Abstract
Binge eating disorder is characterized by excessive, uncontrollable consumption of palatable food within brief periods of time. Excessive intake of palatable food is thought to be driven by hedonic, rather than energy homeostatic mechanisms. However, reward processing does not only comprise consummatory actions; a key component is represented by the anticipatory phase directed at procuring the reward. This phase is highly influenced by environmental food-associated stimuli which can robustly enhance the desire to eat even in the absence of physiological needs. The opioid system (endogenous peptides and their receptors) has been strongly linked to the rewarding aspects of palatable food intake, and perhaps represents the key system involved in hedonic overeating. Here we review evidence suggesting that the opioid system can also be regarded as one of the systems regulating the anticipatory incentive processes preceding binge eating hedonic episodes.
Introduction
Binge eating disorder (BED) is characterized by recurrent-persistent episodes of excessive and uncontrollable food consumption within a short period of time. Although individuals with BED generally have a higher than average body mass index (BMI), weight and BMI are not diagnostic criteria for BED. The aberrant eating behaviour behind this disorder is characterized by a subjective sense of loss of control, distress, uncomfortable fullness and intense feelings of disgust and embarrassment1, without the inappropriate compensatory behaviours of bulimia nervosa2. Episodes of binge eating, associated with at least three specific features (e.g., eating more rapidly than normal, eating until uncomfortably full, eating a large amount when not hungry, eating alone because of embarrassment, feeling disgusted, depressed, or guilty about overeating), occur both in BED and in bulimia nervosa with an average frequency of at least once a week, over three months2.
The lifetime prevalence of frequent binge eating in the United States is about 1.5% with a median age of onset of about 12.5 years3,4. About 35% of those who regularly binge are overweight or obese5. Additionally, individuals reporting to engage in binge eating behaviours have been shown to regain weight at a faster rate than those who do not6. Interestingly, among those who binge approximately 76% of adults and 85% of adolescents experience psychiatric co-morbidities such as anxiety, mood, and substance use disorders3, as well as other disorders such as obesity, diabetes, and cardiovascular diseases7. Based on the growing evidence of high prevalence and clinical significance8,9, the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) has now designated BED as a psychiatric illness distinct from other eating disorders with a specific formal diagnosis.2 The only pharmacological treatment for BED, lisdexamfetamine dimesylate, has just recently been approved by the Food and Drug Administration (FDA)10. On the other hand, medications that have been reported to reduce binge eating in clinical studies, e.g. topiramate11,12, are associated with a variety of adverse side effects, which may result in discontinuation of therapy13.
Unlike individuals with bulimia nervosa, individuals with BED typically do not show marked or sustained dietary restriction designed to influence body weight and shape between binge-eating episodes. They may, however, report frequent attempts at dieting. Therefore, a widely accepted hypothesis about the etiology of binge eating is based on the sequential access to foods with different hedonic value, as occurs in restraint restrained eaters14,15. Highly palatable foods are typically rich in sucrose and fat and they are commonly perceived as ‘forbidden’ between binges because they are calorie-dense16,17. Therefore, selection of low energy- dense foods in chronic dieters18–20 may instead increase craving for more appetitive palatable foods, and makes individuals more vulnerable to behavioural excess and overeating21. Highly palatable foods are more likely to be consumed for reasons beyond hunger, and their high palatability makes it more difficult to limit and to control their intake22,23.
The opioid system (endogenous peptides and their receptors) has been strongly linked to rewarding impact of palatable food intake, and it represents one of the key systems that regulates hedonic overeating 24. Opioid receptor agonist administration increases food intake while opioid receptor antagonists decrease it25. Among the different opioid receptor subtypes, μ-opioid receptors have been strongly involved in the modulation of hedonic feeding in general and more specifically in pathological overeating observed in BED24. Motivated behaviours are not only characterized by hedonic mechanisms leading to consummatory episodes. Anticipatory incentive processes by which an individual comes to expect contact with palatable food, e.g. the exposure to cues associated with that specific food, also play a fundamental role. A cue may be successfully resisted many times, but on some occasions it may trigger irresistible temptation26,27. Clinical data have suggested that some individuals may attribute a higher motivational value to food-cues compared to others and, therefore, these may be more likely to overeat 28,29. Indeed, food craving has been reported to be a major precipitant of binge episodes30,31.
While emphasizing the results obtained in animal models of binge eating, the present review will focus on the role of the opioid system both in the hedonic component of consummatory behaviour and more specifically in anticipatory incentive processes preceding binge-eating hedonic episodes.
Hedonically-driven eating behaviour
Because consumption of large volumes of palatable food in brief periods of time is the key diagnostic criterion for BED, animal models of this disorder have been developed to mimic this essential maladaptive behavioural outcome. Table 1 summarizes the most predictive binge eating preclinical models, divided into three major classes, as a function of the procedures used to induce binge eating in animals: i) binge eating induced by cycles of food deprivation and renewed access to a sucrose solution; ii) binge eating induced by cycles of stress and food restriction/refeeding; iii) binge eating induced by limiting access to a highly palatable diet.
Table 1.
