Abstract
Two cancer testis antigens, the New York esophageal squamous cell carcinoma-1 (NY-ESO-1) and the melanoma-antigen family A (MAGE-A) represent promising immunotherapy targets due to the low expression of these antigens in non-malignant tissue. To assess over-expression patterns in various cancers, we performed a systematic immunohistochemical analysis for NY-ESO-1 and MAGE-A on tissue array samples of 3668 common epithelial carcinomas (CA) and germ cell tumors of high prevalence and mortality. Here, we find significantly higher expression of MAGE-A (>50% on tumor cells) compared to NY-ESO-1 in several carcinomas including cutaneous squamous cell carcinomas (SCC) (52.8%/2.8%), esophageal SCC (50%/0%), head and neck SCC (41.1%/<1%), bladder urothelial CA (40.4%/8.3%), cervical/anal SCC (37.5%/0%), lung SCC (34%/3.8%), lung adenocarcinomas (27.6%/3.9%), ovarian CA (26.4%/3.6%), endometrial CA (26.3%/1.3%), lung small cell CA (24.4%/2.4%), gastric adenocarcinomas (20%/4%), breast-mucinous CA (19.3%/0), hepatocellular CA (18.8%/1.2%), breast infiltrating ductal CA (16.4%/1.8%), colorectal adenocarcinomas (10.7%/<1%), cholangiocarcinomas (9.8%/0%), thymic CA (9%/4.5%), and mesotheliomas (7.9%/<1%). Furthermore, high-expression of MAGE-A, but not NY-ESO-1 was seen in an independent cohort of metastatic SCC (45.5%/3.6%) and metastatic CA (13.5%/0%) of various primaries with significantly higher expression of MAGE-A in metastatic SCC compared to other metastatic CA. MAGE-A is also more highly expressed in germ cell tumors, seminomas (69%/3.5%) and non-seminomas (40.1%/4.7%). In summary, MAGE-A is more highly expressed than NY-ESO-1 in a majority of human malignancies, and targeting MAGE-A may benefit a large number of patients.
Introduction
The cancer testis antigens (CTA) represent promising immunotherapeutic targets due to the relatively low to negligent expression on normal somatic cells and the over-expression in various cancer histologies1. CTAs are primarily expressed in the spermatogonia or dividing germ cells in the male testis and placental trophoblastic cells, but to our knowledge are not expressed in somatic cells. Two of the most well-studied cancer-testis antigens are the New York esophageal squamous cell carcinoma-1 (NY-ESO-1) and the melanoma-antigen family A proteins (MAGE-A), encoded respectively, by the CTAGB1 and MAGEA family of genes located on the X chromosome2. The over-expression of NY-ESO-1 and MAGE-A can elicit potent T cell responses and therefore represents a promising tumor-specific immunotherapeutic target3. Analysis utilizing immunohistochemistry of tumor tissue enables for the rapid evaluation of expression levels at the time of a microscopic cancer diagnosis and enables for the rapid selection of malignancies that possess the potential to elicit antigen specific anti-tumor T cell responses4,5.
A recent phase I/II clinical trial evaluating the ability of adoptively transferred T cells genetically engineered with a T cell receptor recognizing the NY-ESO-1 antigen resulted in durable tumor regression in approximately half of all the patients treated for metastatic melanoma and metastatic synovial sarcoma6. One of the diagnostic criteria critical for the evaluation of trial eligibility was the high-expression of NY-ESO-1 based on immunohistochemical analysis. Prior studies show that approximately 30% of metastatic melanomas and 75% of synovial sarcomas can over-express NY-ESO-14,5. Additionally, the current literature reports positive NY-ESO-1 IHC expression in bladder urothelial carcinomas (22/72: 31%7; 2/14: 14%8; 6/33: 18%)9, esophageal carcinomas (18/56: 32%)10, hepatocellular carcinomas (25/132: 19%)11, head and neck carcinomas (17/70: 24%)12, non-small cell lung carcinomas (13/52: 25%; 15/130: 12%)13, ovarian carcinomas (62/142: 43%)14, and prostatic adenocarcinomas (7/48: 15%)15. In regard to other tumor histologies, NY-ESO-1 mRNA transcript levels are the only data available16. Thus, a moderate percentage of certain cancers are reported to express NY-ESO by IHC.
A second promising cancer-testis antigen, MAGE-A, is also reported to be over-expressed in various cancers and is comprised of 12 different genes at the Xq28 location with 50 – 80% sequence homology between the different protein products. There are several cancer vaccine and adoptive cell therapy clinical trials currently targeting MAGE-A17. Most of the existing data shows over-expression of MAGE-A through gene-expression analysis, however, one study does report 38% (MAGE-A4) and 63% (MAGE-A9) IHC expression in non-muscle invasive bladder urothelial carcinoma18,19. Although the MAGE-A family of proteins represent promising immunotherapeutic targets, the IHC expression pattern of MAGE-A in a variety of different cancers still remains to be explored.
