Abstract
Purpose of review
To explore new data from recent studies addressing the role of co-infections in immune activation in HIV-1 infected patients, with a focus on Immune Reconstitution Inflammatory Syndrome (IRIS), an aberrant inflammatory response occurring shortly after antiretroviral therapy (ART) initiation.
Recent findings
Chronic HIV infection is associated with a number of co-infections that contribute to immune activation in various settings including early after ART initiation in the most noticeable form of IRIS and also in chronic treated infection, with chronic viral infections like CMV and HCV or HBV contributing to immune activation and also morbidity and mortality.
Expanding on older studies, the role of T cells in IRIS has been further elucidated with evidence of more pronounced effector activity in IRIS patients that may be leading to excessive tissue pathology. Newer studies are also continuing to shed light on the role of myeloid cells in IRIS as well as the contribution of antigen load in the syndrome. In addition, preliminary data are beginning to suggest a possible role of inflammasome formation in IRIS. In cryptococcal IRIS, the role of activated immune cells (T cell and myeloid) and biomarkers were evaluated in more detail at the site of infection (CSF). Finally, important differences of patients developing IRIS versus those who die from TB despite ART initiation were reported, a distinction that may have important implications for participant selection in studies aiming to prevent IRIS with immunosuppressive agents.
Summary
Better understanding of the role of opportunistic infections at ART initiation and IRIS pathogenesis will assist in improved strategies for prevention and treatment. The long-term consequences of IRIS remain unclear. Chronic viral coinfections with herpesviruses and HCV are important factors in persistent immune activation in chronic treated HIV.
Keywords: Immune reconstitution inflammatory syndrome, Tuberculosis, Biomarkers, Myeloid cells, Innate immunity
Introduction
Chronic HIV infection more than any other infectious disease is a disease of co-infections, as the HIV virus cripples the immune system and allows reactivation of dormant pathogens or increases susceptibility to exogenous pathogen[1]. These co-infections fuel further the immune activation that characterizes HIV infection, even after initiation of anti-retroviral therapy (ART). From the pre-ART era it was apparent that opportunistic infections including malignancies triggered by infectious agents like Kaposi’s sarcoma and EBV associated lymphomas were the hallmark of HIV [2]. Although in the current era morbidity and mortality by opportunistic diseases has decreased, albeit still significant [3,4], infections like Cytomegalovirus (CMV), tuberculosis, hepatitis C and B, Human Papillomavirus (HPV) are still at center stage of health issues in treated chronic HIV-infected persons [5] and remain important factors for the pathogenesis of serious non-AIDS events [6] as discussed in other reviews in this issue of Current.
Despite concrete evidence that HIV infection should be treated upon diagnosis even at CD4 T cell counts above 500 cells/μL [7], the sobering reality is that many patients present late at diagnosis or treatment initiation in both resource limited and resource rich settings [8]. According to US statistics, 23% of patients get diagnosed with HIV at stage 3 (AIDS) [9] and recent studies from South Africa [10] and Europe [11] report similar statistics with a persistently high proportion of patients presenting late for therapy initiation. The state of advanced immunosuppression in which HIV infected individuals present with at diagnosis makes these patients highly vulnerable to a variety of opportunistic infections, in particular those that live in endemic areas.
In this issue of Current dedicated to Immune Activation in HIV, several reviews have discussed co-infections mostly highlighting their important roles in serious non-AIDS events as well as the role of co-infections in sexual and vertical transmission. In this review the discussion will focus on acute inflammation and immune activation following antiretroviral therapy initiation in the form of immune reconstitution inflammatory syndrome or IRIS and briefly review recent data on contribution of chronic viral infection in immune activation in treated patients.
Co-infections in HIV
Co-infections are a main factor driving increased morbidity and mortality in HIV infected patients. Estimates from the World Health Organization (WHO) indicate that approximately one fourth of the 1.5 million deaths of HIV infected patients is attribute to tuberculosis [12]. Moreover, in both Europe and Northern America approximately 10% of patients present for therapy initiation with an AIDS defining illness most of them representing infections [11]. Among people with HIV in the United States, about 25% are co-infected with HCV, and near 10% are co-infected with HBV, while these percentages are higher in areas where preventive measures and therapy options are less advanced [13–16]. Cryptococcal antigen prevalence among persons with CD4+ counts below 100 cells/mm3 averages 7.2% (95% CI, 6.8–7.6%) in 37 studies of 14,815 patients from low and middle-income countries [17]. Given the immune suppression state of HIV patients, these and other co-infections can present with a wide range of clinical syndromes with atypical manifestations that pose challenges in diagnosis and clinical management, representing a substantial burden for the public health system.
With respect to chronic viral infections, CMV seroprevalence in HIV-infected population is high ranging from 75% to 90% [18]. In Africa, estimates of HSV-1 and HSV-2 seroprevalence among adult HIV-infected individuals vary from 65 to 90% and rates > 85% have also been reported for CMV and EBV in HIV-infected adult individuals from several African countries [19]. HIV-1 infected individuals have higher rates of HSV-2 antibodies compared to HIV-1 uninfected persons; 85% among HSV-2 and HIV-1 co-infected individuals in sub-Saharan Africa, 65% among men who have sex with men (MSM) in San Francisco and 80% of HIV-1 infected men in combined data from a US national survey [20]. It is thus apparent that the majority of HIV-infected persons have a co-infection at a minimum at some point during their course.
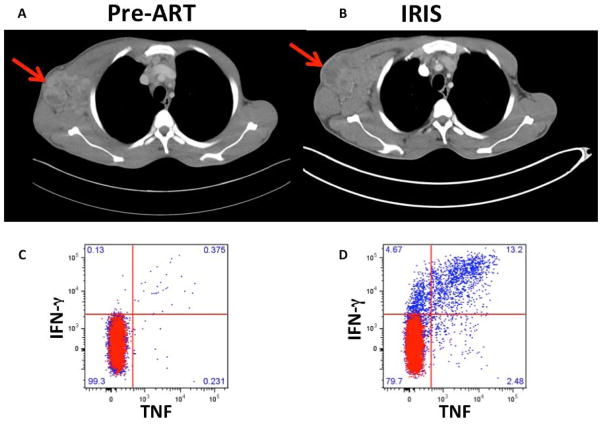
A particularly intriguing pathology related to immune activation in HIV patients with co-infections is a reaction referred to as immune reconstitution inflammatory syndrome (IRIS), which can affect approximately 20% of these patients, despite effective viral suppression induced by ART [21,22]. The initial description of IRIS preceded combination ART. A cluster of Mycobacterium avium complex (MAC) lymphadenitis, in some patients with fever, was described in an Australian cohort treated with zidovudine monotherapy and was thought to be immune restoration disease as many patients also converted their skin test (DTH) to reactive [23]. IRIS is considered an aberrant immune response to a known and treated (paradoxical) or occult pre-ART (unmasking) pathogen and despite overall control of the pathogen (cultures in paradoxical are frequently negative) it is characterized by tissue-destructive inflammation and arises as functional CD4 (+) T cells against the opportunistic pathogen emerge [24,25]. Mycobacterial IRIS is commonly manifested by necrotizing lymphadenopathy (Figure 1A and B), worsening systemic symptoms including fevers or respiratory symptoms [26]. Cryptococcal IRIS frequently affects the CNS and can lead to inflammation and increased intracranial pressure and can be life threatening [27].
Figure 1.
Computerize Tomography (CT) images and CD4 T cell responses of a patient with paradoxical TB-IRIS. A) Chest CT image prior to ART initiation demonstrating significant axillary lymph node enlargement with areas of necrosis (arrow). B) CT images after 2 weeks of ART demonstrating worsening lymphadenopathy (arrow) despite negative TB cultures of lymph node aspirate. C & D) TB-specific CD4 T cell production of TNF and IFNγ after ex vivo stimulation with PPD pre-ART and at the IRIS event respectively showing dramatic increase of TNF and IFNγ production at the IRIS timepoint.
