Abstract
BACKGROUND
The objective of this systematic review and meta-analysis were to evaluate the effectiveness of high fluid intake for the prevention of incident and recurrent kidney stones, as well as its adherence and safety.
METHODS
A literature search was performed encompassing 1980 through July 2014. Studies that reported relative risks, odds ratios, or hazard ratios comparing the risk of kidney stone events in patients with high vs inadequate fluid intake were included. Pooled risk ratios (RRs) and 95% confidence intervals (CIs) were calculated using a random-effect, generic inverse variance method.
RESULTS
Nine studies (2 randomized controlled trials [RCTs] with 269 patients; 7 observational studies with 273,685 individuals) were included in the meta-analysis. Pooled RRs of kidney stones in individuals with high-fluid intake were 0.40 (95% CI 0.20–0.79) and 0.49 (0.34–0.71) in RCTs and observational studies, respectively. High fluid intake was significantly associated with reduced risk of recurrent kidney stones: RRs 0.40 (95% CI 0.20–0.79) and 0.20 (0.09–0.44) in RCTs and observational studies, respectively. Adherence and safety data on high fluid intake treatment were limited; 1 RCT reported no withdrawals due to adverse events.
CONCLUSION
This analysis demonstrated a significantly reduced risk of incident kidney stones among individuals with high fluid consumption. High fluid consumption also reduced the risk of recurrent kidney stones. Furthermore, the magnitude of risk reduction was high. Although increased water intake appears to be safe, future studies on its safety in patients with high risk of volume overload or hyponatremia may be indicated.
Keywords: meta-analysis, fluid intake, kidney stones, water, hyponatremia
INTRODUCTION
Kidney stones are a very common urologic problem. Their incidence is rising, with an estimated global prevalence of 10%–15% [1–4]. In the United States, ~13% of men and 7% of women will develop a kidney stone during their lifetime [1,4]. In addition, once someone has a kidney stone, the likelihood of having another episode is about 50% within 5 years [1].
Increased fluid intake has been universally suggested as a simple and inexpensive strategy for kidney stone prevention [5–7]. Some studies specifically target water, while some include other beverages that contain largely free water. In this paper we use the word fluid to encompass both. High fluid intake to increase urine flow rate can lower the supersaturation of calcium oxalate, calcium phosphate and uric acid, hence reducing the risk for kidney stone formation [6,8]. Wang et al [7] performed a comprehensive review of the potential salutary relationship of high water intake and kidney disease in general, and concluded that available studies support water therapy to prevent and reduce the recurrence of kidney stones. However, a recent and rigorous systematic review drew no firm conclusions regarding the benefit of water therapy for the primary or secondary prevention of stones [9]. Importantly, only a single RCT was used in the final analysis. Nevertheless, recently published guidelines by both the American Urological Association (AUA) and American College of Physicians (ACP) recommend sufficient water intake to achieve a urine volume at or above 2.0–2.5 L/d in order to prevent recurrent kidney stones [5,6]. These recommendations were graded as weak due to low-quality evidence. In addition, the data regarding the protective effects of high water intake on incident and recurrent kidney stone episodes are conflicting. Two studies have suggested no significant reduction in kidney stone risk among individuals with high fluid consumption [10,11]. Conversely, several studies have reported that sufficient fluid intake to achieve an adequate urine volume is an effective strategy to prevent kidney stones [12–19]. Data regarding adherence and safety of high fluid intake treatment are also limited.
Thus the objectives of this systematic review and meta-analysis were to comprehensively accumulate all available data and pool the results in order to: 1) evaluate the evidence for and significance of any treatment effect of high fluid intake on the incidence of kidney stones, and 2) assess the adherence and safety of high fluid intake to prevent kidney stones.
MATERIALS AND METHODS
Search Strategy
Two investigators (WC and SR) independently searched published studies and conference abstracts indexed in MEDLINE and EMBASE encompassing 1980 through 28 July 2014 using the search strategy described in supplementary item 1. A manual search for additional relevant studies using references from retrieved articles was also performed.
Inclusion Criteria
The inclusion criteria were as follows: (1) randomized controlled trials (RCTs) or observational studies (case-control, cross-sectional or cohort studies) published as original studies or conference abstracts that evaluated the effects of high fluid intake and the risk of kidney stones in adults (age≥ 18 years old), (2) studies that provided data to calculate odds ratios, relative risks, hazard ratios or standardized incidence ratios with 95% confidence intervals (CIs), and (3) a reference group composed of subjects with low fluid intake.
