ABSTRACT
This study was undertaken to evaluate the pattern of antibiotic prescriptions in a secondary health care setting in Kyrgyzstan. A retrospective analysis was performed of antibiotic prescriptions in 251 inpatient records of patients admitted to the Sokuluk Territorial Hospital. A total of 19 different antibiotics were prescribed. Penicillin G (24.9%), gentamicin (16.1%), metronidazole (15.6%) and cefazolin (14.5%) were those most frequently prescribed. The major indications for antibiotics were diseases of the respiratory system (28.0%), injury, poisoning and certain other consequences from external causes (25.5%), and diseases of the digestive system (14.3%). Almost three-quarters of the antibiotics were used parenterally, 252 of which (58.9%) were administered intramuscularly and 70 (16.4%) intravenously. Forty-five percent of the patients received two antibiotics, and 12.0% received three antibiotics during their stay at the hospital. Antibiotic therapy proved inappropriate for 184 patients (73.3%). The most common reason given for inappropriateness was the unjustified (not indicated) use of antibiotics in 143 (48.6%) cases. There was a significantly higher inappropriate choice of antibiotics in gynecology (OR=2.70, 95% CI=1.02–7.69) when compared with that in other wards. Although antibiotics were prescribed in all cases post-operatively, none of those patients were given pre-operative prophylactic antibiotics when indicated. We concluded that antibiotic prescriptions were seriously inappropriate in the Kyrgyz Republic with prescribing patterns failing to strictly adhere to the national guidelines. Adoption of an international standard and locally conformable guidelines of antibiotic use can help correct such problems.
Key Words: Hospital, Antibiotic use, Prescribing practice, Quality
INTRODUCTION
The worldwide emergence of antimicrobial resistance is a major public health problem that significantly impacts patient treatment and outcomes. The relationship between antimicrobial use and antimicrobial resistance is complex, with a growing body of data strongly suggesting that higher levels of antimicrobial usage are associated with increased levels of antimicrobial resistance.1,2)
Patients in hospitals nowadays are older, more severely ill, and more immunocompromised than was the case two or three decades ago, and are predisposed to contracting bacterial infections requiring frequent antimicrobial therapy.3) With the increase in antimicrobial prescriptions, prescribing errors have also become more common. These include treatments of colonization, suboptimal empiric therapy, inappropriate combination therapy, dosing, as well as duration errors and mismanagement of apparent antibiotic failures. Studies have shown an inappropriate prescribing of antimicrobials for prophylaxis as well as treatment.4-7) Inadequate consideration of the potential antimicrobial resistance, tissue penetration, drug interactions, side effects, and cost are among the factors which influence the prescription pattern and effectiveness of antimicrobial therapy.8)
In developing countries, antibiotics are prescribed for 44–97% of hospitalized patients often unnecessarily or inappropriately.9-13) Several socio-economic and behavioral factors are thought to contribute to the inappropriate use of antibiotics and, consequently, to the increased incidence of bacterial resistance in developing countries.14) The spread of antibiotic resistance in those countries is associated with complex and interconnected factors, such as excessive and unnecessary prescribing of antibiotics, increased self-prescribing by the people, poor quality of available antibiotics, failure to implement simple infection control practices, and the dearth of routine susceptibility testing and surveillance.15) The lack of funds combines with other factors such as ignorance, inadequate education, inaccessibility to proper health and diagnostic facilities.16) Though the reported factors are complex, an excessive and inappropriate prescribing of antibiotics is at least partially responsible for increased rates of resistance worldwide. In Post-Soviet Central Asian countries, antibiotics are prescribed in 36.6 to 40.0% of cases in outpatient settings.17) An analysis of antibiotics consumption by the population of Kyrgyzstan has demonstrated that 46.8% of them use antibiotics as self-medication, the major share of which consists of out-dated drugs such as chloramphenicol and oletetrin (oleandomycin/tetracycline) as well as gentamicin, which is a popular injectable drug.18) The main reason for self-medication is the sale of antibiotics without a prescription.9)
Given that background, this study was conducted in a secondary care hospital in Kyrgyzstan to assess the pattern of antibiotic prescriptions in terms of the prevalence of a variety of antibiotic uses including frequency, doses, intervals, routes of administration and the appropriateness of the choice of antibiotics.
