Abstract
This paper explores the proton pumping function of cytochrome c oxidase [ferrocytochrome-c:oxygen oxidoreductase (EC 1.9.3.1)] based upon redox linkage at the "high-potential" CuB center. A model is proposed that is derived from a redox-linked ligand exchange mechanism previously described for the CuA site. Qualitative analysis of this mechanism indicates that such a mechanism is feasible. However, the relatively short distance between CuB and cytochrome a3 implies that the uncoupling electron transfers are quite facile. In addition, the position of the CuB center with respect to the inner mitochondrial membrane argues against redox linkage at the CuB site.
Full text
PDF
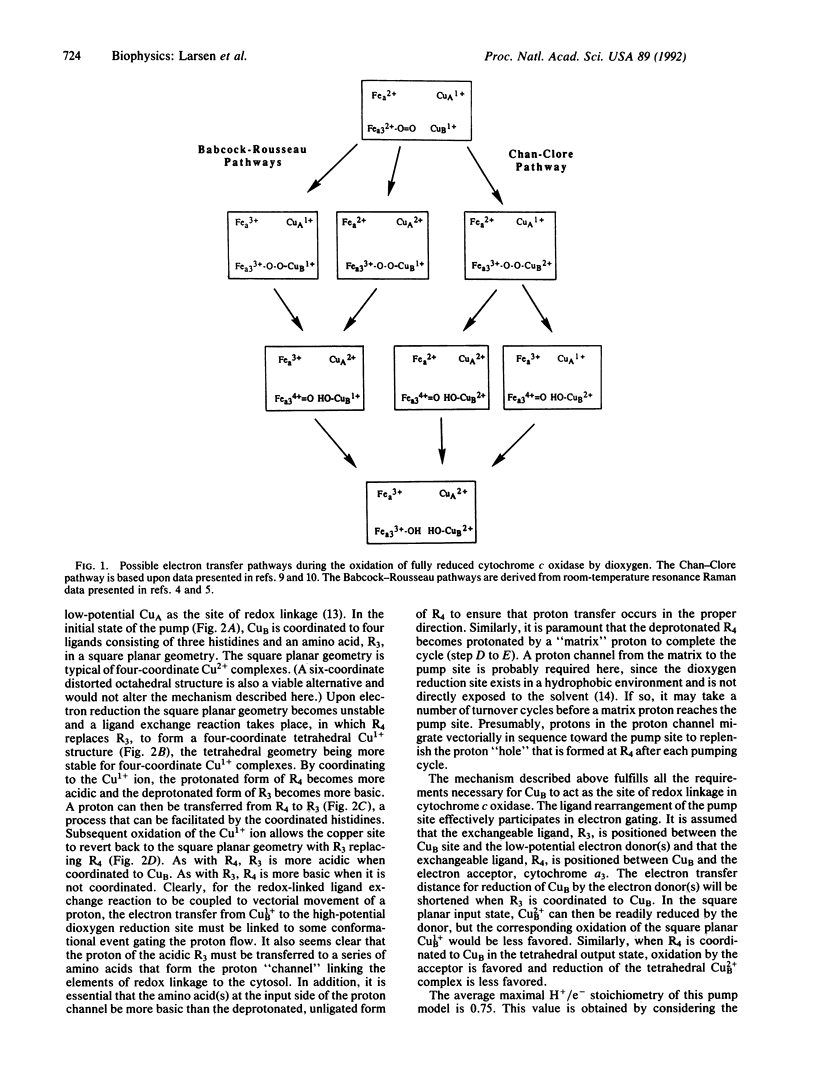
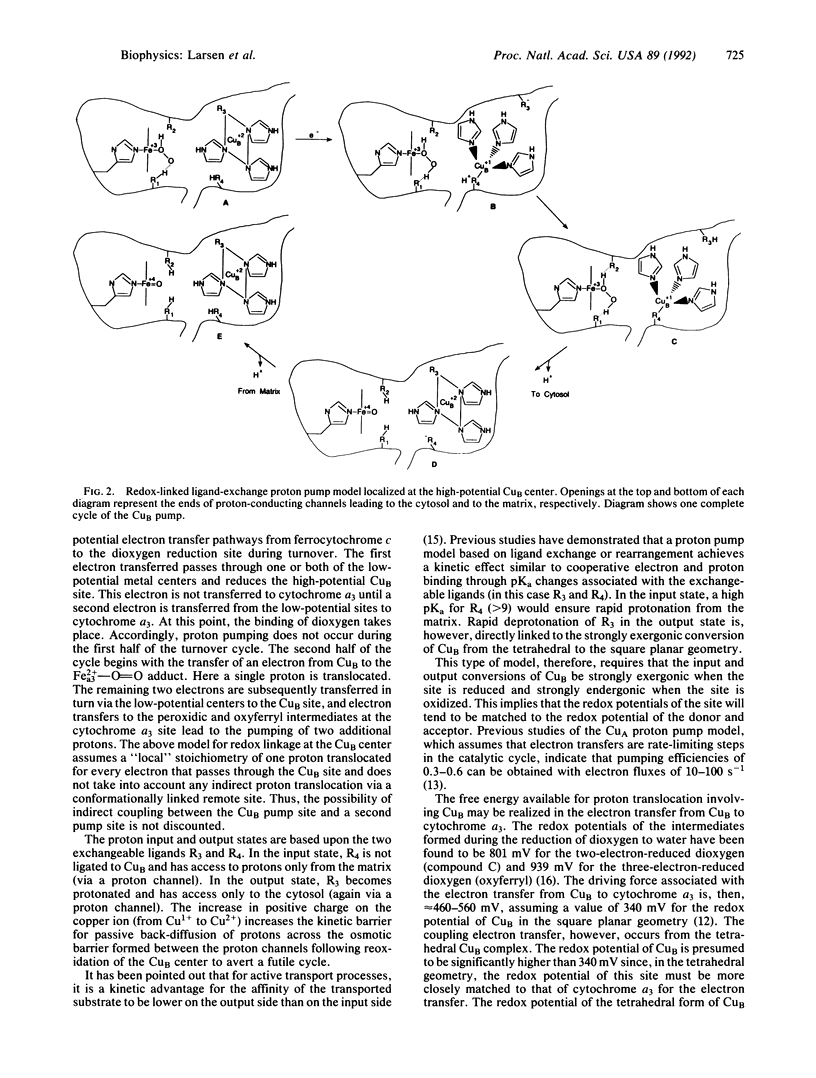


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair D. F., Gelles J., Chan S. I. Redox-linked proton translocation in cytochrome oxidase: the importance of gating electron flow. The effects of slip in a model transducer. Biophys J. 1986 Oct;50(4):713–733. doi: 10.1016/S0006-3495(86)83511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter K. R., Antalis T. M., Palmer G., Ferris N. S., Woodruff W. H. Spectroscopic characterization of compound C and related derivatives of cytochrome oxidase. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1652–1655. doi: 10.1073/pnas.78.3.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. I., Li P. M. Cytochrome c oxidase: understanding nature's design of a proton pump. Biochemistry. 1990 Jan 9;29(1):1–12. doi: 10.1021/bi00453a001. [DOI] [PubMed] [Google Scholar]
- Einarsdóttir O., Choc M. G., Weldon S., Caughey W. S. The site and mechanism of dioxygen reduction in bovine heart cytochrome c oxidase. J Biol Chem. 1988 Sep 25;263(27):13641–13654. [PubMed] [Google Scholar]
- Gelles J., Blair D. F., Chan S. I. The proton-pumping site of cytochrome c oxidase: a model of its structure and mechanism. Biochim Biophys Acta. 1986;853(3-4):205–236. doi: 10.1016/0304-4173(87)90002-4. [DOI] [PubMed] [Google Scholar]