Here, we evaluated the expression of NY-ESO-1 and MAGE-A by IHC in 3668 common carcinomas and germ cell tumors. We report that NY-ESO-1 is not highly expressed in most carcinomas, but MAGE-A in contrast, is more widely expressed in several carcinomas and germ cell tumors including esophageal squamous cell carcinomas (SCC), bladder urothelial CA, head and neck/cervix/anal SCC, lung SCC and adenocarcinomas, lung small cell carcinoma, ovarian CA, endometrial CA, hepatocellular CA, gastric adenocarcinomas, colorectal adenocarcinomas, and breast ductal carcinomas. Based on this data, the development of targeted immunotherapeutics against MAGE-A may provide benefit for large populations of patients and IHC evaluation for MAGE-A expression during diagnosis is a reliable modality to report over-expression of MAGE-A at the protein and tissue level.
Materials and Methods
Tumors
The cohort of tumor samples embedded in tissue arrays include 50 gastric adenocarcinomas, 156 bladder urothelial CA, 181 lung adenocarcinomas, 250 ovarian CA, 76 endometrial CA, 60 renal papillary CA, 32 non-seminoma germ-cell tumor, 193 pancreatic adenocarcinoma, 36 cutaneous SCC, 80 lung SCC, 22 thymic CA, 113 seminoma germ-cell tumor, 158 head and neck SCC, 28 cutaneous merkel cell CA, 250 prostate adenocarcinoma, 549 breast infiltrating ductal CA, 41 lung small cell CA, 80 liver hepatocellular CA, 189 mesotheliomas, 466 colorectal adenocarcinomas, 24 cervical and anal SCC, 26 esophageal SCC, 121 thyroid papillary CA, 85 breast lobular CA, 32 breast mucinous CA, 61 cholangiocarcinomas, 37 thyroid follicular CA, 242 renal clear cell CA, and 30 renal chromophobe CA. These samples were generated primarily from primary sites of disease20. For evaluation of metastatic disease, sections from patients with metastatic carcinomas that were referred to NIH for evaluation for protocol eligibility were assessed for NY-ESO-1 and MAGE-A. Overall, 37 entire slide sections of metastatic carcinomas were stained as follows: pancreas adenocarcinoma (n=10), colon adenocarcinoma (n=9), bladder urothelial carcinoma (n=6) cholangiocarcinoma (n=2), esophagus adenocarcinoma (n=2), breast infiltrating ductal CA (n=1), soft tissue (n=1), gastric adenocarcinoma (n=1), ovarian carcinoma (n=3), and unknown (n=2). Data for one colon adenocarcinoma and one cholangiocarcinoma was not available for NY-ESO staining. Additionally, 28 metastatic squamous cell carcinomas from various primary sites were also evaluated as follows: cervix (n=9), unknown (n=7), lung (n=3), anus (n=3), head and neck (n=3), esophagus (n=1), pancreas (n=1), and skin (n=1); Data was not available for MAGE-A staining for tumors from the following primary sites: unknown (n=2), head and neck (n=2), cervix (n=1), and pancreas (n=1).
Immunohistochemistry
Deparaffinization of paraffin-embedded tissue sections (5 μm) was accomplished through xylene and graded alcohols. NY-ESO-1 (Invitrogen/Life Technologies, Grand Island, NY, 1:100 dilution) and MAGE-A (Santa-Cruz Biotech, Dallas, TX, Clone 6C1, SC-20034, Dilution 1:100) immunohistochemical staining was performed following heat-induced epitope retrieval, using target retrieval solution, low pH (DAKO)21. Slides were incubated in Tris with 3% goat serum for 15 min and then incubated for 1–2 h with the primary antibodies at room temperature. A horseradish peroxidase/3,3′-diaminobenzidine polymer-based detection system (DAKO; Envision+) with the use of an automated slide stainer (DAKO; Autostainer) was utilized for detection. Standard tissue sections of 37 metastatic squamous cell carcinomas and 28 metastatic carcinomas from various primaries were used for NY-ESO-1 and MAGE-A staining. The remaining cases of carcinomas (n=3668) were performed on tissue arrays. Arrays were constructed by using 6–10 mm2 of representative tumor tissue from each case with 20–60 specimens per slide20. NY-ESO-1 and MAGE staining intensity was assessed by at least two pathologists (SPK, MMM) and was scored as positive or negative depending on the clear presence of tumor cell staining that was cytoplasmic for NY-ESO-1 and cytoplasmic and nuclear for MAGE-A. Intensity of staining was also assessed and both weak and strongly intense staining was considered positive. The percentage of staining neoplastic cells was estimated for each case and sections showing >50% of the tumor cells staining positive were categorized as high-expressers. A consensus opinion was achieved for any discordance between the pathologists (<5% of cases).
Statistics
Fisher’s exact test utilizing Graphpad prism software was performed to determine if a significant difference existed between positive staining patterns for MAGE-A and NY-ESO-1 on identical tissue arrays. Values of P < 0.05 were considered significant.