MATERIALS AND METHODS
Sokuluk Territorial Hospital is a 240-bed public secondary-care institution offering all medical specialties and serving a population of about 144, 000. In total, there were 7695 admissions in 2007, 4976 of which were patients with antibiotics prescribed on their inpatient records. Patients with antibiotic prescriptions who were admitted to hospital units of internal medicine, surgery, traumatology, gynecology, infectious disease, intensive care (ICU) and pediatrics were eligible for inclusion in the study. Patients admitted to neurology and cardiology units were excluded because of their infrequent use of antibiotics. Patients who died during their hospital stay or those who underwent incomplete treatment were also excluded. From the final eligible list of 4432 inpatient records, we randomly selected 251 for retrospective analyses.
Variables
Demographic variables were analyzed, including sex, age, comorbidity, length of stay, ward as well as variables for prescribing antibiotics such as dosage, duration (interval between start and stop dates), and reason for switching or stopping.
Quality evaluation
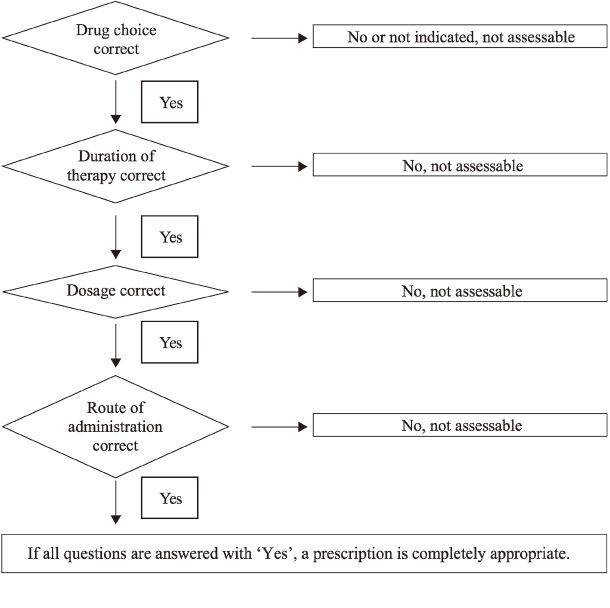
Fig. 1 shows the quality of antibiotic use that was assessed according to the method of Gyssens et al.19) and their original criteria in order to be able to evaluate each parameter of importance associated with antibiotics use. The following classification was used: appropriateness of the prescription, inappropriateness due to improper dosage, intervals and routes of administration; inappropriateness due to duration or to a less effective antibiotic; antibiotic not indicated, and records insufficient for categorization.
Fig. 1.
Assessment criteria for quality-of-use evaluation or antibiotics prescription.
Abstracts for review were compiled using clinical information from existing medical records. Prescriptions were considered therapeutic if (a) the medical record contained information that the antibiotic was prescribed for therapy, or (b) an infectious disease was diagnosed, or (c) clinical signs of infection, e.g., fever, were present on the day that antibiotic therapy was initiated. Antibiotics were classified as prophylactic if (a) the medical record stated that the antibiotic was prescribed for prophylaxis or (b) it was given for only one day relative to the timing of a surgical intervention. In all other cases, prescriptions were denoted as being of unknown indication.
Patient treatment was prescribed by the chief of each ward. Junior doctors working in the wards were required to follow the prescription of the ward chief. In total, 35 doctors working under different ward chiefs were responsible for the overall treatment of those patients.
Three investigators and two specialists from the hospital (an expert physician and a clinical-pharmacologist) and one from the department of basic and clinical pharmacology of the Kyrgyz State Medical Academy independently reviewed each medical record. Assessments of the individual reviewers were summarized in a combined evaluation when at least two of the three reviewers evaluated the prescription as appropriate, not indicated or inappropriate. The antibiotic therapy was reviewed to assure compliance with the recommendations of the national guidelines. The national antibiotic prescription guidelines were drawn up by a local team of physicians and clinical pharmacologists based on international guidelines adapted to the local conditions. However, since there was no guideline on antibiotics used in surgery, we had to use the guideline on antibiotic prophylaxis during surgery of the Smolensk Institute of Antimicrobial Chemotherapy (Russia) for our main criteria.20) The Anatomical Therapeutic Chemical Classification System (ATC) was used for the classification of antibiotics, while the International Classification of Diseases was used for classifying diseases.