Summary of the most predictive binge eating preclinical models, divided into three major classes, as a function of the procedures used to induce binge eating in the animal.
| Features | Binge-eating episodes | |
|---|---|---|
| History of dieting and sucrose exposure model | 12-h food deprivation is followed by 12-h access to sucrose solution79,80. | After few days, subjects escalate sucrose intake during the first hour of access. |
| History of dieting and stress model | Repetition of cycles of food-restriction and refeeding. At the end of each cycle, subjects are exposed to a stressor81–86. | After three restriction/refeeding stress cycles, stressed subjects escalate their palatable food intake. |
| The limited access model | Subjects are never food deprived. They are given sporadic, time limited access to either vegetable fat87,88 or sucrose diet17,19,37,89–91. | Subjects with sporadic access to palatable food escalate their intake. |
Abundant evidence implicates the brain opioid systems in the regulation of food intake and the rewarding impact of palatable food intake24. Hedonic pleasure reactions have been operationalized in animals through ‘liking’ reactions to sweetness, which are affective orofacial expressions (tongue and lateral tongue protrusions) that are homologous in human infants, monkeys, horses and rats32. The neural mechanisms associated with hedonic responses to palatable foods have been investigated by using selective μ-opioid receptor ligands to identify ‘hotspots’ in the basal ganglia, especially in the ventral pallidum (VP) and the nucleus accumbens (NAc)33,34. It has been shown that the ability of μ-opioid receptor agonists to increase food intake is restricted to a specific portion of the NAc shell (the rostrodorsal quadrant of NAc medial shell), which shows high μ-opioid receptor density33. Recently, it has been shown that within the same μ-opioid receptor hotspot stimulation of δ-opioid receptor a subtype of opioid receptor having a prominent role in emotional processing, can also amplify hedonic reactions to sweetness35.
The NAc sends projections to the VP, which also projects back to the NAc, and each structure is embedded in complex mesocortico-limbic circuits involving the lateral hypothalamus, the ventral tegmental area, the prefrontal cortex and the amygdala. Each of these areas is fundamental in reward and motivational processes. Similar to the NAc, the VP has been shown to contain a hedonic hotspot, specifically in the posterior portion34.
Based on these mechanisms, μ-opioid receptor agonists have been demonstrated to increase the intake of palatable food with a high sugar or fat content and to increase the consumption of more preferred food when presented at the same time with less preferred food36. μ-opioid receptor antagonists, on the other hand, reduce binge episodes for highly palatable food17,19,37–39, confirming the critical involvement of this system in the hedonic and consummatory aspects of ingestive behaviour. For example, the preferential μ/k opioid-receptor antagonist nalmefene, an effective and approved treatment for heavy alcohol drinking40, has been successfully tested in a binge-eating paradigm17. In this study, adolescent female Wistar rats were food deprived for 2 h a day and then offered 10-min access to a feeder containing chow followed sequentially by 10-min access to a highly preferred, but macronutrient-comparable, sucrose-rich diet. Those exposed to chow and high sucrose diet developed experience-dependent binge-like hyperphagia of the diet as well as anticipatory hypophagia of the less preferred alternative. ‘Binges’ were reduced dose-dependently by systemically injected nalmefene17, supporting the hypothesis that the endogenous opioid system promotes hedonic intake. Using the binge-eating procedure described above, the behavioural effects of a novel, selective μ-opioid receptor antagonist GSK1521498, currently in clinical development for the treatment of compulsive eating disorders and obesity, was tested in comparison with naltrexone (NTX), a preferential μ-opioid receptor antagonist clinically approved for alcoholism37. Both GSK1521498 and NTX reduced binge-like palatable food hyperphagia and food intake after instrumentally working to obtain it, confirming the key role that the opioid system plays in hedonic eating behaviour. The same compound, GSK1521498, was tested for 4 weeks in binge-eating obese subjects and resulted in reduced hedonic preference, specifically for higher concentrations of sugar and fat, and markedly reduced calorie intake in an ad libitum buffet, particularly for more palatable foods39.
Incentive salience in eating behaviour
Reward processing comprises two dissociable components: an anticipatory (or appetitive) phase, which is directed at procuring the reward, and a consummatory phase. The anticipatory phase can be associated with stimuli (contextual, visual, auditory or food-associated) or the food per se. These stimuli can have a great impact on the eating behaviour, as they can increase it. It has been shown, for example, that learned contextual cues potentiate eating in rats41.
Berridge's group has also demonstrated that hedonic reactions to palatable food can be dissociated from the motivation process regulating its intake (‘wanting’), showing that food intake can be stimulated without enhancing ‘liking’ reactions42.
In animal studies, food-associated cues have been operationalized and thoroughly studied in the context of both Pavlovian and instrumental conditioning43. After repeated pairings of a cue, such as the presentation of a stimulus light (conditioned stimulus, CS) with the delivery of palatable food (unconditioned stimulus, US), the learned cue itself becomes salient, triggers intense urges to obtain the associated reward, and also acts as a conditioned reinforcer able to maintain instrumental seeking even in absence of food presentation. Importantly, cues paired with the delivery of palatable food become capable of promoting consumption even when the animals are not deprived (CS-potentiated feeding)44. Even in fully sated rats, CSs strongly promote feeding compared to neutral stimuli45,46. Both associative learning and prediction contribute to motivation for rewards. The conditioned stimuli gain salience and elicit incentive motivation even in absence of physiological needs.
Evidence from the influence of Pavlovian stimuli on instrumental behaviour is provided by experiments based on the specific Pavlovian-instrumental transfer (PIT) effect where appetitive CSs (associated with positive reinforcers such as food) can greatly enhance instrumental responding for the same reinforcer when presented unexpectedly (independent of the instrumental response). Training consists of three phases: in the first, a Pavlovian association is acquired between a cue and a reinforcer; in the second, an instrumental response is trained for the same reinforcer (without any cue); in the third, the cue is presented during the performance of instrumental behaviour in extinction (without any reinforcer). Such a procedure has shown that appetitive Pavlovian stimuli can greatly enhance instrumental responding for the same reinforcer (specific PIT effect). PIT has been interpreted as evidence that CSs can exert a motivational influence over instrumental performance47 (Figure 1A).