Study eligibility was independently determined by the 2 investigators as noted previously. Differing decisions were resolved by mutual consensus. The quality of each study was evaluated by using the Jadad quality-assessment scale [20] for RCTs and the Newcastle-Ottawa quality assessment scale [21] for observational studies.
Data Extraction
A standardized data collection form was used to extract the following information: last name of first author, title of article, study design, year of study, country of origin, year of publication, sample size, definition of high fluid intake, method used to diagnose kidney stone and recurrent stone events, adherence and withdrawal, mean duration of follow-up and adjusted effect estimate with 95% CI.
Statistical Analysis
Review Manager 5.2 software (The Cochrane Collaboration, Oxford, UK) was used for data analysis. Point estimates and standard errors were extracted from individual studies and were combined by the generic inverse variance method of DerSimonian and Laird [22]. Given the high likelihood of between study variances, a random-effect model was used rather than a fixed-effect model. Statistical heterogeneity was assessed using Cochran’s Q test. This statistic was complemented with the I2 statistic, which quantifies the proportion of the total variation across studies that is due to heterogeneity rather than chance. An I2 of 0%–25% represents insignificant heterogeneity, 26%–50% low heterogeneity, 51%–75% moderate heterogeneity and >75% high heterogeneity [23]. The presence of publication bias was assessed by funnel plots of the logarithm of odds ratios vs their standard errors [24].
RESULTS
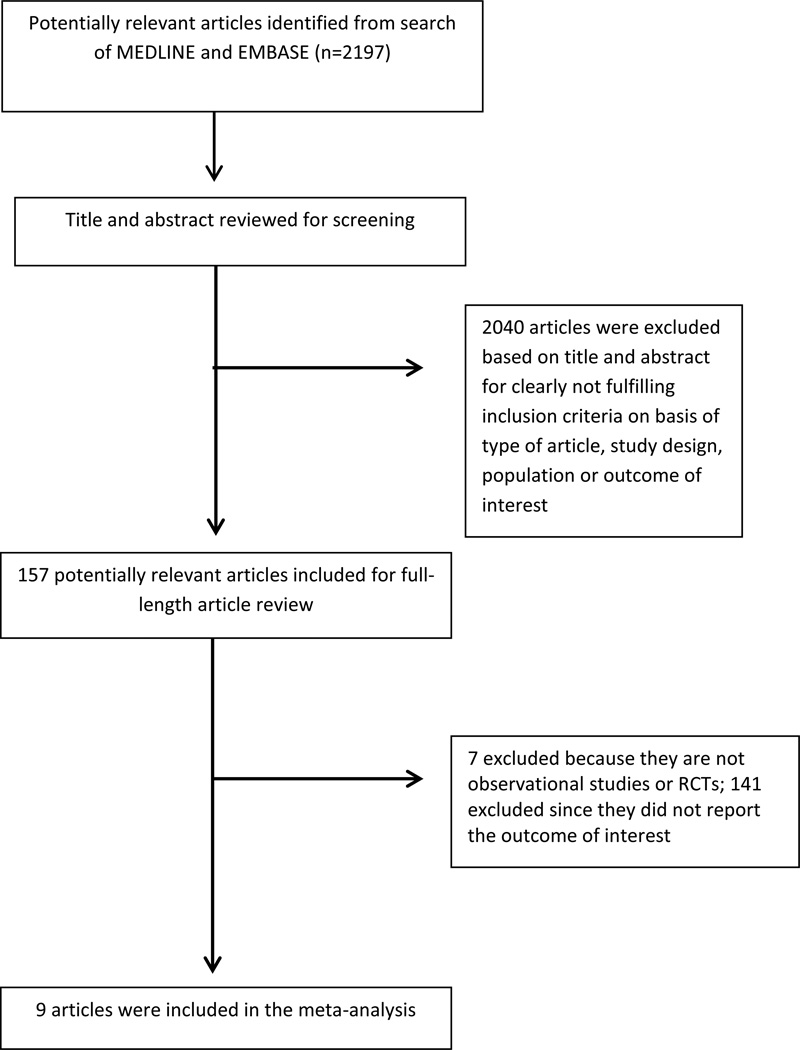
The search strategy yielded 2197 potentially relevant articles: 2040 were excluded based on the title and abstract indicating that they clearly did not fulfill inclusion criteria on the basis of article type, study design, population, or outcome of interest (fig. 1). The remaining 157 articles underwent full-length review, with 148 excluded because they were not RCTs or observational studies (n=7), or did not report outcomes of interest (n=141). Nine articles met our inclusion criteria [10–17], including 2 RCTs with 269 patients [10,11] and 7 observational studies (6 cohort studies [13–17] and 1 cross-sectional study [10]). Thus a total of 273,685 individuals were included in this analysis regarding the risk of kidney stones in individuals with high fluid consumption. Tables 1 and 2 contain detailed characteristics and quality assessment of all RCTs and observational studies included, respectively.
