ABSTRACT
The incidence of cardiovascular events in hypertensive patients is clearly related to a left ventricular mass during treatment, and a regression of left ventricular hypertrophy is associated with a better prognosis. This is the case even independently of changes in other risk factors, including blood pressure. Evidence indicates that lifestyle modifications such as dietary salt restriction and weight loss are effective means in preventing the development of hypertension and reducing blood pressure and left ventricular mass in hypertensive patients. Salt restriction may also reduce the long-term risk of cardiovascular events. It has been recognized that the primary targets of current antihypertensive drugs are the renin-angiotensin-aldosterone system, calcium homeostasis, the ionic transport mechanisms in the kidneys, and the sympathetic nervous system. Clinical as well as experimental studies have demonstrated the cardioprotective effects of antihypertensive drugs independently of their blood pressure lowering effects. Hypertension is often complicated by other disease states including diabetes, dyslipidemia, and ischemic heart disease. Some of the drugs used for the treatment of such complications are also shown to produce cardioprotective effects in addition to their original effects. We ought to better understand these pleiotropic effects for the most effective treatments of hypertension and its complications.
Key Words: Cardioprotection, Lifestyle modification, Pharmacotherapy, Hypertensive heart disease
INTRODUCTION
Heart failure is a final common consequence of various forms of heart disease, and is a leading cause of mortality worldwide. Hypertension remains one of the most common causes of cardiac failure. In the presence of a chronic pressure overload, such as arterial hypertension, a parallel addition of sarcomeres occurs together with an increase in myocyte width, which in turn increases wall thickness. In the development of hypertensive heart disease, myocyte hypertrophy is also associated with apoptosis, collagen deposition, and ventricular fibrosis, along with an impairment of coronary hemodynamics as well, thus profoundly influencing the functional properties of the left (and right) ventricle.
For many years, the occurrence of heart failure has been attributed to a progressive impairment of systolic function. More recently, however, it has been observed that a considerable number of cases with typical symptoms of congestive heart failure present a normal or only mildly impaired systolic function, referred to as diastolic heart failure.1) In hypertensive patients with left ventricular hypertrophy (LVH), abnormalities in both myocardial relaxation and passive filling have been detected. Diastolic heart failure may be observed in approximately one-half of all heart failure cases. Whether it may also be associated with a lower mortality rate compared to other forms of heart failure is still controversial; however, it is clearly associated with a high morbidity. It is conceivable that the early recognition and appropriate therapy of diastolic dysfunction can prevent further progression to diastolic heart failure and death.
The development of LVH in hypertension does not depend exclusively on the level of blood pressure (BP), but may also be modulated by several neurohumoral factors and by the aortic properties. Numerous studies have demonstrated that long-term antihypertensive treatment may be associated with regression of LVH. Accumulating evidence indicates that a reduction of LVH with antihypertensive treatment is associated with an improvement in outcomes and a decrease in the risk of cardiovascular morbidity and mortality.
This review provides an overview of the cardioprotective mechanisms of lifestyle modifications and pharmacologic interventions on cardiac remodeling and dysfunction in hypertensive heart disease.
LIFESTYLE MODIFICATIONS
Salt restriction
In most cases, hypertension results from multiple genetic, dietary, and metabolic interactions rather than from monogenic mutations, which can only be identified in less than 1% of patients with hypertension.2) Several candidate genes have been identified whose products increase renal salt retention and the risk of hypertension.2) Recent epidemiological studies have confirmed a positive correlation between salt intake and elevated blood pressure in up to half of patients with hypertension.3) Of note, high salt intake has also been shown to cause vascular remodeling and an increase in the left ventricular (LV) mass, as well as to increase the incidence of stroke, independently of blood pressure elevation.3) A relationship between salt sensitivity and increased long-term mortality that is independent of blood pressure status has been documented by Weinberger et al.4)
Both dietary salt restriction and weight loss have been shown to reduce LV mass as well as blood pressure.5-7) In addition, in the Treatment of Mild Hypertension Study (TOMHS), nutritional-hygienic (NH) intervention with the emphasis on a reduction of dietary sodium and weight loss was as effective as NH intervention plus pharmacological treatment in reducing LV mass, despite a smaller decrease in blood pressure in the NH intervention-only group.8) This suggests that in mildly hypertensive patients, the effects of sodium restriction and weight loss may be more significant in reducing LV mass rather than BP changes. Moreover, observational follow-up of the trials of hypertension prevention (TOHP) has suggested that sodium reduction may also reduce the long-term risk of cardiovascular events.9)
Although the mechanism by which salt intake affects cardiovascular function remains unclear, it may be related in part to changes in arterial compliance, a known marker of cardiovascular morbidity and mortality.10) Moreover, mechanical stretch and fluid shear stress were shown to activate Rac1, a member of the Rho family of GTPases, in the kidney, and Rac1-induced activation of renal mineralocorticoid receptors may lead to the development of salt-sensitive hypertension.11) Insulin resistance is also closely related to the salt sensitivity of blood pressure,12) with salt loading being shown to result in the development of insulin resistance in young adult black subjects.13) Reduced systemic insulin resistance resulting from dietary salt restriction may thus contribute to the attenuation of hypertension by such a manipulation in hypertensive patients.
Exercise training
Exercise training lowers BP, both in normotensive14) and hypertensive subjects.15) Moreover, exercise and cardiorespiratory fitness have been associated with a reduced risk of cardiovascular events in apparently healthy individuals16) as well as in subjects with multiple coronary risk factors.17) The underlying mechanisms by which exercise training produces preventive cardiovascular effects have not been fully elucidated. Moreover, improvements in lipid profile, glucose metabolism, overweight, nitric oxide bioavailability, and sympathetic nerve activity may contribute to the beneficial effect.
