Abstract
Population-based studies suggest that black patients with multiple myeloma (MM) have a higher mortality rate than white patients; however, other studies suggest this disparity is related to socioeconomic status (SES) rather than race. To provide clarity on this topic, we reviewed 562 patients diagnosed with MM at our institution. Patients with high-SES had a median OS of 62.8 months (mos) (95% CI 43.1–82.6 mos), compared to 53.7 mos (45.2–62.3 mos) and 48.6 mos (40.4–56.8 mos) for the middle and low-SES, respectively (p =0.015). After controlling for race, age, year of diagnosis, severity of comorbidities, stem cell transplant utilization, and insurance provider, patients with low-SES had a 54 percent increase in mortality rate relative to patients with high-SES. To support our findings, we performed a similar analysis of 45,505 patients with MM from the Surveillance Epidemiology and End Results-18 (SEER) database. Low-SES is independently associated with poorer OS in MM.
Keywords: multiple myeloma, cancer health disparities, socioeconomic status, race
INTRODUCTION
Multiple myeloma (MM) is an incurable hematologic malignancy which causes lytic skeletal lesions, renal failure, hypercalcaemia, anemia, and recurrent infections due to aberrant immune function. MM is the second most common hematologic malignancy in the United States (US)1, but is the most common in blacks, who have a nearly twofold increase in incidence compared to the general population2. Advances in MM therapies have improved overall survival (OS)3, however, not all population subgroups have benefited equally from these advances3,4.
Population-based studies utilizing data from US cancer registries suggest that black patients with MM have a much higher mortality rate than their white counterparts5,6. However, in several studies which controlled for treatment, OS was similar or even superior among black patients7–10. This discrepancy suggests that the poorer outcomes observed in black patients are attributable to factors other than race, such as socioeconomic status (SES).
SES is commonly conceptualized as the social standing or class of an individual. Different methodologies have been used to evaluate SES, but it is often measured by income, education, or occupation, either as singular variables or in combination. Commonly, SES is broken into tertiles and classified as low-SES, middle-SES, and high-SES.
The risk of multiple myeloma is significantly increased in individuals with low-SES11–13, however, the impact of SES on MM prognosis is unclear. Some studies have observed poorer survival among patients in lower-SES groups14–17, while others have not18–20. Unfortunately, the current studies have differed significantly in methodology, making their results difficult to compare, and none have included data on treatments for MM such as stem cell transplant (SCT).
Thus, to provide clarity on this topic, we tested the hypothesis that lower SES is associated with poorer OS in MM patients using two separate cohorts.
MATERIALS AND METHODS
We performed retrospective chart review of 652 consecutive adult patients (age 18+) diagnosed with MM from January 2000 through December 2009 at the Washington University School of Medicine (WUSM). This research was performed under the guidance of the Human Research Protection Office (HRPO) at Washington University School of Medicine. Patients were eligible for analysis if their home address within 6 months of diagnosis was available. Patients were followed for survival through December 2013.
Independent variables analyzed were: SES, race, age at diagnosis, year of diagnosis, sex, severity of comorbidities, SCT utilization, and primary insurance provider at diagnosis. SES was approximated by median household income (MHI) of each patient’s census tract from the American Community Survey (2012 5-year estimates); patients were divided into tertiles based on MHI and classified as low-SES, middle-SES, or high-SES. Race was patient reported; patients were classified as white, black, or other. The severity of comorbidities was scored with the ACE-27 as none, mild, moderate, or severe21. Primary insurance provider was classified as: private insurance, Medicare, Medicaid, or no insurance.
Surveillance Epidemiology and End Results-18 (SEER-18) Database
To validate our results, we performed a similar analysis using the Surveillance Epidemiology and End Results (SEER)-18 registries database based on the November 2012 submission22. The SEER-18 registries database is a collection of data from 18 tumor registries across the US, covering approximately 28 percent of the total population.
Case listings were extracted using the SEERStat software for 46,361 consecutive adult patients (age 18+) diagnosed with MM from January 2000 through December 2009. Autopsy or death certificate only cases were excluded. As each patient’s census tract was not available, patients were eligible for analysis if the county of their home address at diagnosis was available. Patients were followed for survival through December 2011.
Independent variables analyzed were: SES, race, age at diagnosis, year of diagnosis, and sex. Comorbidities and SCT utilization were not available for analysis. SES was approximated by MHI of each patient’s county from the 2000 US Census; patients were divided into tertiles based on MHI and classified as low-SES, middle-SES, or high-SES. Race was patient reported; patients were classified as white, black, or other.
