ABSTRACT
The Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study) is a long-term cohort study to investigate the interactions among genotypes, lifestyles, and lifestyle-related diseases, especially cancer. This article reports the outline of the baseline survey of the Daiko Study, one site of the J-MICC Study. That survey was conducted between June 9, 2008 and May 31, 2010 at the Daiko Medical Center of Nagoya University in Nagoya, Japan. Subjects were registered residents of Nagoya City aged 35 to 69 years who had not participated in other J-MICC sites. Recruitment was mainly announced through leaflets distributed in mailboxes citywide, personal communications, and regional information, such as posters in public or commercial facilities. Participants provided blood plasma, serum, buffy coat, urine, and data on health check-ups. They also completed a self-reported questionnaire on lifestyle, disease history, family history, and for women, reproductive history. As of the end of September 2010, 4 out of 5,172 registered participants had withdrawn from the study, leaving data from 5,168 participants (1,467 males and 3,701 females) available for analysis. Mean age ± standard deviation (SD) was 52.5 ± 10.3 years. Current smokers accounted for 24.1% (n=354) of males and 6.9% (n=256) of females. Current drinkers included 74.9% (n=1,099) of males and 45.9% (n=1,699) of females. Lifestyle data and specimens were successfully collected to examine any associations among disease biomarkers, lifestyles, and genotypes.
Key Words: Cohort study, Baseline survey, Nagoya City, Community-based study, J-MICC Study
INTRODUCTION
The Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study) is a long-term cohort study investigating the interactions among genotypes, lifestyles, and lifestyle-related diseases, especially cancer.1-3) Twelve affiliated universities/institutes have participated in the study.4-5) Details of the J-MICC Study have been previously described.1)
The Nagoya University Graduate School of Medicine team enrolled participants from two regions: the West-Central areas of Shizuoka Prefecture5) and Nagoya City. The study profile of the baseline survey in the former has been previously published.5) The latter was the Daiko Study, which was named after the enrollment locale, the Daiko Medical Center of Nagoya University. Participant recruitment started at the Center on June 9, 2008 after a pilot enrollment of 2 participants on May 30, 2008, and ended on May 31, 2010. The study was expected to enroll 5,000 participants. The Center is located in the Higashi Ward of Nagoya, located in the central area of Honshu Island in Japan. Nagoya is Japan’s fourth largest city, with over 2,250,000 citizens.6) This paper describes the study profile of the baseline survey and the characteristics of participants in the Daiko Study.
METHODS
Recruitment and participants
The J-MICC Study and Daiko Study were both approved by the Ethics Review Committee of the Nagoya University School of Medicine (approved numbers were 253 and 618, respectively). Subjects were residents with a certificate of residence, excluding foreign resident registrations, in Nagoya City aged 35 to 69 years at the time of enrollment, who had not participated in other J-MICC Study sites.7) The existence of certificates of residence will be confirmed in a follow-up period to identify any who were deceased. As the results of inquiries about a certificate of residence, participants without such a certificate will be excluded from the cohort.
Participants were recruited mainly through a citywide mailbox distribution of leaflets, personal communications, and regional information (e.g., posters in public or commercial facilities). We distributed leaflets throughout the entire area (16 wards) of Nagoya at least once, and repeatedly in 7 wards (Higashi, Moriyama, Chikusa, Kita, Meito, Tenpaku, and Atsuta) near the Daiko Medical Center. The main channel of personal communication was that by participants who were asked to hand out leaflets to family members and acquaintances. Recruitment of the study was also reported in a newspaper on October 20, 2008, and via a TV program on June 28, 2009.
Enrollment took place 4 days per week between June and December 2008 and 3 days per week between January 2009 and May 2010. In total, 273 days, including a pilot enrollment day in May 2008, were opened. Enrollment stopped from mid-March to mid-April 2010, since the expenditures were not sanctioned by the research grant in the term due to the end and beginning of the fiscal year.
Although recruitment was set at a maximum of 25 individuals per day, we enrolled 26 individuals in 10 days; 27 in 8 days; and 28 in 4 days. On 199 out of 201 enrollment days (November 8, 2008 to May 31, 2010) participants were asked how they learned about the study.