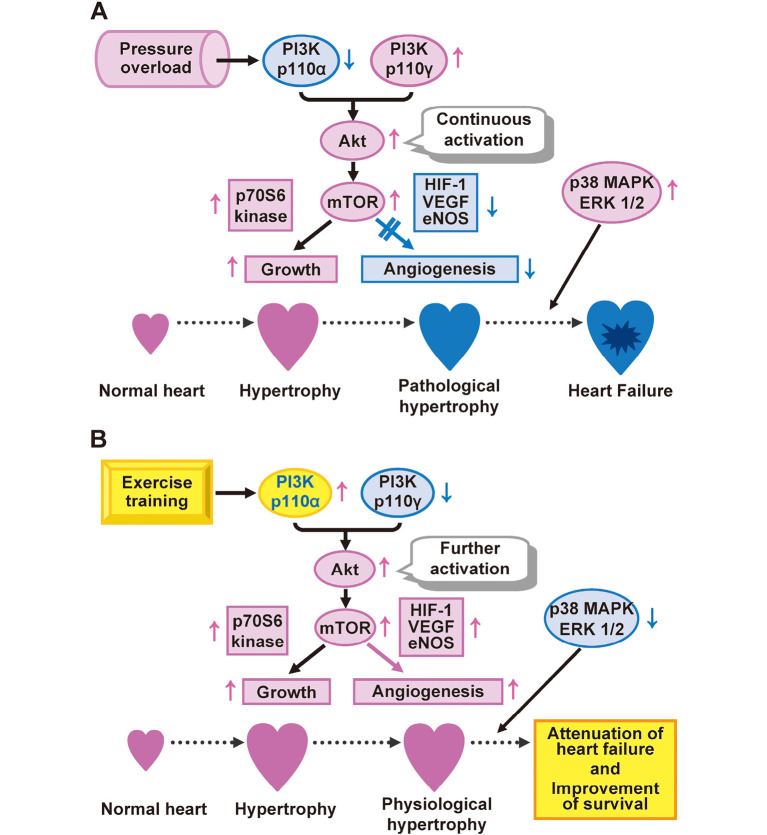
The clinical efficacy of exercise training in individuals with heart failure is well documented. Studies have suggested that carefully designed programs of exercise in patients with heart failure are generally safe and may improve exercise tolerance, vascular endothelial function, central cardiac function, and an overall quality of life.18,19) Exercise training also appears to improve survival in patients20) or animal models21,22) with heart failure. We have shown that exercise training exerts a beneficial effect on cardiac remodeling and attenuated heart failure in rats with salt-sensitive hypertension.22) It also promoted coronary angiogenesis mediated by the phosphatidylinositol 3-kinase (p110α)-Akt-mammalian target of rapamycin signaling, and it attenuated LV concentricity and inhibited myocardial fibrosis, leading to the preservation of cardiac function and improved survival (Fig. 1A,B). Given that the balance between cardiac growth and angiogenesis is a key determinant of cardiac function, it may prove advantageous to stimulate angiogenesis as part of a general strategy to prevent or reverse heart failure. It may also be possible to treat hypertension and heart failure with a combination of antihypertrophic (antihypertensive drugs) and proangiogenic (exercise training) protocols, with combined therapy being more effective than either treatment alone.
Fig. 1.
Akt-mTOR-dependent cardiac growth and coronary angiogenesis as a determinant of cardiac function. (A) PI3K p110α is reduced in the overloaded heart, and in contrast, PI3K p110γ is increased in such hearts. In addition, an increase in the phosphorylation levels of Akt and mTOR in those hearts is not accompanied by an increase in myocardial capillary density. Furthermore, the activations of p38 MAPK and ERKs are found in the overloaded heart, indicating the development of pathological hypertrophy, leading to the progression to heart failure. (B) Exercise training ameliorates the isoform shift of PI3K and inhibits the activations of p38 MAPK and ERKs, leading to the prevention of a decrease in myocardial capillary density as well as to a further increase in the extent of Akt and mTOR phosphorylation. A reduction in the imbalance between cardiac growth and angiogenesis induced by exercise training contributes to the attenuation of heart failure and to a consequent improvement in the survival of hypertensive rats. Thus, the relative balance between cardiac growth and coronary angiogenesis, rather than the extent of hypertrophy, is a critical determinant of physiological versus pathological cardiac hypertrophy.
Abbreviations: mTOR, mammalian target of rapamycin; MAPK, mitogen-activated protein kinase; ERKs, extracellular-signal-regulated kinases; PI3K, phosphatidylinositol 3-kinase; HIF-1, hypoxia-inducible factor 1; VEGF, vascular endothelial growth factor; eNOS, endothelial nitric oxide synthase.
Pharmacotherapies
AT1 receptor blockers/ACE inhibitors
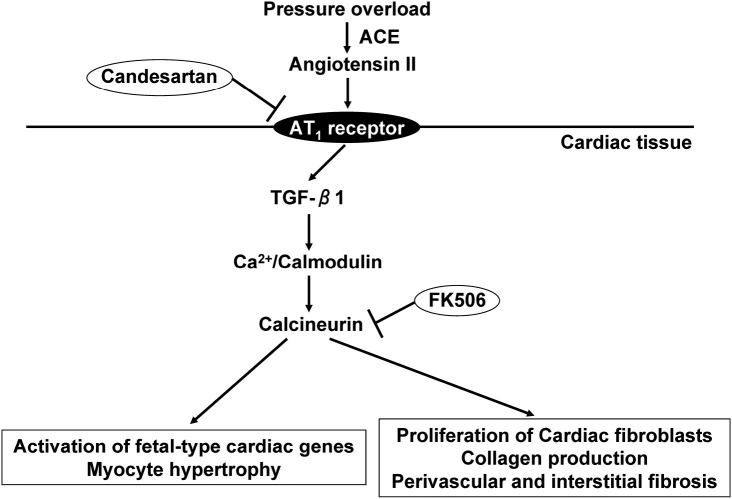
The Losartan Intervention For Endpoint reduction in hypertension study (LIFE) showed that in the presence of a similar BP reduction, the greater regression of LVH with losartan compared with atenolol was accompanied by a reduced incidence of cardiovascular events.23) Moreover, a non-antihypertensive dose of an angiotensin-converting enzyme (ACE) inhibitor reversed LVH in aortic banded rats.24) The antihypertrophic effect of an angiotensin II type 1 (AT1) receptor blocker was greater than that of hydralazine, despite the superior antihypertensive effect of hydralazine, in spontaneously hypertensive rats.25) These observations suggest that blood pressure reduction alone is not sufficient to prevent target organ damage, and that the additional control of local or neurohumoral factors might also be required. We have shown that the AT1 receptor blocker candesartan attenuates the development of cardiac hypertrophy and fibrosis while also reducing cardiac calcineurin activity, in a manner independent of its antihypertensive effect, in Dahl salt-sensitive hypertensive rats,27) suggesting that calcineurin contributes to AT1 receptor–mediated angiotensin II signaling in vivo (Fig. 2). Our recent study26) also suggested that cystein protease cathepsins are likely to trigger and promote cardiac remodeling, and that AT1 receptor blockade with olmesartan attenuates cathepsin expression and activity by inhibiting the production of superoxide by NADPH oxidase, thereby attenuating cardiac remodeling and dysfunction, in Dahl salt-sensitive hypertensive rats.