Figure 1.
Outline of search methodology
Table 1.
Main characteristics of randomized controlled trials included in the meta-analysis for risk of symptomatic kidney stones
| Borghi et al[12] | Sarica et al[11] | |
|---|---|---|
| Country | Italy | Turkey |
| Study design | Prospective randomized controlled trial | Prospective randomized controlled trial |
| Year | 1996 | 2006 |
| Patients, n | 199 (134 men; 65 women) with history of kidney stones |
70 (44 men; 26 women) with history of kidney stones |
| Exposure definition | Water intake to achieve urinary output ≥2 L/d | Fluid intake to achieve urinary output >2.5 L/d |
| Exposure measurement |
24-hour urine collection results in record | Detailed history at follow-up |
| Outcome definition | Recurrent renal stones:
|
Recurrent renal stones |
| Outcome ascertainment |
Self-report and detailed history during follow-up for symptomatic stones and appearance of silent stones at yearly follow-up |
Detailed history, urologic examination, plain abdominal X-ray and renal tomography, kidney sonography at 3, 6 and 12 months after stone disintegration |
| Relative risk (95% CI) (a reference group composed of subjects with low water intake) |
0.45 (0.24–0.84) | 0.15 (0.02–1.07) |
| Baseline urine volume, L |
High water intake: 1.1 Control: 1.0 |
N/A |
| Stone types | Calcium nephrolithiasis (calculus found at chemical examination to be composed of pure calcium oxalate or mixed with traces of calcium phosphate) |
Calcium oxalate in all patients; other types of calculi excluded from study |
| No. stones/patient | N/A
|
N/A |
| Withdrawal rates | 9.5% similar in both high water intake and no treatment. |
No withdrawals due to adverse events |
| Follow-up, y | 5.0 | 2.5 |
| Confounder-adjusted | N/A | N/A |
| Quality assessment (Jadad quality- assessment scale)[20] |
2 of 5 | 3 of 5 |
N/A, not available.
Table 2.
Main characteristics of observational studies included in the meta-analysis for risk of kidney stones
| Hosking et al[16] | Curhan et al[13] | Curhan et al[14] | Curhan et al[25] | Taylor et al[17] | Daudon et al[15] |
Linder et al[10] | |
|---|---|---|---|---|---|---|---|
| Country | USA | USA | USA | USA | USA | France | USA |
| Study design | Retrospective cohort |
Prospective cohort |
Prospective cohort |
Prospective cohort |
Prospective cohort |
Retrospective cohort |
Cross-sectional study |
| Year | 1983 | 1997 | 1997 | 2004 | 2004 | 2005 | 2013 |
| Participants, n | 108 (83 men; 25 women) with history of kidney stones |
37,999 men n Health Professionals Follow-up Study |
91,731 women in Nurses’ Health Study I with no history of kidney stones |
96,245 women in Nurses’ Health Study II |
45,619 men in Health Professionals Follow-up Study |
181 (127 men; 54 women) with ≥1 episode of kidney stones |
1802 health care professionals |
| Exposure definition |
Fluid intake to increase urine volume >1 L/d |
Fluid intake ≥2,544 mL/d |
Fluid intake >2,592 mL/d |
Fluid intake ≥2,769 mL/d |
Fluid intake >2,517 mL/d |
Fluid intake to achieve urine output ≥2 L/d |
Fluid intake >1.8 L/d |
| Exposure measurement |
24-h urine collections recorded during follow-up |
Supplementary questionnaire and random sample of control subjects to provide 24-h urine collection |
Questionnaire | Questionnaire | Self- administered food-frequency questionnaires |
24-h urine collection results in record |
Information on quantity of daily water collected via questionnaires |
| Outcome definition |
Evidence of stone growth or new stone formation |
Incident of urinary stone accompanied by pain/hematuria |