Figure 1.
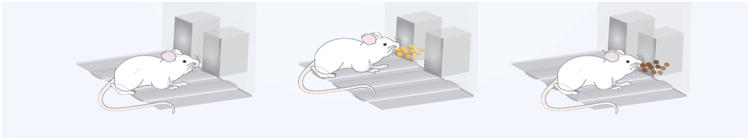
Incentive salience in eating behaviour has been operationalized in the context of both Pavlovian and instrumental conditioning through the use of:
A) Specific Pavlovian-Instrumental Transfer, which consists of three phases: 1, a Pavlovian association between cue-food; 2, an association between instrumental response-food; 3, the instrumental response in the presence of the cue.
B) Second-Order Schedule of Reinforcement, which consists of two temporarily distinct phases: 1, during the first interval, each 10th active lever press (ALP) is associated with a brief 1-sec CS presentation; 2, during the interval(s) following the first one, the 10th active lever press is associated with a 20-sec CS and food delivery.
C) Anticipatory Negative Contrast, which develops in a procedure consisting of: 1, two-hour food deprivation; 2, 10-min brief access to a standard chow diet; 3, 10-min brief access to a highly palatable food.
An additional useful procedure for measuring the impact a CS may have over instrumental performance is the second-order schedule of reinforcement, where a stimulus that acquires its reinforcing properties by being paired with other, generally primary, reinforcers such as food or drugs, can act as a conditioned reinforcer48 to enhance and maintain high levels of instrumental responses over protracted periods of time even in the absence of the primary reinforcer. This procedure, used previously to study the seeking of a sexual reward as well as cocaine, heroin, and alcohol seeking, has been adapted in order to measure, in terms of instrumental responses, the motivation for the opportunity to binge on a palatable food, as well as the impact that ingestion of that food has on subsequent food seeking49. The procedure has temporarily distinct intervals that enable the separate assessment of motivational influence of the food-related CS over instrumental response before and after food ingestion, respectively. This allows the dissociation between pharmacological effects on response to food cues (during the interval before food ingestion) and effects on hedonic impact of the food reward (during the interval(s) after food ingestion)37,50 (Figure 1B).
A different way to study anticipatory incentive processes preceding consummatory behaviours mediated by hedonic mechanisms consists of analysing the phenomenon known as ‘anticipatory negative contrast’51. Evidence suggests that binge hyperphagia of ‘forbidden’ foods is linked with the refusal of otherwise acceptable alternatives in humans52. The phenomenon has been operationalized in pre-clinical models by exposing the animals to less preferred, but perhaps healthier, foods followed by highly palatable foods for restricted periods of time17,37,53: as described above, 10-min brief access to a standard chow diet, followed by a 10-min access to a highly palatable food17,37, promotes binge eating of the highly palatable food, and self-restriction of the otherwise acceptable chow diet (Figure 1C).
The role of the opioid system in incentive motivation for palatable food
The mesolimbic dopamine system has long been implicated in the motivational aspects of feeding behaviour. Exposure to either drugs or palatable food as well as to food-associated stimuli promotes dopamine release in the striatum. Dopamine has been demonstrated to be fundamental in the stimulus–reward learning that is specifically associated with the attribution of incentive salience to reward cues54. It is widely accepted, although quite simplistic, that motivated behaviours for food are dopamine-mediated and that hedonic reactions to food are opioid-mediated. Indeed, dopaminergic manipulations within or outside the NAc shell hotspot consistently fail to enhance positive hedonic reactions to sweet tastes55,56 but potently alter motivated ‘wanting’ for the food rewards42.
Although there is much less consensus, it has been proposed that opioid mechanisms can also regulate incentive motivational processes that underlie the propensity to seek palatable foods.
Striatum
It has been shown that opioid receptor agonists in the NAc increase motivation for food57. Recently, selective stimulation of the three major subtypes of opioid receptors via agonist microinjections [μ (DAMGO), δ (DPDPE), or κ (U50488H)] in the NAc shell hotspot has been employed, to construct anatomical maps for functional localization of consequent changes in hedonic ‘liking’ (assessed by affective orofacial reactions to sucrose taste) versus ‘wanting’ (assessed by changes in food intake). In line with results from other groups that demonstrated that the NAc shell contributes not only to the hedonic impact of sensory pleasure, but also to the incentive motivation to consume foods57, δ- and μ-opioid receptor stimulation enhanced the ‘wanting’ to eat more food. The real distinction between ‘wanting’ and ‘liking’ emerged from the effects of μ- and κ-opioid receptor stimulation: although they both increased the ‘liking’, only the μ-opioid receptor stimulation increased the incentive motivation for food.
In contrast, opioid receptor antagonists have been shown to reduce the anticipatory incentive processes preceding the consummatory episodes of highly palatable food(see Table 2 for a summary). The non-selective opioid receptor antagonist, nalmefene, for example, blocked the anticipatory negative contrast in the binge-eating procedure as well as highly palatable food binge eating17. Additionally, the μ-opioid receptor antagonist GSK1521498 exerted a more specific effect on the impact of the hedonic value of the food and intake than did NTX, reducing the anticipatory chow hypophagia, before the highly palatable food was available for ingestion. Although the paradigm used did not include any discrete stimulus (i.e. a light), several cues might have served as conditioned stimuli predictive of imminent preferred food availability, including the test environment, the deprivation period, or even the preceding first feeder (chow) presentation.