Results
Assessment of NY-ESO-1 Expression in Common Carcinomas
We assessed the expression of NY-ESO-1 on epithelial cancers with the highest prevalence and mortality and found that staining of neoplastic cells with the NY-ESO-1 antibody was predominantly restricted to the cytoplasm (Figure 1 and Figure 2). We included weak and focal staining patterns as positive but still did not detect high-percentages of positive cases in the 3668 tumor samples we analyzed. An additional category was developed for sections that had >50% of the tumor cells stain positive (weak or strong). Specifically, we detected no NY-ESO-1 expression in 24 cervical/anal SCC, 26 esophageal SCC, 121 thyroid papillary CA, 85 breast lobular CA, 32 breast mucinous CA, 61 cholangiocarcinomas, 37 thyroid follicular CA, 242 renal clear cell CA, and 30 renal chromophobe CA (Table 1). On the other hand, positive NY-ESO-1 staining was detected in the following: gastric adenocarcinomas: 14% (7/50), bladder urothelial CA: 10.3% (16/156), lung adenocarcinomas: 8.3% (15/181), ovarian CA: 7.2% (18/250), endometrial CA: 6.6% (5/76), renal papillary CA: 6.6% (4/60), germ cell tumor, non-seminoma: 6.3% (2/32), pancreatic adenocarcinoma: 5.7% (11/193), cutaneous squamous cell carcinoma: 5.5% (2/36), lung squamous cell carcinoma: 5% (4/80), thymic CA: 4.5% (1/22), germ cell tumor, seminoma: 4.4% (5/113), head and neck SCC: 3.8% (6/158), cutaneous merkel cell CA: 3.6% (1/28), prostatic adenocarcinoma: 2.8% (7/250), breast infiltrating ductal CA: 2.6% (14/549), lung small cell CA: 2.4% (1/41), hepatocellular CA: 1.2% (1/80), mesothelioma: 1% (2/189), and colorectal adenocarcinoma: <1% (1/466) (Table 1). High expression of NY-ESO-1 as defined by >50% cytoplasmic tumor cell staining was seen in only a few histologies (Table 1).
Figure 1.
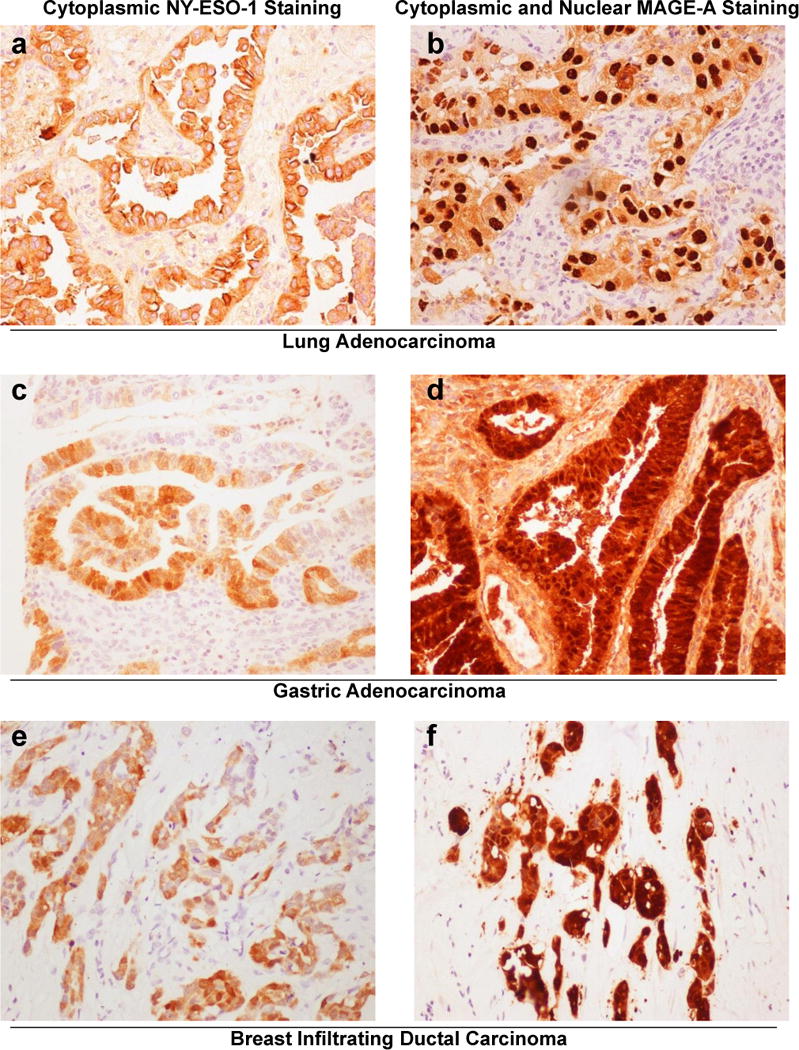
Representative images of NY-ESO-1 and MAGE-A immunohistochemical staining patterns. (a,b) Lung adenocarcinomas, (a) NY-ESO-1 staining and (b) MAGE-A staining for lung adenocarcinomas. (c,d) Gastric adenocarcinomas, (c) NY-ESO-1 staining and (d) MAGE-A staining for gastric adenocarcinomas. (e,f) Breast infiltrating ductal carcinomas, (e) NY-ESO-1 staining and (f) MAGE-A staining in breast infiltrating ductal carcinomas. (All images × 200)
Figure 2.
