Introduction
The maintenance of immune tolerance to self is an essential mechanism to prevent untoward immune responses and autoimmunity. The breakdown of tolerance is often a complex process that is challenging to dissect. Despite this challenge, there have been major advances of our understanding of autoimmunity through the study of monogenic forms of the disease 1. This approach has identified a number of key regulators of immune tolerance with direct relevance to human disease including FoxP3 2–4, CTLA4 5, 6, and AIRE 7, 8. Aire is best known as a critical transcriptional regulator that functions by promoting the display of a wide array of tissue specific antigens (TSA’s) in the thymus. Encounters with TSAs by developing T cells leads to negative selection of autoreactive T cells that recognize TSAs with high affinity 9, 10. Despite the fact that AIRE was identified more than 15 years ago, we continue to learn more about how this key tolerance regulator operates and how it impacts the immune system. Here we highlight some of the recent advances that have expanded our understanding of the biology of Aire.
Autosomal Dominant AIRE
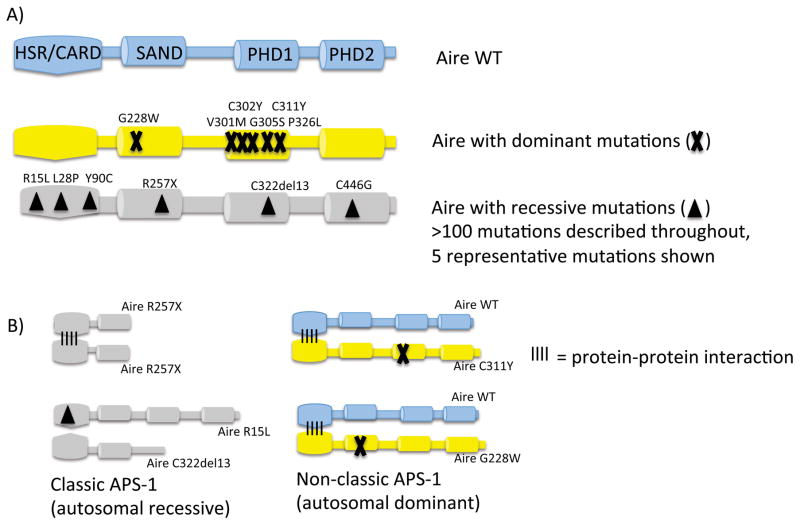
AIRE was originally identified as the culprit mutated gene in patients with an autosomal recessive form of autoimmunity called Autoimmune Polyglandular Syndrome Type 1 (APS1) 7, 8. Although patients classically present with rare AIRE mutations on both alleles, it has now become increasingly appreciated that there are subjects that harbor single point mutations on one allele that have increased susceptibility to autoimmunity 11–13. These patients often present later in life and only with a portion of the features of APS1. To date, these autosomal dominant mutations appear to cluster in the SAND domain and the PHD1 domain of AIRE (Figure 1). Aire has a number of subdomains that include HSR/CARD, SAND, PHD1, and PHD2. Of note, the HSR/CARD domain appears to be involved in promoting Aire to multimerize to itself 14 and thus one can envision a model where point mutations in other domains of Aire can result in interference of the activity of the multimerized Aire complex. The PHD1 domain of Aire functions as a histone code reader (see below) while the SAND domain appears to be involved in promoting a protein-protein interaction with a transcriptional repressive complex (see below). These recent findings raise several interesting questions. First, how widespread could autosomal dominant AIRE mutations be in the general population? The recent study by Husebye and colleagues provides evidence that this may be more frequent then previously thought with a frequency of nearly 1/1000 individuals harboring a potential point mutation in the PHD1 domain. More study will be needed to determine if rare variants in AIRE such as this are major contributors with subjects that have autoimmunity, especially in kindreds with a strong family history. Second, how do these mutations operate at a molecular level to interfere with AIRE activity? Work by our group demonstrated that a particular point mutation in the SAND domain interferes globally with Aire function by severely reducing Aire-dependent thymic TSA expression 12. Similar data for the effect of PHD1 domains in vivo is lacking and it will be interesting to see if such mutations result in a global interference of Aire’s activity or if there is some predilection for a subset of Aire-target genes.
Figure 1.
Disease-causing Aire mutations in patients with classic and non-classic APS-1. A) The wildtype (WT) Aire protein with its domains (top). Dominant Aire mutations (X) and 5 representative recessive Aire mutations (triangle) from more than 100 recessive mutations described are shown below. Dominant mutations cluster in the PHD1 domain whereas recessive mutations are found throughout the Aire protein, including the HSR/CARD multimerization domain. B) Mutant Aire proteins and how they can cause disease. Classic APS-1 can occur with homozygous Aire R257X mutations (upper le]). These mutant Aire proteins can multimerize but lack critical Aire domains. Alternatively, compound heterozygotes with HSR/CARD domain mutant (R15L) that prevents multimerization and truncation mutant (C322del13) (lower left) can also lead to classic APS-1. Non-classic APS-1 occurs with one copy of an Aire C111Y or G228W mutation (right).