Table 2.
Summary of the drugs targeting the opioid system tested on binge-eating paradigms, their effects, and the relative references.
| Drugs | Effects | Reference |
|---|---|---|
| Nalmefene | ↓binge-like eating; ↑negative contrast | Cottone et al.17 |
| GSK1521498 | ↓binge-like eating; ↑negative contrast; ↓CS-controlled food seeking; ↓attentional bias for food-related stimuli | Giuliano et al.37; Ziauddeen et al.39 |
| Naltrexone | ↓binge-like eating; ↑negative contrast | Giuliano et al.37; Ziauddeen et al.39; Katsuura et al.53 |
| DAMGO | ↑ liking; ↑ wanting | Peciña et al.26; Zhang at al.57; Mahler et al.65,67; DiFeliceantonio et al.66 |
| DPDPE | ↑ liking; ↑ wanting | Zhang at al.57 |
| U50488H | ↑liking | Zhang at al.57 |
| Naltrindole | ↑liking | Katsuura et al.53 |
| β- Funaltrexamine | ↓binge-like eating; ↑negative contrast | Katsuura et al.53 |
A different anticipatory contrast paradigm has been developed by Katsuura and Taha53, in which separate groups of rats were presented sequentially with 4% sucrose and then either 20% or 0% sucrose (Group 1: 4-20%, Group 2: 4-0%). Similar to the paradigm described above17,37, daily training in this paradigm produced robust intake of 20% sucrose (binge) preceded by learned hypophagia during access to 4% sucrose (anticipatory negative contrast). The authors then tested the effects of NTX, naltrindole (a δ-opioid receptor antagonist) and β-funaltrexamine (a μ-opioid receptor antagonist) in the NAc shell on sucrose consumption. NTX and β-funaltrexamine infused into the NAc shell significantly reduced sucrose intake in both groups, but the suppressive effects were strongly selective and dependent upon the relative value of sucrose solutions within each group. Thus, they reduced sucrose consumption in the 4-0% group, but they decreased the 20%, and not the 4%, sucrose solution consumption in the 4-20% group. Although the authors interpreted the results as a demonstration that endogenous opioid signaling promoted consumption of the preferred food, since the μ-opioid receptor antagonists tested blocked the learned hypophagia (anticipatory negative contrast) and reduced the sucrose hyperphagia (binge); therefore, it would have been interesting to test the compounds on a group of animals exposed to 4% sucrose during both phases of the session, rather than 4-20%. In that case, a specific effect of the μ-opioid receptor antagonists on the anticipatory negative contrast could have resulted in an increase of 4% sucrose solution intake in the first phase associated with a decrease of the 20% sucrose solution intake in the second phase.
External food-related cues precipitate a desire for food items, resulting in food craving independently of energy-homeostatic needs. In this context, opioid receptor antagonists have been tested in animal models aiming at investigating the role of a conditioned stimulus on instrumental response. In a study comparing dopamine and μ-opioid receptor stimulation in enhancing cue-triggered motivation for reward in PIT26, it was shown that opioid stimulation caused increased cue-triggered ‘wanting’ as well as ‘linking’ at nearly all NAc sites (see Table 2 for a summary).. Thus, μ-opioid receptor stimulation has been shown possibly to have effects functionally identical to dopamine stimulation: they both elevated ‘wanting’. Additionally, the μ-opioid receptor antagonist GSK1521498 has been tested in comparison with NTX on food seeking under second-order schedule of chocolate-flavoured pellet reinforcement, in which a CS associated with chocolate ingestion supports high levels of instrumental-seeking behaviour over delays to the delivery of a large chocolate reward37. Although both compounds reduced food intake, only GSK1521498 reduced the seeking responses for chocolate before its delivery for ingestion, suggesting the additional effect the opioid system has on incentive motivational mechanisms controlling food seeking. The higher potency of GSK1521498 compared with NTX has been hypothesized to be due to its increased selectivity at μ-opioid receptors and/or its specific action on appetitive processes underlying food selection. Several putative neural sites at which μ-opioid receptor antagonism may cause decreases in the propensity to seek food have been hypothesized. It has been shown that the dopaminergic transmission in the NAc has a major role in incentive motivational processing for food34. Therefore, μ-opioid receptors localized on the GABAergic interneurons in the VTA may provide one site at which GSK1521498 might act to decrease dopamine release in the NAc to reduce food seeking and incentive motivation for food.
Additional studies confirmed the role of the striatum and the basal ganglia, specifically putamen and pallidum which are brain regions involved in the motivational mechanisms underlying eating behaviour, in cue-induced responses for highly palatable food38. For example, a 28 day treatment with GSK1521498 in obese individuals with moderate binge eating and was associated with reduction in pallidum/putamen responses to pictures of high-calorie food and a reduction in motivation (measured as grip force) to view images of high-calorie food, confirming its potential as a treatment aiming at reducing compulsive food seeking behaviour.