Understanding key immune determinants of the clinical outcomes in co-infections with HIV, both early after ART initiation and during chronic ART, is critical for driving development of innovative therapeutic strategies.
Recent findings in IRIS
Tuberculosis
Studies in the past had highlighted an important role for mycobacterial antigen-driven expansion of TB-specific CD4 T cells in TB IRIS [28–30] (Figure 1C and D). The granulocytic activity of NK cells was also described as important predictor of paradoxical TB IRIS [31]. In a recent study, the role of T cells was further explored by Haridas et al who found that high pre-ART T cell frequencies of HLA-DR, CD45RO, CCR5 and OX40 expressing CD4 T cells, and Fas effector memory CD8 T cells were associated significantly with TB-IRIS development [32]. Interestingly, after ART treatment initiation, the CD4 T cell memory was skewed towards effector-memory phenotype [32]. In another study, it was postulated that low CD8+ T cell activation (HLA-DR+/CD38+) could be a predisposing factor of both early and late onset TB-IRIS events and that late but not early onset IRIS showed a shift from memory to effector CD8+ and CD4+ T cells after ART initiation [33]. Wilkinson KA et al, studied also the effector function of TB-specific CD4 T cells and demonstrated an increase in IFN-gamma response as well as in perforin 1 and granzyme B expression in heat-killed H37Rv stimulated human PBMCs from paradoxical TB-IRIS patients compared to HIV-TB co-infected non-IRIS patients [34] further highlighting the effector function of TB-specific cells.
Many cytokines that were elevated in patients with TB IRIS had long suggested an important role for innate cells of myeloid origin [22,35], and preliminary studies had pointed to monocytes as cells of interest in IRIS pathogenesis [36,37]. In a newer study by Andrade et al, the role of monocytes in TB IRIS was further delineated [38]. Specific pro-inflammatory biomarkers of innate and myeloid cell activation appeared to be elevated in TB-IRIS patients and more importantly a clear activation of innate responses was seen in TB-HIV co-infected patients pre-ART who had received <4 weeks of anti-TB therapy. In addition, the potential predictive value of CD14++CD16- monocyte frequency and plasma levels of CRP, TNF, IL-6, tissue factor and soluble (s) CD14 for TB-IRIS were highlighted in this and other studies [38–40]. The monocytes seem to be directly involved in production of pro-inflammatory cytokines such as TNF and IL-6 [39]. These cytokines, core participants in immunopathology of IRIS, could also potentially represent important targets for intervention as suggested in a recent case series of patients with mycobacterial IRIS that was refractory to corticosteroids, who were treated with infliximab [41]. Furthermore, IL-18 and CXCL10 have been presented as candidate biomarkers for predicting both paradoxical and unmasking TB-IRIS [40], suggesting a potential role of the inflammasome, which was further supported in a study of gene array analysis pre/post ART in TB patients who developed paradoxical TB-IRIS [42]. This observation should be further followed up by studies dissecting the mechanisms and assessing how a high antigen load during T cell functional recovery may trigger inflammasome activation.
In a novel study that focused primarily on a mouse model of TB studying the role of IL-27 in TB, in vitro Mycobacterium tuberculosis (Mtb) stimulated PBMCs from patients with paradoxical TB-IRIS we found to transcribe more IL-27p28 than PBMCs from HIV/TB co-infected patients without IRIS [43]. Plasma IL-27p28 subunit level was higher in those who developed IRIS compared with those who did not before ART initiation, suggesting a potential role of IL-27 in development of IRIS [43]. This is of particular interest as IL-27 is produced by myeloid cells, and may represent an important immune checkpoint in tuberculosis [44].
In a prospective cohort study at 22 public clinics and the main public hospital in Gaborone, Botswana, the immunological profile at baseline and week-4 after ART initiation in ART-naive adults (aged ≥21 years) with advanced HIV (CD4 cell counts ≤125 cells per μL) and pulmonary tuberculosis was assessed [45]. A main comparison was made between tuberculosis-associated IRIS or early mortality with those who survived without an IRIS diagnosis (controls) in the 6 months after ART initiation. Patients with paradoxical TB-IRIS had decreased pre-ART inflammatory profile but the groups of IRIS and deaths were distinguished by sharply divergent recovery of the adaptive immune system (TB-IRIS patients had similar increases in CD4 cell count and purified protein derivative-specific immune responses with controls, whereas those who died had minimal immune recovery despite virological control during ART). These data suggest that patients who die of TB despite ART initiation are distinct from patients who go on to develop TB-IRIS and may not benefit or may be harmed by strategies to immunosuppress HIV/TB co-infected patients but may in fact benefit for immune boosting strategies [45]. The results of the ongoing randomized controlled clinical trial of prednisone for prevention of TB-IRIS will probably shed light on this specific issue [Preventing TB-IRIS in high-rick patients: a randomized placebo-controlled trial of prednisone (pred-ART), ClinicalTrials.gov Identifier: NCT01924286].
A consistent observation has been that IRIS is more common when TB was pre-treated for a shorter interval prior to ART initiation suggesting that longer treatment of TB could ameliorate the complication of IRIS [39,46,47]. Several randomized controlled trials in mostly resource limited settings though have showed that overall mortality was higher with delaying ART in patients with lower CD4 T cell counts despite the higher incidence of IRIS [48–50]. A recent published systematic review and meta-analysis assessed the effect of early initiation of ART (within 2–4 weeks of TB treatment) on TB/HIV co-infection outcome. Early treatment was shown to be beneficial by reducing all-cause mortality, but it did increase the risk for TB-IRIS and death related to TB-IRIS in these patients [51].
Finally, in the international multi-center randomized study conducted by the INSIGHT START group, initiation of ART in naïve-treated HIV+ individuals with CD4+ T cell count more than 500 cells/mm3 compared to deferred initiation in 350 cells/mm3 resulted in reduced risk for tuberculosis infection both in high and low-moderate income countries [52]. In addition, higher percentage of patients achieved long-term low HIV RNA load as well as higher mean CD4+ count. All these factors could influence the predisposition for paradoxical or unmasking IRIS associated with TB infection if widespread early initiation of ART is achieved.
Cryptococcus
Many studies have focused on exploring specific immune responses in cryptococcal meningitis (CM), which remains the leading cause of mortality among Immune reconstitution inflammatory syndrome cases and a significant cause of mortality in HIV infected individuals.
In a study conducted in sub-Saharan Africa, an area that holds the highest burden of CM related death among the HIV population, Boulware et al. showed definitively that deferring ART treatment for 5 weeks after CM diagnosis compared with early initiation at 1 to 2 weeks significantly improves survival [53]. Interestingly, the hazard ratio was even higher among patients with fewer white cells in their cerebrospinal fluid at randomization (<5 per cubic millimeter). Cryptococcal IRIS events did not differ significantly between the earlier-ART group and the deferred-ART group. Cryptococcal meningitis–related deaths occurred between 2 and 5 weeks after diagnosis (10 in the earlier-ART group and 3 in the deferred ART group) and were judged to be caused by the initial cryptococcosis rather than separate, distinct cryptococcal-IRIS events even though they hypothesized that earlier ART is most harmful in high-risk persons with a predisposition to cryptococcal IRIS (i.e., those with low CSF WCC) [53]. In their cohort, the COAT Team provided evidence that early ART initiation in CM increased CSF cellular infiltrate (CSF WCC ≥5/μL) by day 14 as well as CSF interleukin-13, sCD14, sCD163, and CCL3/MIP-1α, biomarkers of macrophage/microglial activation, and T helper 2 responses [54].