Informed consent process
Potential candidates who called, faxed, or e-mailed the survey office received a set of documents on the study via mail. We set up appointments for them to visit the Daiko Medical Center. On the day of the visit, up to 9 candidates per session watched a 10-minute video outlining the J-MICC/Daiko Study. Each person was individually asked for written informed consent, which was provided by those who understood the study outline. The agreement included: 1) permission to use information on lifestyle, disease history, and family history collected from the self-administered questionnaire; 2) permission to use laboratory data obtained from the health checkup, 3) donation of blood and urine specimens, 4) permission to do genotyping from the donated blood, and 5) follow-up until 2025 for cancer diagnosis and/or death from any cause. Those who agreed to all of the above were registered.
Lifestyle data
The questionnaire in the Daiko Study covered employment, hobbies, mental health status, dental health, long-term care of family member(s) at home, exposure to insecticides, pollinosis, and staying and/or walking in forested areas (shinrin-yoku). It also included questions common to those in the J-MICC Study.1) Participants were requested to complete all questions at home. On enrollment day, trained staff went over the questionnaires with participants face-to-face to ensure completeness, consistency, and accuracy. Participants were free to refuse to answer specific questions.
Biospecimens
From those fasting overnight, peripheral blood was drawn in the morning with three 7-ml vacuum tubes; 2 tubes for serum and 1 with EDTA-Na for plasma and buffy coat. The serum was first separated for participants’ health checkup and then distributed among 10 tubes (300 μl per tube). We separated plasma into 8 tubes (300 μl per tube), and buffy coat into 2 (300 μl per tube). Four serum tubes, 4 plasma tubes, and 1 buffy coat tube were stored for the common use of J-MICC Study. The remainder plus 1 tube to extract DNA were stored for proper Daiko Study, as were 2 tubes with 1 ml urine that we collected. All tubes were stored at –80°C at the Nagoya University Graduate School of Medicine.
For health checkups of participants, blood was tested for total cholesterol, HDL cholesterol, triglycerides, creatinine, uric acid, AST, ALT, and γ-GTP. Blood tests were conducted at SRL Co., Ltd., Tokyo. Urinalyses (e.g., protein, glucose, occult blood, urobilinogen, bilirubin) were conducted with Uropaper III “Eiken” (Otsuka Pharmaceutical Co., Ltd., Tokyo). Anti-H. pylori antibody was examined with urine (Rapiran™). Participants’ blood pressure was measured twice, in a seated position using a standard automated blood pressure measurement monitor, HEM-1000 (Omron, Japan). They were given a 5-minute rest before the first measurement and another two-minute rest before the second. Anthropometric data included body measurements (height, weight, and abdominal circumference). Results were sent to participants within 2 weeks, and have been used for research purposes.
Staff
On each enrollment day, 13 or 14 staff members were engaged with enrollments and health checkups. A total of 74 staff members (29 researchers, 5 technicians, 22 nurses, and 18 assistants) contributed to the study. Two individuals per enrollment day performed office work (e.g., answered the phones and responded to those who came to the reception desk). Five to 6 individuals performed tasks related to data collection (e.g., explaining the outline of the study, confirming informed consent, measuring height and weight, checking questionnaires, and consulting for the health checkup). Two people drew blood and measured blood pressure, 2 examined urine samples, and 2 dispensed blood samples.