- Han S. H., Ching Y. C., Rousseau D. L. Cytochrome c oxidase: decay of the primary oxygen intermediate involves direct electron transfer from cytochrome a. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8408–8412. doi: 10.1073/pnas.87.21.8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Ching Y. C., Rousseau D. L. Ferryl and hydroxy intermediates in the reaction of oxygen with reduced cytochrome c oxidase. Nature. 1990 Nov 1;348(6296):89–90. doi: 10.1038/348089a0. [DOI] [PubMed] [Google Scholar]
- Larsen R. W., Li W., Copeland R. A., Witt S. N., Lou B. S., Chan S. I., Ondrias M. R. Room temperature characterization of the dioxygen intermediates of cytochrome c oxidase by resonance Raman spectroscopy. Biochemistry. 1990 Oct 30;29(43):10135–10140. doi: 10.1021/bi00495a018. [DOI] [PubMed] [Google Scholar]
- Malatesta F., Antonini G., Sarti P., Vallone B., Brunori M. Modulation of cytochrome c oxidase activity by an electrical transmembrane gradient. Ann N Y Acad Sci. 1988;550:269–276. doi: 10.1111/j.1749-6632.1988.tb35342.x. [DOI] [PubMed] [Google Scholar]
- Nagai K., Kitagawa T., Morimoto H. Quaternary structures and low frequency molecular vibrations of haems of deoxy and oxyhaemoglobin studied by resonance raman scattering. J Mol Biol. 1980 Jan 25;136(3):271–289. doi: 10.1016/0022-2836(80)90374-5. [DOI] [PubMed] [Google Scholar]
- Ogura T., Takahashi S., Shinzawa-Itoh K., Yoshikawa S., Kitagawa T. Observation of the Fe4+ = O stretching Raman band for cytochrome oxidase compound B at ambient temperature. J Biol Chem. 1990 Sep 5;265(25):14721–14723. [PubMed] [Google Scholar]
- Oliveberg M., Brzezinski P., Malmström B. G. The effect of pH and temperature on the reaction of fully reduced and mixed-valence cytochrome c oxidase with dioxygen. Biochim Biophys Acta. 1989 Dec 7;977(3):322–328. doi: 10.1016/s0005-2728(89)80087-8. [DOI] [PubMed] [Google Scholar]
