Abstract
Objectives
To examine the association between sleep complaints, use of sleep promoting medications and persistent severe fatigue.
Design
Analysis of data derived from the National Health Aging Trends Study (NHATS) Participants and Setting: A representative sample of Medicare beneficiaries >= 65 years old living in contiguous United States.
Measurements
Difficulty initiating sleep, difficulty staying asleep, use of sleep promoting medications, demographic characteristics, presence of pain, use of pain medications, depression, chronic medical disease, physical activity level, short physical performance battery score measured at baseline. The outcome of interest was persistent severe fatigue, i.e. fatigue which limits the daily activities of study participants that is reported at baseline and 12-month follow up.
Results
A total of 8245 and 7075 participants completed the study at baseline and 12-month follow up respectively. Severe fatigue was reported by 31% of participants at baseline and 31% at follow up, and persistent severe fatigue was reported by 19% of participants. In a logistic regression model, difficulty staying asleep ‘some nights’ and ‘most nights or every night’ (OR (CI) = 1.32 (1.08 – 1.60) and (1.40 (1.09 – 1.79) respectively), as well as use of sleep promoting medications ‘most nights or every night’ (OR (CI) 1.35 (1.08–1.67)) independently predicted persistent sever fatigue.
Conclusion
The results indicate increased risk for persistent severe fatigue among older adults with difficulty staying asleep and those who use sleep promoting medications. These findings underscore the significance of sleep problems and also present potential targets for interventional studies that aim to improve fatigue among older adults.
Keywords: Difficulty falling asleep, Difficulty staying asleep, Use of sleep promoting medications, Persistent fatigue
INTRODUCTION
Aging is associated with decline in physiological reserve which results in decrease in the ability to maintain homeostasis after a physiological stress.1 This inability to maintain homeostasis makes older adults vulnerable to adverse outcomes after stressful events, functional decline and development of frailty. 2–4 Fatigue related symptoms have been described to predict functional decline5;6,7,8;9 frailty2 as well as mortality10, suggesting that fatigue may be one of the manifestations of significant decrease in physiological reserve. Several studies have described a high prevalence of fatigue among older adults, 11–15 and multiple factors which include chronic medical, neurological and psychiatricdisorders11;12;15;16 have been implicated in the pathogenesis of fatigue in old age. Associations between sleep problems and fatigue related symptoms have also been previously reported. For example, among patients with chronic medical problems such as multiple sclerosis 17, cancer18, end-stage kidney disease19 and osteoarthritis20 associations between fatigue and sleep problems have been described. Fatigue has also been reported as a manifestation of sleep disorders such as obstructive sleep apnea 21 and insomnia22, although this was described mostly among middle-aged sleep clinic population. Similar associations between sleep problems and fatigue among community dwelling older adults have also been reported. 8;9;23
However, most of the studies that described association between sleep problems and fatigue focused on prevalent fatigue, and did not describe whether fatigue related symptoms are transient or persistent. Given previous reports that indicated that fatigue related symptoms may be variable over time, 16;24;25 it would be important to examine if sleep problems are associated with persistent fatigue. The current study aims to advance knowledge in the field by examining if sleep complaints at baseline predict persistent fatigue which limits the activities of participants. The objectives of the study are to examine the associations between difficulty falling asleep, difficulty staying asleep and use of sleep promoting medications, and persistent severe fatigue (i.e. fatigue which limits activities of individuals). It is hypothesized that sleep complaints as well as use of sleep promoting medications at baseline predict persistence of severe fatigue at follow up.
METHODS
Data for analysis in the current study is derived from the National Health Aging Trends Study (NHATS), which is sponsored by the National Institute on Aging (grant number NIA U01AG032947) through a cooperative agreement with the Johns Hopkins Bloomberg School of Public Health. Participants of NHATS are a representative sample of Medicare beneficiaries >= 65 years old living in contiguous United States, and the study is designed to investigate trends and dynamics of late life functioning at baseline and during follow up.26 Study methods used in NHATS have been previously described in detail. 26 In short, using a stratified 3-stage sampling design, 12411 Medicare beneficiaries >= 65 years of age were randomly selected, out of which 8245 participants at baseline and 7075 participants at 12-month follow up completed the study. Based on the sampling design and non-response rate, appropriate sampling weights were developed and these weights are used in the current analysis26. Both demographic and clinical data were collected in NHATS and the following variables are used in the current analysis.