Amygdala
The amygdala is the brain area hypothesized to encode the association of initially motivationally neutral environmental stimuli with motivationally relevant outcomes in a Pavlovian manner58. This structure is divided into several subnuclei, including the central (CeA) and the basal and the lateral nuclei often group as the basolateral amygdala (BLA), and it has connections with both the hypothalamus, striatum and medial and orbital prefrontal cortical areas58. The CeA has been hypothesized to mediate more generalized associations based upon the motivational valence of the reinforcer 59,60, whereas the BLA has been shown to be required for selective cuing effects related to the identity of a particular outcome61.
Incentive specific rewards has been shown to increase when the mesocortico-limbic brain systems are activated62. Although Petrovich and colleagues reported that enhancement of eating by an appetitive CS is dependent on the integrity of the BLA, but not CeA63,64, specific stimulation of –the μ-opioid receptor circuit in the CeA has been shown to produce elevation of incentive salience in rats. Specifically, μ-opioid receptor stimulation (using DAMGO infusions) in the CeA caused elevated incentive motivation in subjects naturally attracted by a predictive cue (sign-trackers) and in those naturally attracted by a reward contiguous goal cue (goal-trackers)65,66 but also elevation in ‘wanting’ under PIT67. These findings may have strong clinical implications in compulsive pursuit disorders involving intense motivations for a specific target, which is the case in binge eaters that want food and perhaps a particular food.
Cortex
It has been shown that the medial prefrontal cortex (mPFC), projecting to the amygdala and the lateral hypothalamus, is activated selectively by a cue that stimulates eating behaviour in sated rats68. Moreover, it has been shown that the mPFC mediates enhanced food consumption driven by contextual conditioned cues69.
μ-Opioid receptors within the mPFC have been shown to mediate an important function in overeating70. Naltrexone microinfused into the mPFC selectively reduced the consumption and the motivation to obtain highly palatable food, but not standard chow19.
In humans, the μ-opioid receptor antagonist GSK1521498 was tested for 4 weeks in obese adults with moderate to severe binge eating and resulted in a significant reduction in attentional bias for food-related stimuli, quantified using objective indices of cognitive prioritisation of food (e.g. the visual dot probe task) and shown to be associated with the activation of the lateral PFC71, supporting the central role of μ-opioid receptors in reward-related cognitive functions72. Additionally, a functional magnetic resonance imaging study showed that naltrexone decreased the response in the anterior and dorsal anterior cingulate cortex, an area involved in the processing of rewarding stimuli including food, to the rewarding sight and taste of chocolate73.
Conclusions
Recent evidence suggests that loss of control over food intake is the primary indicator of BED severity. Deficits in cognitive74 function and inhibitory control75 are considered a possible risk and maintenance factor for binge eating, and so are the hypersensitivity to motivational stimuli with high incentive salience producing a bias in attentional processing toward drug-related cues76. Obese versus lean individuals report greater sensitivity to reward77 and elevated responses to food cues in regions of the brain that encode the sensory properties of food. The opioid system has been extensively demonstrated to be involved in the hedonic and consummatory aspects of ingestive behaviour. Here in reviewing the existing literature we discuss the importance of the opioid system in mediating the impact of palatable food-conditioned stimuli on the incentive motivation for food. Drugs inhibiting opioid system activity may have utility in –treatments intended to reduce maladaptive, palatability-driven eating behaviour by reducing the motivational properties of stimuli that elicit the binge eating strongly associated with obesity20,78.
Acknowledgments
CG was funded by Medical Research Council Programme Grant (no. G1002231) and PC was funded by the National Institute on Drug Abuse (NIDA/NIH, no. DA030425) and the National Institute of Mental Health (NIMH/NIH, no. MH091945). The Authors would like to thank Prof. Barry Everitt for constructive and helpful comments on the manuscript and Dr. David Belin for the generous contribution of generating the illustration.
References
- 1.Stein RI, Kenardy J, Wiseman CV, Dounchis JZ, Arnow BA, Wilfley DE. What's driving the binge in binge eating disorder?: A prospective examination of precursors and consequences. Int J Eat Disord. 2007;40(3):195–203. doi: 10.1002/eat.20352. [DOI] [PubMed] [Google Scholar]
- 2.Association AP. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) American Psychiatric Pub; 2013. [Google Scholar]
- 3.Hudson JI, Hiripi E, Pope HG, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry. 2011;68(7):714–723. doi: 10.1001/archgenpsychiatry.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devlin MJ. Is there a place for obesity in DSM-V? Int J Eat Disord. 2007;40(Suppl):S83–S88. doi: 10.1002/eat.20430. [DOI] [PubMed] [Google Scholar]
- 6.Pacanowski CR, Senso MM, Oriogun K, Crain AL, Sherwood NE. Binge Eating Behavior and Weight Loss Maintenance over a 2-Year Period. J Obes. 2014;2014:e249315. doi: 10.1155/2014/249315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.TODAY Study Group. Wilfley D, Berkowitz R, et al. Binge eating, mood, and quality of life in youth with type 2 diabetes: baseline data from the today study. Diabetes Care. 2011;34(4):858–860. doi: 10.2337/dc10-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Striegel-Moore RH, Franko DL. Epidemiology of binge eating disorder. Int J Eat Disord. 2003;34(Suppl):S19–S29. doi: 10.1002/eat.10202. [DOI] [PubMed] [Google Scholar]
- 9.Striegel-Moore RH, Franko DL. Should Binge Eating Disorder Be Included in the DSM-V? A Critical Review of the State of the Evidence. Annu Rev Clin Psychol. 2008;4(1):305–324. doi: 10.1146/annurev.clinpsy.4.022007.141149. [DOI] [PubMed] [Google Scholar]
- 10. [Accessed June 11, 2015];Press Announcements - FDA expands uses of Vyvanse to treat binge-eating disorder. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm432543.htm.