Fig. 2.
AT1 receptor-mediated angiotensin II signaling in the heart. In hypertensive rats, the cardiac renin-angiotensin system is activated in the overloaded heart. Both the AT1 receptor blocker candesartan and the calcineurin inhibitor FK506 inhibit calcineurin in the heart, as well as the development of cardiac hypertrophy and fibrosis, in a manner independent of its antihypertensive effect. However, FK506 does not affect ACE, AT1 receptor, or TGF-β1 gene expression in those rat hearts, suggesting that calcineurin may be located downstream from TGF-β1 in AT1 receptor-mediated angiotensin II signaling.
Abbreviations: AT1, angiotensin II type 1; ACE, angiotensin-converting enzyme; transforming-growth factor-β1, TGF-β1.
Selective MR antagonists
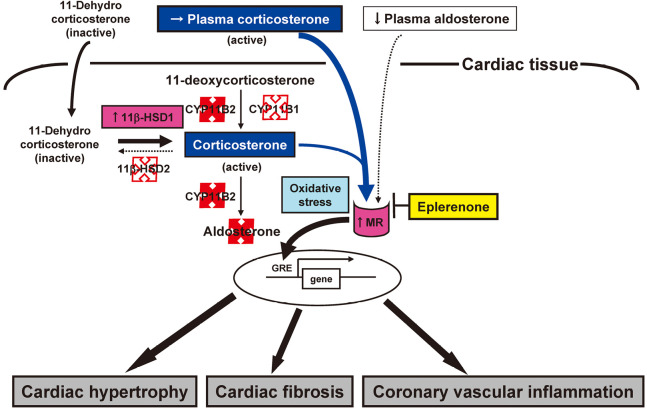
Selective mineralocorticoid receptor (MR) blockade with eplerenone has provided substantial cardiovascular protection in mice suffering chronic pressure overload.28) Thus, overactivity of the renin-angiotensin-aldosterone system (RAAS) is thought to be a risk factor for cardiovascular disease. Studies have clearly demonstrated an aldosterone breakthrough phenomenon during long-term inhibition of the renin-angiotensin-system (RAS). The Randomized Aldactone Evaluation Study (RALES)29) and the Eplerenone Post-AMI Heart Failure Efficacy and Survival Study (EPHESUS)30) have shown that the addition of MR antagonists to the standard treatment with ACE inhibitors or AT1 receptor blockers substantially increases the survival and reduces the hospitalization rate of individuals with severe heart failure. Moreover, our recent pilot study has demonstrated that adding spironolactone to ACE inhibitors ameliorated LV diastolic dysfunction and reduced chamber stiffness in association with a regression of myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy.31) Therefore, given the clinical relevance of the aldosterone breakthrough, adding MR antagonists to RAS inhibitors should prove to be a useful strategy in terms of organ protection. In most patients with essential hypertension or heart failure, plasma aldosterone levels are within the normal range until they receive diuretics. Indeed, in both the RALES and EPHESUS trials, patients’ aldosterone levels were normal and sodium status was unremarkable. These results underscore the importance of MR activation by ligands other than aldosterone. Both ACE inhibitors and AT1 receptor blockers have generally been found to be less effective in hypertensive patients with low renin and aldosterone levels (typically blacks and Japanese) than in hypertensive patients with high renin and aldosterone levels (typically whites). We first demonstrated that selective MR blockade with eplerenone exerts cardioprotective effects in Dahl salt-sensitive hypertensive rats, a model of low-renin, low-aldosterone hypertension.32) The beneficial cardiac effects of eplerenone in this model may be associated with an inhibition of aldosterone-independent MR activation (Fig. 3).11,32)
Fig. 3.
A hypothesis for MR activation and its blockade in the setting of reduced RAAS. Selective MR blockade with eplerenone attenuates cardiac remodeling and failure as well as coronary vascular inflammation, independent of its antihypertensive effect, in rats with salt-sensitive hypertension. The expression of 11β-HSD1 gene is upregulated, 11β-HSD2 and CYP11B1 genes are expressed at only minimal levels, and the expression of CYP11B2 gene is not detected in the hearts of such rats. Corticosterone, an endogenous glucocorticoid in rodents, manifests the same affinity for the MR as does aldosterone. Glucocorticoid-mediated MR activation may thus contribute to cardiac and coronary vascular injury in this model of low-renin, low-aldosterone hypertension. Increased oxidative stress appears to be a driver for activation of the glucocorticoid-MR complex in the cardiovascular system of these animals.
Abbreviations: MR, mineralocorticoid receptor; RAAS, renin-angiotensin-aldosterone system; 11β-HSD1 and 2, 11β-hydroxysteroid dehydrogenase types 1 and 2, respectively; GRE, glucocorticoid response element.
Calcium channel blockers
Calcium channel blockers (CCBs) have been used in the treatment of hypertension or heart failure due to their vasodilating effects, resulting in a reduction of myocardial oxygen demand. However, recent clinical studies have shown that the long-term administration of short-acting CCBs may increase the mortality of patients with heart failure.33) Although amlodipine, as a long-acting, dihydropyridine-based L-type CCB, was expected to produce more beneficial results, the Prospective Randomized AmlodIpine Survival Evaluation II (PRAISE II) trial failed to demonstrate its benefit in patients with systolic heart failure of a non-ischemic origin. However, long-term administration of amlodipine prevented the transition to diastolic heart failure at both depressor and subdepressor doses in Dahl salt-sensitive hypertensive rats.34) Amlodipine did not decrease the collagen content, but attenuated myocardial stiffness by inhibiting the phenotype shift from type III to type I collagen. Thus, amlodipine may exert beneficial effects via the amelioration of collagen remodeling in the treatment of diastolic heart failure. These observations are consistent with our recent results showing that nifedipine is effective in preventing cardiac remodeling and diastolic heart failure in this model of hypertension.35)
Accumulating evidence has suggested that an upregulation of T-type Ca2+ channels has been associated with both LVH and hypertensive diastolic heart failure.36) We compared the effects of benidipine, a blocker of T-type and L-type Ca2+ channels, with those of nitrendipine, an L-type CCB, in Dahl salt-sensitive hypertensive rats. Our results showed that benidipine reduced LV diastolic stiffness and mortality to a greater extent than did nitrendipine, effects likely attributable predominantly to the promotion of coronary angiogenesis rather than to an attenuation of interstitial fibrosis.37) Benidipine may thus be more effective than a purely L-type CCB in preventing hypertensive diastolic heart failure.