Exposure variables: At baseline, 3 sleep related questions were used in NHATS and these include: (1) “How often does it take you more than 30 minutes to fall asleep at night?”; (2) “How often do you have trouble falling back asleep on nights after waking up from sleep?”; (3) How often do you take medication to help you sleep?”. Responses to these questions include every night, most nights, some nights, rarely and never; and based on participants’ response the following 3 categories were created: (1)”Never or rarely”, (2) “Some Nights” and (3) “Most Nights or Every Night”. For the purpose of this analysis, difficulty falling asleep and difficulty staying asleep are defined by positive responses (“Some Nights” or “Most Nights or Every Night”) to the questions “How often does it take you more than 30 minutes to fall asleep at night?” and “How often do you have trouble falling back to sleep on nights after waking up from sleep?” respectively.
Outcome variable
Persistent severe fatigue (PSF) is the outcome variable and is derived from participants’ responses to the following two questions: “In the last month, did you have low energy or were you easily exhausted?”, and for participants who responded “Yes”, this question is followed by: “In the last month, did your low energy or exhaustion ever limit your activities?”. For the purpose of this analysis, severe fatigue is defined as low energy or exhaustion which limits activities. Participants who endorsed severe fatigue at baseline and 12-months follow up are considered to have PSF. Fatigue related questions in NHATS were presented to study participants living in the community and those living in residential care facilities who completed the sample person interview26.
Additional baseline characteristics of study participants included in the analysis are age, gender, marital status, years of education, total family income, smoking history, level of physical activity (physical exercise), presence of pain, use of pain medications, depression, self-reported current medical problems, self-reported current height and weight, and results of short physical performance battery tests (SPPB). 27 Because information for total family income was available for 45% of participants only, hot-deck imputation was used to create imputed total income data by NHATS, and these imputed income values were used in the current analysis. Low income is defined as total family income which is less than the poverty threshold based on the United States 2011 poverty guideline28. Level of physical activity is determined based on the question “In the last month, do you ever spend time on vigorous activities that increased your heart rate and made you breath harder? These included things like working out, swimming, running, or biking or playing a sport, and participants who responded “No” to this question were determined to have low physical activity. Presence of pain was determined based on two questions: “In the last month, have you been bothered by pain”, followed by “In the last month, has pain ever limited your activities?” for participants who answered “Yes” to the first part of the pain question. Participants who endorsed pain which limited their activities were determined to have severe pain, while those who reported pain that did not limit their activities were considered to have mild pain. Depression symptoms were determined based on response to Patient Health Questionnaire-2 (PHQ-2), and a score of 3 or more is considered to indicate significant depression symptoms.29 Results for short physical performance battery (NHATS) was derived from the sum of scores for balance stands, walking speed, and repeated chair stands tests and ranged from 0–12.27 In addition, participants are asked whether they have been told to have chronic medical conditions such as hypertension, diabetes mellitus, heart disease, heart attack, osteoporosis, arthritis, lung disease, stroke, cancer, dementia and the sum of these conditions is used to indicate chronic disease burden. Body mass index was calculated using self-reported height and weight and the following formula: Weight in Kg divided by (height in meters)2.
Statistical Analysis
Demographic, sleep and other clinical characteristics of participants by fatigue status (PSF vs. No PSF) are described using Chi-square analysis and Student’s t-test for categorical and continuous variables, respectively. Unadjusted and adjusted logistic regression (LR) models were created to analyze the association between PSF and sleep complaints. Demographic and clinical characteristics were considered for inclusion in the LR model if they show statistically significant relationship with PSF on bi-variable analysis at P< .05, or if deemed biologically relevant. Interaction terms were created and included in the model if they show significance at P <0.1. Sequential imputations using chained equations were performed to account for missing data. Stata statistical software for survey data (version 13) was used for data analysis and P value of .05 is considered to indicate statistical significance.