Statistical analysis
The Statistical Package for Social Science (SPSS) programs version 17.0 for Windows (SPSS Inc., Chicago, IL, USA) was used to analyze the collected data. Descriptive statistics, such as frequency and percentage were used to present qualitative data. Quantitative data were presented as the mean (± standard deviation). The Odds Ratios (ORs) and 95% confidence intervals (CIs) were used employing a logistic regression model to examine the association between antibiotics and their appropriate use. A P-value of <0.05 was considered statistically significant.
The study was approved by the Bioethics Committee of the Kyrgyz Republic Ministry of Health of (N6/26.03.09).
RESULTS
The demographic characteristics of the respondents are shown in Table 1. About 55.4% of them were female, and over half belonged to the 15–60 year age group. The distribution of patients was almost homogenous in all wards, with gynecology being the highest with 56 (22.3%) admissions.
Table 1.
Demographic and clinical characteristics of patients (n=251)
| Characteristics | Number | Percentage |
|---|---|---|
| Sex | ||
| Male | 112 | 44.6 |
| Female | 139 | 55.4 |
| Age (years) | ||
| 0–14 | 72 | 28.7 |
| 15–60 | 145 | 57.8 |
| Ward | ||
| Internal medicine | 43 | 17.1 |
| Traumatology | 27 | 10.8 |
| ICU | 20 | 8.0 |
| Pediatrics | 30 | 12.0 |
| Gynecology | 56 | 22.3 |
| Surgery | 38 | 15.1 |
| Infectious diseases | 37 | 14.7 |
| Diagnosis upon admissiona | ||
| Respiratory tract infection | 75 | 29.9 |
| Consequences of external causes | 39 | 15.5 |
| Diseases of digestive system | 36 | 14.3 |
| Diseases of genitourinary system | 35 | 13.9 |
| Pregnancy, puerperum and childbirth | 20 | 8.0 |
| Infectious and parasitic diseases | 17 | 6.8 |
| Diseases of circulatory system | 8 | 3.2 |
| Others | 21 | 8.4 |
| Length of stay (days) | Mean±SD | 9.6 ± 4.7 |
| Antimicrobial prescriptions per patient | Mean±SD | 2 ± 0.6 |
| Combination therapy of 2 or 3 antibiotics | 126 | 50.2 |
a International Classification of Diseases – 10
Patients admitted with antibiotics regimens included infections of all organ systems. Most patients were diagnosed with diseases of the respiratory system (n=75, 29.9%), followed by injury, poisoning and other consequences from external causes (n=39, 15.5%), diseases of the digestive (n=36, 14.3%) and genitourinary systems (n=35, 13.9%).
Antibiotic therapy
The frequency of prescriptions of antibiotics regarded as ‘single’ or in ‘group’ is shown in Table 2. The most commonly used groups were penicillins (primarily penicillin G and ampicillin), which accounted for 155 (36.2%) of the total number. Aminoglycosides were ranked second, comprising 86 (20.1%) of prescriptions, and were mostly used in the departments of gynecology and internal medicine. Cephalosporins were ranked third at 77 (18.0%); two antibiotics of this group, i.e., cefazolin at 61 (14.3%) followed by ceftriaxone at 16 (3.7%) were also used.
Table 2.
Most frequently used antibiotics (n=428)
| Antibiotic (group)a | Number | Percentage |
|---|---|---|
| β-lactam antibacterials; penicillins (J01C) | 155 | 36.2 |
| Aminoglycosides (J01G) | 86 | 20.1 |
| Cephalosporins and related substances (J01DA) | 77 | 18.0 |
| Metronidazole (J01XD01) | 66 | 15.4 |
| Tetracyclins (J01A) | 16 | 3.7 |
| Quinolons (J01MA) | 6 | 1.4 |
| Other antibioticsb | 22 | 5.1 |
| Total | 428 | 100.0 |
| Antibiotics (single) | ||
| Penicillin G | 105 | 24.5 |
| Gentamicin | 68 | 15.9 |
| Metronidazole | 66 | 15.4 |
| Cefazolin | 61 | 14.3 |
| Ampicillin | 47 | 11.0 |
| Ceftriaxone | 16 | 3.7 |
| Kanamicin | 15 | 3.5 |
| Doxycycline | 14 | 3.3 |
| Nitrofurantoin | 7 | 1.6 |
| Furazolidone | 7 | 1.6 |
| Ciprofloxacin | 6 | 1.4 |
| Other antibioticsc | 16 | 3.7 |
| Total | 428 | 100.0 |
a Grouping was based on ‘Anatomical Therapeutic Chemical Classification System’
b Other antibiotics included Nitrofurantoin, Furazolidon, Rifampicin, Chloramphenicol, Lincomycin, TMP-SMX
c Amoxicillin, Amoxicillin/clavulanate, Streptomycin, Tetracycline, Rifampicin, Chloramphenicol, Lincomycin, TMP-SMX
A total of 19 different antibiotics were used in all the wards. Only 88 (20.6%) of antibiotics were prescribed in oral form, while most others were prescribed by the parenteral route, 252 (58.9%) of which were administered intramuscularly and 70 (16.4%) intravenously (Table 3). The practice of administering antibiotics via a drainage tube after surgeries was still extant in 17 (4.0%) cases. Overall, 65 patients (38.7%) had their antibiotics switched to another type because of ineffectiveness or adverse reactions, but no bacteriological results were available. No parenteral antibiotics were switched to an oral form.