New molecular insights into Aire’s functional activity
Properties of Aire-dependent TSA expression
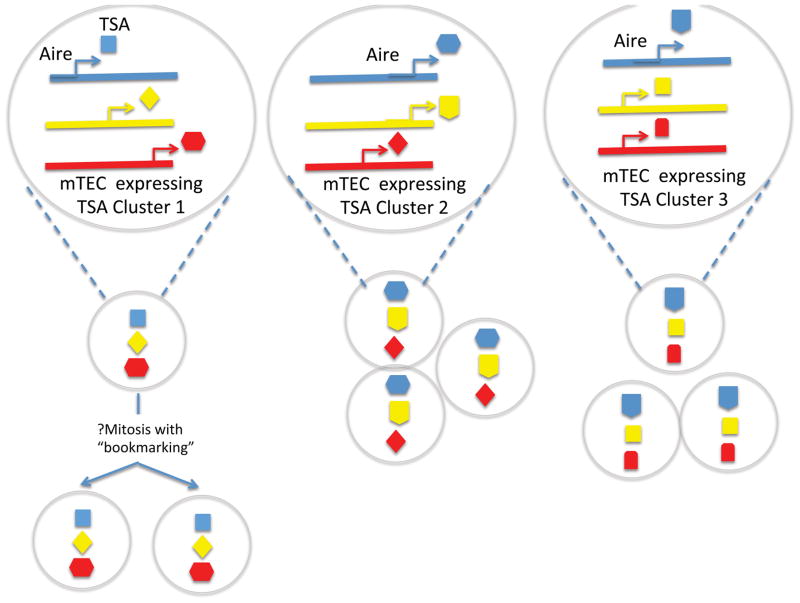
Aire is highly expressed in thymic medullary epithelial cells (mTEC’s) where as outlined above it promotes the expression of thousands of TSAs 15. How Aire impacts transcription of such a large number of genes, most of which are subject to strict spatio-temporal regulation in peripheral tissues, has been of great interest. Despite the fact that Aire was identified more than 15 years ago, progress on the exact mechanisms by which it does this has been technically challenging. There currently is not a robust transfection model or a cell line to assay its activity that is identical to that which occurs in mTEC’s. Furthermore, the number of mTEC’s available from a thymus is relatively small (<50,000 in a single murine thymus). Recently, we have learned a large amount about the properties of TSA expression in the thymus and the target genes of Aire through sophisticated transcriptional analyses of cell sorted mTEC’s that now includes single cell RNA-sequencing analyses 15–17. The overall features of TSA-expression within mTEC’s appear to have the pattern of being both ordered and stochastic (Figure 2). On an individual cell level, each mTEC will randomly express a given TSA gene; however, within that given mTEC one frequently observes that a particular set of other genes will often be co-expressed in that cell. At one level, these co-expressed genes within a given cell are often clustered at a positional level on the same chromosome 16. At another level, these co-expressed genes are also clustered between chromosomes (interchromosomal clustering) 17. Many of these co-expressed genes frequently do not show other commonalities such as being part of a similar transcriptional program or being expressed in the same peripheral tissue. Despite this, within individual cells the TSA transcript areas do show evidence of increased chromatin accessibility. These recent findings complement a series of similar observations in the past that show that individual mTEC’s have a somewhat random pattern of expressing TSA’s that are clustered in the genome 18–20 such that on a population level a broad array of self is being constantly displayed to the thymic T cell repertoire by a diverse pool of mTEC’s that harbor different TSA’s on an individual cell level. In line with these observations, it also has become clear that TSA-expression within mTEC’s does not follow the same rules as that which occurs in peripheral organ tissues. In support of this notion, is the recent observation that transcription of insulin occurs in the thymus of mice deficient for the key pancreatic islet regulator Pdx1 which do not produce pancreatic insulin 21.
Figure 2.
Ordered stochasticasticity of Aire regulated gene expression. Aire-regulated TSA expression in single mTEC does not occur randomly but occur in microclusters of co-expression. Interchromosomal clusters of TSA expression are shown, although clustering of genes located in close proximity in the genome has also been demonstrated. “Bookmarking”, or the maintenance of gene-expression programs after cell division, may result in the clonal expansion of mTECs expressing TSA microclusters (left).
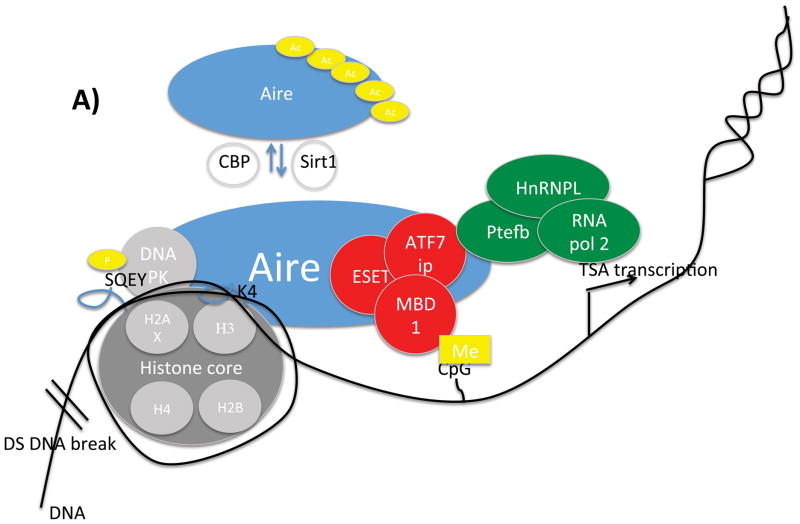
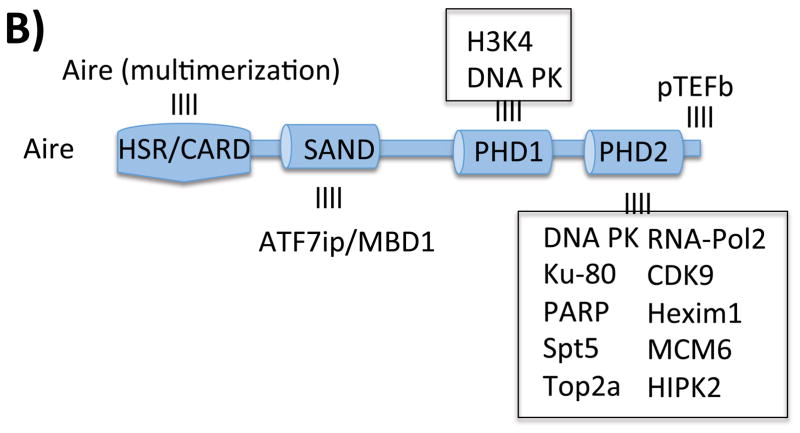
Given these unique properties of Aire-dependent TSA expression, the molecular basis of Aire’s function in mTECs has been the subject of intense interest. A picture has emerged in which Aire partners with multiple other proteins to exert its effect on a broad number of target genes 22–25. Using various approaches including co-IP, yeast two hybrid, and RNAi knockdown approaches, intensive efforts have now identified dozens of Aire’s interacting partners. These partners initially could be broadly classified into 4 functional groups: nuclear transport, chromatin binding/structure, transcription, and pre-mRNA processing 22. More recent reports have suggested additional partners and/or functions as well as provided confirmation of earlier findings (Figure 3A). For instance, interacting proteins have been described that help Aire to recognize and target TSA genes25–30, release stalled RNA polymerase to allow RNA elongation24, 31–33, and regulate Aire itself 34–38. Additionally, inroads have been made into mapping the regions of Aire that bind to interacting partners 25, 38, 39 (Figure 3B). These recent developments in understanding how Aire functions at a molecular level are described in detail below.
Figure 3.