Incident of symptomatic kidney stone |
Incident of symptomatic kidney stone |
Incident of kidney stone accompanied by pain/hematuria |
Recurrent renal stones: symptomatic and asymptomatic |
Self-report history of kidney stones |
| Outcome ascertainment |
Medical record review for documented passage of gravel or radiologic evidence of new growth/formation of stones |
Questionnaire; confirmed medical records |
Questionnaire; confirmed medical records |
Questionnaire; confirmed medical records |
Questionnaire; confirmed medical records |
Medical record review for spontaneous passage, urologic procedures, evidences of new stones by imaging studies |
Electronic surveys on history of urolithiasis |
| Adjusted odds or hazard ratio (95% CI) (a reference group composed of subjects with low water intake) |
0.13 (0.28–0.61) |
0.58 (0.42–0.79) |
0.61 (0.48–0.78) |
0.68 (0.56–0.83) |
0.71 (0.59–0.85) |
0.30 (0.18–0.47) |
0.87 (0.63–1.20) |
| Baseline urine volume, L |
Recurrent stones: 1.6 No recurrence: 1.6 |
N/A | N/A | N/A | N/A | Recurrent stones: 1.4 No recurrence: 1.6 |
N/A |
| Stone types | N/A | Of 148 records with stone composition report, 127 (86%) had stone containing ≥50% calcium oxalate |
Of 78 records with stone composition report,60 (77%) had stone containing ≥50% calcium oxalate |
Of 243 records with stone composition report, 191 (79%) had stone containing ≥50% calcium oxalate |
Of 148 records with stone composition report, 127 (86%) had stone containing ≥50% calcium oxalate |
Calcium oxalate nephrolithiasis as main component of stones, with <50% of calcium phosphate in mixed stones |
N/A |
| Mean stones/patient, n |
N/A | N/A | N/A | N/A | N/A | Prereferral period: 4.8 (SEM 0.5) Follow-up period: 1.1 (SEM 0.1) |
Non-OR worker: 1.3 (SD 1.3) OR worker:1.8 (SD 1.9) Physician OR: 1.8 (SD 1.8) |
| Withdrawal rates |
N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Follow-up (y) | 5.2 | 8 | 12 | 8 | 14 | 3.0 | N/A |
| Confounder adjusted |
N/A | Age, BMI, thiazide use; intake of alcohol, supplementary calcium; other dietary intake of listed variables |
Age, BMI; intake of alcohol, supplemental calcium, dietary calcium, animal protein, potassium, sodium, sucrose |
Age, BMI; intake of alcohol, vitamin B6, vitamin C, supplemental calcium, dietary calcium, animal protein, potassium, sodium, sucrose, magnesium, phosphorus, phytate |
Age, BMI, thiazide use; intake of alcohol, calcium supplement, animal protein, calcium, potassium, sodium, vitamin C, magnesium |
N/A | Working in OR, stress level, BMI, prior bowel resection, DM, family history of urolithiasis |
| Quality assessment (Newcastle- Ottawa scale)[21] |
Selection: 3 stars Comparability: 1 star Outcome:3 stars |
Selection: 3 stars Comparability: 2 stars Outcome: 3 stars |
Selection: 2 stars Comparability: 2 stars Outcome: 3 stars |
Selection: 2 stars Comparability: 2 stars Outcome: 3 stars |
Selection: 2 stars Comparability: 2 stars Outcome: 3 stars |
Selection: 4 stars Comparability: 1 star Outcome: 3 stars |
Selection: 4 stars Comparability: 2 stars Outcome: 3 stars |
BMI, body mass index; DM, diabetes mellitus; OR, operating room; SD, standard deviation; SEM, standard error of mean
Definition of High Water/Fluid Intake
Of the 9 studies, only one by Borghi and colleagues [12] specifically defined high fluid intake as water consumption. The other 8 studies [10,11,13–17] specified only high fluid intake, but not the type. Out of the 9 studies, four [11,12,15,16] used 24-hour urine volume as surrogate for daily water/fluid intake as described in Tables 1 and 2.