Table 3.
Route of administration of antibiotics (n=428)
| Route of administration | Number | Percentage |
|---|---|---|
| Oral | 88 | 20.6 |
| Intramuscular | 252 | 58.9 |
| Intravenous | 70 | 16.4 |
| Into drainage tube | 17 | 4.0 |
| Intrabone | 1 | 0.2 |
| Total | 428 | 100.0 |
Forty-five percent of patients received two antibiotics and 12.0% received three at the same time during their hospital stay.
Quality of antibiotic treatment
Overall, 251 medical records containing 428 antibiotic prescriptions were reviewed. Unfortunately, some records failed to provide sufficient information for a proper assessment of the inappropriateness of the antibiotics used, e.g., in microbiological test results or the presence of surgical site infection. Antibiotic therapy was found to be inappropriate in 184 patients (73.3%), and in 21 (8.4%) it was impossible to identify the appropriateness of therapy. The most common reason for inappropriateness was the unjustified (not indicated) use of antibiotics, which was found in 143 (48.6%) cases (Table 4). The second most common reason was the use of ineffective antibiotics against the bacterial infections to be expected 97 (32.9%) infections. No antibiotic prophylaxis was used in surgical, gynecologic or traumatologic wards during an actual procedure. A total of 65 surgeries performed among observed cases included: clean operations – 27 (41.5%), clean-contaminated – 8 (12.3%), contaminated – 6 (12.2%), and dirty – 24 (36.9%). In all the above cases, antibiotics were administered postoperatively until discharge even to patients without signs of infection after clean and clean-contaminated surgeries, or even to dirty types where the choice of antibiotics was incorrect.
Table 4.
Reasons for inappropriateness of antibiotic therapies (n=294)
| Reason for inappropriatenessa | Number of patients | Percentage | p-value | ORb (95% CIc) for inappropriate therapy |
|---|---|---|---|---|
| Unjustified use | 143 | 48.6 | 0.022 | 1.05 (1.01–1.09) |
| Wrong spectrum/Inadequate used | 97 | 32.9 | 0.106 | 0.96 (0.92–1.01) |
| Inappropriate dose | 18 | 6.1 | 0.014 | 1.24 (1.04–1.46) |
| Inappropriate duration | 7 | 2.4 | 0.261 | 0.92 (0.80–1.06) |
| Improper dosage interval | 29 | 9.9 | 0.001 | 1.49 (1.17–1.89) |
a More than one reason may apply to each patient
b OR: odds ratio; reference category is ‘other reasons’
c CI: confidence interval
d If chosen antibiotic was completely different for that spectrum
Among different antibiotic groups, cephalosporins and quinolones were used least inappropriately (OR=0.35; 95% CI=0.21–0.59; OR=0.62, 95% CI=0.11–3.45, respectively), whereas antibiotics such as penicillins, aminiglycosides, and furazolidone were used most inappropriately (Table 5).
Table 5.