RESULTS
Participation rate
By the end of May 2010, 5,172 participants had been recruited, after a total of 2,216,900 leaflets were distributed throughout Nagoya. They accounted for 0.49% of the 1,063,091 registered residents of Nagoya City aged 35 to 69 years on October 1, 2009.6) Both males (0.42%) and females (0.80%) in the 65–69 year age group had the highest participation rate, and males (0.19%) and females (0.62%) in the 40–44 year age group had the lowest.6)
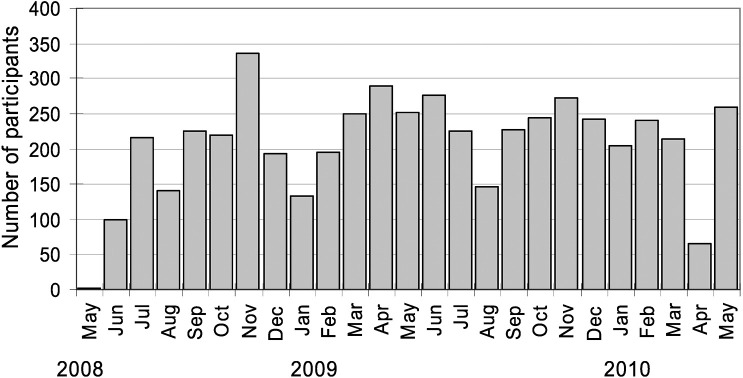
The largest number of participants were from the Moriyama Ward (n=999), while the highest participation rate was from the Higashi Ward (2.4% of residents aged 35–69 years on October 1, 2009).6) Registration of participants by month is shown in Figure 1. Excluding start-up enrollment days in June 2008, the mean number of participants per enrollment day was 19.3. In both 2008 and 2009, participation in August was low. We drew the largest number of participants in November 2008, the month after information about the study appeared in the newspaper.
Fig. 1.
Number of participants per month, including two from a pilot enrollment in May 2008. The survey was suspended from mid-March to mid-April 2010.
In the total enrollment period, only fewer than 10 candidates refused to participate after they received an explanation of the outline of the study when they visited the Center for participation.
Utility of media for recruitment
Table 1 shows the percentage of media used by participants to know about the study. The majority (65.6%) learned about the Daiko Study by posted leaflets. Personal communications, such as the hearsay from former participants, attracted 25.7% of participants.
Table 1.
Sources of information in the Daiko Study
| Media | n | (%) |
|---|---|---|
| Leaflets distributed to mailboxes | 2,722 | (65.6) |
| Personal communications | 1,065 | (25.7) |
| Leaflets (Other) | 76 | (1.8) |
| Newspaper | 64 | (1.5) |
| Web page of Daiko Study | 24 | (0.6) |
| Postings at Daiko Medical Center | 15 | (0.4) |
| Other | 104 | (2.5) |
| No answer | 158 | (3.8) |
* 4152 participants were questioned during 199 out of 201 enrollment days from November 8, 2008 to May 31, 2010.
* 76 participants had duplicate responses.
Gender and age distribution
Data from 4 participants who withdrew from the study at the end of September 2010 were deleted, and their samples were disposed of. Data from the remaining 5,168 participants (1,467 males and 3,701 females) were used in the analyses. Mean age ± standard deviation (SD) was 52.5 ± 10.3 years.
The distribution of participants by age and gender is shown in Figure 2. In all age groups, there were more than twice as many females as males, with more males in the older age groups. Female participants were evenly distributed across all age groups.
Fig. 2.
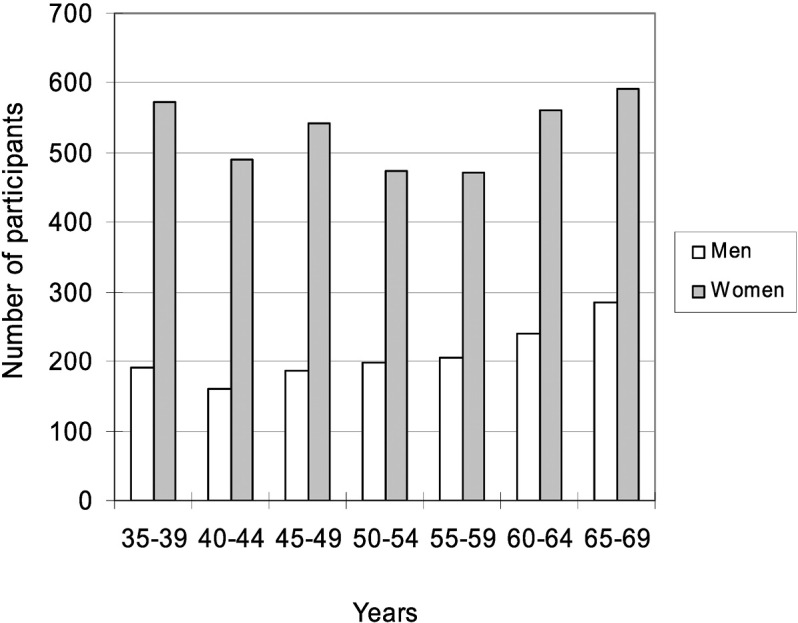
Number of participants by gender and age group
Smoking status and alcohol consumption
Smoking status by gender and age group is shown in Table 2. Current smokers accounted for 24.1% (n=354) of males and 6.9% (n=256) of females. Alcohol consumption is presented in Table 3. Drinkers were defined as those who consumed alcohol at least once per month. 74.9% of male participants (n=1,099) were current drinkers; for females, the figure was 45.9% (n=1,699).
