Abstract
A stereoselective analytical method was developed and validated for the quantification of bupropion, and principle metabolites hydroxybupropion, erythrohydrobupropion and threohydrobupropion in human plasma. Separation of individual enantiomers (R)-bupropion, (S)-bupropion, (R,R)-hydroxybupropion, (S,S-hydroxybupropion), (1S,2S)-threohydrobupropion, (1R,2R)-threohydrobupropion, (1R,2S)-erythrohydrobupropion, and (1S,2R)-erythrohydrobupropion was achieved utilizing an α1-acid glycoprotein column within a 12-minute run time. Chromatograph separation was significantly influenced by mobile phase pH and variability between columns. Analytes were quantified by positive ion electrospray tandem mass spectrometry following plasma protein precipitation with 20% trichloroacetic acid. Identification of erythrohydrobupropion enantiomer peaks and threohydrobupropion enantiomer peaks was achieved by sodium borohydride reduction of enantiopure (R)- and (S)-bupropion. Initial assay validation and sensitivity determination was on AB Sciex 3200, 4000 QTRAP, and 6500 mass spectrometers. Accuracy and precision were within 15% for each analyte. The assay was fully validated over analyte-specific concentrations using an AB Sciex 3200 mass spectrometer. Intra- and inter-assay precision and accuracy were within 12% for each analyte. The limits of quantification for bupropion (R and S), hydroxybupropion (R,R and S,S), threohydrobupropion (1S,2S and 1R,2R), and erythrohydrobupropion (1R,2S and 1S,2R) were 0.5, 2, 1, and 1 ng/mL, respectively. All analytes were stable following freeze thaw cycles at −80°C and while stored at 4°C in the instrument autosampler. This method was applicable to clinical pharmacokinetic investigations of bupropion in patients. This is the first chromatographic method to resolve erythrohydrobupropion and threohydrobupropion enantiomers, and the first stereoselective LC-MS/MS assay to quantify bupropion, and principle metabolites hydroxybupropion, erythrohydrobupropion, and threohydrobupropion in human plasma.
Keywords: bupropion, hydroxybupropion, erythrohydrobupropion, threohydrobupropion, LC-MS/MS, stereoselectivity
1. Introduction
Bupropion is an antidepressant that is efficacious in the treatment of major depressive disorder, relapse prevention, and smoking cessation. It is available in immediate release, twice daily sustained release (SL), and once-daily extended release (XL) formulations. Clinically, bupropion is dosed as a racemic mixture, (R,S)-bupropion, and is metabolized to three main metabolites: hydroxybupropion, erythrohydrobupropion, and threohydrobupropion. Clinical pharmacokinetics of (R,S)-bupropion have been well-described in achiral assays showing that metabolite concentrations exceed those of the parent drug [1].
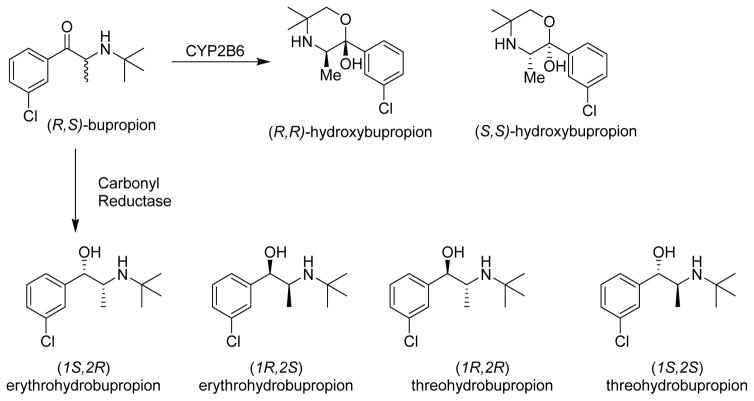
Each of these metabolites, however, actually exist as enantiomeric or diastereomeric pairs because biochemical metabolism creates a new chiral center on the parent molecule (Figure 1). (R,S)-bupropion undergoes stereoselective oxidative metabolism by cytochrome P450 2B6 (CYP2B6) to form (R,R)- and (S,S)-hydroxybupropion [2]. Additionally, bupropion is stereoselectively reduced by carbonyl reductase(s) to form erythrohydrobupropion and threohydrobupropion [3]. The formation of threohydrobupropion is dependent on 11β-hydroxysteroid dehydrogenase 1 reduction of (R)-bupropion in vitro [4]; however, the enzyme that forms erythrohydrobupropion has yet to be elucidated.
Figure 1.
Oxidative and reductive metabolic pathways of (R,S)-bupropion
Clinical bupropion disposition is remarkably stereoselective [5–9]. Peak plasma concentrations and area under the curve values were 3-fold greater for (R)- than for (S)-bupropion, and bupropion apparent oral clearance was 3- and 2-fold greater for (S)- than for (R)- and (R,S)-bupropion, respectively. Plasma area under the curve and elimination half-life were significantly less for (S,S)- than (R,R)-hydroxybupropion. (S,S)-hydroxybupropion was formation rate limited, whereas (R,R)-hydroxybupropion was elimination rate limited. Moreover, while (R)- and (S)-bupropion have similar antidepressant activity [10], only (S,S)-hydroxybupropion, but not (R,R)-hydroxybupropion is pharmacologically active. Accordingly, (S,S)-hydroxybupropion is now considered the most active metabolite of bupropion, and the mediator of pharmacological effect, although it is quantitatively the minor enantiomer of hydroxybupropion. Of note, changes in clinical (S,S)-hydroxybupropion concentrations would not be revealed by an achiral assay.
Achiral LC-MS/MS bioanalytical methods which simultaneously measure bupropion, hydroxybupropion, erythrohydrobupropion, and threohydrobupropion in whole blood [11], plasma and urine [12–15], and breast milk[16], from patients receiving oral bupropion, have been previously described. Additionally, stereoselective LC-UV analytical methods were developed for bupropion and hydroxybupropion enantiomers utilizing achiral-chiral chromatography [17], with ovomucoid [18], cyclobond I 2000 [9], cellulose [19], and α1-acid glycoprotein[20] columns, electrokinetic chromatography [21], and isothiocyanate-based chiral derivatization [22]. Previously, our group utilized an α1-acid glycoprotein chiral column in a validated LC-MS/MS assay for bupropion and hydroxybupropion enantiomers in plasma [5]. Nevertheless, erythrohydrobupropion and threohydrobupropion, like hydroxybupropion, exist as enantiomeric pairs and are pharmacologically active [23]. Since there is not an assay reported for erythrohydrobupropion and threohydrobupropion enantiomers in plasma, their clinical disposition, pharmacogenetics, and overall impact on bupropion clinical pharmacology remain unknown. Therefore, a stereoselective bioanalytical method for bupropion, hydroxybupropion, erythrohydrobupropion, and threohydrobupropion in human plasma was developed and validated.
Materials and Methods
2.1 Materials
Standard methanolic solutions of rac-bupropion HCl and rac-hydroxybupropion were from Cerilliant Corporation (Round Rock, TX). rac-bupropion-d9 hydrochloride, rac-hydroxybupropion-d6, rac-threohydrobupropion and rac-erythrohydrobupropion maleate 1:1 mixture, rac-erythrohydrobupropion-d9, and rac-threohydrobupropion-d9, (R)-bupropion tartaric acid, and (S)-bupropion tartaric acid were from Toronto Research Chemicals (Toronto, ON, Canada). Acid citrate dextrose human plasma was obtained from the American Red Cross (St. Louis, MO). HPLC-grade methanol, trichloroacetic acid, ammonium formate, formic acid, and sodium borohydride were from Sigma Aldrich (St. Louis, MO). All solutions were prepared in ultrapure water (18.2 M ·cm) from a Milli-Q Gradient A10 Filtration System (EMD Millipore, Billerica, MA).
2.2 Calibrator, quality control (QC), and internal standard (IS) solutions
For reference or internal standards obtained in salt form, all reported concentrations reflect the concentration of free analyte, and individual enantiomer. Individual working stock solutions of bupropion (R and S) and hydroxybupropion (R,R and S,S) were prepared by diluting certified authentic solutions. Three working stock concentrations of bupropion (R and S) were prepared at 50, 500, and 5,000 ng/mL and two working stock concentrations of hydroxybupropion (R,R and S,S) were prepared at 500 and 50,000 ng/mL and immediately stored at −80°C. The rac-erythrohydrobupropion and rac-threohydrobupropion (1:1) powder was accurately weighed and prepared as a 1.0 mg/mL stock solution containing 500 μg/mL rac-erythrohydrobupropion and 500 μg/mL rac-threohydrobupropion in DMSO. Two working stock solutions were prepared in water at 5,000 ng/mL and 500 ng/mL erythrohydrobupropion (1R,2S) and (1S,2R) and threohydrobupropion (1S,2S) and (1R,2R). Deuterated internal standards bupropion-d9 (R and S), hydroxybupropion-d6 (R,R and S,S), erythrohydrobupropion-d9 (1R,2S) and (1S,2R), and threohydrobupropion-d9 (1S,2S) and (1R,2R) were individually prepared as 500 μg/mL DMSO stock solutions and stored at −80°C. A working stock of 5,000 ng/mL bupropion-d9 (R and S), 25,000 ng/mL hydroxybupropion-d6 (R,R and S,S), 12,500 ng/mL erythrohydrobupropion-d9 (1R,2S) and (1S,2R), and 12,500 ng/mL threohydrobupropion-d9 (1S,2S) and (1R,2R) was divided into 50 μL aliquots which were stored at −80°C. On the day of an experiment, a fresh working stock of 200 ng/mL bupropion-d9 (R and S), 1000 ng/mL hydroxybupropion-d6 (R,R and S,S), 500 ng/mL erythrohydrobupropion-d9 (1R,2S) and (1S,2R), and 500 ng/mL threohydrobupropion-d9 (1S,2S) and (1R,2R) was prepared.