Inappropriateness of antimicrobial therapy in different groups of antibiotics
| Antibiotic group | IAPa use n (%) |
APb use n (%) |
p-value | ORc (95% CId) for inappropriate therapy |
|---|---|---|---|---|
| β-lactam antibacterials;penicillins (J01C) | 116 (74.8) | 28 (18.1) | 0.036 | 1.69 (1.03–2.78) |
| Aminoglycosides (J01G) | 67 (77.9) | 12 (14.0) | 0.018 | 2.22 (1.12–4.17) |
| Cephalosporins and related substances (J01DA) | 43 (55.8) | 32 (41.6) | <0.001 | 0.35 (0.21–0.59) |
| Metronidazole (J01XD01) | 38 (57.6) | 17 (25.7) | 0.690 | 0.88 (0.48–1.61) |
| Tetracyclins (J01A) | 10 (62.5) | 4 (25.0) | 0.911 | 0.93 (0.29–2.94) |
| Ciprofloxacin (J01MA02) | 3 (50.0) | 2 (33.3) | 0.586 | 0.62 (0.11–3.45) |
| Nitrofurantoin (J01XE01) | 3 (42.8) | 4 (57.2) | 0.055 | 0.23 (0.05–1.03) |
| Furazolidone (G01AX06) | 7 (100.0) | 0 | 0.99 | (NCf) |
| Other antibioticse | 5 (62.5) | 3 (37.5) | 0.681 | 1.47 (0.18–14.28) |
| Total | 326/68.2 | 102/23.8 | <0.001 | 1.09 (1.04–1.14) |
a IAP: inappropriate
b AP: appropriate
c OR: odds ratio; reference category is ‘all other groups of antibiotics studied’
d CI: confidence interval
e Other antibiotics included: Rifampicin, Chloramphenicol, Lincomycin, TMP-SMX
f NC: not calculable
Antibiotics were used least inappropriately in internal medicine and infection wards (OR=0.59, 95% CI=0.27–1.28; OR=0.21, 95% CI=0.10–0.46). Other wards, such as traumatology, ICU, gynecology and surgery posed a higher risk of inappropriate antibiotic use (ORs ranging from 1.30 to 6.67) (Table 6).
Table 6.
The inappropriateness of antimicrobial therapy by medical specialties (n=251)
| Ward | IAPa use n (%) |
APb use n (%) |
p-value | ORc (95% CId) for inappropriate therapy |
|---|---|---|---|---|
| Internal medicine | 31 (72.0) | 11 (25.6) | 0.180 | 0.59 (0.27–1.28) |
| Traumatology | 24 (88.9) | 1 (3.7) | 0.069 | 6.67 (0.86–50.0) |
| ICUe | 15 (75.0) | 3 (15.0) | 0.689 | 1.30 (0.36–4.76) |
| Pediatrics | 23 (76.6) | 7 (23.3) | 0.452 | 0.70 (0.28–1.75) |
| Gynecology | 36 (64.3) | 5 (8.93) | 0.046 | 2.70 (1.02–7.69) |
| Surgery | 34 (89.5) | 3 (7.89) | 0.084 | 2.94 (0.87–10.0) |
| Infection | 21 (56.8) | 16 (43.2) | <0.001 | 0.21 (0.10–0.46) |
| Total | 184 (73.3) | 46 (18.3) | 0.090 | 0.89 (0.78–1.02) |
a IAP: inappropriate
b AP: appropriate
c OR: odds ratio; reference category is ‘all other wards studied’
d CI: confidence interval
e ICU: intensive care unit
DISCUSSION
The major finding of the present study was the presence of high levels of the inappropriate use of antibiotics. Although the principles of antimicrobial prescription have been well established internationally for many years, their inappropriate use is still rampant, especially in developing countries.21,22)
It has been demonstrated that penicillins, aminoglycosides, and cephalosporins were the most frequently used antibiotics in such hospitals. Penicillins and cephalosporins have continued to be a mainstay of therapy in hospitals because of their broad spectrum of activity, clinical efficacy and favorable tolerability profiles.23-25) However, recent surveys in Europe and the US have found that the most frequently prescribed antibiotics were fluoroquinolones, penicillins, and aminoglycosides.26-28) Physicians in our hospital were willing to prescribe injectable antibiotics. As fluoroquinolones were recomended in tablet forms, physicians did not prescribe them, even though they were inexpensive. Cefazolin has been widely prescribed in hospitals, even in the ICU (a very novel finding of this study). Physicians have prescribed cefazolin for patients with a wide range of diseases, such as those of the respiratory tract and gynecologic and abdominal infections, while it is mostly indicated for antibiotic prophylaxis. Despite these considerations, many physicians still believe that cefazolin is a very “strong” and “broad-spectrum” drug, a conviction which in turn influences their prescribing practices. On the other hand, Kyrgyz hospitals, due to their restricted budgets, prefer to purchase cheap generic antibiotics; thus, penicillin G and cefazolin are prescribed even for severe infections.