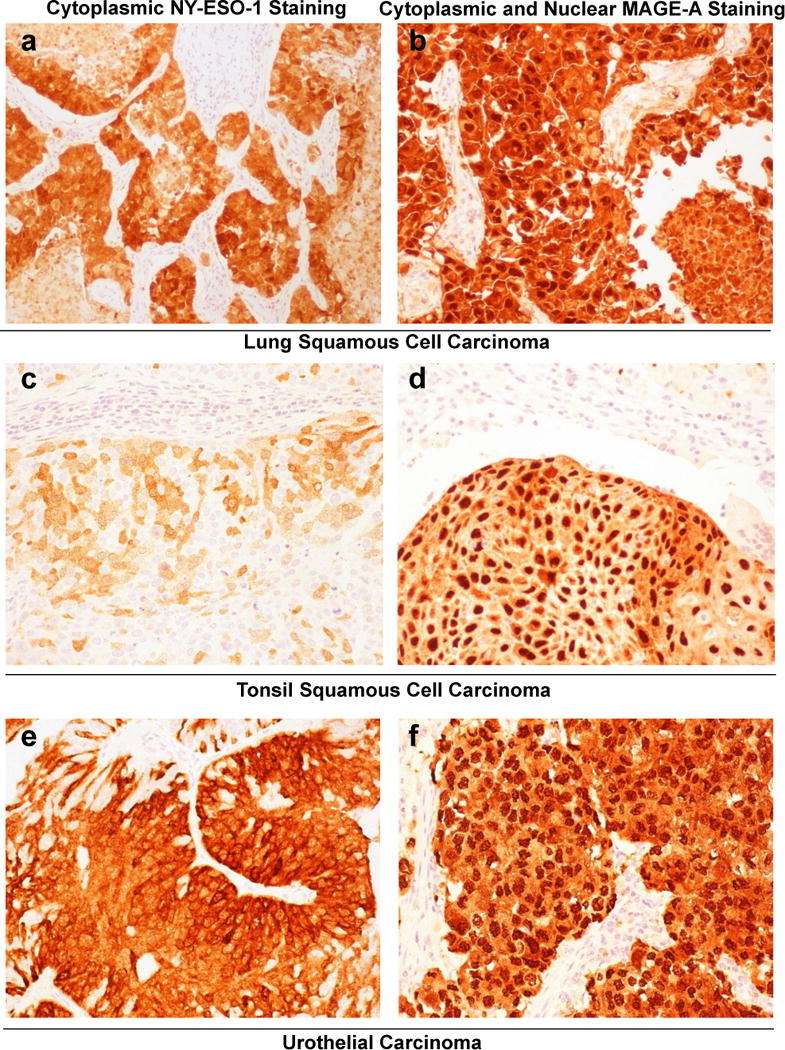
Representative images of NY-ESO-1 and MAGE-A immunohistochemical staining patterns. (a,b) Lung squamous cell carcinomas, (a) NY-ESO-1 staining and (b) MAGE-A staining for lung squamous cell carcinomas. (c,d) Tonsilar squamous cell carcinomas, (c) NY-ESO-1 staining and (d) MAGE-A staining for tonsilar squamous cell carcinomas. (e,f) Bladder, urothelial carcinomas, (e) NY-ESO-1 staining and (f) MAGE-A staining in urothelial carcinomas. (All images × 200)
Table 1.
NY-ESO-1 immunohistochemical staining in germ cell tumors and common carcinomas.