Initially, calibration standards containing the same analyte enantiomeric concentration in human plasma were prepared at 0.25, 0.5, 1, 2.5, 5, 10, 25, 50, 100, 250, 500, 1000 ng/mL along with quality control standards (QC) at 2.5, 50, 500 ng/mL to evaluate instrument performance on AB Sciex 3200, 4000, and 6500 mass spectrometers. For the subsequent validation using analyte-specific concentrations, all calibrators and QC samples at the enantiomeric concentrations described in Table 1 were prepared in individual 50 mL volumetric flasks. Each calibration or QC was subsequently aliquoted (0.25 mL) into 1.5 mL tubes and stored at −80°C until analysis. These calibrator and QC samples were used for the final assay validation on the AB Sciex 3200 mass spectrometer.
Table 1.
Final assay calibration standard and quality control concentrations
| Analyte | Enantiomeric calibrator concentrations (ng/mL) | Quality control
concentrations (ng/mL)a
|
||||
|---|---|---|---|---|---|---|
| QC 1 | QC 2 | QC 3 | QC 4 | QC 5 | ||
| bupropion (R and S) | 0.5, 1, 2.5, 5, 7.5, 10, 25, 50, 75, 100 | 2.5 | 10 | 25 | 50 | |
| hydroxybupropion (R,R and S,S) | 2, 5, 10, 25, 50, 250, 500, 750, 1000, 1250, 1500, 2000, 2500, 3000 | 5 | 25 | 250 | 1000 | 2000 |
| threohydrobupropion (1S,2S) and (1R,2R) | 1, 2.5, 5, 10, 25, 50, 75, 100, 150, 200, 400, 600 | 2.5 | 10 | 50 | 200 | |
| erythrohydrobupropion (1R,2S and (1S,2R) | 1, 2.5, 5, 10, 25, 50, 75, 100, 150, 200, 400, 600 | 2.5 | 10 | 50 | 200 | |
Analyte-specific calibrator and QC concentrations chosen based on a preliminary analysis of patient plasma from a clinical study in patients receiving 300 mg oral bupropion daily at steady-state.
2.3 Sample Preparation
Calibration standards, QCs, and patient samples were thawed at room temperature, vortexed, and transferred (200 μL) to a 1.0 mL DeepWell 96-well plate (Nunc, Thermofisher, Pittsburgh, PA). Freshly prepared internal standard aqueous solution (10 μL) containing 200 ng/mL bupropion-d9 (R and S), 1000 ng/mL hydroxybupropion-d6 (R,R and S,S), 500 ng/mL erythrohydrobupropion-d9 (1R,2S) and (1S,2R), and 500 ng/mL threohydrobupropion-d9 (1S,2S) and (1R,2R) was added to each well. Next, 40 μL of 20% aqueous trichloroacetic acid was added and the plate shaken for 5 min. The plate was centrifuged at 6,100g at 4°C for 15 min, and the supernatant (100 μL) was transferred to a MicroWell 96-well plate (Nunc, Thermofisher, Pittsburgh, PA) containing 10 μL of a 3M aqueous ammonium formate solution, pH 6.9. The final sample pH was 3.
2.4 Syntheses of erythrohydrobupropion and threohydrobupropion from racemic bupropion and individual bupropion enantiomers
rac-bupropion HCl, (R)-bupropion tartaric acid, and (S)-bupropion tartaric acid (1 mg, 0.004 mmol 1.0 equiv) were individually dissolved in methanol (150 μL) in 1 dram vials and stirred in an ice bath at 0°C for 10 min. Subsequently, sodium borohydride (0.291 mg 2.0 mmol, 2 equiv) was added to the reaction mixture by adding 153 μL of a 1.9 mg/mL sodium borohydride methanolic solution, and the reaction mixture stirred for 30 min at 0°C. The ice bath was removed and the mixture was allowed to warm to room temperature. An aliquot of each reaction mixture was diluted in 20 mM ammonium formate, pH 5.0 and subsequently injected on the LC-MS/MS system with the chromatographic method described in section 2.6.
2.5 Instrumentation
AB Sciex 3200
HPLC-ESI-MS/MS was conducted on a Shimadzu (Columbia, MD) HPLC system composed of two LC-20AD pumps with a CTO-20A column oven, DGU-20A3 degasser, FCV-11AL solvent selection valve, and CBM 20A controller connected to a MPS 3C temperature regulated autosampler equipped with an active wash station (Gerstal, Linthicum, MD) all interfaced with an API Sciex 3200 triple quadrupole mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA). Analyst 1.4.2 software (AB Sciex, Foster City, CA) was utilized for instrument control, data acquisition, and analyte mass spectrometric parameter optimization.
AB Sciex 4000
LC-MS/MS analysis was performed on a Shimadzu HPLC system composed of two LC-20AD XR pumps, DGU20A3 degasser, CTO-20A column oven, FCV-11AL solvent selection valve, SIL-20AC XR temperature regulated autosampler, and an external Valco divert valve installed between the LC and mass spectrometer. The LC system was coupled to an API 4000 linear ion trap triple quadrupole (QTRAP) tandem mass spectrometer operated with Analyst 1.5.2.
AB Sciex 6500
LC-MS/MS analysis was performed on a Shimadzu HPLC system composed of two LC-20AD XR pumps, DGU20A5R degasser, CBM-20A system controller, CTO-20C column oven, FCV-11AL solvent selection valve, and a SIL-20AC XR temperature regulated autosampler. The LC system was coupled to an API 6500 triple quadrupole tandem mass spectrometer operated with Analyst 1.6.2.
Multiquant 3.0.1(AB Sciex) was utilized for peak integration, generation of calibration curves, and data analysis.
2.6 Chromatographic Conditions
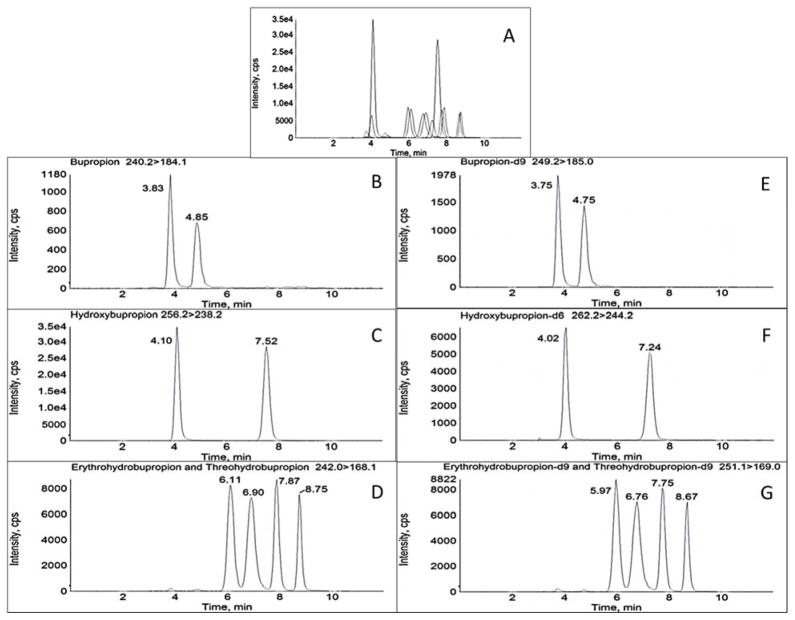
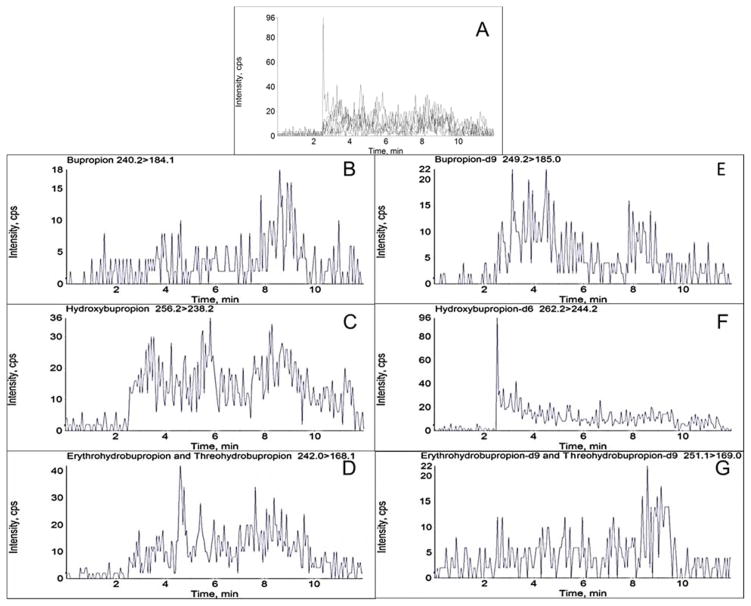
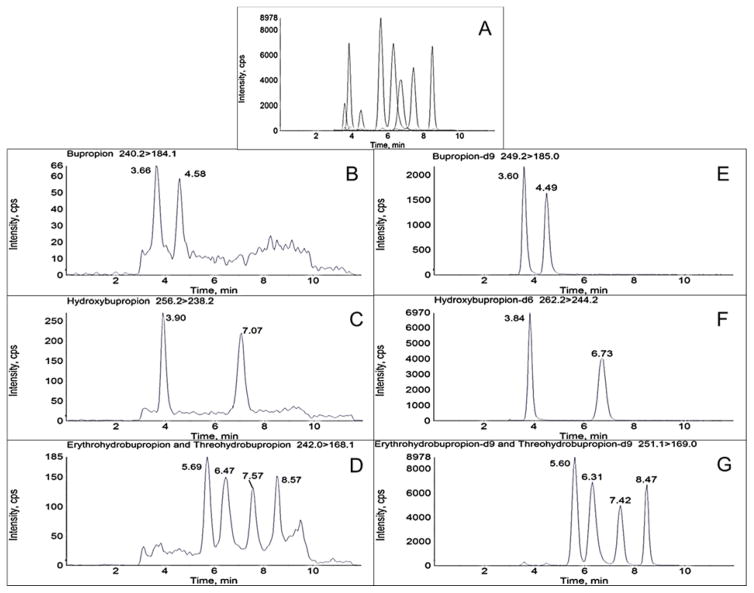
Chiral chromatographic separation of analytes and respective internal standards was achieved utilizing a chiralpak α1-acid glycoprotein (AGP) column (100 x 2.0 mm, 5μm, Chiral Technologies, Westchester, PA) equipped with a chiral AGP guard cartridge (10 x 2.0 mm, Sigma Aldrich, St. Louis, MO). A 0.25 μM inline filter was additionally added prior to the sample entering the column. The flow rate was 0.22 mL/min with a mobile phase consisting of 20 mM aqueous ammonium formate, pH 5.0 (A) and methanol (B). The time program to achieve the separation was as follows: 10% B for 0.5 min, linear gradient to 20% B until 1 min, held at 20% B until 5 min, linear gradient to 50% B until 8 min, and then re-equilibrated to initial conditions until 12 min. The column oven was at ambient temperature and the autosampler was at 4°C. Flow was directed into the mass spectrometer at 2.5 min and diverted to waste at 11.5 min. Under these conditions, retention times (min) for the analytes were: (R)-bupropion 3.83, (S)-bupropion 4.85, (R,R)-hydroxybupropion 7.52, (S,S-hydroxybupropion) 4.10, (1S,2S)-threohydrobupropion 6.11, (1R,2R)-threohydrobupropion) 6.90, (1R,2S)-erythrohydrobupropion 7.87, and (1S,2R)-erythrohydrobupropion 8.75 (Figure 2).