Statistical Analysis
All data was analyzed using IBM SPSS Statistics version 21. Demographics were summarized using descriptive statistics; the relationship between independent variables was analyzed using T-tests or chi-squared tests. Univariate survival analysis was performed by Kaplan-Meier survival curves and Cox proportional hazards modeling; multivariate survival analysis was performed by Cox proportional hazards modeling.
RESULTS
Washington University School of Medicine (WUSM)
Five-hundred-sixty-two patients were eligible for analysis. The median age at diagnosis was 59 years (range 33–91); 55 percent were male; 26 percent were black. The median MHI (approximated by census tract) was $49,464.5 (range 11,917–163,958). The median follow-up was 49 months (range 0–165). Sixty-one percent (343) of patients underwent SCT (341 autologous and 2 syngeneic). Patient characteristics stratified by SES tertile are reported in Table I.
Table I.
Patient characteristics stratified by SES tertile (WUSM)
| Characteristics | Low-SES | Middle-SES | High-SES | All Patients |
|---|---|---|---|---|
| Number of Patients | 187 | 188 | 187 | 562 |
| Median Age at Diagnosis (years) | 58 | 60 | 59 | 59 |
| Race | ||||
| White | 48% | 82% | 88% | 72% |
| Black | 52% | 17% | 8% | 26% |
| Other | 1% | 1% | 4% | 2% |
| Sex | ||||
| Male | 52% | 55% | 57% | 55% |
| Female | 48% | 45% | 43% | 45% |
| Comorbidity Score | ||||
| None | 21% | 26% | 37% | 28% |
| Mild | 33% | 42% | 29% | 35% |
| Moderate | 23% | 18% | 14% | 18% |
| Severe | 9% | 7% | 8% | 8% |
| Unknown | 14% | 7% | 11% | 11% |
| SCT Utilization | ||||
| Yes | 52% | 59% | 72% | 61% |
| No | 48% | 41% | 28% | 39% |
| Insurance Provider | ||||
| Private | 42% | 59% | 69% | 57% |
| Medicare | 35% | 36% | 27% | 33% |
| Medicaid | 10% | 3% | 2% | 5% |
| No Insurance | 13% | 3% | 2% | 6% |
Relationships among baseline characteristics
Black patients were more likely to be in the lowest or middle SES tertiles than white patients (90% compared to 60%, p <0.001), and also were more likely to not have insurance at time of diagnosis (16% compared to 3%, p <0.001). High-SES patients were less likely to have comorbidities at diagnosis than middle-SES and low-SES patients (58% compared to 72% and 76%, p =0.007), and the prevalence of comorbidities increased with age (p <0.001). High-SES patients were more likely to have private insurance at diagnosis than middle-SES and low-SES patients (69% compared to 59% and 42%), and were less likely to have Medicare (27% compared to 36% and 35%), Medicaid (2% compared to 3% and 10%), or no insurance (2% compared to 3% and 13%). Male patients tended to be younger at diagnosis than females, (59.0 years compared to 61.2 years, p=0.019). The average age of patients with Medicare at diagnosis was 69.7 years-old, compared to 54.5 years-old for private insurance, 49.4 years-old for no insurance 49.4, and 47.8 years-old for Medicaid (p< 0.001).
Relationships between baseline characteristics and stem cell transplant utilization
The median age for patients who underwent SCT was 56.9 years (range 33–74), compared to 66.7 years for those who did not (range 35–91) (p <0.001). Patients diagnosed from 2005–2009 were more likely to have undergone SCT than those diagnosed 2000–2004 (69% compared to 50%, p <0.001). High-SES patients were more likely to undergo SCT than middle-SES and low-SES patients (72% compared to 59% and 52%, respectively, p <0.001), as were white patients compared to black patients (67% compared to 45%, p <0.001). Seventy-seven percent of patients without comorbidities underwent SCT while only 23 percent of patients with severe comorbidities did (p <0.001). Eighty-one percent of patients with private insurance at diagnosis underwent SCT, compared to 56 percent of patients with Medicaid, 41 percent with no insurance, and 31 percent with Medicare (p <0.001).