RESULTS
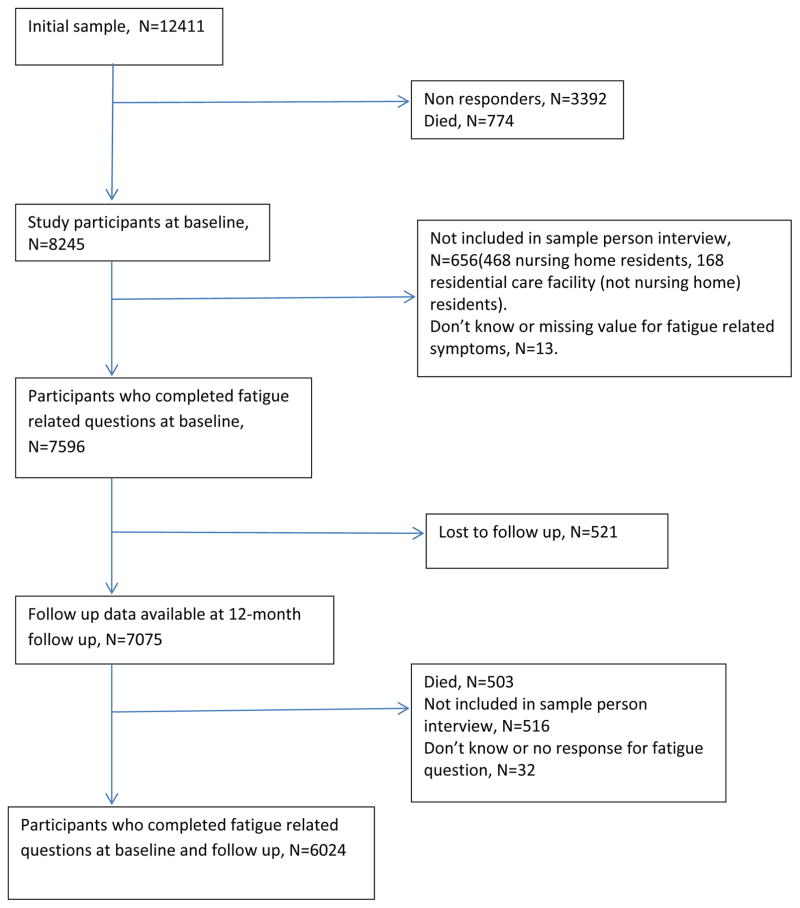
A total of 7596 participants (92%) at baseline and 6037 participants (85%) at 12-month follow up provided response to fatigue related questions, and data on PSF was available for6024 participants. Figure 1 shows the flow of participants through the different stages of the study. Overall, participants for whom data on PSF was not available were older, more likely to be female, more likely to have higher disease burden and depression score (P < .001).
Figure 1.
Diagram showing Flow of Participants during the Study Period
A total of 2371 participants (31%) at baseline and 1878 participants (31%) participants at 12-month follow up reported severe fatigue. Persistent severe fatigue was identified in 1158 participants (19%); while 653 participants (11%) who reported fatigue at baseline did not endorse fatigue at follow up, and 716 (12%) participants reported fatigue for the first time at 12-month follow up. Table 1 and Table 2 show selected demographic and clinical characteristics of study participants by PSF status. As expected, there was statistically significant relationship between PSF and sleep related complaints, increasing age, female gender, decreased physical activity, pain, depressed mood, increased morbidity status and lower SPPB score. In addition, PSF was more prevalent among participants with low socioeconomic status (low income and fewer years of education), while there was no significant difference in PSF occurrence among the different racial groups.
Table 1.