Meya et al. undertook an analysis of CSF from Ugandan patients with HIV-associated cryptococcal meningitis and demonstrated that CM-IRIS was associated with an increasing frequency of CSF CD4+T cells and NK cells expressing PD-L1 (programmed death ligand 1) and migration of intermediate monocytes to the CSF [55]. Jarvis et al. demonstrated that cytokine responses of IFNγ (Th1), IL4 and IL10 (Th2) and IL17 (Th17) in the CSF of patients with HIV-associated CM were linked to increased macrophage activation, more rapid clearance of cryptococci from CSF, and survival at 2 weeks with IL-6 to contribute the most to this protective effect [56]. Strikingly, chemokines consisting primarily of monocyte chemotactic protein-1 (MCP-1) and macrophage inflammatory protein-1α (MIP-1α) were identified as predictive markers of IRIS.
With respect to unmasking CM or TB, in a clinical, open-label, randomized controlled trial conducted in Tanzania and Zambia, investigators tried to assess the effect of advanced health-care delivery strategy as well as the addition of screening for cryptococcus in ART naïve HIV infected individuals’ outcome [17]. Even though most participants presented with advanced stage 3 HIV infection, prompt ART initiation strategy, screening for serum cryptococcal antigen and tuberculosis, supplementation of clinic-based care to the standard care with a short period of adherence support and monitoring in the community reduced all-cause mortality by nearly 30% [17]. Of note, even in the group treated for cryptoccocal infection the adjusted rate of mortality remained higher than cryptoccocal antigen negative individuals.
Viral IRIS
Viral IRIS remains less well investigated and may have different pathogenesis aspects that are not clear to date. Evidence for a role of high antigen load has been observed in both hepatitis flares [57] and in Kaposi sarcoma IRIS after ART initiation [58] but studies looking specifically at viral IRIS pathogenesis have not been published recently.
Gianella et al. investigated CMV vaginal shedding longitudinally among women co-infected with HIV, HSV-2 and CMV, who started ART and were participants in the placebo-controlled trial of HSV-2 suppression with acyclovir in Uganda [59]. Based on their results, younger age as well as higher pre-ART HIV RNA viral load (>100,000 copies/ml), were associated with increased frequency of CMV shedding. Importantly, prevalence of CMV DNA shedding was higher after ART initiation, with peak being observed in months 2–4 post ART suggesting the possibility of an immune reconstitution related reactivation of the virus. CMV can cause clear IRIS events upon ART initiation as highlighted in two recent case reports of acute appendicitis and sialadenitis occurring shortly after ART initiation [60,61].
There is also evidence that ART initiation can increase the rate of HSV-2 shedding and genital ulcer disease during the first 1–3 months of treatment, particularly among women with low CD4+ counts at ART initiation, possibly as a form of immune reconstitution [62]. However, a recently published study conducted in Burkina Faso in women living with HIV-1, coinfected with HSV-2, showed that cervico-vaginal HSV-2 shedding declined while on ART along with a reduction of genital ulcer disease; with the latter being associated mainly with HIV-1 viral suppression [63].
With respect to clinical trials attempting to prevent IRIS, a large randomized double-blinded controlled clinical trial in immunosuppressed (CD4 <100 cells/μL) patients testing maraviroc versus placebo, with the goal to decrease trafficking of effector cells to tissues thus preventing inflammation, failed to prevent IRIS or decrease severity of IRIS events [64]. Of note, maraviroc use did not affect the outcome of opportunistic infections in the cohort, many of who suffered from TB.
Co-infections: ongoing inflammation in chronic HIV infection
The role of co-infections in chronic treated HIV infection and their contribution in morbidity and mortality and pathogenesis of SNAEs has been highlighted in other reviews in this issue of Current. The presence of opportunistic infections at initial presentation of HIV-infected patients is not rare yet their contribution in higher residual chronic inflammation and SNAEs after long-treatment with ART has not been systematically studied.
In a South-African HIV-infected population study, Sullivan et al. showed that patients with active TB infection have elevated levels of soluble inflammatory biomarkers associated with monocyte activation, as well as surface markers of T-cell activation compared to HIV− mono-infected individuals [65]. They also presented that co-expression of CD38 and HLA- DR on CD4+ and CD8+ T cells is higher in HIV infected individuals with latent TB compared to those with no TB and raised again the issue about more widespread treatment of HIV patients with LTBI. The patients with active TB infection had lower CD4 T cell counts so it was unclear if the higher levels of activation could be attributed to lymphopenia, yet the question remains whether these patients have higher residual inflammation and cellular activation after years of successful ART.
CMV has been systematically studied as a contributing co-infection in ageing and cardiovascular disease along with the effect of the virus on immune activation and especially on immune senescence. Lichtner M. et al. demonstrated that HIV-1 infected individuals from ICONA study with CMV IgG seropositivity, without active CMV disease were associated with an increased risk of non-AIDS-defining event/death and an adjusted hazard ratio of 2.27 for cardiovascular and cerebrovascular diseases [18]. The potential role of CMV-specific immune responses (both T cell and antibodies) in cardiovascular disease has been highlighted in several studies in the past [66–68]. In an exposure-matched, small cross-sectional study of low cardiovascular disease risk (nonsmokers), HIV-infected patients, however, increased carotid intima-media thickness was not associated with CMV-seropositivity [69]. The study emphasized the important role of comorbidities but sample size may have been insufficient to detect differences.
The role of CMV has also been implicated in T cell senescence and ageing with accumulation of CD28-CD57+ CD8 T cells [70]. Lee et al. reported that untreated HIV+/CMV+ patients had lower proportions of CD28− CD8+ cells expressing CD57 and were enriched for less well-differentiated CD28− transitional memory (TTR) CD8+ T cells relatively to HIV-uninfected CMV+ persons highlighting important differences on T cells between mono and dually infected persons that may have significance in SNAEs [71]. Unfortunately, they did not study these factors between HIV+/CMV− and HIV+/CMV+ groups. Freeman, Mudd et al, did compare CMV seropositive and CMV seronegative HIV+ patients and detected CD8+ T cell expansion in CMV co-infected patients compared to CMV-, along with higher plasma levels of IP-10, TNF-RII, and D-dimer. In addition, co-infection with HIV and CMV resulted in a significantly lower CD4/CD8 ratio than was seen among HIV-infected CMV-seronegative subjects [72]. Finally, in a small study of HIV+ adults, valganciclovir was efficacious in suppressing CMV DNA and reducing CD8 T cell activation (percent expressing CD38+HLA-DR+), suggesting not only that CMV replication contributes to immune activation in co-infected individuals, but also that pharmacologic suppression of CMV may help reverse this process [73]. It remains though unclear if persistent CMV suppression could have clinical benefits.
Regarding the role of other herpesviruses, several studies have attempted to assess the role of HSV-2 co-infection in HIV disease progression providing evidence that genital shedding is a cofactor for increased HIV replication and that acyclovir/valacyclovir could be helpful in HIV suppression at least at the local mucosal level [25,74–78]. Redd et al. tried to explore the effect of acyclovir on immune activation status in HIV-1/HSV-2 ART-naïve coinfected women and demonstrated a relatively small decrease of sCD14 but at a significantly faster rate in acyclovir treated compared to the untreated group, independently of HIV viral load and CD4+ cell count suggesting a role for monocyte activation during HSV-2 reactivation [79]. In ART treated patients though a randomized controlled trial of valacyclovir for 12 weeks to suppress HSV-2 showed no effect on cellular immune activation or systemic inflammatory markers suggesting that the overall impact on systemic immune activation of treated patients may be small or transient [80]. Prospective clinical trials will contribute to further determine whether HSV suppressive therapies could help in lowering more effectively plasma and genital HIV viral load and local and/or systemic immune activation by using them together with ART in HIV/HSV-2 co-infected patients. Finally, HHV8 may also have an impact in chronic immune activation of chronically treated HIV patients as suggested by a study in 157 patients that reported an independent effect of HHV8 seropositivity on higher CRP and higher CD4 and CD8 T cell activation levels but not on flow dilatation or carotid intima media thickness [81].