We also examined the pharmacological mechanisms underlying the cardioprotection afforded by a combination of the AT1 receptor blocker olmesartan and the CCB azelnidipine, independently of their antihypertensive effects, in Dahl salt-sensitive hypertensive rats.38) The observed cardioprotective effects of the above combination are likely attributable, at least in part, to its prevention of changes in the expression of collagen isoforms and to a decrease in the ratio of elastin to collagen in the failing myocardium as a result of the inhibition of NADPH oxidase-dependent superoxide generation and inflammation.
Thiazide diuretics
Thiazide diuretics such as hydrochlorothiazide (HCTZ) have also been used for the treatment of hypertension by affecting the renal tubular mechanisms of electrolyte reabsorption, directly increasing the excretion of Na+ and Cl–. However, the mechanism underlying the long-term antihypertensive effect of HCTZ has yet to be fully understood. Given that thiazide diuretics reduce morbidity and mortality in hypertensive patients,39) the Seventh Report of the Joint National Committee recommends these agents for use as first-line drugs in the treatment of hypertension.40) The Japanese Society of Hypertension Guidelines for the Management of Hypertension has also recommended diuretics in low doses as first-line drugs for the treatment of hypertension.41) Moreover, it is possible that regimens including putatively safer, non-loop diuretics such as thiazide drugs might prove effective for the maintenance of euvolemia in salt-sensitive and salt-avid individuals.
To elucidate the mechanism underlying the therapeutic effects of combination therapy with losartan and HCTZ, we investigated the antihypertensive and cardioprotective effects of combination therapy with these two drugs, in comparison with those of either drug alone, in Dahl salt-sensitive hypertensive rats.42) The combination of losartan and HCTZ has prevented cardiac hypertrophy and fibrosis as well as markedly reduced blood pressure in these rats. The HCTZ -induced upregulation of angiotensin II type 2 receptor gene expression in the aorta may have contributed to the lowering of blood pressure through the activation of the bradykinin-nitric oxide (NO) pathway. Further attenuation of cardiac remodeling induced by the combination of losartan and HCTZ compared with that elicited by HCTZ monotherapy was likely attributable to the inhibitory effect of losartan on the cardiac RAS. Our results also suggested that combination therapy with an AT1 receptor blocker and a thiazide diuretic is an effective strategy for the management of salt-sensitive hypertension.
β-Adrenergic receptor blockers
β-Adrenergic receptor blockers (β-blockers) are widely used as effective antihypertensive agents, and it has previously been shown that celiprolol, a specific β1-blocker with weak β2-agonistic action, exerts a cardioprotective effect associated with endothelial nitric oxide synthase (eNOS) production in hypertensive rats.43) In addition, the chronic administration of subdepressor doses of betaxolol to Dahl salt-sensitive hypertensive rats has ameliorated perivascular fibrosis and coronary microvascular hyperplasia. The cardioprotective effects of betaxolol have been associated with the stimulation of eNOS production associated with vascular endothelial growth factor and lectin-like oxidized low-density lipoprotein receptor-1, and with the inhibition of adhesion molecules and a signal transduction in the heart.
Bisoprolol exhibits a high selectivity for β1-adrenergic receptors and was found in the Cardiac Insufficiency Bisoprolol Study (CIBIS) II trial to greatly reduce mortality in patients with ischemic or nonischemic heart failure.44) Moreover, the efficacy of this agent was shown to be similar to that of ACE inhibitors for initiating the treatment of chronic heart failure in the CIBIS III trial.45) A recent experimental study has shown that treatment with bisoprolol prevented LV enlargement and LV systolic dysfunction in an animal with dilated cardiomyopathy.46) Bisoprolol also significantly inhibited increases in the levels of oxidative stress and pro-inflammatory cytokines in the myocardium that accompany the development of dilated cardiomyopathy in TO-2 hamsters.
Nicorandil
Nicorandil is a nicotinamide ester with two distinct mechanisms of pharmacological action: It induces the opening of ATP-sensitive potassium (KATP) channels, thereby dilating peripheral and coronary resistance arterioles, and it dilates systemic veins and epicardial coronary arteries through the effects of its nitrate moiety. Nicorandil thus increases coronary blood flow, reduces both preload and afterload, and exerts an anti-anginal effect. This drug also exhibits potentially cardioprotective effects, some of which are likely due to its ability to mimic ischemic preconditioning by opening KATP channels.47)
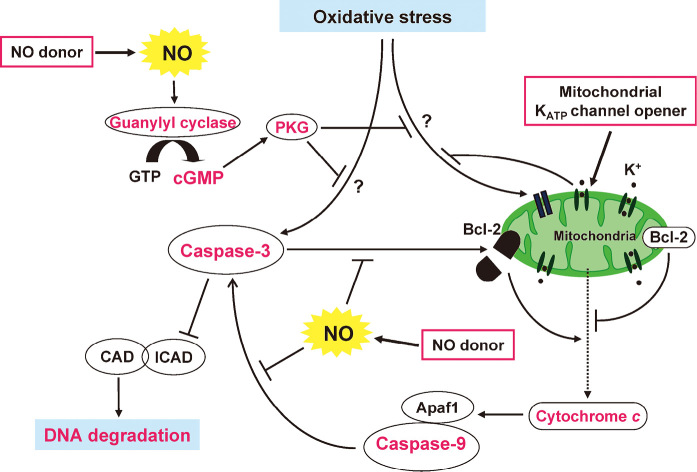
On the other hand, experimental studies have shown that NO protects the heart against ischemia–reperfusion injury, and that NO donors also mimic the effects of preconditioning.48) We have shown that nicorandil inhibits oxidative stress-induced apoptosis by acting as an NO donor as well as by activating mitochondrial KATP channels in rat cardiomyocytes.49) Furthermore, our results have suggested that NO released from nicorandil interrupts apoptotic signaling by both a cGMP-dependent mechanism and probably by the direct inhibition of caspase-3-like activity (Fig. 4).
Fig. 4.
Mechanisms for the nicorandil-induced inhibition of oxidative stress-induced apoptosis in cardiac myocytes. Nicorandil inhibits oxidative stress-induced apoptosis by acting as an NO donor as well as by activating mitochondrial KATP channels in rat cardiomyocytes. NO released from nicorandil interrupts apoptotic signaling both through a cGMP-dependent mechanism and probably by a direct inhibition of caspase-3-like activity. Activation of mitochondrial KATP channels inhibits early apoptotic events, including the loss of mitochondrial membrane potential and cytochrome c release, as well as the subsequent fragmentation of DNA.