- 11.McElroy SL, Guerdjikova AI, Martens B, Keck PE, Pope HG, Hudson JI. Role of antiepileptic drugs in the management of eating disorders. CNS Drugs. 2009;23(2):139–156. doi: 10.2165/00023210-200923020-00004. [DOI] [PubMed] [Google Scholar]
- 12.McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039–1048. doi: 10.1016/j.biopsych.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Yager J. Binge eating disorder: the search for better treatments. Am J Psychiatry. 2008;165(1):4–6. doi: 10.1176/appi.ajp.2007.07101541. [DOI] [PubMed] [Google Scholar]
- 14.Polivy J, Herman CP. Dieting and binging. A causal analysis. Am Psychol. 1985;40(2):193–201. doi: 10.1037//0003-066x.40.2.193. [DOI] [PubMed] [Google Scholar]
- 15.Mela DJ. Determinants of food choice: relationships with obesity and weight control. Obes Res. 2001;9(Suppl 4):249S–255S. doi: 10.1038/oby.2001.127. [DOI] [PubMed] [Google Scholar]
- 16.Yeomans MR, Blundell JE, Leshem M. Palatability: response to nutritional need or need-free stimulation of appetite? Br J Nutr. 2004;92(Suppl 1):S3–S14. doi: 10.1079/bjn20041134. [DOI] [PubMed] [Google Scholar]
- 17.Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2008;33(3):524–535. doi: 10.1038/sj.npp.1301430. [DOI] [PubMed] [Google Scholar]
- 18.De Zwaan M. Binge eating disorder and obesity. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 2001;25(Suppl 1):S51–S55. doi: 10.1038/sj.ijo.0801699. [DOI] [PubMed] [Google Scholar]
- 19.Blasio A, Steardo L, Sabino V, Cottone P. Opioid system in the medial prefrontal cortex mediates binge-like eating. Addict Biol. 2014;19(4):652–662. doi: 10.1111/adb.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanovski SZ, Leet M, Yanovski JA, et al. Food selection and intake of obese women with binge-eating disorder. Am J Clin Nutr. 1992;56(6):975–980. doi: 10.1093/ajcn/56.6.975. [DOI] [PubMed] [Google Scholar]
- 21.Polivy J. The effects of behavioral inhibition: integrating internal cues, cognition, behavior, and affect. Psychological Inquiry. 1998;(9):181–204. [Google Scholar]
- 22.Boggiano MM, Turan B, Maldonado CR, Oswald KD, Shuman ES. Secretive food concocting in binge eating: test of a famine hypothesis. Int J Eat Disord. 2013;46(3):212–225. doi: 10.1002/eat.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witt AA, Lowe MR. Hedonic hunger and binge eating among women with eating disorders. Int J Eat Disord. 2014;47(3):273–280. doi: 10.1002/eat.22171. [DOI] [PubMed] [Google Scholar]
- 24.Nathan PJ, Bullmore ET. From taste hedonics to motivational drive: central μ-opioid receptors and binge-eating behaviour. Int J Neuropsychopharmacol Off Sci J Coll Int Neuropsychopharmacol CINP. 2009;12(7):995–1008. doi: 10.1017/S146114570900039X. [DOI] [PubMed] [Google Scholar]
- 25.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27(8):765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Peciña S, Berridge KC. Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered “wanting” for reward: entire core and medial shell mapped as substrates for PIT enhancement. Eur J Neurosci. 2013;37(9):1529–1540. doi: 10.1111/ejn.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191(3):599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- 28.Dagher A. The neurobiology of appetite: hunger as addiction. Int J Obes 2005. 2009;33(Suppl 2):S30–S33. doi: 10.1038/ijo.2009.69. [DOI] [PubMed] [Google Scholar]
- 29.Robinson MJF, Burghardt PR, Patterson CM, et al. Individual Differences in Cue-Induced Motivation and Striatal Systems in Rats Susceptible to Diet-Induced Obesity. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2015 Mar; doi: 10.1038/npp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng L, Davis C. Cravings and food consumption in Binge Eating Disorder. Eat Behav. 2013;14(4):472–475. doi: 10.1016/j.eatbeh.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Greeno CG, Wing RR, Shiffman S. Binge antecedents in obese women with and without binge eating disorder. J Consult Clin Psychol. 2000;68(1):95–102. [PubMed] [Google Scholar]
- 32.Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24(2):173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- 33.Peciña S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci Off J Soc Neurosci. 2005;25(50):11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci Off J Soc Neurosci. 2007;27(7):1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castro DC, Berridge KC. Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”. J Neurosci Off J Soc Neurosci. 2014;34(12):4239–4250. doi: 10.1523/JNEUROSCI.4458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woolley JD, Lee BS, Fields HL. Nucleus accumbens opioids regulate flavor-based preferences in food consumption. Neuroscience. 2006;143(1):309–317. doi: 10.1016/j.neuroscience.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 37.Giuliano C, Robbins TW, Nathan PJ, Bullmore ET, Everitt BJ. Inhibition of opioid transmission at the μ-opioid receptor prevents both food seeking and binge-like eating. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2012;37(12):2643–2652. doi: 10.1038/npp.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cambridge VC, Ziauddeen H, Nathan PJ, et al. Neural and behavioral effects of a novel mu opioid receptor antagonist in binge-eating obese people. Biol Psychiatry. 2013;73(9):887–894. doi: 10.1016/j.biopsych.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ziauddeen H, Chamberlain SR, Nathan PJ, et al. Effects of the mu-opioid receptor antagonist GSK1521498 on hedonic and consummatory eating behaviour: a proof of mechanism study in binge-eating obese subjects. Mol Psychiatry. 2013;18(12):1287–1293. doi: 10.1038/mp.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gual A, Bruguera P, López-Pelayo H. Nalmefene and its use in alcohol dependence. Drugs Today Barc Spain 1998. 2014;50(5):347–355. doi: 10.1358/dot.2014.50.5.2132323. [DOI] [PubMed] [Google Scholar]