Table 2.
Smoking status by gender and age group
| Years | Smoking Status | |||||
|---|---|---|---|---|---|---|
| Current | Former | Never | ||||
| n | % | n | % | n | % | |
| Male | ||||||
| 35–39 | 52 | 27.2% | 57 | 29.8% | 82 | 42.9% |
| 40–44 | 44 | 27.3% | 46 | 28.6% | 71 | 44.1% |
| 45–49 | 51 | 27.4% | 72 | 38.7% | 63 | 33.9% |
| 50–54 | 47 | 23.9% | 77 | 39.1% | 73 | 37.1% |
| 55–59 | 51 | 24.8% | 97 | 47.1% | 58 | 28.2% |
| 60–64 | 58 | 24.1% | 122 | 50.6% | 61 | 25.3% |
| 65–69 | 51 | 17.9% | 168 | 58.9% | 66 | 23.2% |
| Total | 354 | 24.1% | 639 | 43.6% | 474 | 32.3% |
| Female | ||||||
| 35–39 | 56 | 9.8% | 79 | 13.8% | 437 | 76.4% |
| 40–44 | 26 | 5.3% | 45 | 9.2% | 419 | 85.5% |
| 45–49 | 47 | 8.7% | 66 | 12.2% | 430 | 79.2% |
| 50–54 | 44 | 9.3% | 59 | 12.5% | 370 | 78.2% |
| 55–59 | 33 | 7.0% | 54 | 11.4% | 385 | 81.6% |
| 60–64 | 28 | 5.0% | 43 | 7.7% | 489 | 87.3% |
| 65–69 | 22 | 3.7% | 40 | 6.8% | 528 | 89.5% |
| Total | 256 | 6.9% | 386 | 10.4% | 3,058 | 82.6% |
*Data were not available on one female participant
Table 3.
Alcohol consumption by gender and age group
| Years | Alcohol consumption | |||||
|---|---|---|---|---|---|---|
| Current | Former | Never | ||||
| n | % | n | % | n | % | |
| Male | ||||||
| 35–39 | 128 | 67.0% | 2 | 1.0% | 61 | 31.9% |
| 40–44 | 127 | 78.9% | 3 | 1.9% | 31 | 19.3% |
| 45–49 | 140 | 75.3% | 6 | 3.2% | 40 | 21.5% |
| 50–54 | 153 | 77.7% | 5 | 2.5% | 39 | 19.8% |
| 55–59 | 149 | 72.3% | 8 | 3.9% | 49 | 23.8% |
| 60–64 | 183 | 75.9% | 9 | 3.7% | 49 | 20.3% |
| 65–69 | 219 | 76.8% | 12 | 4.2% | 54 | 18.9% |
| Total | 1,099 | 74.9% | 45 | 3.1% | 323 | 22.0% |
| Female | ||||||
| 35–39 | 307 | 53.7% | 15 | 2.6% | 250 | 43.7% |
| 40–44 | 244 | 49.8% | 11 | 2.2% | 235 | 48.0% |
| 45–49 | 275 | 50.7% | 9 | 1.7% | 258 | 47.6% |
| 50–54 | 247 | 52.2% | 5 | 1.1% | 221 | 46.7% |
| 55–59 | 206 | 43.6% | 7 | 1.5% | 259 | 54.9% |
| 60–64 | 217 | 38.8% | 9 | 1.6% | 334 | 59.6% |
| 65–69 | 203 | 34.3% | 4 | 0.7% | 384 | 65.0% |
| Total | 1,699 | 45.9% | 60 | 1.6% | 1,941 | 52.5% |
*Data were not available on one female participant.