Aire and it’s binding partners. A) Schematic illustration of a partial set of Aire's interacting partners. Dozens of Aire interacting partners with diverse functions have been identified. Here is shown Aire's ineractions with histone core proteins either directly or through its interactions with DNA-PK; ATF7ip/MBDl/ESET complex that interacts with methylated DNA; and pTEFb, HnRNPL and RNA pol2 to release stalled polyermases. Aire also interacts with Sirtl and CBP which control Aire aceytlation. B) Map of regions of Aire that interact with binding partners.
Targeting Aire to TSA genes
A key to understanding how Aire regulates TSA expression is the molecular mechanism(s) used by Aire to target loci encoding TSAs. Aire appears to be atypical among transcription factors in that it does not have a clear DNA binding motif, but instead recognizes genes that possess silenced chromatin states (Figure 3A) 15, 25–27. Unmethylated histone H3 lysine 4 (H3K4) is a repressive epigenetic mark that is selectively recognized by the first plant homeodomain (PHD1) of Aire (Figure 3B) 26–29. Aire’s binding to H3K4 is not sufficient for Aire to drive TSA expression, however, since global demethylation of H3K4 by overexpression of a H3K4 demethylase does not alter TSA expression 29. Thus, additional mechanisms independent of unmethylated H3K4 are likely important in Aire driven TSA expression.
Concordant with this, Aire associates with additional marks of a repressive chromatin state. First, Aire targets activating ATF7ip-MBD1 (activating transcription factor 7-interacting protein-methyl CpG-binding protein 1) complexes, which are normally associated with gene repression (Figure 3A) 25. ATF7ip-MBD1 associates with the histone methyltransferase ESET-SETDB1 to target it to methylated CpG dinucleotides, which are enriched in the promoters of inactive genes. Additionally, ATF7ip is an essential cofactor in the generation of the repressive trimethylated histone 3 lysine 9 (H3K9me3) epigenetic mark 40. Aire-mediated expression of TSAs require the ATF7ip and MBD1 proteins, since shRNA knockdown of these two proteins in vitro prevented Aire-dependent TSA expression. Furthermore, genetic deletion MBD1 in mice also prevented Aire-dependent TSA expression in mTECs and predisposed mice to autoimmunity. The development of autoimmunity mapped to MBD1 deficiency in the thymus, since transplantation of MBD1 deficient thymus into athymic nude mice was sufficient to cause autoimmune disease development. Second, Aire-regulated TSAs in mTECs are highly associated with the repressive H3K27me3 epigenetic mark 15. Aire does not directly bind H3K27me3 27, but may interact indirectly through chromatin binding proteins, such as CHD6 (Chromdomain Helicase-DNA 6) 22.
DNA-PK, a nuclear kinase with multiple roles including the repair of double- strand DNA breaks and DNA replication, may also play a role in targeting Aire to TSAs (Figure 3A) 30. Double strand DNA breaks are marked by the variant histone H2AX phosphorylated at Ser 139 (γH2AX), and DNA PK appears to target Aire to these (γH2AX marks. 30 Since transient double stranded DNA breaks have been associated with transcription initiation 41, DNA-PK may direct Aire to TSAs poised for transcription 42. mTECs derived from reconstituted SCID mice that carry a mutation in DNA-PK have reduced expression of a number of Aire-dependent TSAs, suggesting that efficient mTEC expression of Aire-dependent TSAs requires DNA-PK. Of note, however, additional roles for DNA-PK in Aire’s function, including relaxation of surrounding chromatin22 and phosphorylation of Aire 37 (see below), have also been proposed. Which of these DNA-PK functions are most relevant to Aire’s function remains to be determined. In any case, the prevailing evidence points to Aire as an unusual transcriptional regulator that recognizes a combination of chromatin signals to target TSA genes in mTECs.
Aire releases stalled RNA polymerase
Another atypical aspect of Aire is that Aire does not act primarily by initiating TSA gene transcription. Rather, Aire promotes TSA expression through the release of stalled RNA polymerase to elongate RNA transcripts. Microarray analysis using mRNA spanning probes showed no differences in transcription initiation within mTECs of wildtype and Aire-deficient mice 33. Instead, RNA lengths were reduced (50–100 bp) in Aire-deficient mTECs, suggesting that Aire has a role in promoting RNA elongation. Furthermore, Aire’s interacting partners also suggest that Aire promotes TSA expression through RNA elongation. Aire interacts with positive transcription elongation factor b (P-TEFb), a protein that controls release of stalled RNA polymerase (Figure 3A) 31, 32. This interaction occurs through Aire’s extreme C-terminus, in which patient mutations that disrupts this interaction can result in multi-organ autoimmunity (Figure 3B) 32. Aire’s interaction with P-TEFb was independently confirmed using an RNAi screening approach for Aire’s functional allies 24. Interestingly, this RNAi screen identified 51 candidates involved in Aire’s transactivating function. While many of these candidates had known roles in RNA elongation, none of them had known roles in transcription initiation 24. Thus, Aire primary effect appears to be in RNA elongation rather than initiation of transcription.
The identification of HnRNPL (heterogenous nuclear ribonucleoprotein L) as an Aire binding partner lends support to Aire’s role in releasing stalled RNA polymerase (Figure 3A) 24. HnRNPL knockdown in mTEC cells decreased transcripts that are controlled by the P-TEFb components CCNT2 and CDK9, suggesting that HnRNPL has a role in RNA elongation24. Furthermore, HnRNPL co-immunoprecipitates with Aire and the P-TEFb components CDK9 and HEXIM1. Finally, the 7SK snRNA, a noncoding RNA that regulates P-TEFb activity, co-precipitated with Aire and HnRNPL knockdown decreased this interaction, suggesting that HnRNPL may facilitate Aire’s interaction with the P-TEFb regulator, 7SK snRNA. Thus, Aire appears to interact with multiple P-TEFb complex members that are important in productive RNA elongation.
In addition to promoting RNA elongation, Aire may also regulate pre-mRNA splicing through P-TEFb and other factors 22, 24, 32. Aire increases the pre-mRNA splicing of a heterologous minigene and the Aire-regulated KRT14 gene, and this increase is P- TEF-b dependent, since inhibition of the kinase subunit of P-TEFb blocked Aire-induced pre-mRNA splicing32. Consistent with a role in pre-mRNA splicing, Aire associates with multiple interacting partners that have known roles in pre-mRNA splicing (e.g. EFTUD2, SNRPB, SRSF1) 12, 22. Rather than initiating transcription, then, Aire primarily functions to promote RNA elongation and splicing of target TSAs.