The Risk of Kidney Stones in Individuals with High Fluid Intake
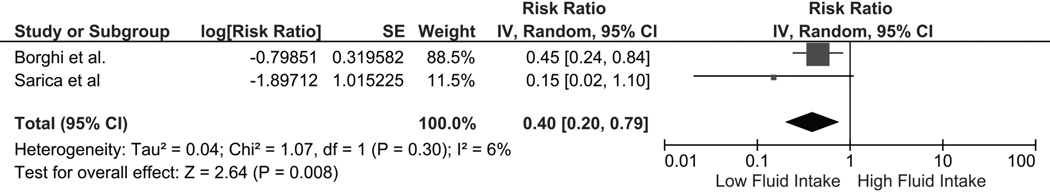
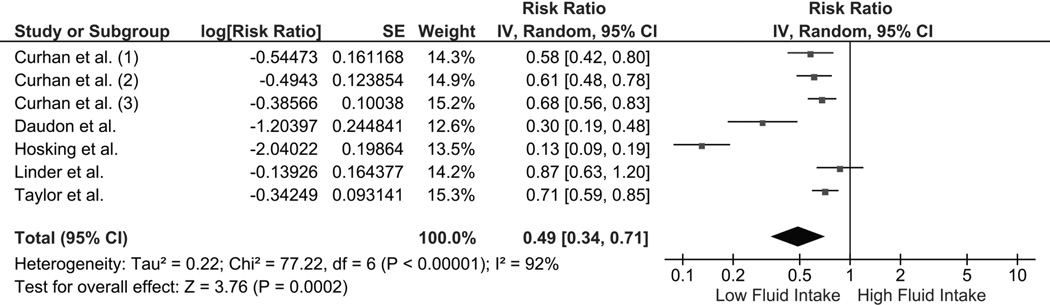
The pooled risk ratio (RR) of kidney stones in individuals with high fluid intake was 0.40 (95% CI 0.20–0.79) in a meta-analysis of the 2 qualifying RCTs (fig. 2). There was no significant statistical heterogeneity (I2 of 6%). When a meta-analysis for observational studies was performed, high fluid intake was also associated with decreased risk of kidney stones (pooled RR 0.49 [95% CI 0.34–0.71]; fig. 3). Statistical heterogeneity was significant for the meta-analysis of observational studies, with an I2 of 92%. A subgroup analysis found that high fluid intake was associated with decreased kidney stone risk in both men (RR 0.67 [95% CI 0.58–0.79]; I2 = 15%) and women (RR 0.65 [95% CI 0.56–0.76]; I2 = 0%).
Figure 2.
Forest plot of included randomized controlled trials comparing risk of kidney stones in individuals with vs without high fluid intake; square data markers, RRs; horizontal lines, 95% CIs, with marker size reflecting statistical weight of study using random-effects meta-analysis. Diamond data markers, overall RRs and 95% CIs for outcomes of interest. IV, inverse variance; SE, standard error.
Figure 3.
Forest plot of included observational studies comparing risk of kidney stones in individuals with vs without high fluid intake; square data markers, RRs; horizontal lines, 95% CIs, with marker size reflecting statistical weight of study using random-effects meta-analysis. Diamond data markers, overall RRs and 95% CIs for outcomes of interest.
Sensitivity Analysis
The benefit of high fluid intake for prevention of kidney stones remained significant in a sensitivity analysis that included only those studies that adjusted for potential confounders [10,13,14,17,25] with a pooled RR of 0.68 (95% CI 0.61–0.76). No significant statistical heterogeneity was apparent between all qualified studies, with an I2 of 5% (Supplementary Fig. 1). A sensitivity meta-analysis was also performed excluding 2 studies by Curhan et al [13] [14] since participants in these 2 studies were likely duplicated in 2 other studies by Curhan et al [25] and Taylor et al [17] that were also included yielding a RR 0.59 [95% CI 0.53–0.66]; I2 = 95%. Another sensitivity meta-analysis was also performed excluding the observational study by Linder et al [10] since it was the only study with a cross-sectional design. The result also remained significant suggesting a benefit of fluid intake (RR 0.57 [95% CI 0.51–0.63]; I2 = 93%).
Another sensitivity meta-analysis was performed for observational studies using high fluid intake (as opposed to strictly water), revealing a pooled RR for kidney stones of 0.49 ([95% CI 0.34–0.71]; I2 = 92%) in the high fluid group. The pooled RR of kidney stones among individuals with high fluid intake remained significant after excluding 2 studies by Curhan et al [13,14] that potentially duplicated participants in 2 other studies by Curhan et al [25] and Taylor et al.[17] remained low at 0.59 ([95% CI 0.53–0.66]; I2 = 95%). A sensitivity meta-analysis for observational studies excluding those 2 [15,16] that used urine volume as a surrogate for high fluid intake also revealed a low RR for stones in the high fluid group of 0.68 ([95% CI 0.61–0.76]). There was no significant heterogeneity with an I2 of 5%. Since only 2 RCT studies were available, on that used fluid and the other water, no further sensitivity analyses were performed to try to separate out the effects of water verus fluid in this group.
The Effect of High Fluid Intake in Individuals with Recurrent Kidney Stones
Two RCTs with 269 patients and 2 observational cohort studies with 289 individuals were included in the data analysis for the risk of kidney stones with high fluid consumption [10,11,14,15]. In a meta-analysis of RCTs, high fluid intake was significantly associated with reduced recurrent kidney stone risk (RR 0.40 [95% CI 0.20–0.79]; I2 = 6%; supplementary fig. 2). In a meta-analysis of observational studies, high fluid intake was also associated with decreased risk of recurrent kidney stones (RR 0.20 [95% CI 0.09–0.44,]; I2 = 86%; supplementary fig. 3).