Hepatitis C is an important co-infection that impacts morbidity and mortality in HIV infection and has implications not only for liver disease including cirrhosis and hepatocellular carcinoma but also for extrahepatic comorbidities in HIV [82,83]. In a recently published retrospective large cohort of 1153 patients, HIV/HCV coinfection was found to be independently associated with cardiovascular events (HR 2.91; CI 95%: 1.19–7.12; P = 0.02) compared to HCV or HIV monoinfection after adjusting for demographic, virologic and traditional cardiac risk factors [84]. This is consistent with a cross-sectional study from the MACS cohort that found HCV/HIV co-infection to be an independent risk factor for subclinical atherosclerosis assessed by non-contrast CT and CT angiography [85]. A small study reported evidence of higher tissue factor activity (measured as microparticles tissue factor), a procoagulant factor, and associated CD4 T cell activation in HCV patients both mono- and HIV-coinfected suggesting a potential mechanism of immune activation and coagulopathy that may predispose to cardiovascular disease [86]. In another prospective study, immune activation markers were compared between HIV monoinfecetd and HIV/HCV coinfected (with and without cirrhosis) ART treated patients with suppressed plasma HIV viremia during 12 months [87]. HIV patients with chronic hepatitis and cirrhosis had higher concentrations of serum sCD14 and IL-6 and incomplete CD4 restoration. Although the percent of activated CD4+ and CD8+ T cells and Treg were higher in HIV-infected patients compared to healthy controls, no significant differences were observed among the groups of HIV patients [87]. In a different study, biomarkers of inflammation including INFa were found to be higher in HCV/HIV co-infected patients compared to HCV mono-infected patients but importantly, some decreased after successful sustained virologic suppression (SVR) of hepatitis C [88]. Decreases in sCD163 and sICAM were also noted 24 weeks after HCV treatment with pegylated interferon/ribavirin in another cohort of 54 patients [89]. It is thus possible that newer, more effective, HCV regimens for HIV patients [90] may favorably impact the chronic immune activation and inflammation of HIV/HCV coinfected patients with a potential subsequent decrease not only of liver morbidity and mortality but also of SNAEs in HCV coinfected persons.
Summary
Co-infections play a fundamental role in immune activation in all stages of HIV infection. This can range from emergence of IRIS early after ART initiation in patients with advanced disease and opportunistic infection, to higher risk of cardiovascular disease in chronically treated HIV patients with CMV or HCV coinfection and to higher HIV transmission potential in patients with HSV shedding. Better understanding of the immune activation pathways involved in HIV coinfections will be instrumental in designing appropriate therapeutic strategies.
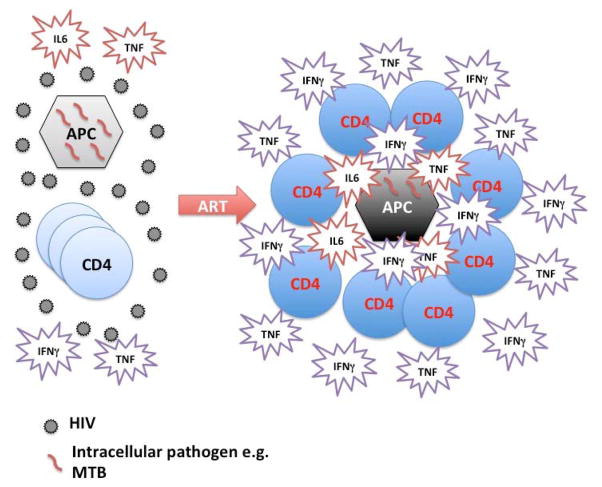
Figure 2.
Chronic HIV infection and CD4 lymphopenia is characterized by defective antigen presenting cell (APC) function as well as deficient in numbers and function pathogen specific CD4 T cells leading to ineffective clearance and pathogen accumulation. ART introduction improves both the function of APC and enhances both the number and function and possibly trafficking of pathogen specific CD4 T cells. This potentially asynchronous recovery of innate and adaptive responses leads at times to a dysregulated hyperinflammatory phenomenon known as IRIS.
Key points.
The vast majority of HIV-infected persons have one or more confections (including CMV and other herpesviruses, HCV or tuberculosis) at least at some point during their course. These coinfections play an important role in chronic immune activation and pathogenesis of SNAEs but the mechanisms are not always clear.
A significant proportion of HIV-infected persons get diagnosed at late stages of disease frequently with an opportunistic disease such as tuberculosis. Earlier initiation of ART could prevent most opportunistic diseases.
IRIS represents a dysregulated inflammatory response that occurs shortly after ART initiation in HIV patients with lymphopenia and infections and is characterized by profound T cell effector function recovery and innate immune activation (Figure 2). Understanding better the mechanisms of IRIS will help design targeted interventions.
Acknowledgments
Funding statement: The work of the authors was supported by the Intramural Research Program, National Institutes of Allergy and Infectious Diseases, National Institutes of Health.
The authors would like to thank Dr Bruno Andrade for helpful comments.
Footnotes
Conflicts of interest. The authors have no conflicts of interest to declare.