Figure 2.
Chromatographic separation of bupropion, hydroxybupropion, erythrohydrobupropion, and threohydrobupropion enantiomers and respective deuterated standards in processed plasma on an AB Sciex 3200 mass spectrometer. Numbers in parenthesis refer to retention times in min. A: Total ion chromatogram. B–G: Representative MRM chromatograms B: (R)-bupropion (3.83), (S)-bupropion (4.85). C: (R,R)-hydroxybupropion (7.52), (S,S)-hydroxybupropion (4.10). D: (1S,2S)-threohydrobupropion (6.11), (1R,2R)-threohydrobupropion) (6.90), (1R,2S)-erythrohydrobupropion (7.87), and (1S,2R)-erythrohydrobupropion (8.75). E: (R)-bupropion-d9 (3.75), (S)-bupropion-d9 (4.75). F: (R,R)-hydroxybupropion-d6 (7.24), (S,S)-hydroxybupropion-d6 (4.02). G: (1S,2S)-threohydrobupropion-d9 (5.97), (1R,2R)-threohydrobupropion-d9) (6.76), (1R,2S)-erythrohydrobupropion-d9 (7.75), and (1S,2R)-erythrohydrobupropion-d9 (8.67). Concentrations correspond to Calibration level 6 (10 ng/mL R- or S-bupropion; 250 ng/mL (R,R)- or (S,S)-hydroxybupropion; 50 ng/mL (1R,2S)- or (1S,2R)-erythrohydrobupropion; 50 ng/mL (1S,2S)- or (1R,2R)-threohydrobupropion)
2.7 Mass Spectrometry Conditions
AB Sciex 3200
The mass spectrometer was operated with a turbo spray ion source in the positive mode (ESI+) with multiple reaction monitoring (MRM). Global parameters were optimized across all analytes: curtain gas 30 psig, ion spray voltage 5000 V, source temperature 650°C, Gas 1 40 psig, and Gas 2 40 psig. Dwell times were 500 msec. Optimized analyte- and internal standard-specific MRM transitions, declustering potential, entrance potential, collision energy, and exit potential are described in Table 2.
Table 2.
Analyte and internal standard MS/MS optimized parameters on AB Sciex 3200, 4000 QTRAP, and 6500 mass spectrometers.
| Analyte | MRM transition (m/z) | Declustering potential | Entrance potential | Collision energy | Exit potential |
|---|---|---|---|---|---|
| AB Sciex 3200 | |||||
| bupropion | 240.2 > 184.1 | 31 | 3.5 | 15 | 14 |
| bupropion-d9 | 249.2 > 185.0 | 36 | 5.5 | 17 | 14 |
| hydroxybupropion | 256.2 > 238.2 | 36 | 3.5 | 17 | 20 |
| hydroxybupropion-d6 | 262.2 > 244.2 | 36 | 5 | 17 | 20 |
| erythrohydrobupropion | 242.0 > 168.1 | 31 | 4 | 21 | 14 |
| erythrohydrobupropion-d9 | 251.1 > 169.0 | 41 | 8 | 25 | 3 |
| threohydrobupropion | 242.0 > 168.1 | 31 | 4 | 21 | 14 |
| threohydrobupropion-d9 | 251.1 > 169.0 | 41 | 8 | 25 | 3 |
| AB Sciex 4000 QTRAP | |||||
| bupropion | 240.0 > 184.0 | 56 | 10 | 19 | 28 |
| bupropion-d9 | 249.3 > 185.1 | 61 | 10 | 19 | 12 |
| hydroxybupropion | 256.1 > 238.1 | 46 | 10 | 17 | 6 |
| hydroxybupropion-d6 | 262.3 > 131.1 | 61 | 10 | 43 | 22 |
| erythrohydrobupropion | 242.1 > 168.1 | 66 | 10 | 23 | 26 |
| erythrohydrobupropion-d9 | 251.2 > 169.2 | 106 | 10 | 27 | 28 |
| threohydrobupropion | 242.1 > 168.1 | 66 | 10 | 23 | 26 |
| threohydrobupropion-d9 | 251.2 > 169.2 | 106 | 10 | 27 | 28 |
| AB Sciex 6500 | |||||
| bupropion | 240.2 > 184.1 | 31 | 3.5 | 15 | 14 |
| bupropion-d9 | 249.2 > 185.0 | 36 | 5.5 | 17 | 14 |
| hydroxybupropion | 256.2 > 238.2 | 36 | 3.5 | 17 | 20 |
| hydroxybupropion-d6 | 262.2 > 244.2 | 36 | 5.0 | 17 | 20 |
| erythrohydrobupropion | 242.0 > 168.1 | 31 | 4.0 | 21 | 14 |
| erythrohydrobupropion-d9 | 251.1 > 169.0 | 41 | 8.0 | 25 | 3 |
| threohydrobupropion | 242.0 > 168.1 | 31 | 4.0 | 21 | 14 |
| threohydrobupropion-d9 | 251.1 > 169.0 | 41 | 8.0 | 25 | 3 |
AB Sciex 4000
Global parameters were optimized across all analytes: curtain gas 20 psig, ion spray voltage 5500 V, source temperature 450°C, Gas 1 30 psig, and Gas 2 40 psig. Dwell times were 500 msec.
AB Sciex 6500
Global parameters were optimized across all analytes: curtain gas 20 psig, ion spray voltage 5000 V, source temperature 600°C, Gas 1 40 psig, and Gas 2 40 psig. Dwell times were 500 msec.
2.8 Method Validation
Validation experiments and run acceptance criteria were based on the United States Food and Drug Administration Bioanalytical Method Validation Guidance [24].
2.8.1 Accuracy and precision
The accuracy and precision for each analyte in pooled blank human plasma were determined at the QC concentrations. Intra-assay and inter-assay accuracy and precision were calculated at five samples per concentration. Accuracy is expressed as a percentage of the nominal concentration and precision is expressed as percent coefficient of variation (%CV). Acceptance criteria was defined as variation and deviation ≤15% for all samples.
2.8.2 Selectivity
Individual lots of human plasma were prepared as described in section 2.3 without internal standard and were confirmed to be negative for all analytes and their respective internal standards. Eight individual lots of blank human plasma were pooled to generate the matrix for preparing calibration and quality control samples. At the lower limit of quantification, there was no significant matrix interference for each analyte.
2.8.3 Ion suppression
To investigate any loss of signal due to matrix effect on ion suppression, a solution of all analytes and internal standards 200 ng/mL bupropion (R and S), 200 ng/mL bupropion-d9 (R and S), 5,000 ng/mL hydroxybupropion (R,R and S,S), 5,000 ng/mL hydroxybupropion-d6 (R,R and S,S), 500 ng/mL erythrohydrobupropion (1R,2S) and (1S,2R), 500 ng/mL erythrohydrobupropion-d9 (1R,2S) and (1S,2R), 500 ng/mL threohydrobupropion (1S,2S) and (1R,2R), and 500 ng/mL threohydrobupropion-d9 (1S,2S) and (1R,2R) was infused into the mass spectrometer while simultaneously injecting a processed blank human plasma sample.