Cancer history
Forty-four males (3.0%) and 166 females (4.5%) reported a history of cancer in the self-administrated questionnaire; 7 males (0.5%) and 24 females (0.6%) were receiving treatment at the time of enrollment. Twenty-seven participants (0.5%) did not respond to the question on cancer history. Colorectal cancer was the most common cancer in men (n=12), followed by cancers of the bladder (n=7) and stomach (n=6). In females, breast cancer (n=64) was most frequently reported, followed by uterine (n=39) and colorectal cancer (n=17).
Laboratory data
Data on blood tests came from 5,164 participants after the exclusion of 4 individuals who withdrew from the study and 4 with unsuccessful blood draws. The mean, standard deviation, minimum and maximum values from the selected laboratory tests, as well as the percentages of those with measurements outside the normal range, are presented according to gender in Tables 4 and 5. The cutoff points for laboratory data were arbitrarily determined, and were the same as those in the Shizuoka Area of the J-MICC Study,5) except that the cutoff point for creatinine in females differed. The blood samples were first used for health check-ups, and then the remainder was stored for research proposes. Therefore, samples from 5,152 participants were available for DNA analysis, because the quantities of blood samples from another 12 participants were insufficient for research purposes.
Table 4.
Body mass index, blood pressure, and blood tests in males
| n | Mean | SD | Min | Max | Ranges out of standard values | ||||
|---|---|---|---|---|---|---|---|---|---|
| Range | % | Range | % | ||||||
| BMI | 1,467 | 23.2 | 3.1 | 15.7 | 52.8 | < 18.5 | 3.7 | 25.0 ≤ | 24.7 |
| DBPa) | 1,467 | 78.8 | 12.3 | 43.5 | 132.5 | 90.0–94.5 | 8.5 | 95.0 ≤ | 9.9 |
| SBPa) | 1,467 | 126.6 | 18.7 | 83.5 | 216.5 | 140.0–159.5 | 16.1 | 160 ≤ | 6.1 |
| T-C | 1,467 | 204.1 | 32.5 | 102 | 347 | 220–279 | 28.6 | 280 ≤ | 1.6 |
| HDL-C | 1,467 | 56.4 | 14.1 | 26 | 160 | 40–49 | 27.9 | < 40 | 7.6 |
| TG | 1,467 | 128.1 | 100.4 | 18 | 1,268 | 150–199 | 11.3 | 200 ≤ | 14.1 |
| AST | 1,467 | 23.1 | 13.4 | 12 | 415 | 40–49 | 1.9 | 50 ≤ | 1.8 |
| ALT | 1,467 | 23.3 | 32.0 | 4 | 1,111 | 40–49 | 4.3 | 50 ≤ | 5.0 |
| γGTP | 1,467 | 46.2 | 56.0 | 9 | 714 | 70–99 | 7.4 | 100 ≤ | 7.0 |
| UA | 1,467 | 6.0 | 1.2 | 0.8 | 10.4 | < 3.0 | 0.5 | 7.0 ≤ | 19.8 |
| Creatinine | 1,467 | 0.8 | 0.2 | 0.5 | 6.8 | 1.10–1.29 | 1.8 | 1.30 ≤ | 0.4 |
BMI: body mass index (kg/m2); DBP: diastolic blood pressure (mmHg); SBP: systolic blood pressure (mmHg); T-C: total cholesterol (mg/dl); HDL-C: HDL cholesterol (mg/dl); TG: triglyceride (mg/dl); AST (U/l); ALT (U/l); γGTP (U/l); UA: uric acid (mg/dl); and creatinine (mg/dl)
a) Average of two measurements
Table 5.