Evaluation for Publication Bias
Overall, assessment of publication bias was limited due to the small number of included studies. Funnel plots to evaluate publication bias of the RCTs and observational studies are summarized in supplementary figs. 4–7. The plots were slightly suggestive of a publication bias in favor of positive studies regarding the reduced risk of kidney stones and recurrent kidney stones.
Adherence and Withdrawal in Individuals with High Fluid Intake
Two studies (RCTs) provided data on withdrawals with high fluid intake [11,12]. One RCT reported similar dropout rates (9.5%) in participants on high fluid intake vs no treatment [11]. The other RCT reported no withdrawals due to adverse events with high water intake [12].
DISCUSSION
This meta-analysis revealed a significant association between high fluid intake and a lower risk of incident kidney stones, with 0.40-fold (RCTs) and 0.59-fold (observational studies) decreased risk. High fluid intake provided the same benefit in men and women. In addition, high fluid intake reduced the risk of recurrent kidney stones (RR 0.40). Overall withdrawal rates of individuals on high fluid intake therapy due to adverse events appeared to be low and not different from rates in controls not on fluid therapy.
Increasing the amount of daily water consumption should protect against stone formation by increasing urine flow rate and volume, which in turn decreases urinary solute concentration and supersaturation [7]. However, although individual studies support the benefit of increased fluid consumption to prevent kidney stones, the magnitude of the effect and amount of water necessary is not readily apparent due to the heterogeneity of the available data. Importantly, this meta-analysis confirmed the effectiveness of fluid therapy for primary and recurrent stone prevention. Although the type of fluid intake in most studies was not clearly defined [10,11,13–17], both increased water intake [26] and increased general fluid intake [27] resulted in higher urine output. A number of studies [11,12,15] in this meta-analysis demonstrated that high fluid consumption to achieve a minimum urine volume of 2.0–2.5 L/d (as recommended by AUA and ACP guidelines [5,6]) significantly reduced the risk of incident and recurrent kidney stones. Since the difference between 24-hour urine volume and actual fluid intake is approximately 0.9 L [28], the available data analyzed here support that the minimal amount of fluid intake needed per day for stone prevention is about 2.9 L or more, depending on daily fluid loss.
A few studies have suggested that even small increases in fluid intake can decrease the risk of kidney stones [16,18]. Strauss et al [18] conducted a prospective cohort study that demonstrated even a small increase in urine volume of 320 mL/d significantly reduced recurrent kidney stone formation. Moreover, a study by Hosking et al [16] also demonstrated that those who did not recur increased urine volume on average ~ 0.5L to a mean of 2.1L, while those who did recur maintained a steady urine volume of ~ 1.7L. These findings support the concept of individualized methods to monitor and increase water intake to prevent kidney stone recurrence. Recently, a study by Sawyer et al [29] demonstrated a strong relationship between body weight and urinary calcium excretion in stone formers. An individualized goal urine output was suggested using statistical modelling in order to achieve a minimum urinary calcium concentration. This individualized approach towards the amount of water intake needed to reduce stone risk makes intuitive sense. However, while awaiting its validation in future clinical studies, however, current evidence clearly supports increasing fluid intake in order to achieve a minimum urine volume of 2.0–2.5 L/d to prevent kidney stone recurrence.
Although fluid drinking seems to be safe, compulsive water drinking of 10–15 L/d in psychogenic polydipsia can result in hyponatremia [30]. Moreover, symptomatic hyponatremia can also be caused by an acute 3–4 L of water load [31]. The present systematic review found that there are limited data on adherence and safety of high fluid intake. Two RCTs suggested no difference in complications or withdrawals in patients on high vs routine fluid intake. In addition, data on the safety of high water intake for kidney stone prevention in individuals with volume-sensitive conditions such as heart failure, moderate–severe chronic kidney disease and cirrhosis are lacking. Future studies assessing the safety of high fluid intake in these populations to prevent kidney stones are warranted.