2.8.4 Stability
Stability experiments utilizing low and high QC samples were performed after 3 subsequent freeze/thaw cycles at −80°C, processed as described in section 2.3, and analyzed by LC-MS/MS. Additionally, the stability of the analytes in solution were assessed by storing fresh processed samples (no freeze/thaw) in the autosampler at 4°C over a period of 48 hours.
2.9 Clinical Samples
The validated bioanalytical method was applied to clinical study approved by the Washington University in St Louis institutional review board. Venous blood samples were obtained immediately before and 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 10, 12, 16, 20, and 24 hr after a daily steady-state dose of 300 mg extended release bupropion. Samples were centrifuged to obtain plasma, which was stored at −80°C until analysis.
3. Results and Discussion
3.1 Method Development
The stereoselective chromatographic separation of bupropion, hydroxybupropion, erythrohydrobupropion, and threohydrobupropion was initially attempted with a lux cellulose-3 column (250 x 4.6 mm 5 μM, Phenomenex, Torrence, CA). The method involved an isocratic elution with a mobile phase consisting of 62% 5 mM ammonium bicarbonate, pH 9.0–9.2, 25% methanol, and 13% acetonitrile at a flow rate of 1.0 mL/min. Under these conditions, there was adequate resolution of bupropion (R and S), erythrohydrobupropion enantiomers, and threohydrobupropion enantiomers; however hydroxybupropion enantiomers could not be resolved. Separation was next attempted with a previously published stereoselective chromatographic method for bupropion and hydroxybupropion enantiomers using an α1-acid glycoprotein column [5]. However, incomplete resolution was observed with erythrohydrobupropion and threohydrobupropion enantiomers. The previous method utilized a 20 mM ammonium formate buffer at pH 5.7, which is above the buffering capacity of ammonium formate. By incrementally lowering the pH of the 20 mM ammonium formate buffer, the erythrohydrobupropion and threohydrobupropion enantiomers began to resolve while the bupropion and hydroxybupropion enantiomer retention times significantly decreased and retained baseline resolution. The optimal pH for chromatographic resolution of all the analytes ranged from pH 4.8–5.1, and required optimization for each individual HPLC column.
Plasma sample preparation was initially performed according to the previously published method [5], using solid phase extraction with Oasis MCX 96 well plates (Waters, Milford, MA). Samples were eluted with 80:18:2 methanol:water:ammonium hydroxide and subsequently acidified with 2 M ammonium formate, pH 4. This method was suboptimal due to inadequate chromatographic separation of erythrohydrobupropion and threohydrobupropion and their enantiomers, due primarily to the high percentage of organic solvent. Therefore, the goal was to develop a sample preparation method which ultimately yielded a predominantly aqueous injectate. Because bupropion is chemically unstable and racemizes (42%, 62%, and >94% after 2, 4, and 24 hours, respectively, in phosphate buffer pH 7.4 at 25°C, and also at pH 4 and 5) [25,26], but does not undergo racemization at low pH (pH 1.2 at 25°C for 50 days) [26], trichloroacetic acid was chosen to precipitate plasma proteins. However, injecting a sample directly in an acidic pH abolished chromatographic resolution. Therefore, 3 M aqueous ammonium formate, pH 6.9 was added to increase the final sample pH to 3.
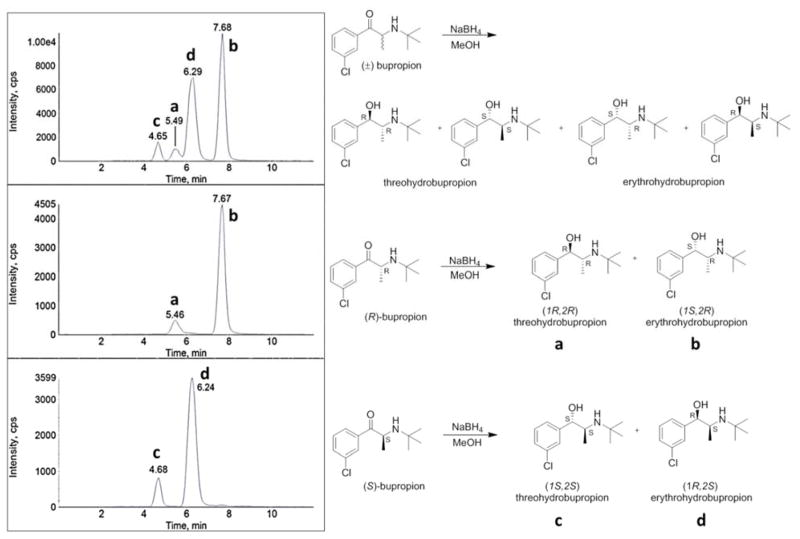
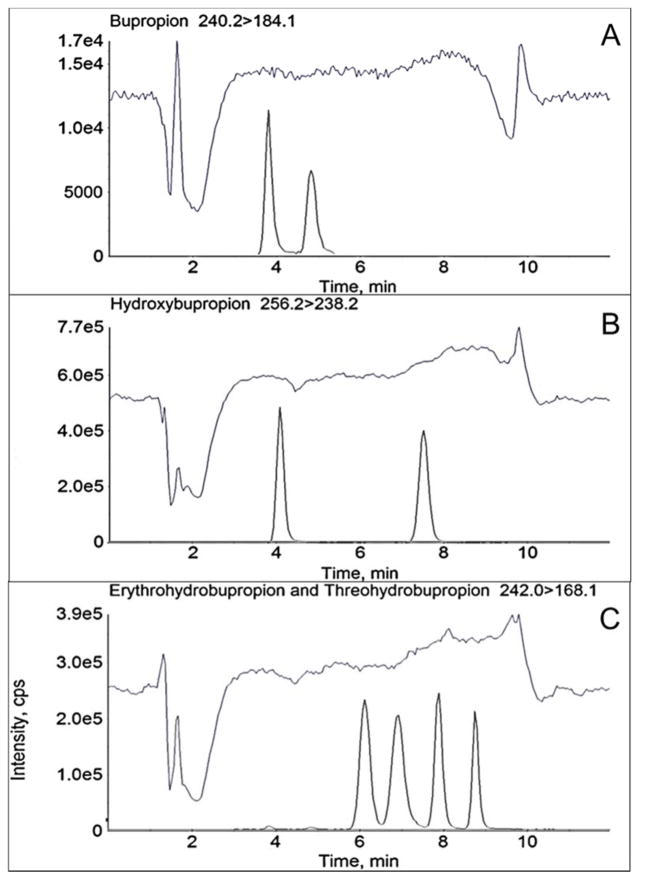
3.2 Peak identification of erythrohydrobupropion and threohydrobupropion enantiomers
Stereoselective chromatographic separation of rac-erythrohydrobupropion and rac-threohydrobupropion produced four distinct peaks, two enantiomers for rac-erythrohydrobupropion and two enantiomers for rac-threohydrobupropion (Figure 2D). Since authentic standards for each erythrohydrobupropion enantiomer and each threohydrobupropion enantiomer were not commercially available, each corresponding enantiomer peak was stereochemically identified. The assignment of each individual enantiomer peak was determined by sodium borohydride reduction of (R)- and (S)-bupropion and comparing their respective product retention times to the product retention times of a rac-bupropion sodium borohydride reduction. Carbonyl reduction of rac-bupropion yields both rac-erythrohydrobupropion and rac-threohydrobupropion metabolites as expected (Figure 3A). Individual reduction of (R)-bupropion or (S)-bupropion will yield one erythrohydrobupropion enantiomer and one threohydrobupropion enantiomer because the chiral center at C2 is fixed, and sodium borohydride reduction of ketone functional groups is not stereoselective. The reaction mixtures from the small scale syntheses were diluted in 20 mM ammonium formate, pH 4.8 and analyzed by LC-MS/MS. It was determined that reduction of (R)-bupropion produced the second threohydrobupropion and second erythrohydrobupropion peaks, which structurally correspond to (1R,2R)-threohydrobupropion and (1S,2R)-erythrohydrobupropion (Figure 3B). The (S)-bupropion reduction produced the first threohydrobupropion and first erythrohydrobupropion peaks, which structurally correspond to (1S,2S)-threohydrobupropion and (1R,2S)-erythrohydrobupropion (Figure 3C).
Figure 3.
Identification of erythrohydrobupropion enantiomer peaks and threohydrobupropion enantiomer peaks. Representative MRM chromatograms from the sodium borohydride reduction of: A: racemic (R,S)-bupropion, B: (R)-bupropion, and C: (S)-bupropion. Experiments were performed utilizing an AB Sciex 3200 instrument. Threohydrobupropion enantiomers elute at 4.65 (c) and 5.49 (a) min, respectively. Erythrohydrobupropion enantiomers elute at 6.29 (d) and 7.68 (b) min, respectively. To accurately identify each of the enantiomers, retention times of the peaks in panel A (a–d) were aligned with the retention times of the peaks in panel B (a and b) and C (c and d), respectively.
3.3 Method Validation
3.3.1 General development on AB Sciex 3200, 4000, and 6500 mass spectrometers
Analytical method validation was initially conducted on three mass spectrometers (AB Sciex 3200, 4000 QTRAP, and 6500) to assess linearity and sensitivity. Initially, calibrator concentrations and QCs were prepared over a broad range and analyzed on each instrument. Calibration curves were analyzed by least squares regression analysis, with choice of model (linear or quadratic) and weighting (1/x or 1/x2) optimized for each analyte and instrument.