Univariate survival analysis
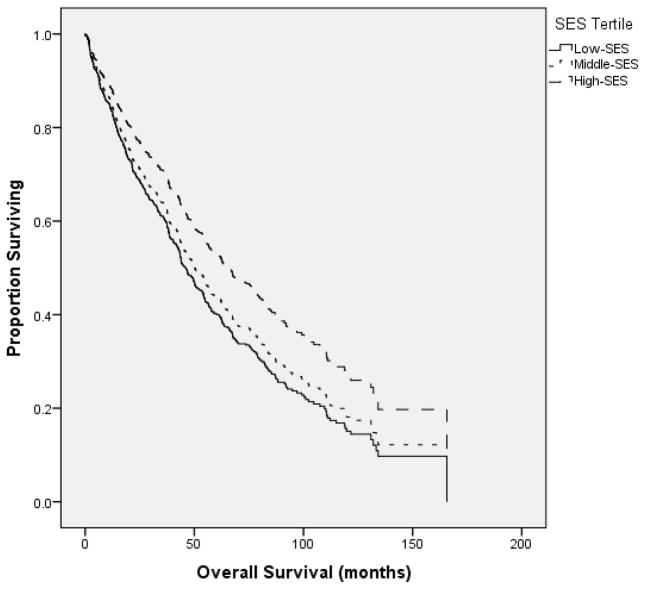
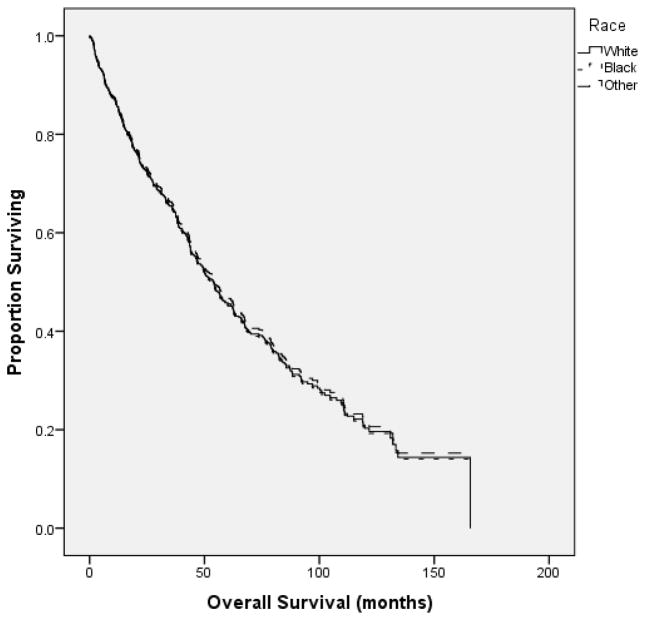
The median OS for all patients was 53.7 months (mos) (95% CI 47.2–60.3 mos). Patients in the high-SES tertile (≥ $57,177 MHI) had a median OS of 62.8 mos (43.1–82.6 mos), compared to 53.7 mos (45.2–62.3 mos) and 48.6 mos (40.4–56.8 mos) for the middle ($41,401–$57,177 MHI) and low-SES tertiles (≤ $41,400 MHI), respectively (p =0.015) [Figure 1]. OS was similar between white and black patients [Figure 2].
Figure 1.
Patients in the highest SES tertile (≥ $57,177 median household income) had a median OS of 62.8 months (mos) (95% CI 43.1–82.6 mos), compared to 53.7 mos (45.2–62.3 mos) and 48.6 mos (40.4–56.8 mos) for the middle and lowest tertiles, respectively (p =0.015).
Figure 2.
In univariate analysis, there was no difference in OS based on race.
Patients with no comorbidities had a median survival of 66.1 mos (95% CI 54.9–77.3 mos), compared to 61.6 mos (47.1–76.2 mos), 43.5 mos (35.9–51.1 mos), and 18.5 mos (8.7–28.3 mos) for mild, moderate, and severe comorbidities, respectively (p <0.001). Patients who underwent ASCT had a median OS of 75.6 mos (95% CI 64.3–86.9 mos), compared to 21.8 mos (17.3–26.2 mos) for those who did not (p <0.001). Patients with private insurance at diagnosis had a median survival of 67.5 mos (95% CI 55.4–79.6 mos), compared to 59 mos (528.4-89-5 mos), 38.3 mos (26.2–50.4 mos), and 37.5 mos (30.5–44.6 mos) for patients with no insurance, Medicaid, and Medicare, respectively (p <0.001).
Age at diagnosis was also associated with OS [HR 1.03/year (95% CI 1.02–1.05)], and patients 65-years-old or older at diagnosis had a median survival of 37.3 mos (95% CI 27.1–47.7 mos), compared to 62.8 mos (55.6–70.0 mos) for patients under 65 at diagnosis (p <0.001). No relationship between sex and OS was observed.
Multivariate survival analysis
In a multivariate model of SES, age at diagnosis, year of diagnosis, race, comorbidity score, SCT utilization, and insurance provider at diagnosis all variables other than insurance provider were independently associated with survival. SES was associated with OS [HR 1.54 (95% CI 1.13–2.09) for low-SES relative to high-SES]; black patients had a reduced mortality rate compared to whites [HR 0.57 (95% CI 0.42–0.76]. The results of the multivariate survival analysis are summarized in Table II.