There are several limitations of the present analysis. First, the RCTs included in this meta-analysis were graded as low-quality evidence by the Jadad quality assessment scale [10,11,19], as confirmed in the recent AUA and ACP guidelines [5,6]. The quality of evidence in most of the observational studies included were, however, moderately high (as evaluated by Newcastle-Ottawa scale assessing the quality of selection, comparability and outcome of included studies) [21]. Moreover, the meta-analyses of RCTs and observational studies showed a quantitatively significant effect of high fluid intake on primary prevention and reduced risk of recurrence (both ~0.5 fold). Second, several studies defined kidney stone events based on self-report, and this can be subject to error [14]. Third, the majority of kidney stones in all studies included were calcium-based; therefore, it is not known if the beneficial effect of high fluid intake can be generalized to other stone types. Fourth, most studies did not provide data on concurrent use of medications to prevent stones in either the high or low fluid intake groups [10,12,14–16]. However, 2 cohort studies [13,17] did adjust for hydrochlorothiazide use in their final analysis (as shown in Table 2). The RCT by Sarica et al.[11] specifically did not use any stone preventing medications in either their high or low fluid intake groups. These 3 studies suggest a benefit for high fluid intake among incident[13,17] and recurrent kidney stone formers prevention[11], independent of medication use. Finally, there was statistical heterogeneity in the meta-analysis of observational studies regarding assessment of the risk of both incident and recurrent kidney stones. The potential sources of this heterogeneity included differences in diagnostic methodology of kidney stones, different means of defining stone occurrence and recurrence among the included studies, definitions of high fluid intake, fluid type, follow-up duration, and confounder-adjusted methods. The meta-analysis of RCTs, however, which minimized confounder effects, showed positive effects of high fluid intake on kidney stone prevention similar to those seen in the meta-analysis of observational studies.
In summary, this meta-analysis clearly demonstrated a statistically and quantitatively significant reduction in the risk of incident and recurrent kidney stones among individuals who increased fluid intake. This positive effect was apparent in both men and women, and in studies that specifically used water and those that used general fluids. Although evidence was largely limited to common calcium stones, these are the majority formed in the population. Thus this study provides strong evidence in support of the common recommendation to increase water intake in order to reduce stone risk.
Supplementary Material
Acknowledgments
Research work for this review was supported by Otsuka America Pharmaceutical, Inc. Editorial assistance was provided by Catherine Fontana and Geoff Marx of BioScience Communications (New York, NY), which was funded by Otsuka. The investigators also acknowledge support from the Rare Kidney Stone Consortium (U54KD083908), a member of the NIH Rare Diseases Clinical Research Network (RDCRN), funded by the NIDDK and the National Center for Advancing Translational Sciences (NCATS), and the Mayo Clinic O’Brien Urology Research Center (U54 DK100227).
ABBREVIATIONS AND ACRONYMS
- AUA
American Urological Association
- ACP
American College of Physicians
- CI
confidence interval
- RCT
randomized clinical trial
- RR
risk ratio
Footnotes
Nothing to disclose.
Employed by Otsuka America Pharmaceutical, Inc.
Supported by the Mayo Clinic O’Brien Urology Research Center and Rare Kidney Stone Consortium, member of the National Institutes of Health Rare Diseases Clinical Research Network, and funded by the National Institute of Diabetes and Digestive, Kidney Diseases and National Center for Advancing Translational Sciences, and Otsuka America Pharmaceutical, Inc.
The authors declare no conflicts of interest in the conduct or write up of this project.
REFERENCES
- 1.Goldfarb DS. Increasing prevalence of kidney stones in the united states. Kidney Int. 2003;63:1951–1952. doi: 10.1046/j.1523-1755.2003.00942.x. [DOI] [PubMed] [Google Scholar]
- 2.Long LO, Park S. Update on nephrolithiasis management. Minerva Urol Nefrol. 2007;59:317–325. [PubMed] [Google Scholar]
- 3.Lopez M, Hoppe B. History, epidemiology and regional diversities of urolithiasis. Pediatr Nephrol. 2010;25:49–59. doi: 10.1007/s00467-008-0960-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the united states: 1976–1994. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 5.Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD. Dietary and pharmacologic management to prevent recurrent nephrolithiasis in adults: A clinical practice guideline from the american college of physicians. Ann Intern Med. 2014;161:659–667. doi: 10.7326/M13-2908. [DOI] [PubMed] [Google Scholar]