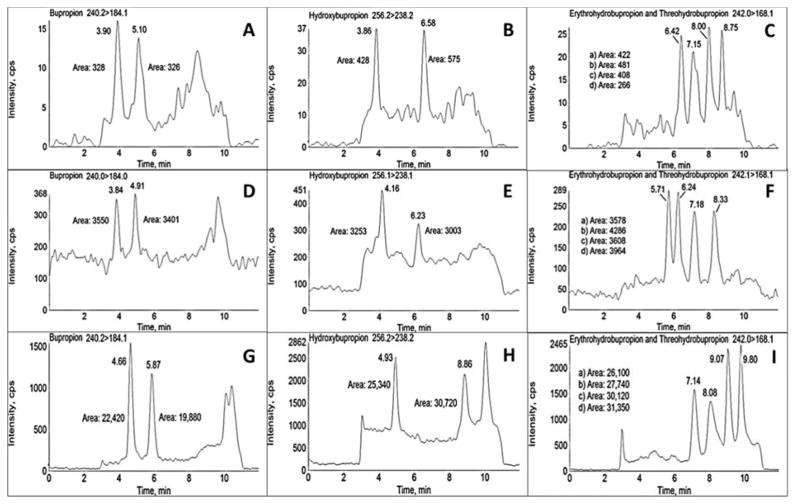
Evaluation of performance across the instruments was investigated by comparing intra-assay (n=3) and inter-assay (n=5) accuracy and precision, as well as sensitivity and linearity. Calibration curves were linear for all analytes on all three instruments (typical r2 values were ≥0.994 for the 3200 (0.5–1,000 ng/mL), 4000 (0.25–1,000 ng/mL), and 6500 (0.25–1,000 ng/mL) instruments, respectively). At each of the QC concentration investigated, accuracy and precision were within 15% (Tables 3–5). The validation results on all three instruments exemplify the robustness of the analytical assay. The sensitivity of each instrument at the lowest calibrator (0.25 ng/mL) is illustrated in Figure 4. Bupropion (R and S) was below the limit of detection on the 3200, although readily quantifiable on the 4000 and 6500 instruments, and for this reason were not used in the calibration curve for each instrument. The 6500 instrument response for bupropion, hydroxybupropion, erythrohydrobupropion, and threohydrobupropion enantiomers was roughly 65-fold and 8-fold higher than the 3200 and 4000 instruments, respectively. Additionally, the 4000 sensitivity was roughly 9-fold higher than the 3200.
Table 3.
Intra-assay and inter- assay accuracy and precision for determination of bupropion and metabolites in human plasmaa on an AB Sciex 3200 mass spectrometer
| Analyte | Nominal concentration (ng/mL) | Intra-assay (n=3) | Inter-assay (n=5) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Calculated Concentration (ng/mL)b | Accuracy (%) | Precision (%) | Calculated Concentration (ng/mL)b | Accuracy (%) | Precision (%) | ||
| (R)-bupropion | 2.5 | 2.5 ± 0.1 | 101 | 5 | 2.3 ± 0.2 | 92 | 7 |
| 25 | 25.0 ± 2.5 | 100 | 10 | 22.3 ± 2.0 | 91 | 9 | |
| 500 | 572 ± 58 | 114 | 10 | 481 ± 63 | 96 | 13 | |
| (S)-bupropion | 2.5 | 2.6 ± 0.2 | 103 | 9 | 2.4 ± 0.2 | 97 | 8 |
| 25 | 24.6 ± 1.8 | 98 | 7 | 22.5 ± 1.1 | 90 | 5 | |
| 500 | 577 ± 32 | 115 | 5 | 521 ± 47 | 104 | 9 | |
| (R,R)-hydroxybupropion | 2.5 | 2.6 ± 0.1 | 104 | 4 | 2.4 ± 0.2 | 97 | 7 |
| 25 | 24.7 ± 1.2 | 99 | 5 | 23.0 ± 0.7 | 92 | 3 | |
| 500 | 519 ± 16 | 104 | 3 | 475 ± 30 | 95 | 6 | |
| (S,S)-hydroxybupropion | 2.5 | 2.2 ± 0.2 | 89 | 10 | 2.3 ± 0.2 | 90 | 8 |
| 25 | 25.3 ± 2.5 | 101 | 10 | 23.1 ± 0.8 | 92 | 3 | |
| 500 | 534 ± 16 | 107 | 3 | 481 ± 40 | 96 | 8 | |
| (1S,2S)-threohydrobupropion | 2.5 | 2.6 ± 0.1 | 106 | 5 | 2.3 ± 0.2 | 94 | 11 |
| 25 | 25.9 ± 3.2 | 104 | 12 | 23.0 ± 0.8 | 92 | 4 | |
| 500 | 523 ± 29 | 105 | 6 | 473 ± 38 | 95 | 8 | |
| (1R,2R)-threohydrobupropion | 2.5 | 2.6 ± 0.1 | 99 | 3 | 2.4 ± 0.2 | 97 | 9 |
| 25 | 24.9 ± 1.9 | 93 | 8 | 22.8 ± 1.3 | 91 | 6 | |
| 500 | 538 ± 39 | 103 | 7 | 479 ± 53 | 96 | 11 | |
| (1R,2S)-erythrohydrobupropion | 2.5 | 2.6 ± 0.2 | 105 | 8 | 2.4 ± 0.3 | 94 | 13 |
| 25 | 25.1 ± 2.1 | 96 | 8 | 22.4 ± 1.4 | 90 | 6 | |
| 500 | 544 ± 23 | 106 | 4 | 481 ± 34 | 96 | 7 | |
| (1S,2R)-erythrohydrobupropion | 2.5 | 2.5 ± 0.3 | 99 | 11 | 2.5 ± 0.4 | 100 | 15 |
| 25 | 24.8 ± 2.7 | 99 | 11 | 23.5 ± 1.0 | 94 | 4 | |
| 500 | 531 ± 51 | 106 | 10 | 481 ± 40 | 96 | 8 | |
Calibration standards were 0.5, 1, 2.5, 5, 10, 25, 50, 100, 250, 500, 1000 ng/mL
Mean ± standard deviation
Table 5.
Intra-assay and inter-assay accuracy and precision for determination of bupropion and metabolites in human plasma on an AB Sciex 6500 mass spectrometer
| Analyte | Nominal concentratio n (ng/mL) | Intra-assay (n=3) | Inter-assay (n=5) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Calculated Concentration (ng/mL) | Accuracy(%) | Precision (%) | Calculated Concentration (ng/mL) | Accuracy (%) | Precision (%) | ||
| (R)-bupropion | 2.5 | 2.2 ± 0.1 | 88 | 5 | 2.3 ± 0.3 | 93 | 11 |
| 25 | 21.1 ± 1.1 | 85 | 5 | 22.5 ± 1.5 | 90 | 7 | |
| 500 | 494 ± 13 | 99 | 3 | 498 ± 35 | 100 | 7 | |
| (S)-bupropion | 2.5 | 2.3 ± 0.2 | 91 | 7 | 2.4 ± 0.3 | 96 | 11 |
| 25 | 21.6 ± 1.4 | 86 | 7 | 22.7 ± 1.7 | 91 | 8 | |
| 500 | 522 ± 11 | 104 | 2 | 534 ± 52 | 107 | 10 | |
| (R,R)-hydroxybupropion | 2.5 | 2.1 ± 0.1 | 84 | 7 | 2.3 ± 0.3 | 91 | 14 |
| 25 | 20.6 ± 1.3 | 82 | 6 | 21.9 ± 1.8 | 88 | 8 | |
| 500 | 518 ± 23 | 104 | 4 | 534 ± 34 | 107 | 6 | |
| (S,S)-hydroxybupropion | 2.5 | 2.3 ± 0.0 | 93 | 2 | 2.3 ± 0.3 | 93 | 11 |
| 25 | 20.9 ± 1.7 | 84 | 8 | 21.7 ± 2.0 | 87 | 9 | |
| 500 | 485 ± 7 | 97 | 1 | 480 ± 28 | 96 | 6 | |
| (1S,2S)-threohydrobupropion | 2.5 | 2.4 ± 0.1 | 94 | 6 | 2.4 ± 0.2 | 96 | 10 |
| 25 | 22.2 ± 1.1 | 89 | 5 | 23.0 ± 1.2 | 92 | 5 | |
| 500 | 457 ± 43 | 91 | 9 | 485 ± 39 | 97 | 8 | |
| (1R,2R)-threohydrobupropion | 2.5 | 2.3 ± 0.2 | 90 | 7 | 2.4 ± 0.2 | 96 | 9 |
| 25 | 21.7 ± 0.7 | 87 | 3 | 22.8 ± 1.2 | 91 | 5 | |
| 500 | 492 ± 9 | 98 | 2 | 493 ± 36 | 99 | 7 | |
| (1R,2S)-erythrohydrobupropion | 2.5 | 2.3 ± 0.1 | 91 | 6 | 2.3 ± 0.3 | 91 | 14 |
| 25 | 22.6 ± 0.6 | 90 | 3 | 22.9 ± 1.6 | 92 | 7 | |
| 500 | 478 ± 15 | 95 | 3 | 485 ± 49 | 97 | 10 | |
| (1S,2R)-erythrohydrobupropion | 2.5 | 2.3 ± 0.2 | 91 | 7 | 2.4 ± 0.3 | 96 | 12 |
| 25 | 21.6 ± 1.2 | 87 | 6 | 22.7 ± 1.5 | 91 | 7 | |
| 500 | 483 ± 6 | 97 | 1 | 489 ± 42 | 98 | 9 | |
Results are the mean ± standard deviation
Figure 4.