Inappropriate intravenous therapy increases the cost of care while also exposing the patient to the risk associated with intravenous catheters.29) “Switch therapy”, i.e., the change from i.v. to oral treatment, has been studied by several investigators over the past few years, and has been shown to save costs, shorten the length of hospital stays, and decrease the adverse reactions of i.v. administration, all with equal therapeutic outcome.30) In our hospital, parenteral administration of antibiotics was more common then oral (79.4% vs 20.5%) and no parenteral drug was switched to oral form. The National guidelines also did not recognize this point. Generally, in our setting, oral antimicrobial agents are promoted for out-patient general practice and parenteral antimicrobial agents for in-patient hospital practice. Factors such as the unavailability of an oral preparation and a patient’s inability to tolerate one may influence the choice of this route. About 65% of such choices may be considered inappropriate in some respects.31) The hospital protocol provides no clear guidelines for the choice of a route which might have been responsible for choosing a parenteral route in the present study.
We found that antibiotic therapy was inappropriate in 73.3% of our patients. Our findings were completely in agreement with the published data indicating that as many as 41% to 91% of all antibiotic prescriptions in hospitals are inappropriate.6) Similar findings were also reported by another study from Brazil where rational antibiotic use was only 45.7%; in another hospital it was a mere 27%;32) and in Indonesia, only 21% of prescriptions were considered to be clearly appropriate.33)
Our quality evaluation confirmed the practice of over-prescription in surgical and gynecology departments and identified major room for improvement in surgical prophylaxis. The high rate of the inappropriate use of antibiotics was found to be due to a lack of antibiotic prophylaxis and the long-term use of antimicrobials in the postoperative period in clean and clean-contaminated surgeries without sign of infection. That differed markedly from the results of other studies. Thus, according to Fonseca, in 78.9% of surgeries, the antibiotic was correctly chosen; in only 15.9% of surgeries was the initial antibiotic administration correctly timed; the use of antibiotics in the post-operative period was appropriate in only 29.8% of cases.32) The Al-Momany study found that 39.4% of patients received antimicrobial prophylaxis for a total duration of 48 hours or less in accordance with the guidelines, while for 58.9%, the duration was longer than recommended.34) Antibiotics were both unjustified and inappropriately administered in 19% of cases in a Turkish hospital.35) Several decades ago it became common surgical practice in the Soviet Union to utilize what have been called by some “preventive” antibiotics, i.e., a post-operative course of antibiotics given for 5–10 days in order to prevent infection.36) It has now been demonstrated that for antibiotics to have a prophylactic effect they must be given within two hours before a surgical incision to be effective.37) As a result, since the 1980s, a dose within two hours before an incision has been the standard of care. Post-Soviet countries just recently began to take steps to resolve this problem, and in Russia an antibiotic policy for surgery was developed in 2003. In Kyrgyzstan, however, there is no such guideline as yet; therefore, physicians continue to use antibiotics as before.
Several limitations of this study need to be mentioned. First, the level of antibiotic use could not be accurately measured due to the absence of accurate medication charts and the poor quality of medication record-keeping in the hospitals. We also encountered difficulties in assessment due to the lack of local data on antibiotic resistance. Furthermore, we were unable investigate the relationship between the adequacy or inadequacy of treatments and clinical outcomes. Finally, we used Gyssen’s method to evaluate our antibiotics. Although Gyssen’s method is the standard for evaluations in prescribing antibiotics, it may have limitations, depending on the location and medical facilities.
In conclusion, our results revealed a significantly high level of the inappropriate use of antibiotics in the Sokuluk Territorial Hospital. Even though surgeons had a tendency to over-prescription and unjustified use of antibiotics, standard antibiotic prophylaxis was still not ensured. The adoption of an international standard and locally conformable guidelines of antibiotic use can help to resolve such problems.
ACKNOWLEDGMENTS
We wish to express our gratitude to the physicians of Sokuluk Territorial Hospital, and to A. Mamasheva for her assistance in collecting data. We are also indebted to Rev. Paul Moore, President of the American nongovernmental organization CitiHope International, for his generous financial support. This study was also supported in part by the “Epidemiological and Clinical Research Information Network (ECRIN),” a non-profit organization
REFERENCES
- 1).Bronzwaer SL, Cars O, Buchholz U, Molstad S, Goettsch W, Veldhuijzen IK, Kool JL, Sprenger MJ, Degener JE. A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg Infect Dis, 2002; 8: 278–282. [DOI] [PMC free article] [PubMed]
- 2).U.S. Congress. Impacts of Antibiotic-Resistant Bacteria. Washington, DC: Office of Technology Assessment1995. Report No.: OTA-H-629.