Table II.
Multivariate Survival Analysis (WUSM)
| 95.0% CI for HR | ||||
|---|---|---|---|---|
|
| ||||
| HR1 | Lower | Upper | p value | |
| SES | 0.022 | |||
| High-SES | 12 | |||
| Middle-SES | 1.25 | 0.95 | 1.65 | 0.114 |
| Low-SES | 1.54 | 1.13 | 2.09 | 0.006 |
| Age (per year) | 1.02 | 1.01 | 1.04 | 0.002 |
| Year of Diagnosis (per year) | 0.95 | 0.91 | 0.99 | 0.031 |
| Race | <0.001 | |||
| White | 12 | |||
| Black | 0.57 | 0.42 | 0.76 | 0.001 |
| Other | 1.97 | 0.92 | 4.24 | 0.083 |
| Comorbidity Score | 0.002 | |||
| None | 12 | |||
| Mild | 1.12 | 0.85 | 1.47 | 0.433 |
| Moderate | 1.34 | 0.97 | 1.84 | 0.074 |
| Severe | 2.17 | 1.43 | 3.28 | <0.001 |
| SCT Utilization | <0.001 | |||
| Yes | 12 | |||
| No | 2.57 | 1.96 | 3.38 | <0.001 |
| Insurance Provider | 0.058 | |||
| Private | 12 | |||
| Medicare | 0.74 | 0.53 | 1.03 | .071 |
| Medicaid | 1.224 | 0.70 | 2.14 | .478 |
| No Insurance | 1.59 | 0.94 | 2.70 | .083 |
Controlled for all other variables within the model
Reference level
To test for immortal time bias with SCT utilization, a similar analysis was performed using only the 518 patients who survived ≥ 6 months and thus would have been able to proceed to ASCT (data not shown). Results of both analyses were similar.
SEER-18 Database
45,505 MM patients were identified for analysis. The median age at diagnosis was 69 years (range 18–85+); 55 percent were male; 18 percent were black. The median MHI (approximated by county) was $44,940 (range 15,810–79,890). The median follow-up was 24 months (range 0–131). Patient characteristics stratified by SES tertile are reported in Table III.
Table III.
Patient characteristics stratified by SES tertile (SEER-18)
| Characteristics | Low-SES | Middle-SES | High-SES | All Patients |
|---|---|---|---|---|
| Number of Patients | 15,238 | 15,146 | 15,121 | 45,505 |
| Median Age at Diagnosis (years) | 69 | 69 | 69 | 69 |
| Race | ||||
| White | 74% | 76% | 80% | 76% |
| Black | 24% | 19% | 12% | 18% |
| Other | 2% | 6% | 8% | 5% |
| Sex | ||||
| Male | 55% | 55% | 55% | 55% |
| Female | 45% | 45% | 45% | 45% |
Relationships among baseline characteristics
Black patients were more likely to be in the low or middle SES tertiles than white patients (78% compared to 65%, p <0.001). The mean age of black patients at diagnosis was 65.3-years-old, compared 68.7-years-old for whites patients (p <0.001). White patients were more likely to be male (56%), while black patients were just as likely to be female as male (p <0.001). Male patients tended to be younger at diagnosis than females (67.3 years compared to 68.8 years, p<0.001).