| Cancer Histology | N | NY-ESO-1 Positive | NY-ESO-1 Positive (>50%) |
|---|---|---|---|
| Adenocarcinoma (stomach) | 50 | 7 (14%) | 2 (4%) |
| Bladder urothelial CA | 156 | 16 (10.3%) | 13 (8.3%) |
| Adenocarcinoma (lung) | 181 | 15 (8.3%) | 7 (3.9%) |
| Ovarian CA | 250 | 18 (7.2%) | 9 (3.6%) |
| Endometrial CA | 76 | 5 (6.6%) | 1 (1.3%) |
| Renal papillary CA | 60 | 4 (6.6%) | 2 (3.3%) |
| Germ cell tumor: non-seminoma | 32 | 2 (6.3%) | 1 (4.7%) |
| Adenocarcinoma (pancreas) | 193 | 11 (5.7%) | 2 (1%) |
| Squamous cell CA (skin) | 36 | 2 (5.5%) | 1 (2.8%) |
| Squamous cell CA (lung) | 80 | 4 (5%) | 3 (3.8%) |
| Thymic CA | 22 | 1 (4.5%) | 1 (4.5%) |
| Germ cell tumor: seminoma | 113 | 5 (4.4%) | 4 (3.5%) |
| Squamous cell CA (head and neck) | 158 | 6 (3.8%) | 1 (<1%) |
| Skin merkel cell CA | 28 | 1 (3.6%) | 1 (3.6%) |
| Adenocarcinoma (prostate) | 250 | 7 (2.8%) | 4 (1.6%) |
| Breast infiltrating ductal CA | 549 | 14 (2.6%) | 10 (1.8%) |
| Lung small cell CA | 41 | 1 (2.4%) | 1 (2.4%) |
| Liver hepatocellular CA | 80 | 1 (1.2%) | 1 (1.2%) |
| Mesothelioma | 189 | 2 (1%) | 1 (<1%) |
| Adenocarcinoma (colon and rectum) | 466 | 1 (<1%) | 1 (<1%) |
| Squamous cell CA (cervix and anal) | 24 | 0 | 0 |
| Squamous cell CA (esophageal) | 26 | 0 | 0 |
| Thyroid papillary CA | 121 | 0 | 0 |
| Breast lobular CA | 85 | 0 | 0 |
| Breast mucinous CA | 32 | 0 | 0 |
| Cholangiocarcinoma | 61 | 0 | 0 |
| Thyroid follicular CA | 37 | 0 | 0 |
| Renal clear cell CA | 242 | 0 | 0 |
| Renal chromophobe CA | 30 | 0 | 0 |
| Total | 3668 | 123 (3.4%) | 66 (1.8%) |
CA = Carcinoma
Assessment of MAGE-A Expression in Common Carcinomas
We next assessed the expression of MAGE-A in our cohorts of various common epithelial cancers and germ cell tumors and found expression of MAGE-A in the nucleus and the cytoplasm of various tumors (Figure 1 and Figure 2). Specifically, we detected MAGE-A staining in seminoma germ cell tumors: 73.5% (83/113), esophageal squamous cell CA: 61.5% (16/26), cutaneous squamous cell CA: 55.6% (20/36), non-seminoma germ cell tumor: 50% (16/32), bladder urothelial CA: 48.1% (75/156), head and neck squamous cell CA: 44.9% (71/158), cervical and anal squamous cell CA: 41.7% (10/24), lung small cell CA: 39% (16/41), lung squamous cell CA: 37.5% (30/80), ovarian CA: 34% (85/250), lung adenocarcinoma: 32% (58/181), endometrial CA: 30.3% (23/76), hepatocellular CA: 26.3% (21/80), breast mucinous CA: 22.6% (7/32), gastric adenocarcinoma: 22% (11/50), breast infiltrating ductal CA: 21.5% (118/549), colorectal adenocarcinoma: 14.4% (67/466), thymic CA: 13.6% (3/22), mesothelioma: 13.2% (25/189), cholangiocarcinoma: 9.8% (6/61), clear cell type renal cell CA: 7.9% (19/242), pancreas adenocarcinoma: 7.8% (15/193), skin merkel cell: CA 7% (2/28), papillary type renal cell: CA 7% (4/60), breast lobular CA: 4.7% (4/85), prostate adenocarcinoma: 3.6% (9/250), and thyroid papillary CA: <1% (1/121) (Table 2). Only two carcinomas did not stain for MAGE-A: thyroid follicular CA (n=37) and renal chromophobe CA (n=30) (Table 2).
Table 2.
MAGE-A immunohistochemical staining in germ cell tumors and common carcinomas.