- 3).Raveh D, Levy Y, Schlesinger Y, Greenberg A, Rudensky B, Yinnon AM. Longitudinal surveillance of antibiotic use in the hospital. QJM, 2001; 94: 141–152. [DOI] [PubMed]
- 4).Erbay A, Colpan A, Bodur H, Cevik MA, Samore MH, Ergonul O. Evaluation of antibiotic use in a hospital with an antibiotic restriction policy. Int J Antimicrob Agents, 2003; 21: 308–312. [DOI] [PubMed]
- 5).Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med, 2003; 163: 972–978. [DOI] [PubMed]
- 6).Hogerzeil HV. Promoting rational prescribing: an international perspective. Br J Clin Pharmacol, 1995; 39: 1–6. [DOI] [PMC free article] [PubMed]
- 7).World Health Organization. WHO Global Strategy for Containment of Antimicrobial Resistance. Geneva, Switzerland: Department of Communicable Disease Surveillance and Response 2001. Report No.: WHO/CDS/CSR/DRS/2001.2.
- 8).Cunha BA. Antibiotic Essentials. 2010, Jones and Barlette.
- 9).Chukwuani CM, Onifade M, Sumonu K. Survey of drug use practices and antibiotic prescribing pattern at a general hospital in Nigeria. Pharm World Sci, 2002; 24: 188–195. [DOI] [PubMed]
- 10).Hariharan S, Pillai G, McIntosh D, Bhanji Z, Culmer L, Harper-McIntosh K. Prescribing patterns and utilization of antimicrobial drugs in a tertiary care teaching hospital of a Caribbean developing country. Fundam Clin Pharmacol, 2009; 23: 609–615. [DOI] [PubMed]
- 11).Hu S, Liu X, Peng Y. Assessment of antibiotic prescription in hospitalised patients at a Chinese university hospital. J Infect, 2003; 46: 161–163. [DOI] [PubMed]
- 12).Ider BE, Clements A, Adams J, Whitby M, Muugolog T. Prevalence of hospital-acquired infections and antibiotic use in two tertiary Mongolian hospitals. J Hosp Infect, 2010; 75: 214–219. [DOI] [PubMed]
- 13).Orrett FA. Antimicrobial prescribing patterns at a rural hospital in Trinidad: evidence for intervention measures. Afr J Med Med Sci, 2001; 30: 161–164. [PubMed]
- 14).Okeke IN, Lamikanra A, Edelman R. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg Infect Dis, 1999; 5: 18–27. [DOI] [PMC free article] [PubMed]
- 15).Awad AI, Eltayeb IB, Baraka OZ. Changing antibiotics prescribing practices in health centers of Khartoum State, Sudan. Eur J Clin Pharmacol, 2006; 62: 135–142. [DOI] [PubMed]
- 16).Lindtjorn B. Essential drug list in a rural hospital. Does it have any influence on drug prescription? Trop Doct, 1987; 17: 151–155. [DOI] [PubMed]
- 17).Gulyaev AE, Nurgogin T. It’s time to be healthy. Bulletin, 2001; 14: 24–26 (in Russian).
- 18).Momunova AA. Drug use in diseases of the respiratory system caused by viruses. Centr Asian Medic J, 2003; 9: 208–210 (in Russian).
- 19).Gyssens IC, van den Broek PJ, Kullberg BJ, Hekster Y, van der Meer JW. Optimizing antimicrobial therapy: a method for antimicrobial drug use evaluation. J Antimicrob Chemother, 1992; 30: 724–727. [DOI] [PubMed]
- 20).Strachunski L, Pleshkov V, Zuzova A, et al. Antibiotic prophylaxis in surgery. In: Guideline on antimicrobial chemotherapy, edited by Strachunski L, Belousov Y, Kozlov S. pp. 327–332 (in Russian), 2007, IACMAC, Smolensk.