Aire interacts with its functional regulators
Interestingly, Aire interacts with a number of proteins that exert control over Aire’s function through post-translational modifications 34–38. First, Aire associates with deacetylase and acetyltransferase proteins that act directly upon Aire 34–36. For instance, Aire physically binds to Sirtuin 1 (Sirt1), a protein deacetylase that is highly expressed within mTECs (Figure 3A) 36. Sirt1 deacetylates lysine residues in Aire, a process that activates Aire transcription. Moreover, eptihelial cell-specific ablation of Sirt1 disrupted Aire-dependent TSA expression without affecting Aire expression, which suggests a role for Sirt1 in Aire-regulated TSA expression. At the same time, Aire also interacts with CREB-binding protein (CBP) and p300, both of which have acetyltransferase activity. CBP and p300 add acetyl groups to the Aire protein, a process that downregulates Aire transcriptional activity and alters the profile of TSAs regulated by Aire35. Thus associations with proteins that acetylate and deacetylate Aire appear to regulate Aire’s function.
In addition to factors that modulate acetylation, proteins that mediate phosphorylation are also associated with Aire 37, 38. Aire interacts with DNA dependent protein kinase (DNA PK), which in expressed in mTECs and phosphorylates Aire in vitro 37. Mutation of Aire Thr68 and Ser156, which are targets of DNA-PK phosphorylation, reduces Aire’s ability to promote transcription in in vitro reporter systems. However, the requirement for DNA-PK’s nuclear kinase activity has been called into question, since addition of Nu7441, an inhibitor of DNA-PK catalytic activity, did not abrogate Aire’s effect on TSA expression in 293T cells30. A possible explanation for these discrepant results is that DNA-PK’s catalytic activity is required for a subset of TSA expression, since a limited number of promoters were tested in these studies. At the same time, Aire’s phosphorylation may also be regulated by homeodomain-interacting protein kinase 2 (HIPK2), a protein that partially-colocalizes with Aire in co-transfected cell lines and human thymus 38. In an in vitro reporter system, HIPK2 decreased Aire transactivation activity in a kinase dependent manner; unexpectedly, however, eptihelial cell-specific deletion of HIPK2 affected largely Aire-independent TSAs. Thus, Aire’s association with this kinase may not affect Aire’s function in promoting mTEC TSA expression. Instead, it is possible the HIPK2 may affect a non-Aire, yet-to-be-identified, regulator of mTEC TSA expression.
Aire promotes positive selection of thymic Tregs
Aire’s major influence on the negative selection of autoreactive T cells is well-established 43–46; however, its influence on thymic positive selection of FoxP3+ Treg’s has been more controversial. Ectopic antigen expression in Aire-positive mTECs promotes the development of antigen-specific CD4+ Tregs in the thymus 47 , which, early on, suggested the possibility that Aire-expressing mTECs can promote thymic Treg development. Subsequent studies have provided evidence that Aire may influence thymic Treg development 48–51. A decrease in thymic CD4+ Tregs is particularly evident during an early perinatal time period and decreased absolute numbers of CD4+ Tregs up to Day 10 49. In addition, decreased thymic CD4+ Tregs have also been reported in adult mice 48, 50, however, data are conflicting on this issue 49.
Regardless of whether Aire transiently or permanently influences thymic Treg numbers, Aire appears to influence, at least in part, specific clones of the thymic Treg repertoire. Certain CD4+ Treg clones seem to be entirely dependent on thymic Aire expression for their development. One particular Treg clone, for instance, that was originally isolated from tumor-infiltrating lymphocytes, is normally generated in the Aire-replete thymus. In the Aire deficient thymus, however, this CD4+ Treg clone is entirely absent 50. Thus, thymic development of this particular Treg clone appears to be completely Aire-dependent. Conversely, some Treg clones, appear to develop independently of Aire within the thymus. Perry et al. used limited TCR repertoire approach to demonstrate that the most frequent thymic Treg clones are Aire independent 51. Morisita Horn similarity index analysis of Treg TCR repertoires showed a high degree of similarity between Aire deficient and Aire wildtype mice. On the surface, this finding suggested that Aire does not have a major effect on Treg TCR selection. Remarkably, removal of the three most frequent Treg TCRs from this analysis significantly lowered the similarity, which pointed to the most frequent clones erasing the effects of Aire. These findings suggest that the most frequent Treg TCRs are Aire independent, whereas rare Treg TCRs include those that are Aire dependent.
The factors governing whether a given CD4+ Treg clone is Aire-dependent or independent are not currently known. It is tempting to speculate that the Aire-dependent clones are those that are specific for Aire-dependent TSAs. Further investigation is required to test this hypothesis, however, as the studies of Perry et al. and Malchow et al. did not directly identify the antigen specificity of the Aire-dependent Treg clones. Interestingly, Aire may also indirectly regulate Treg development through its effects on thymic APC migration 48. Aire regulates mTEC expression of XCL1, a chemokine that directs dendritic cells into the thymus. Similar to Aire deficient mice, XCL1 deficient mice also have reduced frequency and absolute numbers of thymic CD4+ CD8- FOXP3+ CD25+ Tregs. Thus, Aire may affect thymic Treg selection by means other than TSA upregulation.
Given that Aire appears to influence the thymic Treg repertoire, an interesting question is how much does a defect in this process contribute to the autoimmunity present in Aire-deficient individuals? Recent work by Yang et al. 49 suggests that a defect in the neonatal output of thymically derived Treg’s in Aire-deficient mice indeed may be a key player in driving autoimmunity in the model. Suggestion that such a mechanism may be in play came from previous work which demonstrated that temporal knockout of Aire in mice after three weeks of age did not predispose to widespread autoimmunity 52. Yang et al. used elaborate adoptive transfers of FoxP3+ Treg’s from neonatal Aire wildtype or knockout donors into Treg ablated recipients to show that Aire wildtype Treg’s could protect but not Aire knockout Treg’s. In contrast to these findings, two independent studies demonstrated that co-transfer of an Aire-deficient thymic lobe and an Aire wildtype thymic lobe into a single nude recipient mouse did not protect against autoimmunity which favors a primary role for Aire in deletion rather than dominant tolerance 44, 53. In addition, previous work has also demonstrated that genetic crossing of the Aire knockout and FoxP3 knockout mouse results in a more severe autoimmune syndrome than either single knockout alone 54. These latter findings strongly argue that a defect in Treg selection is not the sole, critical immune tolerance defect in the Aire-deficient model. Further work will be needed to parse these issues out including more detailed characterization of the Treg TCR specificities that may be absent in the Aire-deficient model and how they potentially contribute to disease when missing.
