Abstract
Background
Intravenous artesunate has replaced quinine as the first-line therapy for severe imported malaria, given its anti-malarial superiority shown in clinical trials conducted in endemic countries. Evidence for red blood cell (RBC) exchange in patients with severe malaria treated with artesunate is lacking. This retrospective cohort study describes the experience at Hospital Clinic of Barcelona with the use of artesunate for severe malaria and the joint use of RBC exchange in selected cases.
Methods
Patients treated for severe malaria at Hospital Clinic of Barcelona between August 2013 and January 2015 were included in this retrospective study. Severe malaria was defined according to WHO criteria. Data were extracted from electronic hospital records. A log-linear mixed model approach was used to estimate parasite clearance times.
Results
Within the study period, 42 patients were diagnosed of malaria at this centre, of which 38 had Plasmodium falciparum (90.5 %). Sixteen patients (42 %) had severe malaria cases and were treated with intravenous artesunate. Four patients underwent RBC exchange within a period of 15 h after the first dose of artesunate (range 9–21 h). The procedure lasted a median of 2 h (IQR 1.8–2 h), using a median of 12 (IQR 11–14) units of packed RBCs to replace a median of 3794 ml (IQR 2977–4343). The technique was well-tolerated without haemodynamic complications. There were no deaths. The regression model showed an estimated time to 95 % decay of 21.6 h (95 % CI 17.3–28.8). When assessing effect modification by RBC exchange, there was no difference in the parasite elimination rate (p = 0.286).
Discussion and conclusion
In this study RBC exchange failed to show benefits in terms of parasite clearance probably due to the small number of patients analysed. The evidence for exchange transfusion remains limited.
Keywords: Malaria, Severe malaria, Plasmodium falciparum, Automated red blood cell exchange, Artesunate
Background
The management of severe imported malaria remains a challenge of consensus, since the elaboration of clinical guidelines always has to deal with the lack of evidence in non-endemic countries. The design of clinical trials randomizing cases of severe imported malaria is unrealistic nowadays, due to the high number of subjects required, the heterogeneous group of affected travellers in terms of parasitaemia and clinical presentation/complications., and ethical problems given the evidence from endemic regions [1, 2]. The last and most important change in guidelines of imported severe malaria is the replacement of quinine by intravenous artesunate (IVA) as the first-line therapy [3–5]. Superior efficacy and reduction of mortality have been shown in clinical trials in Asia and Africa [6, 7] and confirmed in European retrospective studies [8]. Despite international consensus in using IVA for severe malaria, it is not yet available as a licensed drug in all non-endemic countries. So far, it is ordered as compassionate use or as investigational new drug protocol. In this context, it is difficult to collect data to provide evidence of the safety profile or anti-malarial efficacy. Regarding safety profile, some post-introduction data have suggested delayed haemolytic anaemia as an adverse event that should be monitored the next 4 weeks after IVA [9–11]. Better data is needed in terms of parasite response, which is commonly measured through the parasite clearance time (PCT). Although parasite clearance has not been validated as a surrogate endpoint for death from severe malaria, it is an accepted marker of treatment response. PCT50 is defined as the time required to reduce the initial parasitaemia by 50 %, under anti-malarial treatment. Improvement of PCT with IVA versus quinine [5–7] poses a new scenario that questions the role of some adjunctive therapies, such as exchange transfusion and red blood cell (RBC) exchange.