References
- 1.Zanoni BC, Gandhi RT. Update on opportunistic infections in the era of effective antiretroviral therapy. Infect Dis Clin North Am. 2014;28:501–518. doi: 10.1016/j.idc.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE National Institutes of H, Centers for Disease C Prevention America HIVMAotIDSo. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated Guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58:1308–1311. doi: 10.1093/cid/ciu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Djawe K, Buchacz K, Hsu L, Chen MJ, Selik RM, Rose C, Williams T, Brooks JT, Schwarcz S. Mortality Risk After AIDS-Defining Opportunistic Illness Among HIV-Infected Persons-San Francisco, 1981–2012. J Infect Dis. 2015;212:1366–1375. doi: 10.1093/infdis/jiv235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masur H, Read SW. Opportunistic Infections and Mortality: Still Room for Improvement. J Infect Dis. 2015;212:1348–1350. doi: 10.1093/infdis/jiv236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masur H. HIV-Related Opportunistic Infections Are Still Relevant in 2015. Top Antivir Med. 2015;23:116–119. [PMC free article] [PubMed] [Google Scholar]
- 6.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Group ISS, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, Avihingsanon A, Cooper DA, Fatkenheuer G, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. Immediate-ART initiation group with a CD4+ count of more than 500 cells/mm3 provided net benefits over starting such therapy in patients after the CD4+ count had declined to 350 cells/mm3. Overall few TB events occurred, 6 in the immediate and 20 in the deferred arm but the difference was highly significant (P=0.008). This study highlighted that earlier initiation of ART will effectively minimize occurrence of AIDS events. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IeDea Collaborations ARTC. Avila D, Althoff KN, Mugglin C, Wools-Kaloustian K, Koller M, Dabis F, Nash D, Gsponer T, et al. Immunodeficiency at the start of combination antiretroviral therapy in low, middle-, and high-income countries. J Acquir Immune Defic Syndr. 2014;65:e8–16. doi: 10.1097/QAI.0b013e3182a39979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley H, Hall HI, Wolitski RJ, Van Handel MM, Stone AE, LaFlam M, Skarbinski J, Higa DH, Prejean J, Frazier EL, et al. Vital Signs: HIV diagnosis, care, and treatment among persons living with HIV--United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63:1113–1117. [PMC free article] [PubMed] [Google Scholar]
- 10*.Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013: a meta-analysis. Clin Infect Dis. 2015;60:1120–1127. doi: 10.1093/cid/ciu1137. CD4 counts at HIV diagnosis and ART initiation were evaluated between 2002 and 2013 in Sub-Saharan Africa. At the start of the observation period the median CD4 at presentation was 251 cells/μL and at ART initiation 152 cells/μL, and there was no statistically significant change at the end of the observation period. In South Africa specifically, CD4 count at diagnosis increased by 39 cells/year but CD4 counts at treatment initiation remained the same. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mocroft A, Lundgren JD, Sabin ML, Monforte A, Brockmeyer N, Casabona J, Castagna A, Costagliola D, Dabis F, De Wit S, et al. Risk factors and outcomes for late presentation for HIV-positive persons in Europe: results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE) PLoS Med. 2013;10:e1001510. doi: 10.1371/journal.pmed.1001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. Global Tuberculosis Report. 2015. [Google Scholar]
- 13.Phung BC, Sogni P, Launay O. Hepatitis B and human immunodeficiency virus co-infection. World J Gastroenterol. 2014;20:17360–17367. doi: 10.3748/wjg.v20.i46.17360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melhem NM, Rahhal N, Charide R, Kreidieh K, El-Khatib R. Human immunodeficiency virus and viral hepatitis among high-risk groups: Understanding the knowledge gap in the Middle East and North Africa Region. World J Hepatol. 2015;7:2619–2630. doi: 10.4254/wjh.v7.i25.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data - United States and 6 dependent areas -2013. Supplemental report. 2015;20 Available at http://www.cdc.gov/hiv/library/reports/surveillance. [Google Scholar]
- 16.Chen JY, Feeney ER, Chung RT. HCV and HIV co-infection: mechanisms and management. Nat Rev Gastroenterol Hepatol. 2014;11:362–371. doi: 10.1038/nrgastro.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajasingham R, Meya DB, Boulware DR. Integrating cryptococcal antigen screening and pre-emptive treatment into routine HIV care. J Acquir Immune Defic Syndr. 2012;59:e85–91. doi: 10.1097/QAI.0b013e31824c837e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Lichtner M, Cicconi P, Vita S, Cozzi-Lepri A, Galli M, Lo Caputo S, Saracino A, De Luca A, Moioli M, Maggiolo F, et al. Cytomegalovirus coinfection is associated with an increased risk of severe non-AIDS-defining events in a large cohort of HIV-infected patients. J Infect Dis. 2015;211:178–186. doi: 10.1093/infdis/jiu417. In HIV-1 infected individuals from the ICONA study, CMV IgG seropositivity with no acute disease was an independent risk factor with an adjusted hazard ratio of 2.27 for cardiovascular and cerebrovascular diseases. [DOI] [PubMed] [Google Scholar]
- 19.Schaftenaar E, Verjans GM, Getu S, McIntyre JA, Struthers HE, Osterhaus AD, Peters RP. High seroprevalence of human herpesviruses in HIV-infected individuals attending primary healthcare facilities in rural South Africa. PLoS One. 2014;9:e99243. doi: 10.1371/journal.pone.0099243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnabas RV, Celum C. Infectious co-factors in HIV-1 transmission herpes simplex virus type-2 and HIV-1: new insights and interventions. Curr HIV Res. 2012;10:228–237. doi: 10.2174/157016212800618156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;48:101–107. doi: 10.1086/595006. [DOI] [PubMed] [Google Scholar]
- 22.Sereti I, Rodger AJ, French MA. Biomarkers in immune reconstitution inflammatory syndrome: signals from pathogenesis. Curr Opin HIV AIDS. 2010;5:504–510. doi: 10.1097/COH.0b013e32833ed774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.French MA, Mallal SA, Dawkins RL. Zidovudine-induced restoration of cell-mediated immunity to mycobacteria in immunodeficient HIV-infected patients. AIDS. 1992;6:1293–1297. doi: 10.1097/00002030-199211000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Barber DL, Andrade BB, Sereti I, Sher A. Immune reconstitution inflammatory syndrome: the trouble with immunity when you had none. Nature reviews Microbiology. 2012;10:150–156. doi: 10.1038/nrmicro2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang CC, Sheikh V, Sereti I, French MA. Immune reconstitution disorders in patients with HIV infection: from pathogenesis to prevention and treatment. Curr HIV/AIDS Rep. 2014;11:223–232. doi: 10.1007/s11904-014-0213-0. [DOI] [PubMed] [Google Scholar]
- 26.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, Elliott JH, Murdoch D, Wilkinson RJ, Seyler C, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. The Lancet infectious diseases. 2008;8:516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haddow LJ, Colebunders R, Meintjes G, Lawn SD, Elliott JH, Manabe YC, Bohjanen PR, Sungkanuparph S, Easterbrook PJ, French MA, et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10:791–802. doi: 10.1016/S1473-3099(10)70170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bourgarit A, Carcelain G, Martinez V, Lascoux C, Delcey V, Gicquel B, Vicaut E, Lagrange PH, Sereni D, Autran B. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 29.Mahnke YD, Greenwald JH, DerSimonian R, Roby G, Antonelli LR, Sher A, Roederer M, Sereti I. Selective expansion of polyfunctional pathogen-specific CD4(+) T cells in HIV-1-infected patients with immune reconstitution inflammatory syndrome. Blood. 2012;119:3105–3112. doi: 10.1182/blood-2011-09-380840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meintjes G, Wilkinson KA, Rangaka MX, Skolimowska K, van Veen K, Abrahams M, Seldon R, Pepper DJ, Rebe K, Mouton P, et al. Type 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med. 2008;178:1083–1089. doi: 10.1164/rccm.200806-858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pean P, Nerrienet E, Madec Y, Borand L, Laureillard D, Fernandez M, Marcy O, Sarin C, Phon K, Taylor S, et al. Natural killer cell degranulation capacity predicts early onset of the immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients with tuberculosis. Blood. 2012;119:3315–3320. doi: 10.1182/blood-2011-09-377523. [DOI] [PubMed] [Google Scholar]