- Powers L., Chance B., Ching Y., Angiolillo P. Structural features and the reaction mechanism of cytochrome oxidase: iron and copper X-ray absorption fine structure. Biophys J. 1981 Jun;34(3):465–498. doi: 10.1016/S0006-3495(81)84863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Rousseau D. L., Sassaroli M., Ching Y. C., Dasgupta S. The role of water near cytochrome a in cytochrome c oxidase. Ann N Y Acad Sci. 1988;550:223–237. doi: 10.1111/j.1749-6632.1988.tb35338.x. [DOI] [PubMed] [Google Scholar]
- Tanford C. Mechanism of free energy coupling in active transport. Annu Rev Biochem. 1983;52:379–409. doi: 10.1146/annurev.bi.52.070183.002115. [DOI] [PubMed] [Google Scholar]
- Van Wart H. E., Zimmer J. Resonance Raman evidence for the activation of dioxygen in horseradish oxyperoxidase. J Biol Chem. 1985 Jul 15;260(14):8372–8377. [PubMed] [Google Scholar]
- Varotsis C., Babcock G. T. Appearance of the v(FeIV = O) vibration from a ferryl-oxo intermediate in the cytochrome oxidase/dioxygen reaction. Biochemistry. 1990 Aug 14;29(32):7357–7362. doi: 10.1021/bi00484a001. [DOI] [PubMed] [Google Scholar]
- Wikström M., Casey R. P. What is the essential proton-translocating molecular machinery in cytochrome oxidase? J Inorg Biochem. 1985 Mar-Apr;23(3-4):327–334. doi: 10.1016/0162-0134(85)85042-x. [DOI] [PubMed] [Google Scholar]
- Wikström M. Energy-dependent reversal of the cytochrome oxidase reaction. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4051–4054. doi: 10.1073/pnas.78.7.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström M. How does cytochrome oxidase pump protons? A "cooperative proton pump" model. Ann N Y Acad Sci. 1988;550:199–206. doi: 10.1111/j.1749-6632.1988.tb35336.x. [DOI] [PubMed] [Google Scholar]
- Yoshikawa S., Choc M. G., O'Toole M. C., Caughey W. S. An infrared study of CO binding to heart cytochrome c oxidase and hemoglobin A. Implications re O2 reactions. J Biol Chem. 1977 Aug 10;252(15):5498–5508. [PubMed] [Google Scholar]