Different strategies have been proposed as adjunctive therapies, mainly helping pharmacologic treatment to reduce parasitaemia. Whole blood exchange transfusion has been suggested and used with this purpose. Its theoretical benefits are based on the removal of: (i) infected circulating red blood cells (RBCs) (those sequestered in microvasculature are not affected); (ii) rigid non-infected RBCs, with a rheological effect; (iii) plasma toxins, minimizing systemic inflammatory response and sequestration. It has demonstrated a rapidly reduction in parasite biomass [12], but has not shown effectiveness to improve survival [13]. RBC exchange has been advocated as a better alternative to whole blood exchange transfusion in the adjunctive treatment of severe imported malaria [14–16]. RBC exchange does not remove plasma toxins, but has significant advantages over exchange transfusion in terms of speed, efficiency, haemodynamic stability and retention of plasma components such as clotting factors and may thus represent an improvement in adjunctive therapy for severe malaria [17]. Although RBC exchange it is recommended by some national apheresis societies [18], conclusive evidence is lacking. Some case reports and patient cohorts have been reported, but mainly in conjunction with quinine [14, 19], or using exchange transfusion or RBC exchange [20].
This study reports our experience with the use of artesunate for treating severe malaria and the use of RBC exchange in selected cases treated at Hospital Clinic of Barcelona in Spain between August 2013 and January 2015.
Methods
Study setting and design
This retrospective cohort study includes all patients with severe malaria treated with intravenous artesunate at Hospital Clínic, in Barcelona, Spain, between August 2013 and January 2015. Hospital Clínic is a national reference centre for Tropical Imported Diseases. In 2013, artesunate became available in Hospital Clínic for the treatment of patients with severe malaria. Patients with severe malaria are initially managed in the intensive care unit and subsequently, when they have improved, transferred to a conventional ward.
Study procedures
Patients were diagnosed with severe malaria if their thick or thin blood film showed asexual stages of Plasmodium falciparum and fulfilled one or more WHO criteria (Table 1). Parasite counts were calculated as a percentage of the number of parasitized RBC in a thin blood film. Blood films were performed during the clinical management of the patient when considered by the treating physician and until no parasites were observed.
Table 1.
Severity criteria in Plasmodium falciparum malaria
| Type | Criteria | Definition |
|---|---|---|
| Clinical | Impaired consciousness | Any impairment in consciousness, not related to other causes (hypoglycaemia, concomitant infection) |
| Prostration | Generalized weakness so that the patient is unable to sit, stand or walk without assistance | |
| Multiple convulsions | More than two episodes within 24 h | |
| Respiratory failure | PaO2 <60 mmH (FiO2 21 %) | |
| Shock | Systolic blood pressure <80 mmHg | |
| Jaundice | Clinical jaundice plus evidence of other vital organ dysfunction | |
| Bleeding | Spontaneous bleeding without any other potential cause | |
| Analytical and radiological | Hypoglycaemia | <2.2 mmol/l or <40 mg/dl |
| Metabolic acidosis | Plasma bicarbonate <15 mmol/l | |
| Severe normocytic anaemia | Haemoglobin <5 g/dl | |
| Haemoglobinuria | Haemoglobin in urine | |
| Hyperlactataemia | Lactate >5 mmol/l | |
| Renal impairment | Serum creatinine >265 µmol/l | |
| Pulmonary oedema or distress respiratory syndrome | Bilateral alveolar infiltrates in chest x-ray | |
| Parasitological | Hyperparasitaemia | Parasitaemia >2.5 % |
Adapted from World Health Organization [2]
Intravenous artesunate was administered at doses of 2.4 mg/kg at 0, 12, 24 and then every 24 h until parasitaemia was cleared. Intravenous artesunate was followed by a course of oral atovaquone-proguanil (1000/400 mg) during 3 days.
RBC exchange was performed at the decision of the treating physician in conjunction with Haemotherapy specialists. In general, it was considered as an adjunctive treatment in patients who had high baseline parasitaemia (generally above 30 %) and shock, respiratory failure or cerebral malaria. RBC exchange was also performed if patients were severely ill and parasitaemia did not decrease at least 25 % compared to baseline after 8–12 h of artesunate. RBC exchanges were performed in a COBE Spectra blood separator (TerumoBCT, Lakewood, Co, USA) using the RBCX software. Sex, height, weight, patient’s haematocrit, average haematocrit in the replacement red blood cells units, desired end haematocrit for the patient, fluid balance and the fraction of infected RBC remaining at the end were introduced at the start of the procedure. Once the initial data is entered, the replacement volume of RBC to be exchanged proposed by the COBE Spectra software was followed. ACD-A was used as anticoagulant at a infusion rate of 1.2 mL/min/L total blood volume with prophylactic calcium and magnesium infusion administration [21]. Fresh leukoreduced RBC units in SAG-Mannitol additive solution were used as the replacement solution. If possible, peripheral vein accesses were used for the procedure. If not, a double lumen catheter was inserted in the internal jugular vein.