Univariate survival analysis
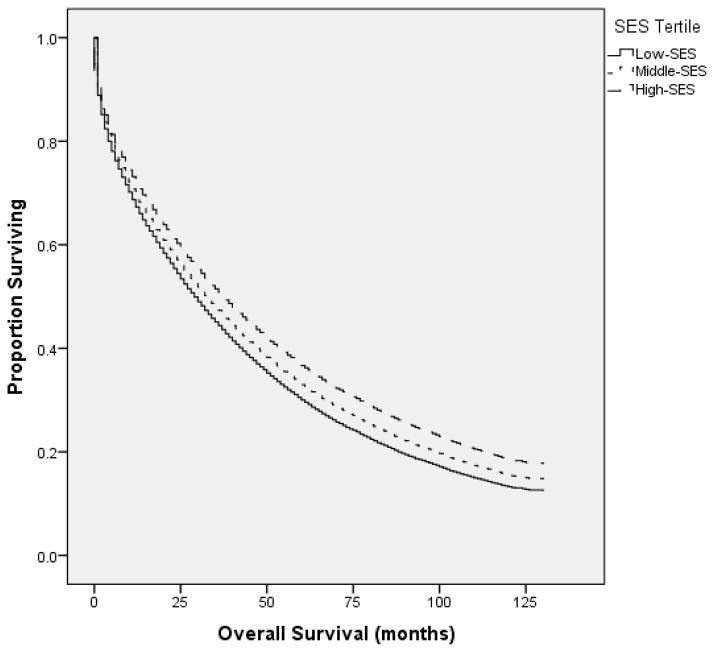
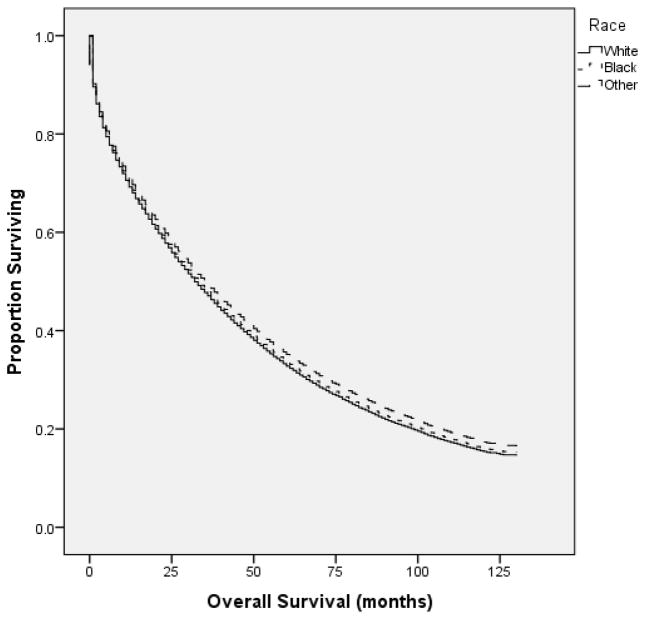
The median OS for all patients was 33.0 mos (95% CI 32.4–33.6 mos). Patients in the high-SES tertile (≥ $50,760 MHI) had a median OS of 38.0 mos (95% CI 36.8–39.2 mos), compared to 32.0 mos (30.9–33.1 mos) and 29 mos (28.1–29.9 mos) for the middle ($42, 071–$50,759 MHI) and low SES tertiles (≤ $42,070 MHI), respectively (p <0.001) [Figure 3]. OS was similar between white and black patients [Figure 4].
Figure 3.
In the SEER-18 registries database, patients in the highest SES tertile (≥ $50,760 median household income) had a median OS of 38.0 mos (95% CI 36.8–39.2 mos), compared to 32 mos (30.9–33.1 mos) and 29 mos (28.1–29.9 mos) for the middle and lowest tertiles, respectively (p <0.001).
Figure 4.
In univariate analysis of the SEER-18 registries database, there was no difference in OS based on race.
Age at diagnosis was associated with OS [HR 1.04/year (95% CI 1.04–1.04)], and patients 65-years-old or older at diagnosis had a median survival of 23 mos (95% CI 22.4–23.6 mos), compared to 62 mos (60.2–63.8 mos) for patients under 65 at diagnosis (p <0.001). No relationship between sex and OS was observed.
Multivariate survival analysis
In a multivariate model of SES, age at diagnosis, year of diagnosis, and race, all four variables were independently associated with survival. SES was associated with OS [HR 1.18 (95% CI 1.15–1.22) for low-SES relative to high-SES; HR 1.10 (95% CI 1.07–1.13) for middle-SES relative to high-SES]. Black patients had a slightly increased mortality rate compared to whites [HR 1.09 (95% CI 1.06–1.12]. The results of the multivariate survival analysis are summarized in Table IV.
Table IV.
Multivariate Survival Analysis (SEER-18)
| 95.0% CI for HR | ||||
|---|---|---|---|---|
|
| ||||
| HR1 | Lower | Upper | p value | |
| SES | <0.001 | |||
| High-SES | 12 | |||
| Middle-SES | 1.10 | 1.07 | 1.13 | <0.001 |
| Low-SES | 1.18 | 1.15 | 1.22 | <0.001 |
| Age (per year) | 1.04 | 1.04 | 1.04 | <0.001 |
| Year of Diagnosis (per year) | 0.97 | 0.96 | 0.97 | <0.001 |
| Race | <0.001 | |||
| White | 12 | |||
| Black | 1.09 | 1.06 | 1.12 | <0.001 |
| Other | 1.02 | 0.97 | 1.07 | 0.516 |
Controlled for all other variables within the model
Reference level
DISCUSSION
In a large study of two separate cohorts of patients with MM, we showed that SES is independently associated with survival. Two potential hypotheses for the disparity in outcomes have been suggested. 1) patients with lower SES delay seeking medical attention and thus are farther advanced at presentation, or 2) patients with lower SES have reduced access to care and/or a lower quality of care for their disease (e.g. are less likely to undergo SCT). The current study found that patients with high-SES were more likely to undergo SCT, but that only partially accounted for the survival advantage associated with high-SES. Additionally, the negative impact of lower SES on survival in MM patients has been observed in studies in England15, Sweden16, and Italy17, all three of which have universal health care systems and therefore the entire population has equal access to health care services. These findings suggest the more likely explanation may be that MM patients with lower SES delay seeking medical attention and thus have more advanced disease at presentation. This hypothesis has not been tested to date, but such associations have been seen in other cancer subtypes23–25.