SEPARATE BOX
In addition to CD4+ FOXP3+ Tregs, CD8+ CD28low Tregs in Aire-deficient mice have also been shown to be defective in their ability to suppress autoimmune colitis in vivo 55. CD8+ CD28low Tregs are a distinct population of T cells that can prevent the development of experimental autoimmune encephalomyelitis (EAE) and experimental colitis 56, 57. CD8+ CD28low Tregs from Aireo/o mice have altered TCR repertoires and decreased suppressive function 55. By immunoscope analysis, the T cell receptor (TCR) gene structure of CD8+ CD28low Tregs from Aireo/o mice was distinct from that of wildtype mice. Furthermore, in the colitis transfer model, cotransfer of CD8+ CD28low Tregs from wildtype mice prevented development of colitis, whereas cotransfer of CD8+ CD28low Tregs from Aireo/o mice did not. Aire thus appears to play an important role in shaping the repertoire of CD8+ CD28low Tregs and the ability of these cells to suppress colitis.
Other Aire functions in mTECs
As noted above, Aire is best known for its role in upregulating mTEC TSA expression to prevent autoimmunity. By upregulating mTEC TSAs, Aire promotes negative selection of effector T cells and development of Tregs. It is now clear, however, that Aire has 1) functions that are independent of its promotion of TSA expression and 2) effects beyond autoimmune disease prevention. These extended roles for Aire are discussed below:
TSA independent functions
An early indication that Aire has TSA-independent functions came from studies using the OTII RIP-mOVA double transgenic system44. In this system, membrane-bound ovalbumin (OVA) is expressed as a neo self-antigen in mTECs under the control of the rat insulin promoter (RIP), and OTII T cells expressing transgenic T cell receptor specific for OVA undergo thymic negative selection upon recognition of OVA. Although negative selection of OTII thymocytes was clearly Aire-dependent, Aire minimally affected mTEC expression of the OVA neo self-antigen 44. Similar results were seen using the OTII RIP-OVAhi system, in which soluble OVA is expressed under the control of RIP 58. These findings collectively suggest that Aire my also regulate T cell negative selection and autoimmunity by TSA-independent mechanisms. Additionally, Aire-deficient mice develop organ specific autoimmunity of the salivary gland and pancreas, and pathogenic T cells appear to be directed against salivary and pancreatic antigens that, unexpectedly, are not Aire-regulated in the thymus 53, 59. Thus, a mechanism distinct from Aire upregulation of TSAs in mTECs is likely to underlie these autoimmune manifestations.
Consistent with these initial observations, TSA-independent roles for Aire have now been delineated. Aire promotes mTEC expression of XCL1, a chemokine critical in recruitment of thymic dendritic cells48, and XCL1 deficiency results in decreased thymic dendritic cell accumulation. Thymic dendritic cells have an important role in mediating thymocyte negative selection, and thymocytes from XCL-deficient mice are sufficient to transfer autoimmune lacrimal disease. As described above, XCL deficiency also impacts Treg development within the thymus48, although Aire/XCL dependent mechanisms are unlikely to account for selection of all Treg TCRs 51.
In addition to upregulating XCL1, Aire also increases expression of ligands of CCR7 and CCR4, chemokines important in thymocyte migration 60. Aire overexpression enhanced thymocyte chemotaxis and emigration via CCR7 and CCR4 chemokine action. In addition to modulating chemokine expression, Aire may also enhance self-tolerance through promoting mTEC apoptosis 61. Aire is expressed in approximately 50% of MHCIIhigh mTECs (MEChi) cells, and Aire deficiency is associated with increased MHCIIhigh mTECs. One potential explanation for this is that Aire in MEChi cells induces apoptosis, and this pro-apoptotic function may enhance self-tolerance by facilitating phagocytosis and cross-presentation of TSAs by thymic antigen-presenting cells 62. In conflict with this interpretation is evidence using an inducible Cre mouse model that suggests that Aire does not affect mTEC lifespan 63. Instead, Aire may be involved with physiologic downregulation of CD80 in mature mTECs63. Thus, Aire’s role as a pro-apoptotic factor remains to be clarified.
Furthermore, three groups have independently reported the existence of post-Aire-expressing mTECs 63–65, a finding potentially at odds with Aire’s role in inducing mTEC apoptosis. Prior to these reports, the most mature mTECs were considered to be MEChi cells, a post-mitotic, Aire-expressing population that also express high levels of CD80 and MHCII. However, recent studies using lineage tracing approaches have delineated a population of post-Aire expressing cells that express low levels of CD80 and MHCII63–65. This population continues to express an array of TSAs, but at quantitatively reduced levels compared to Aire-expressing cells 64. This population may also over-express a subset of TSAs, such as desmoglein, a pemphigus vulgaris-associated antigen 65. Given its continued TSA expression, this post-Aire population likely continues to play a significant role in enforcing self-tolerance. Finally, Aire may play a role in Aire mTEC differentiation. Aire deficiency prevents the expression of epidermal markers of late mTEC maturation, such as CK6, CK10, involucrin, and LEKT1 65, which suggests a role for Aire in end-stage mTEC differentiation. Moreover, Aire deficient mice may have changes in thymic architecture and mTEC morphology 66, which also lends support to Aire’s function in mTEC differentiation.