Data collection
All patients included into the study were identified prospectively in the clinical routine practice. A retrospective review of their electronic records was performed and an anonymized case report form was filled out. This included the following data: demographics and travel history; WHO clinical, analytical and radiological severity criteria; dates and times on symptoms initiation, hospital attendance, blood films performed and treatment commencement; the number of artesunate doses and other antimicrobials previously administered; complications and mortality. Data on RBC exchange was provided by the Department of Hemotherapy and Hemostasis, which keeps a registry of the apheresis performed in the hospital.
Statistical analysis
The data collected were entered into Epi Info 7 (CDC, Atlanta, USA) and then transferred to Stata 13.1 (Stata Corporation, Texas, USA) for the analysis. Categorical variables were described by counts and percentages, whereas median and interquartile ranges (IQR) were used to summarize continuous variables. Comparisons between groups were performed with Fisher exact test and Mann–Whitney test for categorical and continuous variables, respectively.
Parasite clearance times (PCT) were estimated since the administration of the first dose of artesunate. Initially, the parasite loads were calculated from the parasite counts expressed in percentages and the number of RBC per microlitre in each blood film. The variation of parasite loads was analysed using a log-linear mixed-effects regression model incorporating a Gaussian random intercept. This resulted in an estimation of the dynamics of parasitaemia, assuming a single exponential model, allowing calculating the decrease of log parasitaemia per unit of time (hour) or parasite elimination rate. The interaction between RBC exchange and time was included in the model to evaluate the possible modification of the effect of artesunate in clearing parasitaemia.
The average reduction rate per hour was obtained based on the coefficient of the regression model (1- exponential of the coefficient of regression), as well as its 95 % confidence interval. The model estimates obtained were used to calculate the time to 50 % (PCT50 %), 75 % (PCT75 %), 90 % (PCT90 %), 95 % (PCT95 %) and 99 % (PCT99 %) clearance of parasitaemia. 95 % confidence intervals (95 % CI) and p values were obtained for all estimates in the model.
Ethical considerations
The Ethics Committee of Hospital Clínic approved this study. Data collection forms were completely anonymous. As it was a retrospective study, the Ethics Committee approved waiver of individual consent from patients whose routine data was used.
Results
Patients’ baseline characteristics
From August 2013 to January 2015, 42 patients presented with malaria, 38 by P. falciparum (90.5 %), and 16 of those (42.11 %) were identified as severe malaria cases. All 16 were treated with IVA, and 4 additionally with RBC exchange. Their baseline characteristics are summarized and compared in Table 2.
Table 2.