Evaluation of instrument sensitivity at 0.25 ng/mL analyte concentrations. Analysis was performed on AB Sciex 3200 (A–C, top), 4000 QTRAP (D–F, middle), and 6500 (G–I, bottom) instruments. Analytes were (R)- and (S)-bupropion (A,D,G), (R,R)- and (S,S)-hydroxybupropion (B,E,H), (1S,2S)- and (1R,2R)-threohydrobupropion and (1S,2R)- and (1R,2S)-erythrohydrobupropion (C,F,I). Lowercase letters a-d (panels C,F,I) refer to (1S,2S)-threohydrobupropion, (1R,2R)-threohydrobupropion, (1R,2S)-erythrohydrobupropion, and (1S,2R)-erythrohydrobupropion, respectively. Peak areas are shown on each panel. Chromatograms are shown to maximize peak areas; note however the difference in peak intensity (Y axis) ranges. There were small differences in retention times between the three instruments.
3.3.2 Analyte specific validation on an AB Sciex 3200 mass spectrometer
Analyte specific calibrator and QC concentrations (Table 1) were based on a preliminary analysis of plasma samples from a clinical investigation in which patients received 300 mg extended release bupropion once daily, and from previously published clinical studies [12]. Sensitivity down to 0.25 ng/mL was not needed for any analyte, and the upper concentration ranges for bupropion and hydroxybupropion were decreased and increased, respectively. The 3200 instrument was chosen for the final assay development and full validation because it had the requisite sensitivity and the greater sensitivity of the 4000 and 6500 instruments was not needed.
The final assay validation met all acceptance criteria. Accuracy and precision data for each analyte and the QC concentrations are shown in Table 6. For each analyte at each respective concentration, the accuracy and precision was within 12%, including at the limit of quantification. Figure 5 and Figure 6 show the extracted ion chromatograms from a processed plasma sample devoid of analytes or internal standards, and at the lowest calibrator concentration for each analyte, respectively. Extracted ion chromatograms from ion suppression experiments are shown in Figure 7. In each of the chromatograms, ion suppression from matrix components only occurred from 1–3 min and did not interfere with the respective analyte signals. Analyte stability following three subsequent freeze thaw cycles at −80°C and autosampler storage 4°C for 48 hr was evaluated (Table 7). Analyte stability in human plasma ranged from 93–113%, 94–104%, and 88–110% of the nominal concentration after the 1st, 2nd, and 3rd subsequent −80°C freeze thaw cycles, respectively. Additionally, analyte concentrations ranged from 92–115% of the nominal concentration when stored in the autosampler at pH 3 and 4°C for 48 hr. Analyte racemization was also assessed in the autosampler for 21 days at pH 3 at 4°C. All analytes were stable and did not undergo any racemization during the storage and freeze thaw cycles (data not shown). The results of these experiments show that bupropion and metabolite enantiomers are stable after freeze/thaw cycles and storage at pH 3 at 4°C for an extended period of time.
Table 6.
Intra-day and inter-day accuracy and precision for determination of bupropion and metabolites in human plasma with analyte specific calibration rangesa on an AB Sciex 3200 mass spectrometer
| Analyte | Nominal concentration (ng/mL) | Intra-assay (n=5) | Inter-assay (n=5) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Calculated Concentration (ng/mL)b | Accuracy (%) | Precision (%) | Calculated Concentration (ng/mL)b | Accuracy (%) | Precision (%) | ||
| (R)-bupropion | 2.5 | 2.5 ± 0.2 | 99 | 6 | 2.6 ± 0.3 | 106 | 10 |
| 10 | 9.8 ± 0.3 | 98 | 3 | 9.9 ± 0.5 | 99 | 5 | |
| 25 | 25.5 ± 1.0 | 102 | 4 | 25.8 ± 1.2 | 103 | 5 | |
| 50 | 49.1 ± 2.3 | 98 | 5 | 49.6 ± 3.1 | 99 | 6 | |
| (S)-bupropion | 2.5 | 2.1 ± 0.1 | 97 | 6 | 2.5 ± 0.1 | 100 | 3 |
| 10 | 7.8 ± 0.5 | 94 | 6 | 9.4 ± 0.4 | 94 | 5 | |
| 25 | 20.8 ± 0.9 | 100 | 4 | 25.7 ± 0.9 | 103 | 3 | |
| 50 | 41.1 ± 1.7 | 100 | 4 | 49.2 ± 1.9 | 98 | 4 | |
| (R,R)-hydroxybupropion | 5 | 4.8 ± 0.4 | 96 | 9 | 5.1 ± 0.2 | 102 | 3 |
| 25 | 27.7 ± 2.0 | 111 | 7 | 27.6 ± 1.1 | 110 | 4 | |
| 250 | 260 ± 9 | 104 | 4 | 262 ± 12 | 105 | 5 | |
| 1000 | 969 ± 38 | 97 | 4 | 964 ± 42 | 96 | 4 | |
| 2000 | 1990 ± 151 | 100 | 8 | 2035 ± 121 | 102 | 6 | |
| (S,S)-hydroxybupropion | 5 | 5.0 ± 0.3 | 100 | 7 | 5.1 ± 0.3 | 102 | 5 |
| 25 | 26.1 ± 1.2 | 104 | 5 | 26.1 ± 0.1 | 105 | 0 | |
| 250 | 248 ± 10 | 99 | 4 | 246 ± 5 | 98 | 2 | |
| 1000 | 963 ± 33 | 96 | 3 | 955 ± 29 | 96 | 3 | |
| (1S,2S)-threohydrobupropion | 2.5 | 2.5 ± 0.2 | 100 | 9 | 2.5 ± 0.1 | 100 | 5 |
| 10 | 9.7 ± 0.6 | 97 | 6 | 9.8 ± 0.2 | 98 | 2 | |
| 50 | 49.0 ± 2.1 | 98 | 4 | 50.0 ± 2.1 | 100 | 4 | |
| 200 | 200 ± 6 | 100 | 3 | 201 ± 3 | 100 | 1 | |
| (1R,2R)-threohydrobupropion | 2.5 | 2.5 ± 0.2 | 99 | 10 | 2.5 ± 0.0 | 99 | 2 |
| 10 | 9.6 ± 0.5 | 96 | 5 | 9.8 ± 0.5 | 98 | 5 | |
| 50 | 49.2 ± 2.4 | 98 | 5 | 50.8 ± 1.8 | 102 | 3 | |
| 200 | 200 ± 7 | 100 | 4 | 202 ± 8 | 101 | 4 | |
| (1R,2S)-erythrohydrobupropion | 2.5 | 2.5 ± 0.3 | 100 | 12 | 2.6 ± 0.2 | 105 | 8 |
| 10 | 9.6 ± 0.7 | 96 | 7 | 9.8 ± 0.3 | 98 | 3 | |
| 50 | 48.2 ± 2.5 | 96 | 5 | 50.8 ± 2.0 | 102 | 4 | |
| 200 | 197 ± 8 | 99 | 4 | 198 ± 6 | 99 | 3 | |
| (1S,2R)-erythrohydrobupropion | 2.5 | 2.5 ± 0.2 | 100 | 7 | 2.5 ± 0.1 | 100 | 4 |
| 10 | 9.9 ± 0.5 | 99 | 5 | 9.9 ± 0.3 | 99 | 3 | |
| 50 | 48.6 ± 2.0 | 97 | 4 | 50.6 ± 1.7 | 101 | 3 | |
| 200 | 201 ± 6 | 101 | 3 | 203 ± 4 | 101 | 2 | |
Calibration ranges are provided in Table 1
Mean ± standard deviation
Figure 5.
Analysis of analyte- and internal standard free human plasma on an AB Sciex 3200 mass spectrometer. (A) Representative total ion chromatogram and representative MRM chromatograms of: (B) bupropion, (C) hydroxybupropion, (D) erythrohydrobupropion and threohydrobupropion, (E) bupropion-d9, (F) hydroxybupropion-d6, (G) erythrohydrobupropion-d9, and threohydrobupropion-d9.
Figure 6.
Representative MRM chromatograms of a plasma calibration sample at the limit of quantification on an AB Sciex 3200 mass spectrometer (A) Total ion chromatogram, (B) 0.5 ng/mL bupropion (R and S), (C) 2 ng/mL hydroxybupropion (R,R and S,S) (D) 1 ng/mL threohydrobupropion (1S,2S and 1R,2R) and erythrohydrobupropion (1S,2R and 1R,2S). Panels E-G show the deuterated internal standards (E) 10 ng/mL (R)- and (S)-bupropion-d9, (F) 50 ng/mL (R,R)- and (S,S)- hydroxybupropion-d6, (G) 25 ng/mL (1S,2S)- and (1R,2R)-threohydrobupropion-d9 and (1S,2R)- and (1R,2S)-erythrohydrobupropion-d9.
Figure 7.
Evaluation of ionization suppression. Extracted ion chromatograms were acquired after injecting analyte- and internal standard free human plasma while simultaneously infusing a solution of analytes and internal standards into the AB Sciex 3200 mass spectrometer: (A) bupropion, (B) hydroxybupropion, and (C) erythrohydrobupropion and threohydrobupropion. Peak overlays are from a calibration standard to show the respective retention time of each analyte.
Table 7.