Anti-tumor immunity
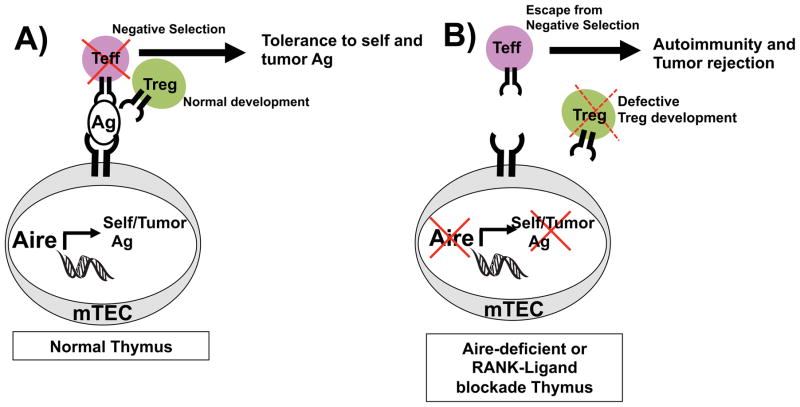
Aire’s role in preventing autoimmunity is evident by the spontaneous development of autoimmunity in mice and humans with Aire mutations. Recently, Aire’s roles in modulating diseases outside of autoimmunity have been delineated. For instance, Aire’s function in preventing effective anti-cancer immunity has recently been clarified (Figure 4). Aire’s role in preventing cancer immunity may be regarded as an extension of its role in preventing autoimmunity. Because many Aire-regulated self-antigens expressed by mTECs are also expressed by tumors, the antigen-specific tolerance induced by Aire also prevents an effective anti-tumor response 67, 68. mTECs express a number of well-known melanoma antigens 67, 68. For instance, Aire upregulates mTEC expression of TRP-1 and consequently the negative selection of TRP-1 specific T cells in mice68. In the setting of Aire deficiency, TRP-1 specific T cells are rescued from negative selection and the T cell immune response against these melanoma antigens is enhanced. With the larger pool of melanoma specific T cells, Aire deficient mice demonstrate reduced melanoma growth and increased survival 67, 68. These findings are likely to translate to patients, since human mTECs express a large number of known melanoma antigens 69. Furthermore, case-control studies have associated melanoma protection with Aire polymorphisms that decrease Aire expression 70.
Figure 4. Aire enforces central tolerance toward self-antigens.
A) In wildtype thymus, Aire in medullary thymic epithelial cells (mTECs), promotes ectopic expression of self-antigens (Ag), which include melanoma Ags. T cells recognizing these antigens undergo negative selection to enforce self-tolerance. B) In Aire deficienct thymus or thymus treated with anti-RANKL antibody, lack of self-antigen expression allows escape of self-reactive T cells from negative selection, which predisposes to autoimmunity.
In addition to melanoma, other cancer types may also be modulated by Aire. In mouse models of primary and transplanted sarcoma, negative regulation of Aire-expressing mTECs enhanced anti-tumor immunity 71. As described above, Aire also regulates Treg TCR clones that were originally isolated from prostate cancer, suggesting a role for Aire in regulating T cell immune response against prostate cancer 50. Thus, Aire is likely to have a broad effect on multiple cancer types.
Recently, our group also provided evidence that modulation of Aire-expressing mTEC’s may be a tractable approach for enhancing tumor-specific immune responses. Aire-expressing mTEC’s have an interesting property of turning over quickly as a cell population. Studies with both genetic and BrdU labeling have demonstrated that in adult mice, mTEC’s have a population half-life of approximately 12–14 days 61, 64. The pool of new Aire-expressing mTEC’s appears to be replenished through a process of mTEC maturation that involves RANK-RANK-Ligand signaling. Taking advantage of this knowledge, Khan et al. demonstrated that in vivo blockade of RANK-Ligand over a two week time window led to selective depletion of Aire-expressing mTEC’s over other thymic epithelial cell populations 72. Treated mice showed evidence of an aquired defect in thymic negative selection and in a TCR transgenic model, treated mice develop an enhanced anti-tumor response in a B16 melanoma model. Importantly, the loss of Aire-expressing cells was transient and the thymus could recover such cells after the removal of anti-RANK-Ligand antibody. Further work will be need to determine if this approach of altering thymic Aire-activity could complement the wide array of current treatments involving peripheral tolerance and checkpoint blockade 73 but this remains an open and exciting possibility.
Aire in GVHD
Aire mediated TSA upregulation in mTECs also impacts the development of chronic graft versus host disease (cGVHD), a major complication of allogeneic hematopoietic stem cell transplantation 74, 75. Autoimmune-like manifestations are a part of cGVHD, and patients who have developed acute graft versus host disease (aGVHD) are strongly predisposed to cGVHD. In aGVHD patients, donor T cell mediate immune destruction of particular recipient tissues, including the thymus. Interestingly, donor T cells in aGVHD appear to selectively target Aire-expressing mTECs within the recipient thymus. As a consequence, mTEC expression of TSAs, especially those restricted to tissues affected in cGVHD, are decreased in aGVHD 75. aGVHD is associated with impaired negative selection of self-reactive T cells, which allows the generation of T cells that can mediate autoimmunity associated with cGVHD74. Understanding that aGVHD induces an Aire-deficient state that predisposes to the generation of autoreactive T cells potentially allows for the development of interventions that may increase Aire expression to prevent cGVHD development.
Aire in tumorogenic keratinocytes
Aire is induced at low levels in mouse and human tumor keratinocytes, a cell type involved in human skin squamous cell carcinoma, and Aire mRNA induction in keratinocytes (but not mTECs) is dependent on keratin 17 (K17) 76. Furthermore, in keratinocytes, Aire protein interacts with K17 protein, and Aire mRNA interacts with ribonucleoprotein hnRNP K in a K17 dependent manner. Global K17 deficiency protected mice from skin tumorigenesis, and this protection was associated with decreased expression of neutrophil activation markers, dermal mast cell density, and expression of other proinflammatory genes. Thus, Aire’s pro-inflammatory role in keratinocytes undergoing tumorigenesis appears to be entirely distinct from its immunoregulatory role in mTECs in dampening anti-tumor immunity, and opens up a new field of investigation.
Conclusions
In the last few years, multiple, sometimes unexpected, roles for Aire beyond its best-known functions have been described. Aire’s role in selecting Tregs, its multiple effects that do not involve upregulation of mTEC TSAs, and the multiple pathologic processes governed by Aire have now been established. Furthermore, molecular insights into Aire’s function are now being clarified and account for some of the fascinating aspects by which it carries out its functional activity. Looking forward there is still much that needs to be learned about the biology by which this critical regulator of tolerance operates and how pathways it controls contribute to human disease.