- 21).Blomberg B. [Antimicrobial resistance in developing countries]. Tidsskr Nor Laegeforen, 2008; 128: 2462–2466. [PubMed]
- 22).Byarugaba DK. A view on antimicrobial resistance in developing countries and responsible risk factors. Int J Antimicrob Agents, 2004; 24: 105–110. [DOI] [PubMed]
- 23).Borg MA, Zarb P, Ferech M, Goossens H. Antibiotic consumption in southern and eastern Mediterranean hospitals: results from the ARMed project. J Antimicrob Chemother, 2008; 62: 830–836. [DOI] [PubMed]
- 24).Mettler J, Simcock M, Sendi P, Widmer AF, Bingisser R, Battegay M, Fluckiger U, Bassetti S. Empirical use of antibiotics and adjustment of empirical antibiotic therapies in a university hospital: a prospective observational study. BMC Infect Dis, 2007; 7: 21. [DOI] [PMC free article] [PubMed]
- 25).Singh J, Burr B, Stringham D, Arrieta A. Commonly used antibacterial and antifungal agents for hospitalised paediatric patients: implications for therapy with an emphasis on clinical pharmacokinetics. Paediatr Drugs, 2001; 3: 733–761. [DOI] [PubMed]
- 26).Al-Niemat SI, Bloukh DT, Al-Harasis MD, Al-Fanek AF, Salah RK. Drug use evaluation of antibiotics prescribed in a Jordanian hospital outpatient and emergency clinics using WHO prescribing indicators. Saudi Med J, 2008; 29: 743–748. [PubMed]
- 27).Dumpis U, Gulbinovic J, Struwe J, Lagergren A, Griskevicius L, Bergman U. Differences in antibiotic prescribing in three university hospitals in the Baltic region revealed by a simple protocol for quality assessment of therapeutic indications. Int J Clin Pharmacol Ther, 2007; 45: 568–576. [DOI] [PubMed]
- 28).Erdeljic V, Francetic I, Macolic Sarinic V, Bilusic M, Huic M, Mercep I, Makar-Ausperger K. [Evaluation of justification for antibiotic use at the Internal Medicine Clinic of the Clinical Hospital in Zagreb]. Acta Med Croatica, 2004; 58: 293–299. [PubMed]
- 29).Finch RG, Metlay JP, Davey PG, Baker LJ. Educational interventions to improve antibiotic use in the community: report from the International Forum on Antibiotic Resistance (IFAR) colloquium, 2002. Lancet Infect Dis, 2004; 4: 44–53. [DOI] [PubMed]
- 30).Vogtlander NP, Van Kasteren ME, Natsch S, Kullberg BJ, Hekster YA, Van Der Meer JW. Improving the process of antibiotic therapy in daily practice: interventions to optimize timing, dosage adjustment to renal function, and switch therapy. Arch Intern Med, 2004; 164: 1206–1212. [DOI] [PubMed]
- 31).Bianco A, Pileggi C, Trani F, Angelillo IF. Appropriateness of admissions and days of stay in pediatric wards of Italy. Pediatrics, 2003; 112: 124–128. [DOI] [PubMed]
- 32).Fonseca LG, de Oliveira Conterno L. Audit of antibiotic use in a Brazilian University Hospital. Braz J Infect Dis, 2004; 8: 272–280. [DOI] [PubMed]
- 33).Hadi U, Duerink DO, Lestari ES, Nagelkerke NJ, Keuter M, Huis In’t Veld D, Suwandojo E, Rahardjo E, van den Broek P, Gyssens IC. Audit of antibiotic prescribing in two governmental teaching hospitals in Indonesia. Clin Microbiol Infect, 2008; 14: 698–707. [DOI] [PubMed]
- 34).Al-Momany NH, Al-Bakri AG, Makahleh ZM, Wazaify MM. Adherence to international antimicrobial prophylaxis guidelines in cardiac surgery: a Jordanian study demonstrates need for quality improvement. J Manag Care Pharm, 2009; 15: 262–271. [DOI] [PMC free article] [PubMed]
- 35).Tourmousoglou CE, Yiannakopoulou E, Kalapothaki V, Bramis J, St Papadopoulos J. Adherence to guidelines for antibiotic prophylaxis in general surgery: a critical appraisal. J Antimicrob Chemother, 2008; 61: 214–218. [DOI] [PubMed]
- 36).Ackerman P, Brachman M, et al. The Basic Infection Control Manual. In: Surgical Site Infection, edited by Edward OR, Nina AS, Ludmila Z. pp. 91–113, 2006, American International Health Alliance, USA.
- 37).Wiley AM, Ha’eri GB. Routes of infection: a study of using “tracer particles” in the orthopedic operating room. Clin Orthop Relat Res, 1979: 150–155. [PubMed]