Stability of bupropion and metabolites in human plasma
| Analyte | Stability in plasma
(%)a (n
= 3) |
||||
|---|---|---|---|---|---|
| Nominal Concentration (ng/mL) | 1st freeze-thaw | 2nd freeze-thaw | 3rd freeze-thaw | 48 hr in 4°C autosampler | |
| (R)-bupropion | 2.5 | 93 ± 11 | 103 ± 11 | 88 ± 11 | 102 ± 3 |
| 50 | 98 ± 2 | 95 ± 1 | 97 ± 3 | 104 ± 2 | |
| (S)-bupropion | 2.5 | 113 ± 1 | 104 ± 4 | 110 ± 8 | 115 ± 3 |
| 50 | 96 ± 2 | 94 ± 1 | 93 ± 1 | 101 ± 5 | |
| (R,R)-hydroxybupropion | 5 | 102 ± 1 | 104 ± 8 | 101 ± 9 | 99 ± 7 |
| 2000 | 96 ± 2 | 97± 2 | 96 ± 2 | 102 ± 2 | |
| (S,S)-hydroxybupropion | 5 | 101 ± 6 | 99 ± 5 | 97 ± 10 | 92 ± 2 |
| 2000 | 96 ± 2 | 98 ± 4 | 97 ± 6 | 100 ± 2 | |
| (1S,2S)-threohydrobupropion | 2.5 | 96 ± 7 | 102 ± 11 | 99 ± 6 | 104 ± 6 |
| 200 | 96 ± 2 | 96 ± 2 | 95 ± 2 | 101 ± 2 | |
| (1R,2R)-threohydrobupropion | 2.5 | 96 ± 7 | 101 ± 9 | 97 ± 11 | 104 ± 10 |
| 200 | 98 ± 4 | 100 ± 3 | 95 ± 1 | 99 ± 3 | |
| (1R,2S)-erythrohydrobupropion | 2.5 | 100 ± 5 | 109 ± 8 | 109 ± 6 | 112 ± 5 |
| 200 | 98 ± 3 | 98 ± 4 | 99 ± 1 | 103 ± 3 | |
| (1S,2R)-erythrohydrobupropion | 2.5 | 93 ± 6 | 102 ± 10 | 92 ± 9 | 100 ± 5 |
| 200 | 97 ± 0 | 98 ± 1 | 96 ± 1 | 102 ± 3 | |
Reported values are the percentage of the initial observed concentration (prepared freshly with no freeze thaw cycles), mean ± standard deviation
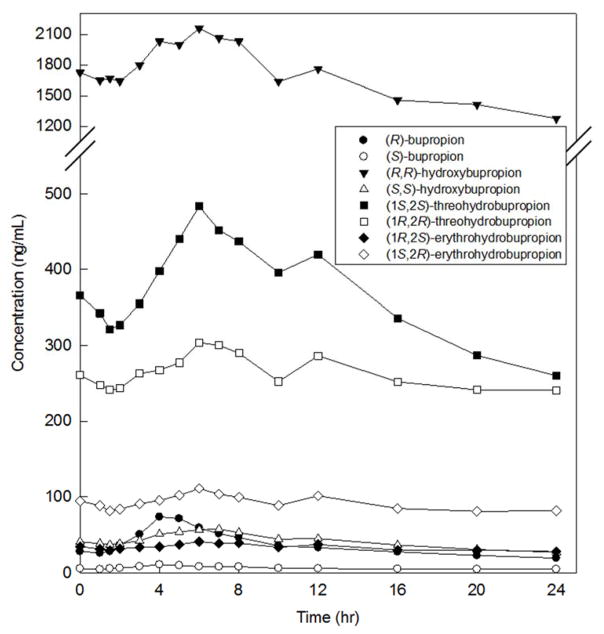
3.4 Method Application
The validated method was successfully used to quantify plasma concentrations of bupropion, hydroxybupropion, erythrohydrobupropion, and threohydrobupropion enantiomers from a patient who received 300 mg oral extended release bupropion once daily (Figure 8). Concentrations were within the calibration ranges. This is the first accurate quantification or erythrohydrobupropion enantiomers and threohydrobupropion enantiomers in human plasma. There was a 1.5- to 2-fold difference in enantiomer concentrations for both erythrohydrobupropion and threohydrobupropion. These differences substantiate the requirement for a stereoselective assay for all bupropion metabolites.
Figure 8.
Plasma concentrations of (R)-bupropion, (S)-bupropion, (R,R)-hydroxybupropion, (S,S)-hydroxybupropion, (1S,2S)-threohydrobupropion, (1R,2R)-threohydrobupropion, (1R,2S)-erythrohydrobupropion, and (1S,2R)-erythrohydrobupropion from a research subject who received 300 mg oral extended release bupropion each morning.
3.5 Method comparison
This is the first stereoselective bioanalytical method to quantify enantiomers of bupropion, and the principle metabolites hydroxybupropion, erythrohydrobupropion, and threohydrobupropion in human plasma. It therefore represents an advance over previous methods, which either analyzed these analytes achirally [3,11–15,27,28], or analyzed only bupropion and hydroxybupropion stereoselectively [5,9,17]. In addition, sample preparation (acid precipitation), enabled in part by changing the chromatographic method, was simpler than the previously reported solid phase extraction [5].
There are three published bioanalytical methods for the stereoselective analysis of bupropion and/or hydroxybupropion enantiomers in plasma from clinical investigations [5,9,17]. Each of the previous methods utilized either liquid-liquid extraction (1.5% v/v isoamyl alcohol in n-heptane) or solid-phase extraction (mixed-mode cation exchange) for sample cleanup. The current method utilizes 20% trichloroacetic acid for plasma protein precipitation, which vastly improved assay efficiency and throughput. One full 96-well plate of samples takes approximately 1 hr to prepare. Total run times for the previous methods ranged from 15–30 min and they only analyzed hydroxybupropion (R,R and S,S) and/or bupropion (R and S) enantiomers. The current improved method analyzes bupropion (R and S), hydroxybupropion (R,R and S,S), threohydrobupropion (1S,2S and 1R,2R), and erythrohydrobupropion (1R,2S and 1S,2R) within 12 min. Previous limits of quantification for bupropion enantiomers were 12.5 ng/mL when analyzed by LC-UV at 254 nm and 0.5 ng/mL when analyzed by LC-MS/MS. For hydroxybupropion enantiomers, previous limits of quantification were either 12.5 and 62.5 ng/mL when analyzed by LC-UV at 214 nm or 2.5 ng/mL when analyzed by LC-MS/MS. In this assay, limits of quantification were 0.5 ng/mL, 2 ng/mL, 1 ng/mL and 1 ng/mL for bupropion (R and S), hydroxybupropion (R,R and S,S), threohydrobupropion (1S,2S and 1R,2R), and erythrohydrobupropion (1R,2S and 1S,2R), respectively. The sensitivity for bupropion and hydroxybupropion enantiomers is comparable to the previously published LC-MS/MS assay, although no attempt was made to maximize sensitivity.
4. Conclusion
The overall goal was to develop and validate a short high-throughput and completely stereoselective method for bupropion and its primary major metabolites. This is the first stereoselective bioanalytical method to quantify enantiomers of bupropion and the principle metabolites hydroxybupropion, erythrohydrobupropion and threohydrobupropion in human plasma, and is therefore an advance over previous methods, The validated method is within full compliance with FDA guidelines for assay validation. As a result of this work, erythrohydrobupropion and threohydrobupropion enantiomers can be resolved chromatographically and the enantiomers of bupropion and major metabolites can all be quantified using a single method.
Table 4.
Intra- assay and inter- assay accuracy and precision for determination of bupropion and metabolites in human plasma on an AB Sciex 4000 QTRAP mass spectrometer
| Analyte | Nominal concentratio n (ng/mL) | Intra-assay (n=3) | Inter-assay (n=5) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Calculated Concentration (ng/mL) | Accuracy (%) | Precision (%) | Calculated Concentration (ng/mL) | Accuracy (%) | Precision (%) | ||
| (R)-bupropion | 2.5 | 2.5 ± 0.3 | 99 | 13 | 2.4 ± 0.2 | 94 | 9 |
| 25 | 23.0 ± 1.1 | 92 | 5 | 23.1 ± 1.5 | 92 | 6 | |
| 500 | 517 ± 39 | 103 | 8 | 510 ± 50 | 102 | 10 | |
| (S)-bupropion | 2.5 | 2.4 ± 0.1 | 94 | 2 | 2.5 ± 0.1 | 99 | 3 |
| 25 | 21.9 ± 0.9 | 88 | 4 | 22.7 ± 1.2 | 91 | 5 | |
| 500 | 532 ± 13 | 106 | 2 | 524 ± 44 | 105 | 8 | |
| (R,R)-hydroxybupropion | 2.5 | 2.3 ± 0.2 | 94 | 7 | 2.4 ± 0.3 | 96 | 12 |
| 25 | 22.0 ± 1.1 | 88 | 5 | 22.8 ± 1.2 | 91 | 5 | |
| 500 | 481 ± 22 | 96 | 5 | 481 ± 35 | 96 | 7 | |
| (S,S)-hydroxybupropion | 2.5 | 2.4 ± 0.3 | 98 | 12 | 2.3 ± 0.3 | 92 | 12 |
| 25 | 23.0 ± 2.5 | 92 | 11 | 22.9 ± 1.3 | 92 | 6 | |
| 500 | 490 ± 43 | 98 | 9 | 471 ± 30 | 94 | 6 | |
| (1S,2S)-threohydrobupropion | 2.5 | 2.4 ± 0.1 | 94 | 6 | 2.4 ± 0.3 | 96 | 11 |
| 25 | 24.3 ± 1.1 | 97 | 5 | 23.1 ± 1.5 | 93 | 7 | |
| 500 | 512 ± 27 | 102 | 5 | 472 ± 50 | 94 | 11 | |
| (1R,2R)-threohydrobupropion | 2.5 | 2.3 ± 0.2 | 90 | 8 | 2.4 ± 0.3 | 97 | 14 |
| 25 | 23.7 ± 2.5 | 95 | 11 | 23.0 ± 1.0 | 92 | 4 | |
| 500 | 519 ± 38 | 104 | 7 | 481 ± 42 | 96 | 9 | |
| (1R,2S)-erythrohydrobupropion | 2.5 | 2.6 ± 0.3 | 105 | 10 | 2.3 ± 0.3 | 90 | 12 |
| 25 | 23.6 ± 2.1 | 94 | 9 | 21.6 ± 1.3 | 87 | 6 | |
| 500 | 480 ± 18 | 96 | 4 | 461 ± 56 | 92 | 12 | |
| (1S,2R)-erythrohydrobupropion | 2.5 | 2.3 ± 0.2 | 94 | 7 | 2.4 ± 0.3 | 95 | 11 |
| 25 | 22.9 ± 2.6 | 91 | 11 | 23.3 ± 1.7 | 93 | 7 | |
| 500 | 490 ± 39 | 98 | 8 | 487 ± 34 | 97 | 7 | |
Results are the mean ± standard deviation
Highlights.