Abbreviations: NO, nitric oxide; KATP, ATP-sensitive potassium; CAD, caspase-activated DNase; ICAD, Inhibitor of CAD; PKG; protein kinase G; APaf1, apoptotic protease activating factor 1.
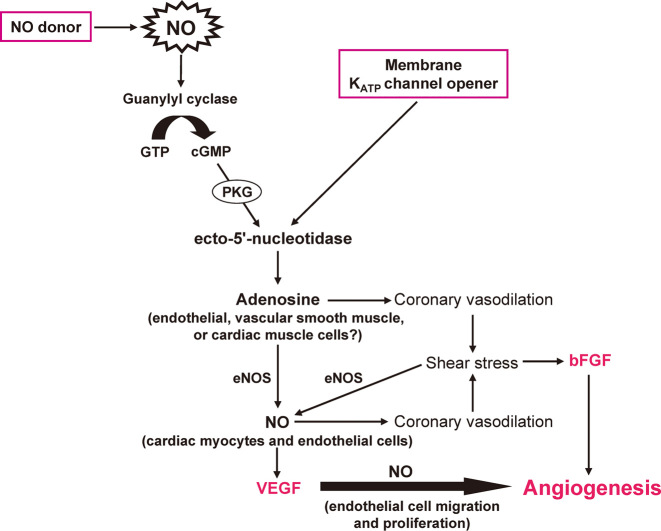
The Impact of Nicorandil in Angina (IONA) study, which included subjects with LV dysfunction (ejection fraction of <45%), demonstrated a significant improvement in clinical outcome in patients with stable angina treated with nicorandil, suggesting the possible efficacy of this drug in the treatment of heart failure.50) Long-term administration of vasodilators increases shear stress, which is thought to be important for vascular growth in the heart. We therefore investigated the effects of nicorandil on vascular growth and gene expression in the failing heart of Dahl salt-sensitive hypertensive rats.51) A non-antihypertensive dose of nicorandil increased the expression of both vascular endothelial growth factor and basic fibroblast growth factor genes in the myocardium, as well as promoted coronary capillary and arteriolar growth, and attenuated the development of heart failure in those rats (Fig. 5). Our results suggested that the chronic administration of nicorandil may prove effective for the treatment of hypertensive heart failure as well as for that of ischemic heart disease.
Fig. 5.
Proposed molecular mechanisms underlying proangiogenic effect of nicorandil. Nicorandil, an activator of KATP channels with a nitrate-like action, may increase the production of interstitial adenosine through a NO- or KATP channel-mediated activation of ecto-5’-nucleotidase, leading to the development of coronary vasodilation. The nicorandil-induced dilation of coronary resistance arterioles increases coronary blood flow and shear stress, the latter of which seems to activate VEGF and bFGF gene expressions and the angiogenic cascade. Such an effect may contribute to the beneficial action of this drug in treating heart failure.
Abbreviations: NO, nitric oxide; PKG, protein kinase G; eNOS, endothelial nitric oxide synthase; KATP, ATP-sensitive potassium; VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor.
Thiazolidinediones
Thiazolidinediones are oral agents for the treatment of type 2 diabetes. These drugs increase the secretion of adiponectin by inducing the activation of the peroxisome proliferator-activated receptor γ in adipocytes.52) Thiazolidinediones also activate the AMP-activated protein kinase (AMPK) in muscle cells,53) although the extent to which their action mediates the clinical effects of these drugs remains unclear. AMPK is an important mediator of adiponectin signaling, and not only improves myocardial glucose and lipid metabolism but also prevents ventricular contractile dysfunction in the ischemic heart.54) Abnormalities of glucose and lipid metabolism in cardiac muscle are also associated with heart failure,55) suggesting that the adiponectin-AMPK axis negatively regulates changes in metabolism that are linked to the progression of cardiac remodeling. We therefore investigated the effects of pioglitazone on cardiac hypertrophy and hypertrophic signaling in Dahl salt-sensitive hypertensive rats.56) Long-term administration of pioglitazone attenuated LV hypertrophy and fibrosis as well as inhibited phosphorylation of mammalian target of rapamycin and p70S6 kinase in the heart of those rats. The beneficial cardiac effects of pioglitazone are likely attributable, at least partly, both to the activation of AMPK signaling through the stimulation of adiponectin secretion and to the inhibition of Akt signaling.
Statins
3-Hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, or statins, have become the most widely administered agents used to reduce serum lipid concentrations and the associated risk of cardiovascular disease. Evidence also suggests that statin therapy prevents cardiac myocyte hypertrophy57) as well as heart failure following experimental myocardial infarction.58) In our recent study, though treatment with pravastatin did not prevent the development of compensated hypertensive LV hypertrophy in Dahl salt-sensitive rats, it did ameliorate LV relaxation abnormality and systolic dysfunction, and blocked the transition to heart failure.59) The beneficial effects of pravastatin were associated with an inhibition of increases both in the activities of matrix metalloproteinases (MMPs) and in the production of superoxide induced in the myocardium of the rats.
Pitavastatin is a synthetic HMG-CoA reductase inhibitor that manifests a cholesterol-lowering effect 10 times greater than that of either pravastatin or simvastatin.60) We have recently demonstrated that pitavastatin ameliorated cardiac remodeling and dysfunction, prevented heart failure, and prolonged survival when administered either before or after the development of LVH in Dahl salt-sensitive hypertensive rats, the effects of which were independent of changes in blood pressure or serum lipid levels.61) Furthermore, the inhibition of the transition from LVH to heart failure by pitavastatin treatment was accompanied by an inhibition of changes in the cardiac endothelin-1 system and MMP activity. Pitavastatin also blocked the translocation of RhoA to the membrane fraction and RhoA activation, as well as the phosphorylation of the mitogen-activated protein kinases extracellular signal-regulated kinase (ERK)-1 and ERK-2, along with an increase in the DNA binding activity of a serum response factor (SRF) in the heart.62) These findings suggested that the effects of pitavastatin on load-induced cardiac hypertrophy and fibrosis are independent of its cholesterol-lowering action and may be mediated, at least in part, through the inhibition of RhoA–ERK–SRF signaling.