- 32**.Haridas V, Pean P, Jasenosky LD, Madec Y, Laureillard D, Sok T, Sath S, Borand L, Marcy O, Chan S, et al. TB-IRIS, T-cell activation, and remodeling of the T-cell compartment in highly immunosuppressed HIV-infected patients with TB. AIDS. 2015;29:263–273. doi: 10.1097/QAD.0000000000000546. In a large cohort of 154 Cambodian severely immunocompromised HIV/TB co-infected patients, development of TB-IRIS was associated with significantly greater pre-ART frequencies of HLA-DR+ CD45RO+CD4, CCR5+CD4, OX40+CD4, and Fas effector memory CD8 T cells, and significantly elevated levels of plasma interleukin IL-6, IL-1beta, IL-8, and IL-10, and viral load. This study highlighted the combination of innate and adaptive (T cell) activation as important features of TB-IRIS pathogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goovaerts O, Jennes W, Massinga-Loembe M, Ondoa P, Ceulemans A, Vereecken C, Worodria W, Mayanja-Kizza H, Colebunders R, Kestens L, et al. Lower Pre-Treatment T Cell Activation in Early- and Late-Onset Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. PLoS One. 2015;10:e0133924. doi: 10.1371/journal.pone.0133924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Wilkinson KA, Walker NF, Meintjes G, Deffur A, Nicol MP, Skolimowska KH, Matthews K, Tadokera R, Seldon R, Maartens G, et al. Cytotoxic mediators in paradoxical HIV-tuberculosis immune reconstitution inflammatory syndrome. J Immunol. 2015;194:1748–1754. doi: 10.4049/jimmunol.1402105. Peripheral blood mononuclear cells isolated from TB-IRIS patients and controls were stimulated ex vivo with heat-killed Mycobacterium tuberculosis H37Rv and production of IFNγ was measured by ELISPOT and RNA from PBMC was isolated. INFg was higher in PBMC derived from IRIS patients compared to controls and RNA analysis pointed perforin 1 and granzyme B as the top genes indicating an important role of the granule exocytosis pathway in IRIS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grant PM, Komarow L, Lederman MM, Pahwa S, Zolopa AR, Andersen J, Asmuth DM, Devaraj S, Pollard RB, Richterman A, et al. Elevated interleukin 8 and T-helper 1 and T-helper 17 cytokine levels prior to antiretroviral therapy in participants who developed immune reconstitution inflammatory syndrome during ACTG A5164. The Journal of infectious diseases. 2012;206:1715–1723. doi: 10.1093/infdis/jis604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tran HT, Van den Bergh R, Vu TN, Laukens K, Worodria W, Loembe MM, Colebunders R, Kestens L, De Baetselier P, Raes G, et al. The role of monocytes in the development of Tuberculosis-associated Immune Reconstitution Inflammatory Syndrome. Immunobiology. 2014;219:37–44. doi: 10.1016/j.imbio.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Tran HT, Van den Bergh R, Loembe MM, Worodria W, Mayanja-Kizza H, Colebunders R, Mascart F, Stordeur P, Kestens L, De Baetselier P, et al. Modulation of the complement system in monocytes contributes to tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2013;27:1725–1734. doi: 10.1097/QAD.0b013e328361648b. [DOI] [PubMed] [Google Scholar]
- 38**.Andrade BB, Singh A, Narendran G, Schechter ME, Nayak K, Subramanian S, Anbalagan S, Jensen SM, Porter BO, Antonelli LR, et al. Mycobacterial antigen driven activation of CD14++CD16- monocytes is a predictor of tuberculosis-associated immune reconstitution inflammatory syndrome. PLoS Pathog. 2014;10:e1004433. doi: 10.1371/journal.ppat.1004433. In a prospective study of culture-positive TB HIV patients in India the role of myeloid cell-derived cytokines and chemokines in TB-IRIS was elucidated and CD14++CD16- monocytes were an independent predictor of paradoxical TB-IRIS and producers of key inflammatory cytokines (IL-1, IL-6 and TNF). This study directly implicated monocytes in TB-IRIS pathogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narendran G, Andrade BB, Porter BO, Chandrasekhar C, Venkatesan P, Menon PA, Subramanian S, Anbalagan S, Bhavani KP, Sekar S, et al. Paradoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in prediction. PLoS One. 2013;8:e63541. doi: 10.1371/journal.pone.0063541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40*.Tan HY, Yong YK, Andrade BB, Shankar EM, Ponnampalavanar S, Omar SF, Narendran G, Kamarulzaman A, Swaminathan S, Sereti I, et al. Plasma interleukin-18 levels are a biomarker of innate immune responses that predict and characterize tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2015;29:421–431. doi: 10.1097/QAD.0000000000000557. Proinflammatory cytokines and chemokines were assessed among HIV patients with 1) paradoxical or unmasking TB-IRIS, 2) TB no IRIS and 3) no TB or IRIS with Interleukin-18 found to be higher both pre-ART and during the IRIS event in patients with IRIS (and confirmed in a second cohort of paradoxical TB-IRIS) suggesting a possible role of IL-18 as a predictor of IRIS. [DOI] [PubMed] [Google Scholar]
- 41*.Hsu DC, Faldetta KF, Pei L, Sheikh V, Utay NS, Roby G, Rupert A, Fauci AS, Sereti I. A Paradoxical Treatment for a Paradoxical Condition: Infliximab Use in Three Cases of Mycobacterial IRIS. Clin Infect Dis. 2015 doi: 10.1093/cid/civ841. A case series of three patients with mycobacterial IRIS refractory to corticosteroids who received infliximab with resolution of symptoms and no effect on ex vivo TB-specific CD4 responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42**.Lai RP, Meintjes G, Wilkinson KA, Graham CM, Marais S, Van der Plas H, Deffur A, Schutz C, Bloom C, Munagala I, et al. HIV-tuberculosis-associated immune reconstitution inflammatory syndrome is characterized by Toll-like receptor and inflammasome signalling. Nat Commun. 2015;6:8451. doi: 10.1038/ncomms9451. Transcriptional profiling of patients with TB who did or did not develop IRIS showed no significant differences at baseline (pre-ART) but as soon as 0.5 weeks post-ART initiation a signature involving TLR and inflammasome genes distinguished the TB-IRIS group. Findings were corroborated by in vitro experiments blocking MyD88 and caspases 1 that led to decreases in IL-1 production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Torrado E, Fountain JJ, Liao M, Tighe M, Reiley WW, Lai RP, Meintjes G, Pearl JE, Chen X, Zak DE, et al. Interleukin 27R regulates CD4+ T cell phenotype and impacts protective immunity during Mycobacterium tuberculosis infection. J Exp Med. 2015;212:1449–1463. doi: 10.1084/jem.20141520. Study mostly focused on mouse TB model highlighting the role of IL-27 in TB immune responses with respect to mycobacterial burden control, homing of TB-specific cells and inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearl JE, Khader SA, Solache A, Gilmartin L, Ghilardi N, deSauvage F, Cooper AM. IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. J Immunol. 2004;173:7490–7496. doi: 10.4049/jimmunol.173.12.7490. [DOI] [PubMed] [Google Scholar]