Demographic and Selected Clinical Characteristics of Study Participants with and without Persistent Fatigue
| No PSF (N= 4866) | PSF N = 1158 |
Total N = 6024 |
P value | |
|---|---|---|---|---|
|
| ||||
| Age Categories in years | ||||
| 65 – 69 | 956 (20) | 194 (17) | 1150 (19) | |
| 70 – 74 | 1056 (22) | 213 (18) | 1269 (21) | |
| 75 – 79 | 986 (20) | 235 (20) | 1221 (20) | |
| 80 – 84 | 945 (19) | 244 (21) | 1189 (20) | |
| 85 – 89 | 549 (11) | 174 (15) | 723 (12) | < .001 |
| 90+ | 374 (8) | 98 (9) | 472 (8) | |
|
| ||||
| Male | 2108 (43) | 404 (35) | 2512 (42) | < .001 |
| Female | 2758 (57) | 754 (65) | 3512 (58) | |
|
| ||||
| Race | ||||
| White | 3360 (69) | 797 (69) | 4169 (69) | |
| Black | 1059 (22) | 246 (21) | 1305 (22) | |
| Hispanic | 272 (6) | 76 (7) | 348 (6) | |
| Other | 175 (4) | 39 (3) | 214 (4) | = .616 |
|
| ||||
| Marital Status | ||||
| Married/living together | 2489 (51) | 533 (46) | 3022 (50) | |
| Not currently married† | 2372 (49) | 624 (54) | 2996 (50) | = .002 |
|
| ||||
| Education | ||||
| >12 years | 2324 (48) | 467 (41) | 2791 (47) | |
| 9 – 12 years | 1951 (40) | 496 (43) | 2447 (41) | |
| <9 years | 551 (11) | 182 (16) | 733 (12) | < .001 |
|
| ||||
| Income | ||||
| Not low | 3774 (78) | 786 (68) | 4560 (76) | |
| Low | 1092 (22) | 372 (32) | 1464 (24) | <.001 |
|
| ||||
| Smoking | ||||
| Never | 2431 (50) | 563 (49) | 2994 (50) | |
| History of smoking | 2084 (43) | 495 (43) | 2579 (43) | |
| Currently smoking | 347 (7) | 100 (9) | 447 (7) | < .001 |
|
| ||||
| Body Mass Index (Kg/m2) | ||||
| 18.5 – 24.9 | 1573 (33) | 341 (30) | 1914 (32) | |
| 25.0 – 29.9 | 1765 (37) | 349 (31) | 2114 (36) | |
| 30.0 – 34.9 | 809 (17) | 221 (19) | 1030 (17) | |
| >= 35 | 538 (11) | 200 (17) | 738 (12) | |
| < 18.5 | 99 (2) | 31 (3) | 130 (2) | < .001 |
|
| ||||
| Health Status | ||||
| Excellent/Very good | 2319 (48) | 149 (13) | 2468 (41) | |
| Good | 1629 (33) | 321 (28) | 1950 (32) | < .001 |
| Poor/Fair | 916 (19) | 687 (59) | 1603 (27) | |
PSF = persistent severe fatigue.
include never married, separated/divorced, widowed
Table 2.