- 41.Petrovich GD, Ross CA, Gallagher M, Holland PC. Learned contextual cue potentiates eating in rats. Physiol Behav. 2007;90(2-3):362–367. doi: 10.1016/j.physbeh.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castro DC, Berridge KC. Advances in the neurobiological bases for food “liking” versus “wanting”. Physiol Behav. 2014;136:22–30. doi: 10.1016/j.physbeh.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickinson A, Mackintosh NJ. Classical conditioning in animals. Annu Rev Psychol. 1978;29:587–612. doi: 10.1146/annurev.ps.29.020178.003103. [DOI] [PubMed] [Google Scholar]
- 44.Crombag HS, Galarce EM, Holland PC. Pavlovian influences on goal-directed behavior in mice: the role of cue-reinforcer relations. Learn Mem Cold Spring Harb N. 2008;15(5):299–303. doi: 10.1101/lm.762508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weingarten HP. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 1983;220(4595):431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- 46.Petrovich GD, Ross CA, Gallagher M, Holland PC. Learned contextual cue potentiates eating in rats. Physiol Behav. 2007;90(2-3):362–367. doi: 10.1016/j.physbeh.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur J Neurosci. 2001;13(10):1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- 48.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 49.Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology (Berl) 2000;153(1):17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- 50.Smith KL, Rao RR, Velázquez-Sánchez C, et al. The Uncompetitive N-methyl-D-Aspartate Antagonist Memantine Reduces Binge-Like Eating, Food-Seeking Behavior, and Compulsive Eating: Role of the Nucleus Accumbens Shell. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2015;40:1163–1171. doi: 10.1038/npp.2014.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flaherty CF, Coppotelli C, Grigson PS, Mitchell C, Flaherty JE. Investigation of the devaluation interpretation of anticipatory negative contrast. J Exp Psychol Anim Behav Process. 1995;21(3):229–247. doi: 10.1037//0097-7403.21.3.229. [DOI] [PubMed] [Google Scholar]
- 52.Pliner P, Peter C, Polivy J. Palatability as a determinant of eating: Finickiness as a function of taste, hunger, and the prospect of good food. In: Capaldi ED, Powley TL, editors. Taste, Experience, and Feeding. Washington, DC, US: American Psychological Association; 1990. pp. 210–226. Vol. [Google Scholar]
- 53.Katsuura Y, Taha SA. Mu opioid receptor antagonism in the nucleus accumbens shell blocks consumption of a preferred sucrose solution in an anticipatory contrast paradigm. Neuroscience. 2014;261:144–152. doi: 10.1016/j.neuroscience.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flagel SB, Clark JJ, Robinson TE, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469(7328):53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci U S A. 2011;108(27):E255–E264. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tindell AJ, Berridge KC, Zhang J, Peciña S, Aldridge JW. Ventral pallidal neurons code incentive motivation: amplification by mesolimbic sensitization and amphetamine. Eur J Neurosci. 2005;22(10):2617–2634. doi: 10.1111/j.1460-9568.2005.04411.x. [DOI] [PubMed] [Google Scholar]
- 57.Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci. 2003;117(2):202–211. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]
- 58.Fernando ABP, Murray JE, Milton AL. The amygdala: securing pleasure and avoiding pain. Front Behav Neurosci. 2013;7:190. doi: 10.3389/fnbeh.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26(3):321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 60.Holland PC, Hsu M. Role of amygdala central nucleus in the potentiation of consuming and instrumental lever-pressing for sucrose by cues for the presentation or interruption of sucrose delivery in rats. Behav Neurosci. 2014;128(1):71–82. doi: 10.1037/a0035445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J Neurosci Off J Soc Neurosci. 2005;25(4):962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berridge KC. From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur J Neurosci. 2012;35(7):1124–1143. doi: 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holland PC, Petrovich GD. A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiol Behav. 2005;86(5):747–761. doi: 10.1016/j.physbeh.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petrovich GD, Ross CA, Mody P, Holland PC, Gallagher M. Central, But Not Basolateral, Amygdala Is Critical for Control of Feeding by Aversive Learned Cues. J Neurosci. 2009;29(48):15205–15212. doi: 10.1523/JNEUROSCI.3656-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahler SV, Berridge KC. Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci Off J Soc Neurosci. 2009;29(20):6500–6513. doi: 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DiFeliceantonio AG, Berridge KC. Which cue to “want”? Opioid stimulation of central amygdala makes goal-trackers show stronger goal-tracking, just as sign-trackers show stronger sign-tracking. Behav Brain Res. 2012;230(2):399–408. doi: 10.1016/j.bbr.