| Cancer Histology | N | MAGE-A Positive | MAGE-A Positive (>50%) |
|---|---|---|---|
| Germ cell tumor: seminoma | 113 | 83 (73.5%)* | 78 (69%)* |
| Squamous cell CA (esophagus) | 26 | 16 (61.5%)* | 13 (50%)* |
| Squamous cell CA (skin) | 36 | 20 (55.6%)* | 19 (52.8%)* |
| Germ cell tumor: non-seminoma | 32 | 16 (50%)* | 13 (40.1%)* |
| Bladder urothelial CA | 156 | 75 (48.1%)* | 63 (40.4%)* |
| Squamous cell CA (head and neck) | 158 | 71 (44.9%)* | 65 (41.1%)* |
| Squamous cell CA (cervix and anal) | 24 | 10 (41.7%)* | 9 (37.5%)* |
| Lung small cell CA | 41 | 16 (39%)* | 10 (24.4%)* |
| Squamous cell CA (lung) | 80 | 30 (37.5%)* | 27 (34%)* |
| Ovarian CA | 250 | 85 (34%)* | 66 (26.4%)* |
| Adenocarcinoma (lung) | 181 | 58 (32%)* | 50 (27.6%)* |
| Endometrial CA | 76 | 23 (30.3%)* | 20 (26.3%)* |
| Liver hepatocellular CA | 80 | 21 (26.3%)* | 15 (18.8%)* |
| Breast mucinous CA | 32 | 7 (22.6%)* | 6 (19.3%)* |
| Adenocarcinoma (stomach) | 50 | 11 (22%)* | 10 (20%)* |
| Breast infiltrating ductal CA | 549 | 118 (21.5%)* | 90 (16.4%)* |
| Adenocarcinoma (colon and rectum) | 466 | 67 (14.4%)* | 50 (10.7%)* |
| Thymic CA | 22 | 3 (13.6%)* | 2 (9%)* |
| Mesothelioma | 189 | 25 (13.2%)* | 15 (7.9%)* |
| Cholangiocarcinoma | 61 | 6 (9.8%)* | 6 (9.8%)* |
| Renal clear cell CA | 242 | 19 (7.9%) | 11 (4.5%) |
| Adenocarcinoma (pancreas) | 193 | 15 (7.8%) | 7 (3.6%) |
| Skin merkel cell CA | 28 | 2 (7%) | 1 (3.5%) |
| Renal papillary CA | 60 | 4 (7%) | 2 (3.5%) |
| Breast lobular CA | 85 | 4 (4.7%) | 2 (2.4%) |
| Adenocarcinoma (prostate) | 250 | 9 (3.6%) | 5 (2%) |
| Thyroid papillary CA | 121 | 1 (<1%) | 1 (<1%) |
| Thyroid follicular CA | 37 | 0 | 0 |
| Renal chromophobe CA | 30 | 0 | 0 |
| Total | 3668 | 815 (22.2%) | 656 (17.9%) |
CA = Carcinoma
= Fisher’s exact test: p < 0.05 for MAGE-A expression compared to NY-ESO-1 expression
We next assessed the percentage of tumors that highly expressed MAGE-A (staining of >50% of tumor cells) and found that the majority of malignancies that expressed MAGE-A were indeed high expressers (Table 2). In summary, MAGE-A is widely and highly expressed in several cancer histologies of high prevalence and mortality. Furthermore, statistical analysis revealed that the majority of cancer types evaluated possess significantly higher MAGE-A expression compared to NY-ESO-1 (Table 2).
NY-ESO-1 and MAGE-A expression in metastatic lesions
In addition to tissue array data that consisted of primary lesions, we evaluated the expression patterns for NY-ESO-1 and MAGE-A in metastatic disease in an independent cohort of patients. We found that NY-ESO-1 staining remained low in metastatic lesions with no staining in the 35 tumor tissue sections from patients with metastatic carcinomas of various primaries (Table 3). For the cohort of metastatic squamous cell carcinomas, 7.1% (2/28) had positive NY-ESO-1 staining with only 3.6% (1/28) having high expression of NY-ESO-1 (Table 3). In contrast, MAGE-A expression was significantly higher compared to NY-ESO-1 in metastatic lesions and comparable to MAGE-A expression measured in the primary lesions from the tissue arrays. We found that MAGE-A was positive in 16% (6/37) of tumor sections examined with high expression positivity in 13.5% (5/37) in metastatic carcinomas. For our cohort of metastatic squamous cell carcinomas, MAGE-A was positive in 50% (11/22) of tumor sections examined with high expression positivity in 45.5% (10/22) (Table 3). Interestingly, MAGE-A expression was significantly higher in metastatic SCC compared to the cohort of metastatic carcinomas that consisted mainly of adenocarcinomas (Table 3). In summary, the expression patterns of MAGE-A and NY-ESO-1 in metastatic lesions are similar to the staining patterns seen in primary lesions with MAGE-A expression being significantly higher than NY-ESO-1 in multiple primary and metastatic malignancies (Table 1, 2, and 3).
Table 3.
NY-ESO-1 and MAGE-A immunohistochemical staining in metastatic carcinomas.
| Cancer Histology | N | NY-ESO Positive | NY-ESO Positive (>50%) |
|---|---|---|---|
| Metastatic carcinomas | 35 | 0 | 0 |
| Metastatic squamous cell carcinomas | 28 | 2 (7.1%) | 1 (3.6%) |
| Cancer Histology | N | MAGE-A Positive | MAGE-A Positive (>50%) |
| Metastatic carcinomas | 37 | 6 (16%) | 5 (13.5%) |
| Metastatic squamous cell carcinomas | 22 | 11 (50%)* ** | 10 (45.5%)* ** |
CA = Carcinoma
= Fisher’s exact test: p < 0.05 for MAGE-A expression compared to NY-ESO-1 expression
= Fisher’s exact test: p <0.05 for Metastatic squamous cell carcinomas compared to metastatic adenocarcinomas
Primary histology for metastatic carcinomas include: pancreas (n=10) colon (n=9), bladder (n=6) bile duct (n=2), esophagus (n=2), breast (n=1), soft tissue (n=1), gastric (n=1), ovary (n=3), unknown (n=2); Data for colon (n=1) and bile duct (n=1) not available for NY-ESO staining.