Clinical and Sleep Characteristics of Study Participants with and without Persistent Fatigue
| No PSF (N= 4866) | PSF (N = 1158) | Total N = 6024 |
P value | |
|---|---|---|---|---|
|
| ||||
| DFA† | ||||
| Rarely/Never | 2819 (58) | 441 (38) | 3260 (54) | |
| Some Nights | 1131 (23) | 311 (27) | 1442 (24) | |
| Most nights/Every night | 901 (19) | 403 (35) | 1304 (22) | <.001 |
|
| ||||
| DSA‡ | ||||
| Rarely/Never | 2959 (61) | 487 (42) | 3446 (57) | |
| Some Nights | 1269 (26) | 354 (31) | 1623 (27) | |
| Most nights/Every night | 624 (13) | 311 (27) | 935 (16) | <.001 |
|
| ||||
| Sleep aid use | ||||
| Rarely/Never | 4010 (82) | 742 (64) | 4752 (79) | |
| Some Nights | 279 (6) | 94 (8) | 373 (6) | < .001 |
| Most nights/Every night | 570 (12) | 316 (27) | 886 (15) | |
|
| ||||
| No significant pain | 2558 (53) | 216 (19) | 2774 (46) | |
| Pain without activity limitation | 1294 (27) | 174 (15) | 1468 (24) | |
| Pain with activity limitation | 1011 (21) | 768 (66) | 1779 (30) | < .001 |
|
| ||||
| Pain Medication | ||||
| Rarely/Never | 3135(64) | 389 (34) | 3524 959) | |
| Sometimes | 717 (15) | 196 (17) | 913 (15) | |
| Often/Always | 1009 (21) | 572 (49) | 1581 (26) | < .001 |
|
| ||||
| Depression score | ||||
| < 3 | 3857 (88) | 479 (55) | 4336 (83) | |
| >= 3 | 528 (12) | 385 (45) | 913 (17) | < .001 |
|
| ||||
| Physical activity | ||||
| Yes | 1883 (39) | 252 (22) | 2135 (35) | |
| No | 2983 (61) | 905 (78) | 3886 (65) | < .001 |
|
| ||||
| Disease Burden | ||||
| 0 – 1 | 1645 (34) | 120 (10) | 1765 (29) | |
| 2. – 3 | 2533 (52) | 585 (51) | 3118 (52) | |
| > 3 | 688 (14) | 453 (39) | 1141 (19) | < .001 |
|
| ||||
| NHATS SPPB score*** | 4.7 (6.0) | 2.3 (5.9) | 4.3 (6.0) | < .001 |
PSF= persistent severe fatigue.
DFA= Difficulty falling asleep.
DSA = Difficulty staying asleep
National Health Aging Trends Study Short Physical Performance Battery Test result (Higher result indicate better function)
Table 3 shows three LR models (unadjusted, partially adjusted and fully adjusted) in which associations between sleep related complaints and PSF are depicted. Because there were only 5098 observations in the fully adjusted LR model, imputations were performed to account for missing data, and results of the fully adjusted LR model shown in Table 3 is obtained using these imputed data. As shown, both difficulty staying asleep and use of medications for sleep often or always at baseline were independent predictors of PSF. Interaction terms for gender and race, by sleep complaints and use of sleep promoting medications did not show significant association with PSF.
Table 3.
Logistic Regression Analysis showing the association between sleep related complaints and persistent fatigue in unadjusted and adjusted models.
| Model 1 Odds Ratio (OR) Confidence Interval (CI) |
Model 2 OR (CI) |
Model 3 OR (CI) |
||
|---|---|---|---|---|
|
| ||||
| Difficulty falling asleep | ||||
| Rarely/Never | 1.0 | 1.0 | 1.0 | |
| Some Nights | 1.44 (1.21 – 1.71) | 1.36 (1.14 – 1.62) | 1.16 (0.94 – 1.36) (P =.163) | |
| Most nights/Every night | 1.71 (1.42 – 2.06) | 1.61 (1.33 – 1.94) | 1.19 (0.93 – 1.52) (P = .164) | |
|
| ||||
| Difficulty staying asleep | ||||
| Rarely/Never | 1.0 | 1.0 | 1.0 | |
| Some Nights | 1.35 (1.21 – 1.71) | 1.36 (1.15 – 1.61) | 1.32 (1.08 – 1.60) (P=.007) | |
| Most nights/Every night | 2.00 (1.64 – 2.43) | 2.02 (1.66–2.47) | 1.40 (1.09 – 1.79) (P =.009) | |
|
| ||||
| Use of Sleep Aids | ||||
| Rarely/Never | 1.0 | 1.0 | 1.0 | |
| Some Nights | 1.37 (1.06–1.77) | 1.42 (1.10 – 1.24) | 1.01(0.75 – 1.30) (P = .917) | |
| Most nights/Every night | 2.44 (2.07 – 2.88) | 2.36 (1.99 – 2.79) | 1.35 (1.08 – 1.67) (P = .009) | |
Model 1 = Unadjusted model. Model 2: Model adjusted for age, gender, race, education, marital status.
Model 3: Model 2 + body mass index, pain, pain medication use, depression status, physical activity level, disease burden and Short Physical Performance Battery test score.