References
- 1.Cheng MH, Anderson MS. Monogenic Autoimmunity. Annu Rev Immunol. 2011 doi: 10.1146/annurev-immunol-020711-074953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 3.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 4.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 5.Kuehn HS, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345:1623–7. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schubert D, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20:1410–6. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dystrophy TF-GACAP-C-E. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. The Finnish-German APECED Consortium. Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 8.Nagamine K, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–8. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 9.Anderson MS, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 10.Metzger TC, Anderson MS. Control of central and peripheral tolerance by Aire. Immunol Rev. 2011;241:89–103. doi: 10.1111/j.1600-065X.2011.01008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cetani F, et al. A novel mutation of the autoimmune regulator gene in an Italian kindred with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, acting in a dominant fashion and strongly cosegregating with hypothyroid autoimmune thyroiditis. J Clin Endocrinol Metab. 2001;86:4747–52. doi: 10.1210/jcem.86.10.7884. [DOI] [PubMed] [Google Scholar]
- 12.Su MA, et al. Mechanisms of an autoimmunity syndrome in mice caused by a dominant mutation in Aire. J Clin Invest. 2008;118:1712–26. doi: 10.1172/JCI34523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oftedal BE, et al. Dominant Mutations in the Autoimmune Regulator AIRE Are Associated with Common Organ-Specific Autoimmune Diseases. Immunity. 2015;42:1185–96. doi: 10.1016/j.immuni.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Pitkanen J, et al. The autoimmune regulator protein has transcriptional transactivating properties and interacts with the common coactivator CREB-binding protein. J Biol Chem. 2000;275:16802–9. doi: 10.1074/jbc.M908944199. [DOI] [PubMed] [Google Scholar]
- 15.Sansom SN, et al. Population and single-cell genomics reveal the Aire dependency, relief from Polycomb silencing, and distribution of self-antigen expression in thymic epithelia. Genome Res. 2014;24:1918–31. doi: 10.1101/gr.171645.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennecke P, et al. Single-cell transcriptome analysis reveals coordinated ectopic gene-expression patterns in medullary thymic epithelial cells. Nat Immunol. 2015;16:933–41. doi: 10.1038/ni.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meredith M, Zemmour D, Mathis D, Benoist C. Aire controls gene expression in the thymic epithelium with ordered stochasticity. Nat Immunol. 2015;16:942–9. doi: 10.1038/ni.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derbinski J, et al. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202:33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villasenor J, Besse W, Benoist C, Mathis D. Ectopic expression of peripheral-tissue antigens in the thymic epithelium: probabilistic, monoallelic, misinitiated. Proc Natl Acad Sci U S A. 2008;105:15854–9. doi: 10.1073/pnas.0808069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinto S, et al. Overlapping gene coexpression patterns in human medullary thymic epithelial cells generate self-antigen diversity. Proc Natl Acad Sci U S A. 2013;110:E3497–505. doi: 10.1073/pnas.1308311110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danso-Abeam D, et al. Aire mediates thymic expression and tolerance of pancreatic antigens via an unconventional transcriptional mechanism. Eur J Immunol. 2013;43:75–84. doi: 10.1002/eji.201242761. [DOI] [PubMed] [Google Scholar]
- 22.Abramson J, Giraud M, Benoist C, Mathis D. Aire's partners in the molecular control of immunological tolerance. Cell. 2010;140:123–35. doi: 10.1016/j.cell.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 23.Gaetani M, et al. AIRE-PHD fingers are structural hubs to maintain the integrity of chromatin-associated interactome. Nucleic Acids Res. 2012;40:11756–68. doi: 10.1093/nar/gks933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giraud M, et al. An RNAi screen for Aire cofactors reveals a role for Hnrnpl in polymerase release and Aire-activated ectopic transcription. Proc Natl Acad Sci U S A. 2014;111:1491–6. doi: 10.1073/pnas.1323535111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waterfield M, et al. The transcriptional regulator Aire coopts the repressive ATF7ip-MBD1 complex for the induction of immunotolerance. Nat Immunol. 2014;15:258–65. doi: 10.1038/ni.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Org T, et al. The autoimmune regulator PHD finger binds to non-methylated histone H3K4 to activate gene expression. EMBO Rep. 2008;9:370–6. doi: 10.1038/embor.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koh AS, et al. Aire employs a histone-binding module to mediate immunological tolerance, linking chromatin regulation with organ-specific autoimmunity. Proc Natl Acad Sci U S A. 2008;105:15878–83. doi: 10.1073/pnas.0808470105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chignola F, et al. The solution structure of the first PHD finger of autoimmune regulator in complex with non-modified histone H3 tail reveals the antagonistic role of H3R2 methylation. Nucleic Acids Res. 2009;37:2951–61. doi: 10.1093/nar/gkp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koh AS, Kingston RE, Benoist C, Mathis D. Global relevance of Aire binding to hypomethylated lysine-4 of histone-3. Proc Natl Acad Sci U S A. 2010;107:13016–21. doi: 10.1073/pnas.1004436107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zumer K, Low AK, Jiang H, Saksela K, Peterlin BM. Unmodified histone H3K4 and DNA-dependent protein kinase recruit autoimmune regulator to target genes. Mol Cell Biol. 2012;32:1354–62. doi: 10.1128/MCB.06359-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oven I, et al. AIRE recruits P-TEFb for transcriptional elongation of target genes in medullary thymic epithelial cells. Mol Cell Biol. 2007;27:8815–23. doi: 10.1128/MCB.01085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zumer K, Plemenitas A, Saksela K, Peterlin BM. Patient mutation in AIRE disrupts P-TEFb binding and target gene transcription. Nucleic Acids Res. 2011;39:7908–19. doi: 10.1093/nar/gkr527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giraud M, et al. Aire unleashes stalled RNA polymerase to induce ectopic gene expression in thymic epithelial cells. Proc Natl Acad Sci U S A. 2012;109:535–40. doi: 10.1073/pnas.1119351109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Incani F, et al. AIRE acetylation and deacetylation: effect on protein stability and transactivation activity. J Biomed Sci. 2014;21:85. doi: 10.1186/s12929-014-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saare M, Rebane A, Rajashekar B, Vilo J, Peterson P. Autoimmune regulator is acetylated by transcription coactivator CBP/p300. Exp Cell Res. 2012;318:1767–78. doi: 10.1016/j.yexcr.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Chuprin A, et al. The deacetylase Sirt1 is an essential regulator of Aire-mediated induction of central immunological tolerance. Nat Immunol. 2015;16:737–45. doi: 10.1038/ni.3194. [DOI] [PubMed] [Google Scholar]