Characteristics of 16 patients with severe malaria treated with artesunate with or without RBC exchange
| Overall (n = 16) | Artesunate only (n = 12) | Artesunate and RBC exchange (n = 4) | p value | |
|---|---|---|---|---|
| Demographics | ||||
| Age | 40 (33–50) | 36 (32–48) | 47 (39–55) | 0.332 |
| Male | 11 (68.75) | 8 (66.67) | 3 (75) | 0.755 |
| Clinical data | ||||
| Number of severity criteria | 3 (1–6) | 1 (1–5) | 7 (6–10) | 0.016 |
| Clinical criteria | ||||
| >2 criteria | 6 (37.5) | 3 (25) | 3 (75) | 0.074 |
| Impaired consciousness | 3 (18.75) | 2 (16.67) | 1 (25) | 0.712 |
| Postration | 8 (50) | 5 (41.67) | 3 (75) | 0.248 |
| Multiples convulsions | 0 (0) | 0 (0) | 0 (0) | – |
| Respiratory failure | 3 (18.75) | 1 (8.33) | 2 (50) | 0.064 |
| Shock | 4 (25) | 3 (25) | 1 (25) | 1 |
| Jaundice | 6 (37.50) | 2 (16.67) | 4 (100) | 0.003 |
| Bleeding | 3 (18.75) | 2 (16.67) | 1 (25) | 0.712 |
| Analytical and radiological criteria | ||||
| ≥2 criteria | 7 (43.75) | 3 (25) | 4 (100) | 0.009 |
| Hypoglycaemia | 2 (12.50) | 1 (8.33) | 1 (25) | 0.383 |
| Metabolic acidosis | 4 (25) | 1 (8.33) | 3 (75) | 0.008 |
| Severe normocytic anaemia | 0 (0) | 0 (0) | 0 (0) | – |
| Haemoglobinuriaa | 4 (26.67) | 2 (16.67) | 2 (66.67) | 0.080 |
| Hyperlactataemiaa | 2 (13.33) | 0 (0) | 2 (66.67) | 0.002 |
| Renal impairment | 6 (37.50) | 2 (16.67) | 4 (100) | 0.003 |
| Pulmonary oedema | 3 (18.75) | 1 (8.33) | 2 (50) | 0.064 |
| Parasitological data | ||||
| Parasitaemia (%) | 12.5 (3.95–32.5) | 7.3 (3.85–19.50) | 46.5 (34–57) | 0.008 |
| Parasitaemia >30 % | 4 (25) | 1 (8.33) | 3 (75) | 0.008 |
| Hyperparasitaemia | 15 (93.75) | 11 (91.67) | 4 (100) | 0.551 |
Categorical variables are described by counts and percentages; continuous variables are expressed as median (interquartile range)
RBC red blood cell
aData available only for 15 participants
The median age was 40 years old (IQR 33–50), with a high proportion of male patients (11/16, 68.75 %). Most patients were travellers who had arrived from sub-Saharan Africa (11/16, 68.70 %), followed by immigrants “visiting friends and relatives”—temporary visitors- (VFR) (3/16, 18.75 %). None of the travellers completed a full course of chemoprophylaxis. Two patients were residents in endemic countries travelling to Spain (2/16, 12.50 %).
Half of the cohort presented three or more severity criteria, the most frequent being hyperparasitaemia (93.75 %), prostration (50 %), renal impairment (37.50 %) and jaundice (37.50 %). The median time to seek for health advice was 5 days (range 2.50–11.70) since the onset of symptoms. The median initial parasitaemia was 12.5 % (range 3.95–32.50 %). Four patients (25 %) presented with an initial parasitaemia above 30 %.
Patients finally treated with RBC exchange had a higher median initial parasitaemia compared to those who were treated with IVA alone (46.50 vs 7.30 %) and presented with more severity criteria (Table 2).
RBC exchange and outcome
Four patients (25 %) received RBC exchange as an adjunctive treatment to IVA. This technique was initiated a median of 15.45 h after the first dose of IVA (IQR 9.05–20.58). The median duration of the technique was 2 h (IQR 1.80–2), using a median of 12 units of packed RBCs (IQR 11–14) to replace a median of 3794 ml (IQR 2977–4343).
The procedure was well tolerated without haemodynamic or hydroelectrolytical complications. The median difference in haematocrit and platelet count before and after RBC exchange was 2 % and −13,500/mm3, respectively. The median difference in blood calcium and potassium before and after the procedure was 0.15 mg/dl and −0.2 mmol/l, respectively. All patients survived and none of them had long-term renal or neurological sequelae. Two cases of delayed haemolysis could be detected: they completely recovered and did not show more complications.