A stereoselective analytical method for bupropion and the principle metabolites was developed and validated on three AB Sciex triple quadrupole mass spectrometers (3200, 4000 QTRAP, and 6500).
Bupropion, hydroxybupropion, erythrohydrobupropion and threohydrobupropion enantiomers were resolved utilizing a α1-acid glycoprotein chiral column.
Each erythrohydrobupropion and threohydrobupropion enantiomer peaks were stereochemically assigned by sodium borohydride reduction of enantiopure (R)-bupropion and (S)-bupropion.
The validated assay was applied to determine plasma concentrations of (R)-bupropion, (S)-bupropion, (R,R)-hydroxybupropion, and (S,S)-hydroxybupropion, (1S,2S)-threohydrobupropion, (1R,2R)-threohydrobupropion, (1R,2S)-erythrohydrobupropion, and (1S,2R)-erythrohydrobupropion from a patient who received extended release (R,S)-bupropion.
Acknowledgments
Supported by National Institutes of Health grants R01DA14211 and U01 FD004899 (EDK) and T32-DA007261 (AMT).
Footnotes
No author has any conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dhillon S, Yang LP, Curran MP. Bupropion: a review of its use in the management of major depressive disorder. Drugs. 2008;68:653–689. doi: 10.2165/00003495-200868050-00011. [DOI] [PubMed] [Google Scholar]
- 2.Coles R, Kharasch ED. Stereoselective metabolism of bupropion by CYP2B6 and human liver microsomes. Pharm Res. 2008;25:1405–1411. doi: 10.1007/s11095-008-9535-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connarn JN, Zhang X, Babiskin A, Sun D. Metabolism of bupropion by carbonyl reductases in liver and intestine. Drug Metab Dispos. 2015;43:1019–1027. doi: 10.1124/dmd.115.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer A, Vuorinen A, Zielinska AE, Strajhar P, Lavery GG, Schuster D, Odermatt A. Formation of threohydrobupropion from bupropion is dependent on 11β-hydroxysteroid dehydrogenase 1. Drug Metab Dispos. 2013;41:1671–1678. doi: 10.1124/dmd.113.052936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coles R, Kharasch ED. Stereoselective analysis of bupropion and hydroxybupropion in human plasma and urine by LC/MS/MS. J Chromatogr B. 2007;857:67–75. doi: 10.1016/j.jchromb.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Kharasch ED, Mitchell D, Coles R. Stereoselective bupropion hydroxylation as an in vivo phenotypic probe for cytochrome P4502B6 (CYP2B6) activity. J Clin Pharmacol. 2008;48:464–474. doi: 10.1177/0091270008314254. [DOI] [PubMed] [Google Scholar]
- 7.Kharasch ED, Mitchell D, Coles R, Blanco R. Rapid clinical induction of hepatic cytochrome P4502B6 activity by ritonavir. Antimicrob Agents Chemother. 2008;52:1663–1669. doi: 10.1128/AAC.01600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joy MS, Frye RF, Stubbert K, Brouwer KR, Falk RJ, Kharasch ED. Use of enantiomeric bupropion and hydroxybupropion to assess CYP2B6 activity in glomerular kidney diseases. J Clin Pharmacol. 2010;50:714–720. doi: 10.1177/0091270009353031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H, Loboz KK, Gross AS, McLachlan AJ. Stereoselective analysis of hydroxybupropion and application to drug interaction studies. Chirality. 2007;19:163–170. doi: 10.1002/chir.20356. [DOI] [PubMed] [Google Scholar]
- 10.Musso DL, Mehta NB, Soroko FE, Ferris RM, Hollingsworth EB, Kenney BT. Synthesis and evaluation of the antidepressant activity of the enantiomers of bupropion. Chirality. 1993;5:495–500. doi: 10.1002/chir.530050704. [DOI] [PubMed] [Google Scholar]
- 11.Denooz R, Mercerolle M, Lachatre G, Charlier C. Ultra-performance liquid chromatography- tandem mass spectrometry method for the determination of bupropion and its main metabolites in human whole blood. J Anal Toxicol. 2010;34:280–286. doi: 10.1093/jat/34.5.280. [DOI] [PubMed] [Google Scholar]
- 12.Benowitz NL, Zhu AZ, Tyndale RF, Dempsey D, Jacob P., 3rd Influence of CYP2B6 genetic variants on plasma and urine concentrations of bupropion and metabolites at steady state. Pharmacogenet Genomics. 2013;23:135–141. doi: 10.1097/FPC.0b013e32835d9ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loboz KK, Gross AS, Ray J, McLachlan AJ. HPLC assay for bupropion and its major metabolites in human plasma. J Chromatogr B. 2005;823:115–121. doi: 10.1016/j.jchromb.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Petsalo A, Turpeinen M, Tolonen A. Identification of bupropion urinary metabolites by liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:2547–2554. doi: 10.1002/rcm.3117. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Vernikovskaya DI, Abdelrahman DR, Hankins GD, Ahmed MS, Nanovskaya TN. Simultaneous quantitative determination of bupropion and its three major metabolites in human umbilical cord plasma and placental tissue using high-performance liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2012;70:320–329. doi: 10.1016/j.jpba.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas JS, Kaplan CP, Barenboim D, Jacob P, 3rd, Benowitz NL. Bupropion in breast milk: an exposure assessment for potential treatment to prevent post-partum tobacco use. Tob Control. 2004;13:52–56. doi: 10.1136/tc.2003.004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suckow RF, Zhang MF, Cooper TB. Enantiomeric determination of the phenylmorpholinol metabolite of bupropion in human plasma using coupled achiral-chiral liquid chromatography. Biomed Chromatogr. 1997;11:174–179. doi: 10.1002/(SICI)1099-0801(199705)11:3<174::AID-BMC681>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 18.Munro JS, Walker TA. Bupropion hydrochloride: the development of a chiral separation using an ovomucoid column. J Chromatogr A. 2001;913:275–282. doi: 10.1016/s0021-9673(01)00639-2. [DOI] [PubMed] [Google Scholar]
- 19.Batra S, Bhushan R. Resolution of enantiomers of bupropion and its metabolites by liquid chromatography. Biomed Chromatogr. 2016 doi: 10.1002/bmc.3572. in press. [DOI] [PubMed] [Google Scholar]
- 20.Hermansson J, Grahn A. Optimization of the separation of enantiomers of basic drugs. Retention mechanisms and dynamic modification of the chiral bonding properties on an alpha 1-acid glycoprotein column. J Chromatogr A. 1995;694:57–69. doi: 10.1016/0021-9673(94)00936-4. [DOI] [PubMed] [Google Scholar]
- 21.Castro-Puyana M, Garcia MA, Marina ML. Enantiomeric separation of bupropion enantiomers by electrokinetic chromatography: quantitative analysis in pharmaceutical formulations. J Chromatogr B. 2008;875:260–265. doi: 10.1016/j.jchromb.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Bhushan R, Batra S. High-performance liquid chromatographic enantioseparation of (RS)-bupropion using isothiocyanate-based chiral derivatizing reagents. Biomed Chromatogr. 2013;27:956–959. doi: 10.1002/bmc.2885. [DOI] [PubMed] [Google Scholar]
- 23.Bondarev ML, Bondareva TS, Young R, Glennon RA. Behavioral and biochemical investigations of bupropion metabolites. Eur J Pharmacol. 2003;474:85–93. doi: 10.1016/s0014-2999(03)02010-7. [DOI] [PubMed] [Google Scholar]
- 24.US Food and Drug Administration. Guidance for industry: Bioanalytical method validation. Center for Drug Evaluation and Research; 2001. [Google Scholar]
- 25.Fang QK, Han Z, Grover P, Kessler D, Senanayake CH, Wald SA. Rapid access to enantiopure bupropion and its major metabolite by stereospecific nucleophilic substitution on an a-ketotriflate. Tetrahedron: Asymmetry. 2000;11:3659–3663. [Google Scholar]
- 26.Khan A, Reinhard JF World Intellectual Property Organization. WO2012118562. patent. 2012
- 27.Borges V, Yang E, Dunn J, Henion J. High-throughput liquid chromatography-tandem mass spectrometry determination of bupropion and its metabolites in human, mouse and rat plasma using a monolithic column. J Chromatogr B. 2004;804:277–287. doi: 10.1016/j.jchromb.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 28.Hsyu PH, Singh A, Giargiari TD, Dunn JA, Ascher JA, Johnston JA. Pharmacokinetics of bupropion and its metabolites in cigarette smokers versus nonsmokers. J Clin Pharmacol. 1997;37:737–743. doi: 10.1002/j.1552-4604.1997.tb04361.x. [DOI] [PubMed] [Google Scholar]