Other covariates that showed positive association include; Pain that limits activity (OR (CI): 3.82 (3.08 – 4.74));
Depression (OR (CI): 3.35 (2.87–3.91)); Number of chronic diseases 2–3: OR (CI) (1.73(1.35 – 2.24); Number of chronic diseases >3: (OR (CI) 3.16 (2.46 – 4.06)); Low physical activity: (OR (CI) 1.27 (1.06–1.52). Covariates that showed negative association include: Black race (OR (CI): 0.63 (0.49–0.79)), Hispanic race: OR (CI): 0.71 (0.50 – 0.99)), 9–12 years of education: OR (CI): 0.83 (0.71 – 0.99)), not married status: score OR (CI): 0.80 (0.66 – 0.97)) and higher short physical performance battery score OR (CI): 0.89 (0.85 – 0.91)).
Other variables that showed statistically significant association with PSF in the fully adjusted model included Black race, Hispanic race (White race was the reference group), 9–12 years of education (> 12 years of education was the reference group), “not married” status, presence of severe pain, use of pain medication often or always, higher depression score (PHQ2 >3), disease burden, lack of physical activity and SPPB score (Table 3). There were no significant associations between PSF and age, gender, smoking, poverty status and body mass index in the fully adjusted model, suggesting that the association between PSF and these variables observed in bi-variable analysis may be explained by the other clinical characteristics of study participants. It is also notable that Black and Hispanic participants were less likely to report PSF than White participants in the fully adjusted model.
DISCUSSION
Results of the current study indicate that difficulty staying asleep, as well as use of sleep promoting medications predict PSF among older adults, independent of known conditions previously described to be associated with fatigue. Although previous studies have described significant association between sleep complaints and prevalent fatigue related symptoms17–20;23;30, results of the current study extend knowledge in the field for the following reasons. Firstly, unlike most previous studies that focused on prevalent fatigue, persistent fatigue which limits activities was used as the outcome variable in the current study. Secondly, use of sleep promoting medications were also independently associated with fatigue and this may indicate the long-term adverse effects of this group of medications31. Last but not least, participants in the present study are a representative sample of older adults living in contiguous United States and this would make generalization of the results possible. To the knowledge of the author, this is the first study to report the association between sleep related symptoms as well as use of sleep promoting medications, and persistent fatigue which limits activities among a representative sample of older adults living in contiguous United States. These results not only underscore the significance of sleep problems, but also present potential targets for future interventional studies that aim improve fatigue related symptoms among older adults. Given the nature of the present study, it was not possible to identify specific sleep disorders that are associated with PSF, and future studies would be needed to determine the role of specific sleep disorders in the pathogenesis of persistent fatigue among older adults.
Previous studies have described possible biological mechanisms involved in the pathogenesis of fatigue and these include pro-inflammatory cytokine activity and hypothalamus-pituitary-adrenal axis dysfunction32. Given previous reports that indicated abnormal HPA axis and cytokine levels among patients with sleep disorders33;34, it is plausible that the association between sleep problems and fatigue symptoms could be mediated by the same mechanism.
Results of the current study is in agreement with previous studies that reported significant association between fatigue, and pain, depression, low physical activity and higher morbidity status16;23. Lack of association between fatigue, demographic variables such as age and sex in the fully adjusted regression model is not consistent with reports from previous studies23. In the current study, covariates which were not included in previous studies such as sleep related variables, pain and use of pain medication were included, and this may explain, at least in part, the non-significant finding between PSF and these variables.
It is notable that associations between fatigue and adverse health outcomes observed in the current study is similar to reports from previous studies that have used different instruments to define fatigue. 35 Given these findings, the questions used in the current study may be suitable for use as a screening tool for fatigue in a busy clinic set up.