Primary histology for metastatic squamous cell carcinomas include: cervix (n=9), unknown (n=7), lung (n=3), anus (n=3), head and neck (n=3), esophagus (n=1), pancreas (n=1), and skin (n=1); Data for unknown (n=2), head and neck (n=2), cervix (n=1), and pancreas (n=1) not available for MAGE-A staining.
Discussion
The cancer testis antigens represent ideal immunotherapeutic targets due to its restricted expression in normal tissue combined with over-expression in malignantly transformed cells. A reliable and reproducible modality to evaluate the expression of these antigens is through standardized immunohistochemical staining. Similar to the reporting of estrogen and progesterone receptor and IHC results for infiltrating ductal carcinomas of the breast22,23, results for MAGE-A and NY-ESO-1 can be readily interpreted and reported in conjunction with a pathologic diagnosis at the time of tissue evaluation. Here, we analyzed MAGE-A and NY-ESO-1 expression in 3668 common epithelial carcinomas and germ cell tumors of high prevalence and mortality in addition to 65 metastatic carcinomas from patients evaluated for protocol placement at the NIH Clinical Center. Decisions to treat patients on protocols targeting NY-ESO-1 and MAGE-A were based on IHC expression patterns. Most published reports describe increased mRNA expression for both NY-ESO-1 and MAGE-A in several different cancer types. Here, we directly compare the two cancer testis antigens utilizing IHC to evaluate for expression patterns across multiple different cancer histologies.
In this study, we show that MAGE-A is more widely expressed than NY-ESO-1 across a wide-range of common carcinomas. Despite the classic view that NY-ESO-1 is a promising immunotherapy target, we find that NY-ESO-1 is not highly expressed on common carcinomas. The highest percentage of NY-ESO-1 positivity is seen in gastric adenocarcinomas with seven out of fifty cases (14%) staining positive. However, only 2 of these tumors (4%) are high expressers as defined by positive staining in the majority of neoplastic cells (>50%). Additional histologies that show positive staining in 5–10% of tumors examined include bladder urothelial CA, lung adenocarcinoma, ovarian CA, endometrial CA, renal papillary CA, pancreas adenocarcinoma, cutaneous and lung squamous cell carcinomas and non-seminoma germ cell tumors (Table 1). However, only a small fraction (1–5%) of these tumors are high expressers of NY-ESO-1 with most of the positivity restricted to focal staining patterns. Thus, only a small percentage of common carcinomas express NY-ESO-1, although some patients may still benefit from immunotherapies targeting this antigen due to the high incidence and mortality of these common malignancies.
We also focused on another well-studied cancer testis antigen, MAGE-A. Here, we surprisingly find that MAGE-A is significantly higher in expression compared to NY-ESO-1 in the majority of cancers examined. The highest expression of MAGE-A is seen in germ cell tumors (seminomas and non-seminomas) and five common carcinomas (esophageal SCC, cutaneous SCC, head and neck SCC, cervical and anal SCC and bladder urothelial CA; Table 2). Our data suggests that cancers with squamous differentiation appear to be associated with increased expression of MAGE-A, but further studies will be needed in order to decipher a direct link. Several other carcinomas also exhibited significant MAGE-A over-expression (>25% of tumors examined) including lung SCC, lung adenocarcinoma, lung small cell carcinoma, ovarian CA, endometrial CA and liver hepatocellular CA. The majority of cases that showed MAGE-A expression were high-expressers with more than 50% of the tumor cells staining positive with microscopic examination revealing strong nuclear and cytoplasmic expression of the protein (Figure 1 and Figure 2).
We further examined a separate cohort of tumor tissue samples of metastatic disease from patients that were screened for eligibility criteria for protocol placement at the NIH Clinical Center. Consistent with the data from the tissue arrays, NY-ESO-1 was not highly expressed and significantly lower compared to MAGE-A in metastatic SCC and other metastatic carcinomas (Table 3). Interestingly, MAGE-A was more highly expressed in metastatic SCC compared to metastatic non-SCC.
In conclusion, immunohistochemical staining for NY-ESO-1 and MAGE-A allows for the rapid evaluation of expression patterns at the time of cancer diagnosis. Our data suggests that only a small percentage of patients with common carcinomas may benefit from targeting NY-ESO-1. On the other hand, MAGE-A is more widely expressed across multiple histologies including squamous cell carcinomas from different sites of origin and represents an immunotherapy target that may benefit a large number of patients with various cancer types. Future studies will need to determine if the degree of MAGE-A expression in tumor tissue will predict responses to MAGE-A targeted immunotherapy trials currently in progress24–26. Additionally, studies to examine the relative importance of antigen expression directly on tumor cells compared to stromal cells may also be important for predicting responses to treatment27.