Body mass index, blood pressure, and blood tests in females
| n | Mean | SD | Min | Max | Ranges out of standard values | ||||
|---|---|---|---|---|---|---|---|---|---|
| Range | % | Range | % | ||||||
| BMI | 3,700 | 21.1 | 3.0 | 11.5 | 41.5 | < 18.5 | 16.3 | 25.0 ≤ | 9.5 |
| DBPa) | 3,701 | 69.6 | 11.4 | 36.0 | 131.0 | 90.0–94.5 | 2.4 | 95.0 ≤ | 2.6 |
| SBPa) | 3,701 | 114.4 | 18.7 | 71.5 | 251.0 | 140.0–159.5 | 7.3 | 160 ≤ | 2.6 |
| T-C | 3,697 | 210.1 | 34.8 | 105 | 388 | 220–279 | 35.4 | 280 ≤ | 2.8 |
| HDL-C | 3,697 | 68.4 | 14.8 | 30 | 140 | 40–49 | 7.8 | < 40 | 0.9 |
| TG | 3,697 | 82.1 | 51.5 | 18 | 1,558 | 150–199 | 4.6 | 200 ≤ | 2.4 |
| AST | 3,697 | 20.2 | 7.4 | 6 | 259 | 40–49 | 0.6 | 50 ≤ | 0.5 |
| ALT | 3,697 | 16.0 | 9.8 | 1 | 195 | 40–49 | 1.2 | 50 ≤ | 0.9 |
| γGTP | 3,697 | 22.1 | 25.1 | 5 | 918 | 70–99 | 1.6 | 100 ≤ | 1.0 |
| UA | 3,697 | 4.3 | 0.9 | 0.4 | 9.8 | < 3.0 | 5.3 | 7.0 ≤ | 0.8 |
| Creatinine | 3,697 | 0.6 | 0.1 | 0.3 | 1.1 | 0.80–0.99 | 1.8 | 1.00 ≤ | 0.1 |
BMI: body mass index (kg/m2); DBP: diastolic blood pressure (mmHg); SBP: systolic blood pressure (mmHg); T-C: total cholesterol (mg/dl); HDL-C: HDL cholesterol (mg/dl); TG: triglyceride (mg/dl); AST (U/l); ALT (U/l); γGTP (U/l); UA: uric acid (mg/dl); and creatinine (mg/dl)
a) Average of two measurements
DISCUSSION
During 2 years, we enrolled 5,172 participants in the Daiko Study, they included 0.49% of the targeted residents. In comparison, the Saga J-MICC Study recruited 19.7% of the targeted residents in Saga City, which is located in the southern part of Japan.4) In the Saga J-MICC Study, all candidates with their name and addresses were contacted by mail. This was an effective approach in Saga City. However, we judged that recruitment mainly via posted leaflets without names and addresses was the best choice for our region, since doing otherwise would involve difficulties in obtaining names and addresses of all candidates in Nagoya City.
The Higashi Ward, in which the Daiko Medical Center is located, had the highest participation rate, indicating that convenience probably played some role. Participation rates also depended on convenience in the Saga J-MICC Study,4) where enrollment places were moved to several different areas of the city.
Personal communication was also an effective means of recruitment. As many as 25.7% of participants learned about the study from former participants. Newspaper also contributed to recruitment; the highest enrollment occurred in the month after a newspaper reported the study. August was the lowest month, most likely due to the hot climate and summer vacations. Although data on why participants were interested in the study was not available, the reports of participants on their own anti-H. pylori antibody tests (an item not included in the usual health check-ups) seemed to encourage them to participate.
In this study, the number of male participants was lower than that among females. The relative small number of male samples would prove to be one of the limitations of stratified analysis by gender or of male-specific cancers in the future.
In conclusion, in the Daiko Study, which was a part of the J-MICC Study, lifestyle data and specimens were successfully collected from more than 5,000 participants. A secondary survey targeting the participants in this study has been planned for 2014. Deaths and cancer occurrences will be followed up until 2025.