Additional possible mechanisms for the disparity in outcome based on SES include: poorer tolerance and adherence to treatment and increased treatment complications among lower SES patients26. Determining the correct mechanisms will be an important area for future research, particularly in the current environment of healthcare reform and the need to reduce health care disparities.
The impact of SES on MM survival in the US has been previously studied but with smaller sample sizes and different methodologies. In 1984 Savage, et al. was the first publication on the topic; they reported on 144 MM patients diagnosed between 1958 and 1980 at two hospitals in New York14. SES variables were obtained from the 1970 Census from each patient’s home census tract and included: median family income, percentage of families living below the poverty level, adult male unemployment rate, low education (percentage of adults with less than 9 years of education), prevalence of separation or divorce, and overcrowding (mean occupancy of more than 1.5 persons/room. In univariate analysis, all socioeconomic variables were significantly associated with survival.
Weston, et al. (1987) reported on 153 MM patients (127 newly diagnosed and 26 previously diagnosed) referred between 1976 and 1982 to Duke University or one of the Veteran’s Administration Hospitals in Durham, North Carolina18. They prospectively collected patient reported SES variables from time of diagnosis, which included: current income, highest income, occupation, type of dwelling, years of education, and crowding. None of the SES variables were significantly associated with survival in univariate analysis. The strength of the study was the use of patient-reported SES, rather than geographical-based surrogates of SES.
Lenhard, et al. (1987) reported on 1,479 newly diagnosed MM patients enrolled into the Centralized Cancer Patient Data System (CCPDS), a NCI supported multi-center collaborative database, between 1977 and 198219. The investigators used the percentage of the population in each patient’s home zip code that were high school graduates during the 1970 census as a surrogate for SES. While there was a trend of poorer survival for patients living in areas with lowest percent of patients, it was not statistically significant. SES levels were not defined using quantiles, which may explain the lack of significance.
Abou-Jawde, et al. (2006) reported on 292 MM patients (168 newly diagnosed and 124 relapsed/refractory) diagnosed from 1997 to 2003 at the Cleveland Clinic, using MHI for each patient’s home zip code as a surrogate for SES20. The authors did not find an association between SES and OS, however, the study controlled for distance traveled to the treatment center, which may also be a function of SES, and thus potentially reduced the ability to observe an effect of SES on OS27.
We found a high-degree of consistency between the results of the two datasets analyzed. In the WUSM dataset we found a 23 and 14 percent OS advantage for high-SES patients over low-SES and middle-SES patients, respectively; In the SEER-18 dataset we found a 24 percent and 16 percent OS advantage for high-SES patients over low-SES and middle-SES patients, respectively. In both datasets black patients were more likely to be low or middle-SES than white patients, males tended to be younger than females, increasing age was associated with poorer survival, and race was not associated with OS in univariate analysis but was in multivariate analysis.
In multivariate survival analysis of the WUSM dataset, white patients had a 76 percent increase in mortality rate compared to black patients, while in multivariate survival analysis of the SEER-18 dataset, black patients had a 9 percent increase in mortality rate compared to white patients. Several possible factors could account for this discrepancy such as the absence of comorbidity, SCT utilization, and insurance data in the multivariate model for the SEER-18 dataset. Alternatively, additional treatment variations or differences in access to care between black and white patients in the SEER-18 dataset could explain the difference; in the WUSM dataset, once patients presented for care, they likely were treated uniformly regardless of race within a single center. An analysis of a dataset such as the SEER-Medicare linked dataset would allow further exploration of this discrepancy by examining patients treated across the country, while accounting for comorbidities.
Overall patients in the WUSM dataset had a better OS than patients in the SEER-18 dataset [53.7 mos (95% CI 47.2–60.3 mos) compared to 33 mos (32.4–33.6mos)], but that is likely attributed to age differences between the two populations, as the median age for WUSM patients was 59 years, compared to 69 years for SEER-18 patients. The median OS of patients <65 years of diagnosis was similar between the two groups [62.8 mos (55.6–70.0 mos) compared to 62 mos (60.2–63.8 mos)]. The discrepancy between median age at diagnosis between the two datasets is likely attributed to referral bias at WUSM. It has been reported that patients treated at NCI-designated Cancer Centers tend to be younger than those treated elsewhere28.