- 45**.Ravimohan S, Tamuhla N, Steenhoff AP, Letlhogile R, Nfanyana K, Bellamy SL, MacGregor RR, Gross R, Weissman D, Bisson GP. Immunological profiling of tuberculosis-associated immune reconstitution inflammatory syndrome and non-immune reconstitution inflammatory syndrome death in HIV-infected adults with pulmonary tuberculosis starting antiretroviral therapy: a prospective observational cohort study. Lancet Infect Dis. 2015;15:429–438. doi: 10.1016/S1473-3099(15)70008-3. Immunological profiling at baseline and week-4 after ART initiation in a large cohort of ART-naive adults with advanced HIV with pulmonary tuberculosis showed that paradoxical TB-IRIS patients had decreased pre-ART inflammatory cytokines like IL-6 with increases after ART that were also associated with CD4 increases similar to those observed in non-IRIS survivors; contrary to patients with early mortality who had high IL-6 at baseline and lower CD4 increases post-ART. This study highlighted important baseline differences of patients at risk for paradoxical TB-IRIS versus at risk of death early post-ART. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luetkemeyer AF, Kendall MA, Nyirenda M, Wu X, Ive P, Benson CA, Andersen JW, Swindells S, Sanne IM, Havlir DV, et al. Tuberculosis immune reconstitution inflammatory syndrome in A5221 STRIDE: timing, severity, and implications for HIV-TB programs. J Acquir Immune Defic Syndr. 2014;65:423–428. doi: 10.1097/QAI.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naidoo K, Yende-Zuma N, Padayatchi N, Jithoo N, Nair G, Bamber S, Gengiah S, El-Sadr WM, Friedland G, Abdool Karim S. The immune reconstitution inflammatory syndrome after antiretroviral therapy initiation in patients with tuberculosis: findings from the SAPiT trial. Annals of internal medicine. 2012;157:313–324. doi: 10.7326/0003-4819-157-5-201209040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, Gengiah T, Nair G, Bamber S, Singh A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. The New England journal of medicine. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Havlir DV, Kendall MA, Ive P, Kumwenda J, Swindells S, Qasba SS, Luetkemeyer AF, Hogg E, Rooney JF, Wu X, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C, Nerrienet E, Madec Y, Marcy O, Chan S, Prak N, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abay SM, Deribe K, Reda AA, Biadgilign S, Datiko D, Assefa T, Todd M, Deribew A. The Effect of Early Initiation of Antiretroviral Therapy in TB/HIV Coinfected Patients: A Systematic Review and Meta-Analysis. J Int Assoc Provid AIDS Care. 2015 doi: 10.1177/2325957415599210. [DOI] [PubMed] [Google Scholar]
- 52.WHO Guidelines Approved by the Guidelines Review Committee, editor. WHO Policy on Collaborative TB/HIV Activities: Guidelines for National Programmes and Other Stakeholders. 2012. [PubMed] [Google Scholar]
- 53**.Boulware DR, Meya DB, Muzoora C, Rolfes MA, Huppler Hullsiek K, Musubire A, Taseera K, Nabeta HW, Schutz C, Williams DA, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014;370:2487–2498. doi: 10.1056/NEJMoa1312884. Cardinal randomized study of 177 patients with HIV and cryptococcal meningitis (CM) that established that earlier initiation of ART 9 within 1–2 weeks of CM treatment was associated with higher 26-week mortality (45%) compared to delayed ART initiation at 5 weeks of diagnosis (30%) particularly in patients with low (<5) white cell count in cerebrospinal fluid (CSF). The excess mortality associated with earlier ART initiation occurred early within 2 to 5 weeks after diagnosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Scriven JE, Rhein J, Hullsiek KH, von Hohenberg M, Linder G, Rolfes MA, Williams DA, Taseera K, Meya DB, Meintjes G, et al. Early ART After Cryptococcal Meningitis Is Associated With Cerebrospinal Fluid Pleocytosis and Macrophage Activation in a Multisite Randomized Trial. J Infect Dis. 2015;212:769–778. doi: 10.1093/infdis/jiv067. CSF from patients from the early ART initiation of the COAT trial (ref #53) had evidence of pleocytosis and increased levels of biomarkers suggestive of macrophage activation (IL-13, sCD14, sCD163, and CCL3/MIP-1alpha), highlighting a potential role of myeloid cell activation in early post-ART mortality in CM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Meya DB, Okurut S, Zziwa G, Rolfes MA, Kelsey M, Cose S, Joloba M, Naluyima P, Palmer BE, Kambugu A, et al. Cellular immune activation in cerebrospinal fluid from ugandans with cryptococcal meningitis and immune reconstitution inflammatory syndrome. J Infect Dis. 2015;211:1597–1606. doi: 10.1093/infdis/jiu664. This study evaluated patients with CM IRIS and showed a distinct pattern of immune cells in peripheral blood versus CSF highlighting the importance of studying the site of infection. An increasing frequency of CSF CD4 T cells, a shift in monocyte phenotype from classic to an intermediate, and increased programmed death ligand 1 expression on natural killer cells characterized CM-IRIS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56*.Jarvis JN, Meintjes G, Bicanic T, Buffa V, Hogan L, Mo S, Tomlinson G, Kropf P, Noursadeghi M, Harrison TS. Cerebrospinal fluid cytokine profiles predict risk of early mortality and immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis. PLoS Pathog. 2015;11:e1004754. doi: 10.1371/journal.ppat.1004754. This study used principal component analysis to discern patterns of chemokines/cytokines that can predict survival and IRIS in patients with CM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andrade BB, Hullsiek KH, Boulware DR, Rupert A, French MA, Ruxrungtham K, Montes ML, Price H, Barreiro P, Audsley J, et al. Biomarkers of inflammation and coagulation are associated with mortality and hepatitis flares in persons coinfected with HIV and hepatitis viruses. J Infect Dis. 2013;207:1379–1388. doi: 10.1093/infdis/jit033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Letang E, Lewis JJ, Bower M, Mosam A, Borok M, Campbell TB, Naniche D, Newsom-Davis T, Shaik F, Fiorillo S, et al. Immune reconstitution inflammatory syndrome associated with Kaposi sarcoma: higher incidence and mortality in Africa than in the UK. AIDS. 2013;27:1603–1613. doi: 10.1097/QAD.0b013e328360a5a1. [DOI] [PubMed] [Google Scholar]
- 59*.Gianella S, Redd AD, Grabowski MK, Tobian AA, Serwadda D, Newell K, Patel EU, Kalibbala S, Ssebbowa P, Gray RH, et al. Vaginal Cytomegalovirus Shedding Before and After Initiation of Antiretroviral Therapy in Rakai, Uganda. J Infect Dis. 2015;212:899–903. doi: 10.1093/infdis/jiv135. CMV vaginal shedding was evaluated in 96 Ugandan women and increased shedding was found from month 2–4 after ART initiation suggesting an immune restoration manifestation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faldetta KF, Kattakuzhy S, Wang HW, Sereti I, Sheikh V. Cytomegalovirus immune reconstitution inflammatory syndrome manifesting as acute appendicitis in an HIV-infected patient. BMC Infect Dis. 2014;14:313. doi: 10.1186/1471-2334-14-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheikh V, Caplan M, Wilson EM, Papadi B, Rosenberg AZ, Higgins J, Driscoll B, Filie AC, Sereti I. Cytomegalovirus immune reconstitution inflammatory syndrome manifesting as sialadenitis in an HIV-infected patient. AIDS. 2013;27:1833–1835. doi: 10.1097/QAD.0b013e328362d8b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tobian AA, Grabowski MK, Serwadda D, Newell K, Ssebbowa P, Franco V, Nalugoda F, Wawer MJ, Gray RH, Quinn TC, et al. Reactivation of herpes simplex virus type 2 after initiation of antiretroviral therapy. J Infect Dis. 2013;208:839–846. doi: 10.1093/infdis/jit252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Low AJ, Nagot N, Weiss HA, Konate I, Kania D, Segondy M, Meda N, van de Perre P, Mayaud P. Herpes simplex virus type-2 (HSV-2) cervico-vaginal shedding among women living with HIV-1 on antiretroviral therapy in Burkina Faso: an 8-year longitudinal study. J Infect Dis. 2015 doi: 10.1093/infdis/jiv495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64**.Sierra-Madero JG, Ellenberg SS, Rassool MS, Tierney A, Belaunzaran-Zamudio PF, Lopez-Martinez A, Pineirua-Menendez A, Montaner LJ, Azzoni L, Benitez CR, et al. Effect of the CCR5 antagonist maraviroc on the occurrence of immune reconstitution inflammatory syndrome in HIV (CADIRIS): a double-blind, randomised, placebo-controlled trial. Lancet HIV. 2014;1:e60–67. doi: 10.1016/S2352-3018(14)70027-X. First randomized placebo controlled trial trying to decrease IRIS incidence or severity with use of maraviroc as an immune modulator in 272 participants with HIV infection and CD4 counts <100 cells/μL. Maraviroc did not affect incidence or severity of IRIS events. [DOI] [PubMed] [Google Scholar]