- 37.Liiv I, et al. DNA-PK contributes to the phosphorylation of AIRE: importance in transcriptional activity. Biochim Biophys Acta. 2008;1783:74–83. doi: 10.1016/j.bbamcr.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rattay K, et al. Homeodomain-interacting protein kinase 2, a novel autoimmune regulator interaction partner, modulates promiscuous gene expression in medullary thymic epithelial cells. J Immunol. 2015;194:921–8. doi: 10.4049/jimmunol.1402694. [DOI] [PubMed] [Google Scholar]
- 39.Yang S, Bansal K, Lopes J, Benoist C, Mathis D. Aire's plant homeodomain(PHD)-2 is critical for induction of immunological tolerance. Proc Natl Acad Sci U S A. 2013;110:1833–8. doi: 10.1073/pnas.1222023110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Binda O. On your histone mark, SET, methylate! Epigenetics. 2013;8:457–63. doi: 10.4161/epi.24451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ju BG, et al. A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science. 2006;312:1798–802. doi: 10.1126/science.1127196. [DOI] [PubMed] [Google Scholar]
- 42.Zumer K, Saksela K, Peterlin BM. The mechanism of tissue-restricted antigen gene expression by AIRE. J Immunol. 2013;190:2479–82. doi: 10.4049/jimmunol.1203210. [DOI] [PubMed] [Google Scholar]
- 43.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–4. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 44.Anderson MS, et al. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–39. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 45.DeVoss J, et al. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203:2727–35. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taniguchi RT, et al. Detection of an autoreactive T-cell population within the polyclonal repertoire that undergoes distinct autoimmune regulator (Aire)-mediated selection. Proc Natl Acad Sci U S A. 2012;109:7847–52. doi: 10.1073/pnas.1120607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aschenbrenner K, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–8. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 48.Lei Y, et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med. 2011;208:383–94. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015;348:589–94. doi: 10.1126/science.aaa7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malchow S, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–24. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perry JS, et al. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity. 2014;41:414–26. doi: 10.1016/j.immuni.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guerau-de-Arellano M, Martinic M, Benoist C, Mathis D. Neonatal tolerance revisited: a perinatal window for Aire control of autoimmunity. J Exp Med. 2009;206:1245–52. doi: 10.1084/jem.20090300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuroda N, et al. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol. 2005;174:1862–70. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- 54.Chen Z, Benoist C, Mathis D. How defects in central tolerance impinge on a deficiency in regulatory T cells 10.1073/pnas.0507014102. PNAS. 2005;102:14735–14740. doi: 10.1073/pnas.0507014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pomie C, et al. Autoimmune regulator (AIRE)-deficient CD8+CD28low regulatory T lymphocytes fail to control experimental colitis. Proc Natl Acad Sci U S A. 2011;108:12437–42. doi: 10.1073/pnas.1107136108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Najafian N, et al. Regulatory functions of CD8+CD28- T cells in an autoimmune disease model. J Clin Invest. 2003;112:1037–48. doi: 10.1172/JCI17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menager-Marcq I, Pomie C, Romagnoli P, van Meerwijk JP. CD8+CD28- regulatory T lymphocytes prevent experimental inflammatory bowel disease in mice. Gastroenterology. 2006;131:1775–85. doi: 10.1053/j.gastro.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hubert FX, et al. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood. 2011;118:2462–72. doi: 10.1182/blood-2010-06-286393. [DOI] [PubMed] [Google Scholar]
- 59.Niki S, et al. Alteration of intra-pancreatic target-organ specificity by abrogation of Aire in NOD mice. J Clin Invest. 2006;116:1292–301. doi: 10.1172/JCI26971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laan M, et al. Autoimmune regulator deficiency results in decreased expression of CCR4 and CCR7 ligands and in delayed migration of CD4+ thymocytes. J Immunol. 2009;183:7682–91. doi: 10.4049/jimmunol.0804133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. 2007;204:2521–8. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kyewski B, Derbinski J. Self-representation in the thymus: an extended view. Nat Rev Immunol. 2004;4:688–98. doi: 10.1038/nri1436. [DOI] [PubMed] [Google Scholar]
- 63.Nishikawa Y, et al. Temporal lineage tracing of Aire-expressing cells reveals a requirement for Aire in their maturation program. J Immunol. 2014;192:2585–92. doi: 10.4049/jimmunol.1302786. [DOI] [PubMed] [Google Scholar]
- 64.Metzger TC, et al. Lineage tracing and cell ablation identify a post-Aire-expressing thymic epithelial cell population. Cell Rep. 2013;5:166–79. doi: 10.1016/j.celrep.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X, et al. Post-Aire maturation of thymic medullary epithelial cells involves selective expression of keratinocyte-specific autoantigens. Front Immunol. 2012;3:19. doi: 10.3389/fimmu.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yano M, et al. Aire controls the differentiation program of thymic epithelial cells in the medulla for the establishment of self-tolerance. J Exp Med. 2008;205:2827–38. doi: 10.1084/jem.20080046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trager U, et al. The immune response to melanoma is limited by thymic selection of self-antigens. PLoS One. 2012;7:e35005. doi: 10.1371/journal.pone.0035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu ML, Nagavalli A, Su MA. Aire deficiency promotes TRP-1-specific immune rejection of melanoma. Cancer Res. 2013;73:2104–16. doi: 10.1158/0008-5472.CAN-12-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gotter J, Brors B, Hergenhahn M, Kyewski B. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. J Exp Med. 2004;199:155–66. doi: 10.1084/jem.20031677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Conteduca G, et al. The role of AIRE polymorphisms in melanoma. Clin Immunol. 2010;136:96–104. doi: 10.1016/j.clim.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 71.Akiyama N, et al. Limitation of immune tolerance-inducing thymic epithelial cell development by Spi-B-mediated negative feedback regulation. J Exp Med. 2014;211:2425–38. doi: 10.1084/jem.20141207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khan IS, et al. Enhancement of an anti-tumor immune response by transient blockade of central T cell tolerance. J Exp Med. 2014;211:761–8. doi: 10.1084/jem.20131889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–14. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dertschnig S, Hauri-Hohl MM, Vollmer M, Hollander GA, Krenger W. Impaired thymic expression of tissue-restricted antigens licenses the de novo generation of autoreactive CD4+ T cells in acute GVHD. Blood. 2015;125:2720–3. doi: 10.1182/blood-2014-08-597245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dertschnig S, et al. Epithelial cytoprotection sustains ectopic expression of tissue-restricted antigens in the thymus during murine acute GVHD. Blood. 2013;122:837–41. doi: 10.1182/blood-2012-12-474759. [DOI] [PubMed] [Google Scholar]
- 76.Hobbs RP, et al. Keratin-dependent regulation of Aire and gene expression in skin tumor keratinocytes. Nat Genet. 2015 doi: 10.1038/ng.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]