The strengths of the current study are: its large sample size, its inclusion of SCT as a predictor of survival, and its inter-dataset reliability. The current study is limited by the use of area-based surrogates of SES rather than patient reported SES. The use of area-based surrogates of SES is common because medical records rarely contain information regarding SES and most US public health surveillance databases contain little or no SES data; only 7 percent of US state cancer registries include data on education and none include data on income.
An additional limitation of this study is the lack of MM staging data. While staging systems for MM prognosis have been developed, they are only useful for prognosis not for risk stratification29. Furthermore, the most commonly used staging system for MM, the International Staging System (ISS), was developed in 2005 and requires a beta-2 microglobulin test. Unfortunately, beta-2 microglobulin tests were not routinely performed on MM patients at our institution prior to 2005, therefore ISS stage would only be available on ~50% of the WUSM dataset. Neither lab values nor MM staging data is available in the SEER-18 dataset.
CONCLUSION
In conclusion, low-SES is independently associated with poorer OS in MM. The most likely hypothesis for this is patients with low-SES delay seeking medical attention and thus are farther advanced at presentation.
There have been many campaigns in recent years to eliminate outcome disparities in cancer based on race and SES, including: increasing early detection, increasing access to care, and improving the quality of treatment, however, none of these campaigns have specifically addressed hematologic malignancies such as multiple myeloma. This paper highlights the need for additional action to eliminate outcome disparities based on SES in hematologic malignancies.
The Affordable Care Act includes many measures with the intent to increase the access and quality of care of all patients, such as an annual checkup at no out-of-pocket cost. Additional measures such as oral chemotherapy parity, which limits the copays insurance companies can charge for oral chemotherapy agents to that of comparable intravenous chemotherapy, should improve access to these agents for lower SES patients. However, increasing access and reducing costs alone will not eliminate all outcome disparities, as they are still prevalent today in countries with long histories of universal health care. The population also needs to be educated on the early signs and symptoms of MM and advised to seek medical care early.
Acknowledgments
We would like to thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Missouri. The Siteman Cancer Center is supported in part by a NCI Cancer Center Support Grant #P30 CA91842.
Funding Support:
None
Footnotes
Conflicts of Interest:
Dr. Keith E. Stockerl-Goldstein is on the Speaker’s Bureau for Celgene and Millennium.
Dr. Tanya M. Wildes’ research is supported by Grant Number KM1CA156708 and K12CA167540 through the National Cancer Institute (NCI) at the National Institutes of Health (NIH) and Grant Number UL1 TR000448 through the Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCI, NCATS or NIH.
The remaining authors have no conflicts of interest to declare.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2006;56(2):106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Blattner WA. Epidemiology of multiple myeloma and related plasma cell disorders: An analytic review. In: Potter M, editor. Progress in Myeloma. New York, NY: Elseveier North Holland; 1980. pp. 1–65. [Google Scholar]
- 3.Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97(19):1407–1427. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with special feature regarding survival. Cancer. 2004;101(1):3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 5.Altekruse SF, Kosary Cl, Krapch M, et al. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Cancer Institute; 2010. [Google Scholar]
- 6.Davey Smith G, Neaton JD, Wentworth D, Stamler R, Stamler J. Mortality differences between black and white men in the USA: contribution of income and other risk factors among men screened for the MRFIT. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Lancet. 1998;351(9107):934–939. doi: 10.1016/s0140-6736(00)80010-0. [DOI] [PubMed] [Google Scholar]
- 7.Verma PS, Howard RS, Weiss BM. The impact of race on outcomes of autologous transplantation in patients with multiple myeloma. Am J Hematol. 2008;83:355–358. doi: 10.1002/ajh.21139. [DOI] [PubMed] [Google Scholar]
- 8.Saraf S, Chen YH, Dobogai LC, et al. Prolonged responses after autologous stem cell transplantation in African-American patients with multiple myeloma. Bone Marrow Transplant. 2006;34:1099–1102. doi: 10.1038/sj.bmt.1705392. [DOI] [PubMed] [Google Scholar]