- 65.Sullivan ZA, Wong EB, Ndung’u T, Kasprowicz VO, Bishai WR. Latent and Active Tuberculosis Infection Increase Immune Activation in Individuals Co-Infected with HIV. EBioMedicine. 2015;2:334–340. doi: 10.1016/j.ebiom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, Hoh R, Martin JN, McCune JM, Waters DD, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006;20:2275–2283. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- 67.Parrinello CM, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, Xue X, Hunt PW, Deeks SG, Hodis HN, et al. Cytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected women. J Infect Dis. 2012;205:1788–1796. doi: 10.1093/infdis/jis276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sacre K, Hunt PW, Hsue PY, Maidji E, Martin JN, Deeks SG, Autran B, McCune JM. A role for cytomegalovirus-specific CD4+CX3CR1+ T cells and cytomegalovirus-induced T-cell immunopathology in HIV-associated atherosclerosis. AIDS. 2012;26:805–814. doi: 10.1097/QAD.0b013e328351f780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goulenok T, Boyd A, Larsen M, Fastenackels S, Boccara F, Meynard JL, Hadour N, Samri A, Desvarieux M, Autran B, et al. Increased carotid intima-media thickness is not associated with T-cell activation nor with cytomegalovirus in HIV-infected never-smoker patients. AIDS. 2015;29:287–293. doi: 10.1097/QAD.0000000000000539. [DOI] [PubMed] [Google Scholar]
- 70.Effros RB. The silent war of CMV in aging and HIV infection. Mech Ageing Dev. 2015 doi: 10.1016/j.mad.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee SA, Sinclair E, Hatano H, Hsue PY, Epling L, Hecht FM, Bangsberg DR, Martin JN, McCune JM, Deeks SG, et al. Impact of HIV on CD8+ T cell CD57 expression is distinct from that of CMV and aging. PLoS One. 2014;9:e89444. doi: 10.1371/journal.pone.0089444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Freeman ML, Mudd JC, Shive CL, Younes SA, Panigrahi S, Sieg SF, Lee SA, Hunt PW, Calabrese LH, Gianella S, et al. CD8 T-Cell Expansion and Inflammation Linked to CMV Coinfection in ART-treated HIV Infection. Clin Infect Dis. 2015 doi: 10.1093/cid/civ840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, Tracy RP, Corey L, Deeks SG. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203:1474–1483. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nagot N, Ouedraogo A, Foulongne V, Konate I, Weiss HA, Vergne L, Defer MC, Djagbare D, Sanon A, Andonaba JB, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–799. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 75.Nagot N, Ouedraogo A, Konate I, Weiss HA, Foulongne V, Defer MC, Sanon A, Becquart P, Segondy M, Sawadogo A, et al. Roles of clinical and subclinical reactivated herpes simplex virus type 2 infection and human immunodeficiency virus type 1 (HIV-1)-induced immunosuppression on genital and plasma HIV-1 levels. J Infect Dis. 2008;198:241–249. doi: 10.1086/589621. [DOI] [PubMed] [Google Scholar]
- 76.LeGoff J, Roques P, Jenabian MA, Charpentier C, Brochier C, Bouhlal H, Gresenguet G, Frost E, Pepin J, Mayaud P, et al. Variability of human immunodeficiency virus-1 in the female genital reservoir during genital reactivation of herpes simplex virus type 2. Clin Microbiol Infect. 2015;21:873 e871–879. doi: 10.1016/j.cmi.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 77.Reynolds SJ, Makumbi F, Newell K, Kiwanuka N, Ssebbowa P, Mondo G, Boaz I, Wawer MJ, Gray RH, Serwadda D, et al. Effect of daily aciclovir on HIV disease progression in individuals in Rakai, Uganda, co-infected with HIV-1 and herpes simplex virus type 2: a randomised, double-blind placebo-controlled trial. Lancet Infect Dis. 2012;12:441–448. doi: 10.1016/S1473-3099(12)70037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, Mujugira A, Baeten JM, Mullins JI, Hughes JP, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Redd AD, Newell K, Patel EU, Nalugoda F, Ssebbowa P, Kalibbala S, Frank MA, Tobian AA, Gray RH, Quinn TC, et al. Decreased monocyte activation with daily acyclovir use in HIV-1/HSV-2 coinfected women. Sex Transm Infect. 2015;91:485–488. doi: 10.1136/sextrans-2014-051867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yi TJ, Walmsley S, Szadkowski L, Raboud J, Rajwans N, Shannon B, Kumar S, Kain KC, Kaul R, Tan DH. A randomized controlled pilot trial of valacyclovir for attenuating inflammation and immune activation in HIV/herpes simplex virus 2-coinfected adults on suppressive antiretroviral therapy. Clin Infect Dis. 2013;57:1331–1338. doi: 10.1093/cid/cit539. [DOI] [PubMed] [Google Scholar]
- 81.Masia M, Robledano C, Ortiz de la Tabla V, Antequera P, Lumbreras B, Hernandez I, Gutierrez F. Coinfection with human herpesvirus 8 is associated with persistent inflammation and immune activation in virologically suppressed HIV-infected patients. PLoS One. 2014;9:e105442. doi: 10.1371/journal.pone.0105442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22:1979–1991. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- 83.Soriano V, Berenguer J. Extrahepatic comorbidities associated with hepatitis C virus in HIV-infected patients. Curr Opin HIV AIDS. 2015;10:309–315. doi: 10.1097/COH.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 84**.Fernandez-Montero JV, Barreiro P, de Mendoza C, Labarga P, Soriano V. Hepatitis C virus coinfection independently increases the risk of cardiovascular disease in HIV-positive patients. J Viral Hepat. 2015 doi: 10.1111/jvh.12447. in a large cohort of 1136 patients (567 HIV-monoinfected, 70 HCV-monoinfected and 499 HIV/HCV-coinfected) followed up for 79.4 +/− 21 months HIV/HCV-coinfected patients had a greater incidence of cardiovascular disease events and/or death than HIV or HCV-monoinfected individuals. After adjustment both HIV/HCV coinfection and hypertension were independently associated with cardiovascular disease events and/or death in HIV-infected patients. [DOI] [PubMed] [Google Scholar]
- 85.McKibben RA, Haberlen SA, Post WS, Brown TT, Budoff M, Witt MD, Kingsley LA, Palella FJ, Jr, Thio CL, Seaberg EC. A Cross-sectional Study of the Association Between Chronic Hepatitis C Virus Infection and Subclinical Coronary Atherosclerosis Among Participants in the Multicenter AIDS Cohort Study. J Infect Dis. 2015 doi: 10.1093/infdis/jiv396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hodowanec AC, Lee RD, Brady KE, Gao W, Kincaid S, Plants J, Bahk M, Mackman N, Landay AL, Huhn GD. A matched cross-sectional study of the association between circulating tissue factor activity, immune activation and advanced liver fibrosis in hepatitis C infection. BMC Infect Dis. 2015;15:190. doi: 10.1186/s12879-015-0920-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marquez M, Romero-Cores P, Montes-Oca M, Martin-Aspas A, Soto-Cardenas MJ, Guerrero F, Fernandez-Gutierrez C, Giron-Gonzalez JA. Immune activation response in chronic HIV-infected patients: influence of Hepatitis C virus coinfection. PLoS One. 2015;10:e0119568. doi: 10.1371/journal.pone.0119568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kushner LE, Wendelboe AM, Lazzeroni LC, Chary A, Winters MA, Osinusi A, Kottilil S, Polis MA, Holodniy M. Immune biomarker differences and changes comparing HCV mono-infected, HIV/HCV co-infected, and HCV spontaneously cleared patients. PLoS One. 2013;8:e60387. doi: 10.1371/journal.pone.0060387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chew KW, Hua L, Bhattacharya D, Butt AA, Bornfleth L, Chung RT, Andersen JW, Currier JS. The effect of hepatitis C virologic clearance on cardiovascular disease biomarkers in human immunodeficiency virus/hepatitis C virus coinfection. Open Forum Infect Dis. 2014;1:ofu104. doi: 10.1093/ofid/ofu104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tang L, Kottilil S. Treatment of hepatitis C in patients with HIV. Lancet HIV. 2015;2:e308–309. doi: 10.1016/S2352-3018(15)00128-9. [DOI] [PubMed] [Google Scholar]