- 6.Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlaga BR, Monga M, Penniston KL, Preminger GM, Turk TM, White JR. Medical management of kidney stones: Aua guideline. J Urol. 2014;192:316–324. doi: 10.1016/j.juro.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Wang CJ, Grantham JJ, Wetmore JB. The medicinal use of water in renal disease. Kidney Int. 2013;84:45–53. doi: 10.1038/ki.2013.23. [DOI] [PubMed] [Google Scholar]
- 8.Dawson CH, Tomson CR. Kidney stone disease: Pathophysiology, investigation and medical treatment. Clin Med. 2012;12:467–471. doi: 10.7861/clinmedicine.12-5-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao Y, Wei Q. Water for preventing urinary stones. Cochrane Database Syst Rev. 2012;6:CD004292. doi: 10.1002/14651858.CD004292.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Linder BJ, Rangel LJ, Krambeck AE. The effect of work location on urolithiasis in health care professionals. Urolithiasis. 2013;41:327–331. doi: 10.1007/s00240-013-0579-2. [DOI] [PubMed] [Google Scholar]
- 11.Sarica K, Inal Y, Erturhan S, Yagci F. The effect of calcium channel blockers on stone regrowth and recurrence after shock wave lithotripsy. Urol Res. 2006;34:184–189. doi: 10.1007/s00240-006-0040-x. [DOI] [PubMed] [Google Scholar]
- 12.Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: A 5-year randomized prospective study. J Urol. 1996;155:839–843. [PubMed] [Google Scholar]
- 13.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. Family history and risk of kidney stones. J Am Soc Nephrol. 1997;8:1568–1573. doi: 10.1681/ASN.V8101568. [DOI] [PubMed] [Google Scholar]
- 14.Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126:497–504. doi: 10.7326/0003-4819-126-7-199704010-00001. [DOI] [PubMed] [Google Scholar]
- 15.Daudon M, Hennequin C, Boujelben G, Lacour B, Jungers P. Serial crystalluria determination and the risk of recurrence in calcium stone formers. Kidney Int. 2005;67:1934–1943. doi: 10.1111/j.1523-1755.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- 16.Hosking DH, Erickson SB, Van den Berg CJ, Wilson DM, Smith LH. The stone clinic effect in patients with idiopathic calcium urolithiasis. J Urol. 1983;130:1115–1118. doi: 10.1016/s0022-5347(17)51711-5. [DOI] [PubMed] [Google Scholar]
- 17.Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: New insights after 14 years of follow-up. J Am Soc Nephrol. 2004;15:3225–3232. doi: 10.1097/01.ASN.0000146012.44570.20. [DOI] [PubMed] [Google Scholar]
- 18.Strauss AL, Coe FL, Deutsch L, Parks JH. Factors that predict relapse of calcium nephrolithiasis during treatment: A prospective study. Am J Med. 1982;72:17–24. doi: 10.1016/0002-9343(82)90566-6. [DOI] [PubMed] [Google Scholar]
- 19.Embon OM, Rose GA, Rosenbaum T. Chronic dehydration stone disease. Br J Urol. 1990;66:357–362. doi: 10.1111/j.1464-410x.1990.tb14954.x. [DOI] [PubMed] [Google Scholar]
- 20.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 21.Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 25.Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses' health study ii. Arch Intern Med. 2004;164:885–891. doi: 10.1001/archinte.164.8.885. [DOI] [PubMed] [Google Scholar]
- 26.Buckley CM, Hawthorne A, Colyer A, Stevenson AE. Effect of dietary water intake on urinary output, specific gravity and relative supersaturation for calcium oxalate and struvite in the cat. Br J Nutr. 2011;106(Suppl 1):S128–S130. doi: 10.1017/S0007114511001875. [DOI] [PubMed] [Google Scholar]
- 27.Chung BD, Parekh U, Sellin JH. Effect of increased fluid intake on stool output in normal healthy volunteers. J Clin Gastroenterol. 1999;28:29–32. doi: 10.1097/00004836-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Xu H, Zisman AL, Coe FL, Worcester EM. Kidney stones: An update on current pharmacological management and future directions. Expert Opin Pharmacother. 2013;14:435–447. doi: 10.1517/14656566.2013.775250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawyer MD, Anderson CB, Viprakasit DP, Dietrich MS, Herrell SD, Miller NL. An individualized weight-based goal urine volume model significantly improves expected calcium concentrations relative to a 2-l goal urine volume. Urolithiasis. 2013;41:403–409. doi: 10.1007/s00240-013-0573-8. [DOI] [PubMed] [Google Scholar]
- 30.Hariprasad MK, Eisinger RP, Nadler IM, Padmanabhan CS, Nidus BD. Hyponatremia in psychogenic polydipsia. Arch Intern Med. 1980;140:1639–1642. [PubMed] [Google Scholar]
- 31.Kawai N, Baba A, Suzuki T, Shiraishi H. Roles of arginine vasopressin and atrial natriuretic peptide in polydipsia-hyponatremia of schizophrenic patients. Psychiatry Res. 2001;101:39–45. doi: 10.1016/s0165-1781(00)00243-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.