Parasite clearance times
The values of parasitaemia at each timepoint for each patient were analysed to estimate the PCT (Fig. 1). Table 3 summarizes the estimates from the mixed effects regression model including the effect of time on parasite clearance, thus obtaining the parasite elimination rate. The results of the model suggested that parasitaemia significantly decreased in relation with time. According to the estimated coefficient, the average reduction rate was 12 % per hour (95 % CI 10–14, p < 0.0001). Patients who received RBC exchange as an adjunctive therapy had a higher baseline parasitaemia compared to those who had IVA alone (p < 0.0001). When assessing effect modification by RBC exchange in the model, there was not any difference in the parasite elimination rate (coefficient for interaction 0.03, 95 % CI −0.02; 0.08, p = 0.2861).
Fig. 1.
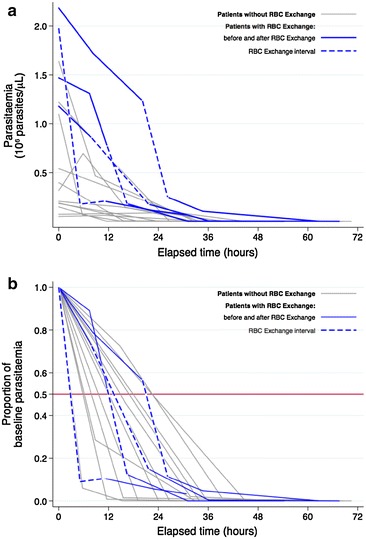
a Parasitaemia dynamics during the first 72 h under treatment with artesunate expressed as parasite load. b Parasitaemia dynamics expressed as the proportion of the baseline parasitaemia
Table 3.
Mixed-effects regression model to estimate the changes in parasitaemia in 16 patients with severe malaria
| Parameter | Coefficient | 95 % confidence interval | p value |
|---|---|---|---|
| Log initial parasitaemia/µLa | 12.02 | 11.24; 12.79 | <0.0001 |
| RBC exchangeb | 2.47 | 1.43; 3.51 | <0.0001 |
| Parasite elimination rate (/h)c | −0.13 | −0.15; −0.10 | <0.0001 |
RBC red blood cell
aLog initial parasitaemia for individuals who did not receive RBC exchange (intercept of the regression model)
bDifference in log initial parasitaemia for individuals who received RBC exchange vs those who did not
cDifference in log parasitaemia per unit increase of time (hour)
Based on the estimates of this model, the mean time to 50 % (PCT50), 90 % (PCT90), 95 % (PCT95) and 99 % (PCT99) parasite clearance were calculated (Table 4). PCT were calculated for the whole cohort without stratifying between those who had RBC exchange or not, since there was no evidence of an interaction between this therapy and time. Interestingly, 95 % of initial parasitaemia was cleared in a mean time of less than 24 h (21.60, 95 % CI 17.30–28.80), and almost the whole initial parasitaemia was cleared in less than 36 h (33.21, 95 % CI 26.60–44.30).
Table 4.
Mean parasite clearance times estimated from the mixed-effects model in 16 patients with severe malaria
| % | All patients (95 % CI) n = 16 | Artesunate only (95 % CI) n = 12 | Artesunate and RBC exchange (95 % CI) n = 4 |
|---|---|---|---|
| PCT 50 | 5.0 (4.0–6.7) | 5.0 (4.0–6.7) | 6.3 (4.6–9.7) |
| PCT 75 | 10.0 (8.0–13.3) | 10.0 (8.0–13.3) | 12.6 (9.3–19.4) |
| PCT 90 | 16.6 (13.3–22.1) | 16.6 (13.3–22.1) | 20.9 (15.4–32.3) |
| PCT 95 | 21.6 (17.3–28.8) | 21.6 (17.3–28.8) | 27.2 (20.1–42.0) |
| PCT 99 | 33.2 (26.6–44.3) | 33.2 (26.6–44.3) | 41.7 (30.9–64.5) |
Data expressed as hours
PCT parasite clearance time
Discussion
In the present study, it is shown in the largest case series of severe imported malaria that use RBC exchange in a cohort treated with artesunate that IVA reduces quickly parasitaemia and that RBC exchange does not seem to add any benefit in terms of parasite clearance times.