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mahler SV, Berridge KC. What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology (Berl) 2012;221(3):407–426. doi: 10.1007/s00213-011-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci Off J Soc Neurosci. 2005;25(36):8295–8302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petrovich GD, Ross CA, Holland PC, Gallagher M. Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats. J Neurosci Off J Soc Neurosci. 2007;27(24):6436–6441. doi: 10.1523/JNEUROSCI.5001-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mena JD, Sadeghian K, Baldo BA. Induction of hyperphagia and carbohydrate intake by μ-opioid receptor stimulation in circumscribed regions of frontal cortex. J Neurosci Off J Soc Neurosci. 2011;31(9):3249–3260. doi: 10.1523/JNEUROSCI.2050-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Browning M, Holmes EA, Murphy SE, Goodwin GM, Harmer CJ. Lateral Prefrontal Cortex Mediates the Cognitive Modification of Attentional Bias. Biol Psychiatry. 2010;67(10):919–925. doi: 10.1016/j.biopsych.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chamberlain SR, Mogg K, Bradley BP, et al. Effects of mu opioid receptor antagonism on cognition in obese binge-eating individuals. Psychopharmacology (Berl) 2012;224(4):501–509. doi: 10.1007/s00213-012-2778-x. [DOI] [PubMed] [Google Scholar]
- 73.Murray E, Brouwer S, McCutcheon R, Harmer CJ, Cowen PJ, McCabe C. Opposing neural effects of naltrexone on food reward and aversion: implications for the treatment of obesity. Psychopharmacology (Berl) 2014;231(22):4323–4335. doi: 10.1007/s00213-014-3573-7. [DOI] [PubMed] [Google Scholar]
- 74.Van den Eynde F, Guillaume S, Broadbent H, et al. Neurocognition in bulimic eating disorders: a systematic review. Acta Psychiatr Scand. 2011;124(2):120–140. doi: 10.1111/j.1600-0447.2011.01701.x. [DOI] [PubMed] [Google Scholar]
- 75.Manasse SM, Espel HM, Forman EM, et al. The independent and interacting effects of hedonic hunger and executive function on binge eating. Appetite. 2015;89:16–21. doi: 10.1016/j.appet.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addict Abingdon Engl. 2001;96(1):103–114. doi: 10.1080/09652140020016996. [DOI] [PubMed] [Google Scholar]
- 77.Davis C, Strachan S, Berkson M. Sensitivity to reward: implications for overeating and overweight. Appetite. 2004;42(2):131–138. doi: 10.1016/j.appet.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 78.Goldfein JA, Walsh BT, LaChaussee JL, Kissileff HR, Devlin MJ. Eating behavior in binge eating disorder. Int J Eat Disord. 1993;14(4):427–431. doi: 10.1002/1098-108x(199312)14:4<427::aid-eat2260140405>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 79.Avena NM, Rada P, Hoebel BG. Sugar bingeing in rats. Curr Protoc Neurosci Editor Board Jacqueline N Crawley Al. 2006;Chapter 9 doi: 10.1002/0471142301.ns0923cs36. Unit9.23C. [DOI] [PubMed] [Google Scholar]
- 80.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32(1):20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boggiano MM, Chandler PC, Viana JB, Oswald KD, Maldonado CR, Wauford PK. Combined dieting and stress evoke exaggerated responses to opioids in binge-eating rats. Behav Neurosci. 2005;119(5):1207–1214. doi: 10.1037/0735-7044.119.5.1207. [DOI] [PubMed] [Google Scholar]
- 82.Boggiano MM, Chandler PC. Binge eating in rats produced by combining dieting with stress. Curr Protoc Neurosci Editor Board Jacqueline N Crawley Al. 2006;Chapter 9 doi: 10.1002/0471142301.ns0923as36. Unit9.23A. [DOI] [PubMed] [Google Scholar]
- 83.Micioni Di Bonaventura MV, Ciccocioppo R, Romano A, et al. Role of bed nucleus of the stria terminalis corticotrophin-releasing factor receptors in frustration stress-induced binge-like palatable food consumption in female rats with a history of food restriction. J Neurosci Off J Soc Neurosci. 2014;34(34):11316–11324. doi: 10.1523/JNEUROSCI.1854-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD. The role of palatable food and hunger as trigger factors in an animal model of stress induced binge eating. Int J Eat Disord. 2003;34(2):183–197. doi: 10.1002/eat.10168. [DOI] [PubMed] [Google Scholar]
- 85.Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol Behav. 2002;77(1):45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- 86.Piccoli L, Micioni Di Bonaventura MV, Cifani C, et al. Role of orexin-1 receptor mechanisms on compulsive food consumption in a model of binge eating in female rats. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2012;37(9):1999–2011. doi: 10.1038/npp.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65(3):545–553. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- 88.Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int J Eat Disord. 2000;28(4):436–445. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 89.Berner LA, Avena NM, Hoebel BG. Bingeing, self-restriction, and increased body weight in rats with limited access to a sweet-fat diet. Obes Silver Spring Md. 2008;16(9):1998–2002. doi: 10.1038/oby.2008.328. [DOI] [PubMed] [Google Scholar]
- 90.Berner LA, Bocarsly ME, Hoebel BG, Avena NM. Baclofen suppresses binge eating of pure fat but not a sugar-rich or sweet-fat diet. Behav Pharmacol. 2009;20(7):631–634. doi: 10.1097/FBP.0b013e328331ba47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cottone P, Wang X, Park JW, et al. Antagonism of Sigma-1 Receptors Blocks Compulsive-Like Eating. Neuropsychopharmacology. 2012;37(12):2593–2604. doi: 10.1038/npp.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]