Fenofibrates
Fenofibrate, a ligand and activator of peroxisome proliferator-activated receptor-α, is used clinically for the treatment of dyslipidemia. Treatment with fenofibrate also reduced the extent of myocardial inflammation and collagen deposition in rats infused with angiotensin II63) as well as ameliorated both myocardial fibrosis and diastolic dysfunction in rats with mineralocorticoid-dependent hypertension.64) These observations suggest that fenofibrate might exert direct actions on the heart that are independent of its lipid-lowering activity. We have recently shown that treatment with fenofibrate markedly attenuated cardiac hypertrophy, ameliorated both diastolic and systolic dysfunction, and improved the survival rate in Dahl salt-sensitive rats. Fenofibrate also significantly improved the redox status and inhibited the increases in the DNA binding activities of the redox-regulated transcription factors nuclear factor-κB, activator protein-1, early growth response gene-1, surfactant protein 1, and E26 transformation specific-1 in the myocardium as well as the cardiac inflammatory response.65)
CONCLUSIONS
In this review, we discussed the cardioprotective mechanisms of lifestyle modifications and pharmacologic interventions on cardiac remodeling and dysfunction in hypertensive heart disease. Lifestyle modifications such as dietary salt restriction can prevent hypertension and reduce BP and LV mass in hypertensive patients via mechanisms that remain uncertain. Many antihypertensive drugs, including inhibitors of RAAS, exert cardioprotective effects beyond lowering BP in clinical and experimental settings. Hypertension is often complicated by diabetes, dyslipidemia, and ischemic heart disease. Some of the drugs used for the treatment of such complications also possesses cardioprotective effects in addition to their original effects. We should strive to better understand these pleiotropic effects to discover the most effective treatments of complicated hypertension.
ACKNOWLEDGMENTS
We wish to thank Ms. Miwa Takatsu for preparing the manuscript.
REFERENCES
- 1).Bonow RO, Udelson JE. Left ventricular diastolic dysfunction as a cause of congestive heart failure. Mechanisms and management. Ann Intern Med, 1992; 117: 502–510. [DOI] [PubMed]
- 2).Strazzullo P, Galletti F, Barba G. Altered renal handling of sodium in human hypertension: short review of the evidence. Hypertension, 2003; 41: 1000–1005. [DOI] [PubMed]
- 3).Orlov SN, Mongin AA. Salt-sensing mechanisms in blood pressure regulation and hypertension. Am J Physiol Heart Circ Physiol, 2007; 293: H2039–2053. [DOI] [PubMed]
- 4).Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension, 2001; 37: 429–432. [DOI] [PubMed]
- 5).Ferrara LA, de Simone G, Pasanisi F, Mancini M, Mancini M. Left ventricular mass reduction during salt depletion in arterial hypertension. Hypertension, 1984; 6: 755–759. [DOI] [PubMed]
- 6).McCurley JM, Hanlon SU, Wei SK, Wedam EF, Michalski M, Haigney MC. Furosemide and the progression of left ventricular dysfunction in experimental heart failure. J Am Coll Cardiol, 2004; 44: 1301–1307. [DOI] [PubMed]
- 7).Jula AM, Karanko HM. Effects on left ventricular hypertrophy of long-term nonpharmacological treatment with sodium restriction in mild-to-moderate essential hypertension. Circulation, 1994; 89: 1023–1031. [DOI] [PubMed]
- 8).Liebson PR, Grandits GA, Dianzumba S, Prineas RJ, Grimm RH, Jr., Neaton JD, Stamler J. Comparison of five antihypertensive monotherapies and placebo for change in left ventricular mass in patients receiving nutritional-hygienic therapy in the Treatment of Mild Hypertension Study (TOMHS). Circulation, 1995; 91: 698–706. [DOI] [PubMed]
- 9).Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ, 2007; 334: 885–888. [DOI] [PMC free article] [PubMed]
- 10).Sanders PW. Dietary salt intake, salt sensitivity, and cardiovascular health. Hypertension, 2009; 53: 442–445. [DOI] [PMC free article] [PubMed]
- 11).Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, Miyoshi J, Takai Y, Fujita T. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med, 2008; 14: 1370–1376. [DOI] [PubMed]
- 12).Ogihara T, Asano T, Ando K, Sakoda H, Anai M, Shojima N, Ono H, Onishi Y, Fujishiro M, Abe M, Fukushima Y, Kikuchi M, Fujita T. High-salt diet enhances insulin signaling and induces insulin resistance in Dahl salt-sensitive rats. Hypertension, 2002; 40: 83–89. [DOI] [PubMed]
- 13).Falkner B, Hulman S, Kushner H. Hyperinsulinemia and blood pressure sensitivity to sodium in young blacks. J Am Soc Nephrol, 1992; 3: 940–946. [DOI] [PubMed]
- 14).Kingwell BA, Jennings GL. Effects of walking and other exercise programs upon blood pressure in normal subjects. Med J Aust, 1993; 158: 234–238. [DOI] [PubMed]
- 15).Fagard RH. Exercise is good for your blood pressure: effects of endurance training and resistance training. Clin Exp Pharmacol Physiol, 2006; 33: 853–856. [DOI] [PubMed]
- 16).Lee IM, Hsieh CC, Paffenbarger RS, Jr. Exercise intensity and longevity in men. The Harvard Alumni Health Study. JAMA, 1995; 273: 1179–1184. [PubMed]
- 17).Sesso HD, Paffenbarger RS, Jr., Lee IM. Physical activity and coronary heart disease in men: The Harvard Alumni Health Study. Circulation, 2000; 102: 975–980. [DOI] [PubMed]
- 18).Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation, 2003; 107: 1210–1225. [DOI] [PubMed]