The results of this retrospective follow-up study confirm the speed of action of IVA when used for imported severe falciparum malaria, reducing parasite clearance time in a similar way to other cohorts [5–7]. This case series, due to the mathematical population model applied, provides valuable data about parasite kinetics during the response to treatment. Due to the small sample size, the regression model was estimated assuming linear reduction of parasite density and, therefore, the real reduction could be underestimated at early times. The PCT included in this article can be used in the clinical practice by physicians treating imported severe malaria, as a reference for monitoring the microbiological answer to IVA.
Regarding the use of RBC exchange as an adjunctive therapy, it was found that it does not significantly contribute to parasite clearance [20]. However, this conclusion has to be taken very cautiously. First, small sample size and the study design may be responsible for this lack of evidence for the effect of the adjunctive therapy. Furthermore, the endpoint of the study was parasite clearance alone, which does not allow any firm conclusion for other biological plausible benefits of RBC exchange. Finally, the fact that the treatment protocol at this institution indicates RBC exchange for only selected patients may have reduced the effect modification of RBC exchange (selection bias).
In this study, a mixed effects model for log parasitaemia with fixed and random effects for initial parasitaemia, and fixed effects for exchange transfusion and time was used (Table 3). The authors were unable to demonstrate an effect of exchange transfusion on the decay of parasitaemia, as addition of an interaction between exchange transfusion and time was non-significant. This may have been due to the small sample size.
The development of safer apheresis therapies that can be used for severe malaria, such as RBC exchange, may have an impact on the medical management of the disease and there are many aspects of its use as adjunctive therapy susceptible of further investigation. One of the main questions about using RBC exchange for severe malaria together with IVA is which would be the accurate moment to initiate the apheresis. There is no debate that anti-malarial drug is the first step for this medical emergency. Its use is envisioned due to its good safety profile, confirmed by the data in this cohort, and its plausible biological benefits. One of the hypothetical concerns when using RBC exchange immediately after AVI is that the drug could be partially removed within the RBC exchange since artesunate has shown to have high binding to RBC [22].
Therefore, the use of RBC exchange should target to optimize IVA effect, not to replace it, not even diminish it. Since artesunate half-life is less than 15 min and 30–60 min for its metabolite dihydroartemisinin [23], the recommendation would be initiating RBC exchange not earlier than 3–4 h after initiating AVI. Due to its mechanism of action and its stage-specificity, artesunate generates more “once-parasitized” RBC than quinine when used in cases of severe malaria [24]. These RBC are erythrocytes whose parasite has been removed, by the spleen or by artesunate, becoming less mobile, spherical and particulate cell. The “pitting” effect explains disparity between haematocrit decrease and parasite count decay, as well as the rapid effect of artesunate [25]. One of the hypotheses is that once-parasitized RBC are major contributors to the development of post-artesunate delayed haemolysis (PADH) [11]. In this cohort of patients, just two cases of delayed haemolytic anaemia were detected, with no relation to RBC exchange. The authors hypothesize whether RBC exchange may prevent delayed anaemia with IVA.
Conclusions
This case series illustrates the experience with the use of AVI for treating severe malaria, and the use of RBC exchange as adjunctive therapy. No serious adverse effects of RBC exchange transfusion were encountered in this small series. The study provides valuable data about technical procedure of RBC exchange and about monitoring microbiological response to artesunate. Although there remains limited evidence to support RBC exchange transfusion in severe malaria, it may be considered in special circumstances, such as the suspicion of artemisinin resistant hyperparasitemic falciparum malaria, the unavailability of IVA or in settings where parasite clearance may be prolonged, such as splenectomy.
A randomized clinical trial would provide some evidence about the prompt indication and initiation of RBC exchange when using artesunate, but it is currently unaffordable. Further multicentric, cohort studies should be performed to gradually increase the level of evidence to determine which selected patients with imported severe malaria may benefit from this adjunctive treatment and at which moment after artesunate administration will not reduce the drug’s benefit.
