a consultant to the Alzheimer's Association and the AlzRisk Project (www.alzrisk.org) and receives support from NIH/NIEHS (R21ES020404 [PI] and R21ES24700 [PI]). R. Wilson serves as a Consulting Editor for Aging, Neuropsychology, and Cognition, Psychology and Aging, and Neuropsychology; has served as a consultant for Pain Therapeutics, Inc.; and receives research support from the NIH (P30AG010161 [Co-I], RF1AG015819 [Co-I], R01AG017917 [Co-I], R01AG034374 [Co-I], R01AG039478 [Co-I], R01AG036042 [Co-I], R01AG036836 [Co-I], R01AG041797 [Co-I], R01AG042210 [Co-I], and R01NR013151 [Co-I]), the Alzheimer's Association (NIRGD-11-205469), and Zinfandel Pharmaceuticals. U. Bültmann serves on the editorial board of the Journal of Occupational Rehabilitation. D. Evans served on a Data Monitoring Committee for Eli Lily and Company and receives research support from the NIH (AG11101 [PI], AG09966 [PI], AG030146 [PI], AG10161 [Co-I], AG021972 [Co-I], ES10902 [Co-I], NR009543 [Co-I], HL084209 [Co-I], AG036650 [PI], and AG12505 [Co-I]). C. Mendes de Leon serves on the editorial boards of Psychosomatic Medicine and the Journal of Aging and Health and receives research support from the NIH (R01AG032247 [PI], P60MD002249 [Co-PI], R21ES02041 [Co-I], T32AG027708 [PI], and R01AG022018 [Co-I]) and the Center for Medicare and Medicaid Innovation (CMMI MIDS TORP 141747 [Co-I]). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013;80:1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langbaum JB, Fleisher AS, Chen K, et al. Ushering in the study and treatment of preclinical Alzheimer disease. Nat Rev Neurol 2013;9:371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M. Midlife healthy-diet index and late-life dementia and Alzheimer's disease. Dement Geriatr Cogn Dis Extra 2011;1:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol 2005;4:705–711. [DOI] [PubMed] [Google Scholar]
- 5.Daviglus ML, Bell CC, Berrettini W, et al. National Institutes of Health State-of-the-Science Conference statement: preventing Alzheimer disease and cognitive decline. Ann Intern Med 2010;153:176–181. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology 2013;81:314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging 1999;14:245–263. [DOI] [PubMed] [Google Scholar]
- 8.Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology 2003;61:812–816. [DOI] [PubMed] [Google Scholar]
- 9.Finkel D, Andel R, Gatz M, Pedersen NL. The role of occupational complexity in trajectories of cognitive aging before and after retirement. Psychol Aging 2009;24:563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh-Manoux A, Marmot MG, Glymour M, Sabia S, Kivimäki M, Dugravot A. Does cognitive reserve shape cognitive decline? Ann Neurol 2011;70:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marioni RE, Proust-Lima C, Amieva H, et al. Cognitive lifestyle jointly predicts longitudinal cognitive decline and mortality risk. Eur J Epidemiol 2014;29:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vemuri P, Lesnick TG, Przybelski SA, et al. Association of lifetime intellectual enrichment with cognitive decline in the older population. JAMA Neurol 2014;71:1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher GG, Stachowski A, Infurna FJ, Faul JD, Grosch J, Tetrick LE. Mental work demands, retirement, and longitudinal trajectories of cognitive functioning. J Occup Health Psychol 2014;19:231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Then FS, Luck T, Luppa M, König HH, Angermeyer MC, Riedel-Heller SG. Differential effects of enriched environment at work on cognitive decline in old age. Neurology 2015;84:1–8. [DOI] [PubMed] [Google Scholar]
- 15.Evans DA, Beckett LA, Field TS, et al. Apolipoprotein E epsilon4 and incidence of Alzheimer disease in a community population of older persons. JAMA 1997;277:822–824. [PubMed] [Google Scholar]
- 16.Peterson NG, Mumford MD, Borman WC, Jeanneret PR, Fleishman EA, eds. An Occupational Information System for the 21st Century: The Development of the O*NET. Washington, DC: American Psychological Association; 1999:3–8. [Google Scholar]
- 17.Wilson RS, Bennett DA, Beckett LA, et al. Cognitive activity in older persons from a geographically defined population. J Gerontol B Psychol Sci Soc Sci 1999;54:P155–P160. [DOI] [PubMed] [Google Scholar]
- 18.Andel R, Silverstein M, Kåreholt I. The role of midlife occupational complexity and leisure activity in late-life cognition. J Gerontol B Psychol Sci Soc Sci 2015;70:314–321. [DOI] [PubMed] [Google Scholar]
- 19.Smart EL, Gow AJ, Deary IJ. Occupational complexity and lifetime cognitive abilities. Neurology 2014;83:2285–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potter GG, Helms MJ, Plassman BL. Associations of job demands and intelligence with cognitive performance among men in late life. Neurology 2008;70:1803–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 2002;8:448–460. [PubMed] [Google Scholar]
- 22.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA 1994;271:1004–1010. [PubMed] [Google Scholar]
- 23.Mohammed AH, Zhu SW, Darmopil S, et al. Environmental enrichment and the brain. Prog Brain Res 2002;138:109–133. [DOI] [PubMed] [Google Scholar]
- 24.Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE. The role of APOE-epsilon4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology 2003;60:1077–1081. [DOI] [PubMed] [Google Scholar]
- 25.Richards M, Sacker A. Lifetime antecedents of cognitive reserve. J Clin Exp Neuropsychol 2003;25:614–624. [DOI] [PubMed] [Google Scholar]
- 26.Clouston SA, Kuh D, Herd P, Elliott J, Richards M, Hofer SM. Benefits of educational attainment on adult fluid cognition: international evidence from three birth cohorts. Int J Epidemiol 2012;41:1729–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang HX, Wahlberg M, Karp A, Winblad B, Fratiglioni L. Psychosocial stress at work is associated with increased dementia risk in late life. Alzheimers Dement 2012;8:114–120. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz BS, Stewart WF, Bolla KI, et al. Past adult lead exposure is associated with longitudinal decline in cognitive function. Neurology 2000;55:1144–1150. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Bureau of Labor Statistics. American Time Use Survey: 2014 Results [online]. Available at: www.bls.gov/news.release/atus.nr0.htm. Accessed June 30, 2015. [Google Scholar]