ACKNOWLEDGMENTS
The authors wish to thank Ms. Taeko Ito, Ms. Junko Ando, Ms. Mariko Nakano and the entire staff for their cooperation in collecting data. This study was supported, in part, by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (Nos. 17015018 and 221S0001).
REFERENCES
- 1).The J-MICC Study Group. The Japan Multi-institutional Collaborative Cohort Study (J-MICC Study) to detect gene-environment interactions for cancer. Asian Pac J Cancer Prev, 2007; 8: 317–323. [PubMed]
- 2).Naito M, Eguchi H, Okada R, Ishida Y, Nishio K, Hishida A, Wakai K, Tamakoshi A, and Hamajima N, for the J-MICC Study Group. Controls for monitoring the deterioration of stored blood samples in the Japan Multi-institutional Collaborative Cohort Study (J-MICC Study). Nagoya J Med Sci, 2008; 70: 107–115. [PubMed]
- 3).Wakai K, Hamajima N, Okada R, Naito M, Morita E, Hishida A, Kawai S, Nishio K, Yin G, Asai Y, Matsuo K, Hosono S, Ito H, Watanabe M, Kawase T, Suzuki T, Tajima K, Tanaka K, Higaki Y, Hara M, Imaizumi T, Taguchi N, Nakamura K, Nanri H, Sakamoto T, Horita M, Shinchi K, Kita Y, Turin TC, Rumana N, Matsui K, Miura K, Ueshima H, Takashima N, Nakamura Y, Suzuki S, Ando R, Hosono A, Imaeda N, Shibata K, Goto C, Hattori N, Fukatsu M, Yamada T, Tokudome S, Takezaki T, Niimura H, Hirasada K, Nakamura A, Tatebo M, Ogawa S, Tsunematsu N, Chiba S, Mikami H, Kono S, Ohnaka K, Takayanagi R, Watanabe Y, Ozaki E, Shigeta M, Kuriyama N, Yoshikawa A, Matsui D, Watanabe I, Inoue K, Ozasa K, Mitani S, Arisawa K, Uemura H, Hiyoshi M, Takami H, Yamaguchi M, Nakamoto M, Takeda H, Kubo M, and Tanaka H, for the J-MICC Study Group. Profile of Participants and Genotype Distributions of 108 Polymorphisms in a Cross-sectional Study of Associations of Genotypes with Lifestyle and Clinical Factors: A Project in the Japan Multi-institutional Collaborative Cohort (J-MICC) Study. J Epidemiol, 2011; 21: 223–35. [DOI] [PMC free article] [PubMed]
- 4).Hara M, Higaki Y, Imaizumi T, Taguchi N, Nakamura K, Nanri H, Sakamoto T, Horita M, Shinchi K, Tanaka K. Factors influencing participation rate in a baseline survey of a genetic cohort in Japan. J Epidemiol, 2010; 20: 40–5. [DOI] [PMC free article] [PubMed]
- 5).Asai Y, Naito M, Suzuki M, Tomoda A, Kuwabara M, Fukada Y, Okamoto A, Oishi S, Ikeda K, Nakamura T, Misu Y, Katase S, Tokumasu S, Nishio K, Ishida Y, Hishida A, Morita E, Kawai S, Okada R, Wakai K, Tamakoshi A, Hamajima N. Baseline data of Shizuoka area in the Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study). Nagoya J Med Sci, 2009; 71: 137–44. [PMC free article] [PubMed]
- 6).City of Nagoya. The 100th Nagoya statistical year book. Nagoya: 2009. (in Japanese).
- 7).Kataoka R, Kimata A, Yamamoto K, Hirosawa N, Ueyama J, Kondo T, Okada R, Kawai S, Hishida A, Naito M, Morita E, Wakai K, Hamajima N. Association of UGT1A1 Gly71Arg with urine urobilinogen. Nagoya J Med Sci, 2011; 73: 33–40. [PMC free article] [PubMed]