Authors’ contributions
JM and JG designed the study. ACC and JGJ drafted the manuscript and were responsible for data acquisition. ML and JC provided data on the RBC exchange. LQ and JGJ performed the data analysis. All authors critically reviewed all drafts of the manuscript and approved the final version. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Antonia Calvo-Cano, Email: acalvocano@gmail.com.
Joan Gómez-Junyent, Email: gjunyent@hotmail.com.
Miguel Lozano, Email: MLOZANO@clinic.ub.es.
Pedro Castro, Email: PCASTRO@clinic.ub.es.
Joan Cid, Email: JCID@clinic.ub.es.
Jose María Nicolás, Email: NICOLAS@clinic.ub.es.
Llorenç Quintó, Email: llorenc.quinto@isglobal.org.
Maite Martin, Email: MMARTIN@clinic.ub.es.
Jose Muñoz, Email: jose.munoz@isglobal.org.
Joaquim Gascon, Email: JGASCON@clinic.cat.
References
- 1.González A, Nicolás JM, Muñoz J, Castro P, Mas J, Valls ME, et al. Severe imported malaria in adults: retrospective study of 20 cases. Am J Trop Med Hyg. 2009;81:595–599. doi: 10.4269/ajtmh.2009.08-0637. [DOI] [PubMed] [Google Scholar]
- 2.Cramer JP, Lopez-Velez R, Burchard GD, Grobusch MP, de Vries PJ. Treatment of imported severe malaria with artesunate instead of quinine–more evidence needed? Malar J. 2011;10:256. doi: 10.1186/1475-2875-10-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . Guidelines approved by the Guidelines Review Committee. Guidelines for the treatment of malaria. Geneva: World Health Organization; 2015. [Google Scholar]
- 4.Askling HH, Bruneel F, Burchard G, Castelli F, Chiodini PL, Grobusch MP, et al. Management of imported malaria in Europe. Malar J. 2012;11:328. doi: 10.1186/1475-2875-11-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinclair D, Donegan S, Isba R, Lalloo DG. Artesunate versus quinine for treating severe malaria. Cochrane Database Syst Rev. 2012;6:Cd005967. doi: 10.1002/14651858.CD005967.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dondorp A, Nosten F, Stepniewska K, Day N, White N. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–725. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 7.Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet. 2010;376:1647–1657. doi: 10.1016/S0140-6736(10)61924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eder M, Farne H, Cargill T, Abbara A, Davidson RN. Intravenous artesunate versus intravenous quinine in the treatment of severe falciparum malaria: a retrospective evaluation from a UK centre. Pathog Glob Health. 2012;106:181–187. doi: 10.1179/2047773212Y.0000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Published reports of delayed hemolytic anaemia after treatment with artesunate for severe malaria–worldwide, 2010–2012. MMWR Morb Mortal Wkly Rep. 2013;62:5–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Rolling T, Wichmann D, Schmiedel S, Burchard GD, Kluge S, Cramer JP. Artesunate versus quinine in the treatment of severe imported malaria: comparative analysis of adverse events focussing on delayed haemolysis. Malar J. 2013;12:241. doi: 10.1186/1475-2875-12-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoller T, Junghanss T, Kapaun A, Gjorup I, Richter J, Hugo-Persson M, et al. Intravenous artesunate for severe malaria in travelers, Europe. Emerg Infect Dis. 2011;17:771–777. doi: 10.3201/eid1705.101229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller KD, Greenberg AE, Campbell CC. Treatment of severe malaria in the United States with a continuous infusion of quinidine gluconate and exchange transfusion. N Engl J Med. 1989;321:65–70. doi: 10.1056/NEJM198907133210201. [DOI] [PubMed] [Google Scholar]