Strengths of the current study include participants who are a representative sample of older adults living in the contiguous United States. In addition, data collection at baseline and 12-month follow up was conducted using a standard protocol. However, the study also has its limitations. Data on sleep were limited to two questions related to sleep complaints and one question on the use of medications for sleep. Data on objective sleep parameters and names of specific sleep medications were not collected. Although the sleep related questions used in the current study have been used in previous epidemiological studies, they do not provide information on possible underlying etiology of specific sleep problem or specific medication used. As stated above, future studies would be needed to identify specific sleep disorders and/or medications that predict fatigue in old age. Data on daytime sleepiness was not collected in NHATS and it is possible that fatigue symptoms may be taken to mean daytime sleepiness by study participants. However previous studies among patients with sleep disorders have indicated that fatigue related symptoms and daytime sleepiness are well differentiated by patients21; hence, the overlap in reporting of these two symptoms would be minimal.
In conclusion, result of the current epidemiological study indicate that difficulty staying asleep as well as use of sleep promoting medications independently predict persistent fatigue symptom which limit the activities of older adults. These findings not only underscore the importance of sleep related complaints, but also suggest that sleep related issues should be considered as a potential target in future interventional studies that aim to improve fatigue related symptoms among older adults.
Acknowledgments
This work was supported by grant from Deep South Resource Center for Minority Aging Research (P30AG31054) from the National Institute on Aging. The National Health and Aging Trends Study (NHATS) is sponsored by the National Institute of Aging (grant number NIA U01AG032947) through a cooperative agreement with the Johns Hopkins Bloomberg School of Public Health.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the author and has determined that the author has no financial or any other kind of personal conflicts with this paper.
Author Contributions: The author is responsible for the study concept and design, data analysis, interpretation of results and preparation of the manuscript.
Sponsor’s Role: None
References
- 1.Neustadt John, Pieczenik Steve. Organ reserve and health aging. Integrative Medicine. 2008;7:50–52. [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 4.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avlund K, Pedersen AN, Schroll M. Functional decline from age 80 to 85: influence of preceding changes in tiredness in daily activities. Psychosom Med. 2003;65:771–777. doi: 10.1097/01.psy.0000082640.61645.bf. [DOI] [PubMed] [Google Scholar]
- 6.Hardy SE, Studenski SA. Fatigue and function over 3 years among older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1389–1392. doi: 10.1093/gerona/63.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135:313–321. doi: 10.7326/0003-4819-135-5-200109040-00007. [DOI] [PubMed] [Google Scholar]
- 8.Vestergaard S, Nayfield SG, Patel KV, et al. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disability. J Gerontol A Biol Sci Med Sci. 2009;64:76–82. doi: 10.1093/gerona/gln017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardy SE, Studenski SA. Fatigue and function over 3 years among older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1389–1392. doi: 10.1093/gerona/63.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardy SE, Studenski SA. Fatigue predicts mortality in older adults. J Am Geriatr Soc. 2008;56:1910–1914. doi: 10.1111/j.1532-5415.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Addington AM, Gallo JJ, Ford DE, Eaton WW. Epidemiology of unexplained fatigue and major depression in the community: the Baltimore ECA follow-up, 1981–1994. Psychol Med. 31:1037–1044. doi: 10.1017/s0033291701004214. 01. [DOI] [PubMed] [Google Scholar]
- 12.Swain MG. Fatigue in chronic disease. Clin Sci (Lond) 2000;99:1–8. [PubMed] [Google Scholar]
- 13.Liao S, Ferrell BA. Fatigue in an older population. J Am Geriatr Soc. 2000;48:426–430. doi: 10.1111/j.1532-5415.2000.tb04702.x. [DOI] [PubMed] [Google Scholar]