- 19).Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation, 2007; 115: 3086–3094. [DOI] [PubMed]
- 20).O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA, 2009; 301: 1439–1450. [DOI] [PMC free article] [PubMed]
- 21).Chicco AJ, McCune SA, Emter CA, Sparagna GC, Rees ML, Bolden DA, Marshall KD, Murphy RC, Moore RL. Low-intensity exercise training delays heart failure and improves survival in female hypertensive heart failure rats. Hypertension, 2008; 51: 1096–1102. [DOI] [PubMed]
- 22).Miyachi M, Yazawa H, Furukawa M, Tsuboi K, Ohtake M, Nishizawa T, Hashimoto K, Yokoi T, Kojima T, Murate T, Yokota M, Murohara T, Koike Y, Nagata K. Exercise training alters left ventricular geometry and attenuates heart failure in dahl salt-sensitive hypertensive rats. Hypertension, 2009; 53: 701–707. [DOI] [PubMed]
- 23).Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet, 2002; 359: 995–1003. [DOI] [PubMed]
- 24).Baker KM, Chernin MI, Wixson SK, Aceto JF. Renin-angiotensin system involvement in pressure-overload cardiac hypertrophy in rats. Am J Physiol, 1990; 259: H324–332. [DOI] [PubMed]
- 25).Kojima M, Shiojima I, Yamazaki T, Komuro I, Zou Z, Wang Y, Mizuno T, Ueki K, Tobe K, Kadowaki T, Nagai R, Yazaki Y. Angiotensin II receptor antagonist TCV-116 induces regression of hypertensive left ventricular hypertrophy in vivo and inhibits the intracellular signaling pathway of stretch-mediated cardiomyocyte hypertrophy in vitro. Circulation, 1994; 89: 2204–2211. [DOI] [PubMed]
- 26).Cheng XW, Murohara T, Kuzuya M, Izawa H, Sasaki T, Obata K, Nagata K, Nishizawa T, Kobayashi M, Yamada T, Kim W, Sato K, Shi GP, Okumura K, Yokota M. Superoxide-dependent cathepsin activation is associated with hypertensive myocardial remodeling and represents a target for angiotensin II type 1 receptor blocker treatment. Am J Pathol, 2008; 173: 358–369. [DOI] [PMC free article] [PubMed]
- 27).Nagata K, Somura F, Obata K, Odashima M, Izawa H, Ichihara S, Nagasaka T, Iwase M, Yamada Y, Nakashima N, Yokota M. AT1 receptor blockade reduces cardiac calcineurin activity in hypertensive rats. Hypertension, 2002; 40: 168–174. [DOI] [PubMed]
- 28).Kuster GM, Kotlyar E, Rude MK, Siwik DA, Liao R, Colucci WS, Sam F. Mineralocorticoid receptor inhibition ameliorates the transition to myocardial failure and decreases oxidative stress and inflammation in mice with chronic pressure overload. Circulation, 2005; 111: 420–427. [DOI] [PubMed]
- 29).Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med, 1999; 341: 709–717. [DOI] [PubMed]
- 30).Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med, 2003; 348: 1309–1321. [DOI] [PubMed]
- 31).Izawa H, Murohara T, Nagata K, Isobe S, Asano H, Amano T, Ichihara S, Kato T, Ohshima S, Murase Y, Iino S, Obata K, Noda A, Okumura K, Yokota M. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: a pilot study. Circulation, 2005; 112: 2940–2945. [DOI] [PubMed]
- 32).Nagata K, Obata K, Xu J, Ichihara S, Noda A, Kimata H, Kato T, Izawa H, Murohara T, Yokota M. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and failure in low-aldosterone hypertensive rats. Hypertension, 2006; 47: 656–664. [DOI] [PubMed]
- 33).Furberg CD, Psaty BM, Meyer JV. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circulation, 1995; 92: 1326–1331. [DOI] [PubMed]
- 34).Nishikawa N, Masuyama T, Yamamoto K, Sakata Y, Mano T, Miwa T, Sugawara M, Hori M. Long-term administration of amlodipine prevents decompensation to diastolic heart failure in hypertensive rats. J Am Coll Cardiol, 2001; 38: 1539–1545. [DOI] [PubMed]
- 35).Yamada T, Nagata K, Cheng XW, Obata K, Saka M, Miyachi M, Naruse K, Nishizawa T, Noda A, Izawa H, Kuzuya M, Okumura K, Murohara T, Yokota M. Long-term administration of nifedipine attenuates cardiac remodeling and diastolic heart failure in hypertensive rats. Eur J Pharmacol, 2009; 615: 163–170. [DOI] [PubMed]
- 36).Izumi T, Kihara Y, Sarai N, Yoneda T, Iwanaga Y, Inagaki K, Onozawa Y, Takenaka H, Kita T, Noma A. Reinduction of T-type calcium channels by endothelin-1 in failing hearts in vivo and in adult rat ventricular myocytes in vitro. Circulation, 2003; 108: 2530–2535. [DOI] [PubMed]
- 37).Nishizawa T, Cheng XW, Jin Z, Obata K, Nagata K, Hirashiki A, Sasaki T, Noda A, Takeshita K, Izawa H, Shi GP, Kuzuya M, Okumura K, Murohara T. Ca(2+) channel blocker benidipine promotes coronary angiogenesis and reduces both left-ventricular diastolic stiffness and mortality in hypertensive rats. J Hypertens, 2010; 28: 1515–1526. [DOI] [PMC free article] [PubMed]
- 38).Cheng XW, Okumura K, Kuzuya M, Jin Z, Nagata K, Obata K, Inoue A, Hirashiki A, Takeshita K, Unno K, Harada K, Shi GP, Yokota M, Murohara T. Mechanism of diastolic stiffening of the failing myocardium and its prevention by angiotensin receptor and calcium channel blockers. J Cardiovasc Pharmacol, 2009; 54: 47–56. [DOI] [PMC free article] [PubMed]
- 39).ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA, 2002; 288: 2981–2997. [DOI] [PubMed]
- 40).Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA, 2003; 289: 2560–2572. [DOI] [PubMed]
- 41).Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ito S, Iwao H, Kario K, Kawano Y, Kim-Mitsuyama S, Kimura G, Matsubara H, Matsuura H, Naruse M, Saito I, Shimada K, Shimamoto K, Suzuki H, Takishita S, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Ueshima H, Umemura S, Ishimitsu T, Rakugi H. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009). Hypertens Res, 2009; 32: 3–107.