- 13.Riddle MS, Jackson JL, Sanders JW, Blazes DL. Exchange transfusion as an adjunct therapy in severe Plasmodium falciparum malaria: a meta-analysis. Clin Infect Dis. 2002;34:1192–1198. doi: 10.1086/339810. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Fuertes LF, Tapia-Martin M, Angel-Moreno A, Pisos-Alamo E, Losada-Castillo MC, Diaz-Cremades JM, et al. Automated erythrocytapheresis as treatment of severe malaria. Study of 6 patients. Med Clin (Barc) 2008;131:298–301. doi: 10.1016/S0025-7753(08)72263-2. [DOI] [PubMed] [Google Scholar]
- 15.Files JC, Case CJ, Morrison FS. Automated erythrocyte exchange in fulminant falciparum malaria. Ann Intern Med. 1984;100:396. doi: 10.7326/0003-4819-100-3-396. [DOI] [PubMed] [Google Scholar]
- 16.Nieuwenhuis JA, Meertens JH, Zijlstra JG, Ligtenberg JJ, Tulleken JE, van der Werf TS. Automated erythrocytapheresis in severe falciparum malaria: a critical appraisal. Acta Trop. 2006;98:201–206. doi: 10.1016/j.actatropica.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Macallan DC, Pocock M, Robinson GT, Parker-Williams J, Bevan DH. Red cell exchange, erythrocytapheresis, in the treatment of malaria with high parasitaemia in returning travellers. Trans R Soc Trop Med Hyg. 2000;94:353–356. doi: 10.1016/S0035-9203(00)90101-9. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz J, Winters JL, Padmanabhan A, Balogun RA, Delaney M, Linenberger ML, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: the sixth special issue. J Clin Apher. 2013;28:145–284. doi: 10.1002/jca.21276. [DOI] [PubMed] [Google Scholar]
- 19.Auer-Hackenberg L, Staudinger T, Bojic A, Locker G, Leitner GC, Graninger W, et al. Automated red blood cell exchange as an adjunctive treatment for severe Plasmodium falciparum malaria at the Vienna General Hospital in Austria: a retrospective cohort study. Malar J. 2012;11:158. doi: 10.1186/1475-2875-11-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreeftmeijer-Vegter AR, Melo Mde M, de Vries PJ, Koelewijn R, van Hellemond JJ, van Genderen PJ. Manual blood exchange transfusion does not significantly contribute to parasite clearance in artesunate-treated individuals with imported severe Plasmodium falciparum malaria. Malar J. 2013;12:115. doi: 10.1186/1475-2875-12-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cid J, Carbassé G, Andreu B, Baltanás A, Garcia-Carulla A, Lozano M. Efficacy and safety of plasma exchange: ann 11-year single-center experience of 2730 procedures in 317 patients. Transfus Apher Sci. 2014;51:209–214. doi: 10.1016/j.transci.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Vyas N, Avery BA, Avery MA, Wyandt CM. Carrier-mediated partitioning of artemisinin into Plasmodium falciparum-infected erythrocytes. Antimicrob Agents Chemother. 2002;46:105–109. doi: 10.1128/AAC.46.1.105-109.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis TM, Phuong HL, Ilett KF, Hung NC, Batty KT, Phuong VD, et al. Pharmacokinetics and pharmacodynamics of intravenous artesunate in severe falciparum malaria. Antimicrob Agents Chemother. 2001;45:181–186. doi: 10.1128/AAC.45.1.181-186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chotivanich K, Udomsangpetch R, Dondorp A, Williams T, Angus B, Simpson JA, et al. The mechanisms of parasite clearance after antimalarial treatment of Plasmodium falciparum malaria. J Infect Dis. 2000;182:629–633. doi: 10.1086/315718. [DOI] [PubMed] [Google Scholar]
- 25.Angus BJ, Chotivanich K, Udomsangpetch R, White NJ. In vivo removal of malaria parasites from red blood cells without their destruction in acute falciparum malaria. Blood. 1997;90:2037–2040. [PubMed] [Google Scholar]