Footnotes
Disclosure/Conflict of Interest
The authors do not possess any conflicts of interest
References
- 1.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nature reviews Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 2.Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer immunity. 2004;4:1. [PubMed] [Google Scholar]
- 3.Van Der Bruggen P, et al. Tumor-specific shared antigenic peptides recognized by human T cells. Immunological reviews. 2002;188:51–64. doi: 10.1034/j.1600-065x.2002.18806.x. [DOI] [PubMed] [Google Scholar]
- 4.Aung PP, et al. Expression of New York esophageal squamous cell carcinoma-1 in primary and metastatic melanoma. Human pathology. 2014;45:259–267. doi: 10.1016/j.humpath.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai JP, et al. NY-ESO-1 expression in synovial sarcoma and other mesenchymal tumors: significance for NY-ESO-1-based targeted therapy and differential diagnosis. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25:854–858. doi: 10.1038/modpathol.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins PF, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21:1019–1027. doi: 10.1158/1078-0432.CCR-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma P, et al. Frequency of NY-ESO-1 and LAGE-1 expression in bladder cancer and evidence of a new NY-ESO-1 T-cell epitope in a patient with bladder cancer. Cancer immunity. 2003;3:19. [PubMed] [Google Scholar]
- 8.Kurashige T, et al. Ny-ESO-1 expression and immunogenicity associated with transitional cell carcinoma: correlation with tumor grade. Cancer research. 2001;61:4671–4674. [PubMed] [Google Scholar]
- 9.Bolli M, et al. NY-ESO-1/LAGE-1 coexpression with MAGE-A cancer/testis antigens: a tissue microarray study. International journal of cancer Journal international du cancer. 2005;115:960–966. doi: 10.1002/ijc.20953. [DOI] [PubMed] [Google Scholar]
- 10.Akcakanat A, et al. NY-ESO-1 expression and its serum immunoreactivity in esophageal cancer. Cancer chemotherapy and pharmacology. 2004;54:95–100. doi: 10.1007/s00280-004-0768-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhang WM, et al. Correlation of NY-ESO-1 gene and protein expression to metastasis and clinicopathologic features of hepatocellular carcinoma. Ai zheng = Aizheng = Chinese journal of cancer. 2005;24:622–626. [PubMed] [Google Scholar]
- 12.Prasad ML, et al. Expression and significance of cancer testis antigens in primary mucosal melanoma of the head and neck. Head & neck. 2004;26:1053–1057. doi: 10.1002/hed.20112. [DOI] [PubMed] [Google Scholar]
- 13.Jungbluth AA, et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. International journal of cancer Journal international du cancer. 2001;92:856–860. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 14.Odunsi K, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer research. 2003;63:6076–6083. [PubMed] [Google Scholar]
- 15.Fossa A, et al. NY-ESO-1 protein expression and humoral immune responses in prostate cancer. The Prostate. 2004;59:440–447. doi: 10.1002/pros.20025. [DOI] [PubMed] [Google Scholar]
- 16.Nakada T, et al. NY-ESO-1 mRNA expression and immunogenicity in advanced prostate cancer. Cancer immunity. 2003;3:10. [PubMed] [Google Scholar]
- 17.Kageyama S, et al. Adoptive Transfer of MAGE-A4 T-cell Receptor Gene-Transduced Lymphocytes in Patients with Recurrent Esophageal Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21:2268–2277. doi: 10.1158/1078-0432.CCR-14-1559. [DOI] [PubMed] [Google Scholar]
- 18.Bergeron A, et al. High frequency of MAGE-A4 and MAGE-A9 expression in high-risk bladder cancer. International journal of cancer Journal international du cancer. 2009;125:1365–1371. doi: 10.1002/ijc.24503. [DOI] [PubMed] [Google Scholar]
- 19.Sang M, Lian Y, Zhou X, Shan B. MAGE-A family: attractive targets for cancer immunotherapy. Vaccine. 2011;29:8496–8500. doi: 10.1016/j.vaccine.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Miettinen M. A simple method for generating multitissue blocks without special equipment. Applied immunohistochemistry & molecular morphology: AIMM/official publication of the Society for Applied Immunohistochemistry. 2012;20:410–412. doi: 10.1097/PAI.0b013e318245c82f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabe Y, et al. MDM2 antagonist nutlin-3 displays antiproliferative and proapoptotic activity in mantle cell lymphoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:933–942. doi: 10.1158/1078-0432.CCR-08-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammond ME, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Archives of pathology & laboratory medicine. 2010;134:907–922. doi: 10.5858/134.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammond ME, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbst RS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahoney KM, Atkins MB. Prognostic and predictive markers for the new immunotherapies. Oncology. 2014;28(Suppl 3):39–48. [PubMed] [Google Scholar]
- 26.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerkar SP, Restifo NP. Cellular constituents of immune escape within the tumor microenvironment. Cancer research. 2012;72:3125–3130. doi: 10.1158/0008-5472.CAN-11-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]