- 42).Yamada Y, Tsuboi K, Hattori T, Murase T, Ohtake M, Furukawa M, Ueyama J, Nishiyama A, Murohara T, Nagata K. Mechanism underlying the efficacy of combination therapy with losartan and hydrochlorothiazide in rats with salt-sensitive hypertension. Yamada Y, Hypertens Res, 2011; 34: 809–816. [DOI] [PubMed]
- 43).Kobayashi N, Mori Y, Nakano S, Tsubokou Y, Shirataki H, Matsuoka H. Celiprolol stimulates endothelial nitric oxide synthase expression and improves myocardial remodeling in deoxycorticosterone acetate-salt hypertensive rats. J Hypertens, 2001; 19: 795–801. [DOI] [PubMed]
- 44).CIBIS II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet, 1999; 353: 9–13. [PubMed]
- 45).The CIBIS III Investigators. Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence: results of the randomized Cardiac Insufficiency Bisoprolol Study (CIBIS) III. Circulation, 2005; 112: 2426–2435. [DOI] [PubMed]
- 46).Ichihara S, Yamada Y, Ichihara G, Kanazawa H, Hashimoto K, Kato Y, Matsushita A, Oikawa S, Yokota M, Iwase M. Attenuation of oxidative stress and cardiac dysfunction by bisoprolol in an animal model of dilated cardiomyopathy. Biochem Biophys Res Commun, 2006; 350: 105–113. [DOI] [PubMed]
- 47).Patel DJ, Purcell HJ, Fox KM. Cardioprotection by opening of the K(ATP) channel in unstable angina. Is this a clinical manifestation of myocardial preconditioning? Results of a randomized study with nicorandil. CESAR 2 investigation. Clinical European studies in angina and revascularization. Eur Heart J, 1999; 20: 51–57. [DOI] [PubMed]
- 48).Rakhit RD, Edwards RJ, Mockridge JW, Baydoun AR, Wyatt AW, Mann GE, Marber MS. Nitric oxide-induced cardioprotection in cultured rat ventricular myocytes. Am J Physiol Heart Circ Physiol, 2000; 278: H1211–1217. [DOI] [PubMed]
- 49).Nagata K, Obata K, Odashima M, Yamada A, Somura F, Nishizawa T, Ichihara S, Izawa H, Iwase M, Hayakawa A, Murohara T, Yokota M. Nicorandil inhibits oxidative stress-induced apoptosis in cardiac myocytes through activation of mitochondrial ATP-sensitive potassium channels and a nitrate-like effect. J Mol Cell Cardiol, 2003; 35: 1505–1512. [DOI] [PubMed]
- 50).The IONA Study Group. Effect of nicorandil on coronary events in patients with stable angina: the Impact Of Nicorandil in Angina (IONA) randomised trial. Lancet, 2002; 359: 1269–1275. [DOI] [PubMed]
- 51).Xu J, Nagata K, Obata K, Ichihara S, Izawa H, Noda A, Nagasaka T, Iwase M, Naoe T, Murohara T, Yokota M. Nicorandil promotes myocardial capillary and arteriolar growth in the failing heart of Dahl salt-sensitive hypertensive rats. Hypertension, 2005; 46: 719–724. [DOI] [PubMed]
- 52).Tiikkainen M, Hakkinen AM, Korsheninnikova E, Nyman T, Makimattila S, Yki-Jarvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes, 2004; 53: 2169–2176. [DOI] [PubMed]
- 53).LeBrasseur NK, Kelly M, Tsao TS, Farmer SR, Saha AK, Ruderman NB, Tomas E. Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am J Physiol Endocrinol Metab, 2006; 291: E175–181. [DOI] [PubMed]
- 54).Russell RR, 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest, 2004; 114: 495–503. [DOI] [PMC free article] [PubMed]
- 55).Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, Gross RW, Kelly DP. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A, 2003; 100: 1226–1231. [DOI] [PMC free article] [PubMed]
- 56).Kato MF, Shibata R, Obata K, Miyachi M, Yazawa H, Tsuboi K, Yamada T, Nishizawa T, Noda A, Cheng XW, Murate T, Koike Y, Murohara T, Yokota M, Nagata K. Pioglitazone attenuates cardiac hypertrophy in rats with salt-sensitive hypertension: role of activation of AMP-activated protein kinase and inhibition of Akt. J Hypertens, 2008; 26: 1669–1676. [DOI] [PubMed]
- 57).Takemoto M, Node K, Nakagami H, Liao Y, Grimm M, Takemoto Y, Kitakaze M, Liao JK. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J Clin Invest, 2001; 108: 1429–1437. [DOI] [PMC free article] [PubMed]
- 58).Bauersachs J, Galuppo P, Fraccarollo D, Christ M, Ertl G. Improvement of left ventricular remodeling and function by hydroxymethylglutaryl coenzyme a reductase inhibition with cerivastatin in rats with heart failure after myocardial infarction. Circulation, 2001; 104: 982–985. [DOI] [PubMed]
- 59).Ichihara S, Noda A, Nagata K, Obata K, Xu J, Ichihara G, Oikawa S, Kawanishi S, Yamada Y, Yokota M. Pravastatin increases survival and suppresses an increase in myocardial matrix metalloproteinase activity in a rat model of heart failure. Cardiovasc Res, 2006; 69: 726–735. [DOI] [PubMed]
- 60).Suzuki H, Aoki T, Tamaki T, Sato F, Kitahara M, Saito Y. Hypolipidemic effect of NK-104, a potent HMG-CoA reductase inhibitor, in guinea pigs. Atherosclerosis, 1999; 146: 259–270. [DOI] [PubMed]
- 61).Saka M, Obata K, Ichihara S, Cheng XW, Kimata H, Nishizawa T, Noda A, Izawa H, Nagata K, Murohara T, Yokota M. Pitavastatin improves cardiac function and survival in association with suppression of the myocardial endothelin system in a rat model of hypertensive heart failure. J Cardiovasc Pharmacol, 2006; 47: 770–779. [DOI] [PubMed]
- 62).Saka M, Obata K, Ichihara S, Cheng XW, Kimata H, Noda A, Izawa H, Nagata K, Yokota M. Attenuation of ventricular hypertrophy and fibrosis in rats by pitavastatin: potential role of the RhoA-extracellular signal-regulated kinase-serum response factor signalling pathway. Clin Exp Pharmacol Physiol, 2006; 33: 1164–1171. [DOI] [PubMed]
- 63).Diep QN, Benkirane K, Amiri F, Cohn JS, Endemann D, Schiffrin EL. PPAR alpha activator fenofibrate inhibits myocardial inflammation and fibrosis in angiotensin II-infused rats. J Mol Cell Cardiol, 2004; 36: 295–304. [DOI] [PubMed]
- 64).Ogata T, Miyauchi T, Sakai S, Takanashi M, Irukayama-Tomobe Y, Yamaguchi I. Myocardial fibrosis and diastolic dysfunction in deoxycorticosterone acetate-salt hypertensive rats is ameliorated by the peroxisome proliferator-activated receptor-alpha activator fenofibrate, partly by suppressing inflammatory responses associated with the nuclear factor-kappa-B pathway. J Am Coll Cardiol, 2004; 43: 1481–1488. [DOI] [PubMed]
- 65).Ichihara S, Obata K, Yamada Y, Nagata K, Noda A, Ichihara G, Yamada A, Kato T, Izawa H, Murohara T, Yokota M. Attenuation of cardiac dysfunction by a PPAR-alpha agonist is associated with down-regulation of redox-regulated transcription factors. J Mol Cell Cardiol, 2006; 41: 318–329. [DOI] [PubMed]