- 14.Meng H, Hale L, Friedberg F. Prevalence and predictors of fatigue in middle-aged and older adults: evidence from the health and retirement study. J Am Geriatr Soc. 2010;58:2033–2034. doi: 10.1111/j.1532-5415.2010.03088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wijeratne C, Hickie I, Brodaty H. The characteristics of fatigue in an older primary care sample. J Psychosom Res. 2007;62:153–158. doi: 10.1016/j.jpsychores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 16.de Rekeneire N, Leo-Summers L, Han L, Gill TM. Epidemiology of restricting fatigue in older adults: the precipitating events project. J Am Geriatr Soc. 2014;62:476–481. doi: 10.1111/jgs.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strober LB, Arnett PA. An examination of four models predicting fatigue in multiple sclerosis. Arch Clin Neuropsychol. 2005;20:631–646. doi: 10.1016/j.acn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Ancoli-Israel S, Moore PJ, Jones V. The relationship between fatigue and sleep in cancer patients: a review. Eur J Cancer Care (Engl) 2001;10:245–255. doi: 10.1046/j.1365-2354.2001.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jhamb M, Liang K, Yabes J, et al. Prevalence and correlates of fatigue in chronic kidney disease and end-stage renal disease: are sleep disorders a key to understanding fatigue? Am J Nephrol. 2013;38:489–495. doi: 10.1159/000356939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawker GA, French MR, Waugh EJ, Gignac MA, Cheung C, Murray BJ. The multidimensionality of sleep quality and its relationship to fatigue in older adults with painful osteoarthritis. Osteoarthritis Cartilage. 2010;18:1365–1371. doi: 10.1016/j.joca.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Chervin RD. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest. 2000;118:372–379. doi: 10.1378/chest.118.2.372. [DOI] [PubMed] [Google Scholar]
- 22.The International Classification of Sleep Disorders: Diagnostic and Coding Manual. Insomnia. 2005 [Google Scholar]
- 23.Goldman SE, Ancoli-Israel S, Boudreau R, et al. Sleep problems and associated daytime fatigue in community-dwelling older individuals. J Gerontol A Biol Sci Med Sci. 2008;63:1069–1075. doi: 10.1093/gerona/63.10.1069. [DOI] [PubMed] [Google Scholar]
- 24.Schepers VP, Visser-Meily AM, Ketelaar M, Lindeman E. Poststroke fatigue: course and its relation to personal and stroke-related factors. Arch Phys Med Rehabil. 2006;87:184–188. doi: 10.1016/j.apmr.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Nijrolder I, van der Windt DA, van der Horst HE. Prognosis of fatigue and functioning in primary care: a 1-year follow-up study. Ann Fam Med. 2008;6:519–527. doi: 10.1370/afm.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasper JD, Freedman VA. National Health and Aging Trends Study User Guide: Rounds 1 & 2, Final Release. Baltimore: John Hopkins University School of Public Health; 2014. [Google Scholar]
- 27.Kasper Judith D, Freedman Vicki A, Niefeld Marlene R. NHATS Technical Paper #4. Baltimore: Johns Hopkins University School of Public Health; 2012. Construction of performance-based summary measures of physical capacity in the National Health and Aging Trends Study. Available at www.NHATS.org. [Google Scholar]
- 28.US Department of Health and Human Services. 2012 HHS Poverty Guidelines. 2012. [Google Scholar]
- 29.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 30.Wu HS, Davis JE, Natavio T. Fatigue and disrupted sleep-wake patterns in patients with cancer: a shared mechanism. Clin J Oncol Nurs. 2012;16:E56–E68. doi: 10.1188/12.CJON.E56-E68. [DOI] [PubMed] [Google Scholar]
- 31.Vermeeren A. Residual effects of hypnotics: epidemiology and clinical implications. CNS Drugs. 2004;18:297–328. doi: 10.2165/00023210-200418050-00003. [DOI] [PubMed] [Google Scholar]
- 32.Norheim KB, Jonsson G, Omdal R. Biological mechanisms of chronic fatigue. Rheumatology (Oxford) 2011;50:1009–1018. doi: 10.1093/rheumatology/keq454. [DOI] [PubMed] [Google Scholar]
- 33.Basta M, Chrousos GP, Vela-Bueno A, Vgontzas AN. CHRONIC INSOMNIA AND STRESS SYSTEM. Sleep Med Clin. 2007;2:279–291. doi: 10.1016/j.jsmc.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90:3106–3114. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- 35.Whitehead L. The measurement of fatigue in chronic illness: a systematic review of unidimensional and multidimensional fatigue measures. J Pain Symptom Manage. 2009;37:107–128. doi: 10.1016/j.jpainsymman.2007.08.019. [DOI] [PubMed] [Google Scholar]