- 9.Modiano MR, Villar-Werstler P, Crowley J, Salmon SE. Evaluation of race as a prognostic factor in multiple myeloma: An ancillary of southwest oncology group study 8229. J Clin Oncol. 1996;14:974–977. doi: 10.1200/JCO.1996.14.3.974. [DOI] [PubMed] [Google Scholar]
- 10.Rajkumar SV, Greipp PR, Jacobus S, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11(1):29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baris D, Brown LM, Silverman DT, et al. Socioeconomic status and multiple myeloma among US blacks and whites. Am J Public Health. 2000;90(8):1277–1281. doi: 10.2105/ajph.90.8.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koessel SL, Theis MK, Vaughan TL, et al. Socioeconomic status and the incidence of multiple myeloma. Epidemiology. 1996;7(1):4–8. doi: 10.1097/00001648-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Clark DW, MacMahon B. The incidence of multiple myeloma. J Chronic Dis. 1956;4(5):508–515. [PubMed] [Google Scholar]
- 14.Savage D, Lindenbaum J, Van Ryzin J, Struening E, Garrett TJ. Race, poverty, and survival in multiple myeloma. Cancer. 1984;54(12):3085–3094. doi: 10.1002/1097-0142(19841215)54:12<3085::aid-cncr2820541246>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Renshaw C, Ketley N, Moller J, Davies EA. Trends in the incidence and survival of multiple myeloma in South East England 1985–2004. BMC Cancer. 2010;10(74) doi: 10.1186/1471-2407-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristinsson SY, Derolf AR, Edgren G, Dickman PW, Bjorkholm M. Socioeconomic differences in patient survival are increasing for acute myeloid leukemia and multiple myeloma in Sweden. J Clin Oncol. 2009;27(12):2073–2080. doi: 10.1200/JCO.2008.18.2006. [DOI] [PubMed] [Google Scholar]
- 17.Pasqualetti P, Colantonio D, Collacciani A, Casale R. Socioeconomic status and survival in multiple myeloma. Minerva Med. 1990;81:713–716. [PubMed] [Google Scholar]
- 18.Weston B, Grufferman S, MacMillan JP, Cohen HJ. Effects of socioeconomic and clinical factors on survival in multiple myeloma. J Clin Oncol. 1987;5(12):1977–1984. doi: 10.1200/JCO.1987.5.12.1977. [DOI] [PubMed] [Google Scholar]
- 19.Lenhard RE, Jr, Enterline JP, Crowley J, Ho GY. The effects of distance from primary treatment centers on survival among patients with multiple myeloma. J Clin Oncol. 1987;5(10):1640–1645. doi: 10.1200/JCO.1987.5.10.1640. [DOI] [PubMed] [Google Scholar]
- 20.Abou-Jawde RM, Baz R, Walker E, et al. The role of race, socioeconomic status, and distance traveled on the outcome of African-American patients with multiple myeloma. Haematologica. 2006;91(10):1410–1413. [PubMed] [Google Scholar]
- 21.Piccirillo JF, Creech C, Zequeira R, Anderson S, Johnston AS. Inclusion of comorbidity into oncology data registries. J Registry Management. 1999;26(2):66–70. [Google Scholar]
- 22.Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch; SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2012 Sub (1973–2010 varying) - Linked To County Attributes - Total U.S., 1969–2011 Counties. ( www.seer.cancer.gov) released April 2013, based on the November 2012 submission. [Google Scholar]
- 23.Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected finding from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20(4):417–435. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Islami F, Kahn AR, Bickell NA, Schymura MJ, Boffetta P. Disentangling the effects of race/ethnicity and socioeconomic status of neighborhood in cancer stage distribution in New York City. Cancer Causes Control. 2013;24(6):1069–1078. doi: 10.1007/s10552-013-0184-2. [DOI] [PubMed] [Google Scholar]
- 25.Bray C, Morrison DS, McKay P. Socio-economic deprivation and survival of non-Hodgkin lymphoma in Scotland. Leuk Lymphoma. 2008;49(5):917–923. doi: 10.1080/10428190801933377. [DOI] [PubMed] [Google Scholar]
- 26.Gupta S, Wilejto M, Pole JD, Guttmann A, Sung L. Low Socioeconomic Status is Associated with Worse Survival in Children with Cancer: A Systematic Review. PloS One. 2014;9(2):e89482. doi: 10.1371/journal.pone.0089482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ojha RP, Prabhakar D, Evans E, Lowery K, Thertulien R, Fischbach LA. RE 10104: Abou-Jawde et al. The role of race, socioeconomic status, and distance traveled on the outcome of African-American patients with multiple myeloma. Haematologica 2006, 91: 1410–1413. Haematologica. 2007;92(4):e46. doi: 10.3324/haematol.11157. [DOI] [PubMed] [Google Scholar]
- 28.In H, Neville BA, Lipsitz SR, Corso KA, Weeks JC, Greenberg CC. The Role of National Cancer Institute-Designated Cancer Center Status. Ann Surg. 2012;255(5):890–895. doi: 10.1097/SLA.0b013e31824deae6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, and risk stratification, and response assessment of multiple myeloma. Leukemia. 2009;23(1):3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]