ABSTRACT
Coronary calcification is proportional to the extent and severity of atherosclerotic disease, and is a predictor of cardiac events. Furthermore, coronary calcification protruding into the lumen is considered as one type of vulnerable plaque. Optical coherence tomography (OCT) can provide in vivo imaging of the detailed vessel wall structure of the coronary artery with high resolution, as in the histological approach. We analyzed coronary calcification in that fashion using OCT in vivo. This study consisted of 70 superficial coronary calcifications of 39 consecutive patients who underwent percutaneous coronary intervention. After revascularization, OCT was performed in the treated vessel. We analyzed morphologic characteristics and the quantification of OCT-determined coronary calcification. Superficial coronary calcifications were classified into two groups depending on whether they did not intrude the lumen (type I) or did (type II). The distance from the lumen and the volume of each calcification were then measured. Superficial coronary calcifications were classified into two groups; type I, n = 39 (56%) and type II, n = 31 (44%). Type II calcifications were located significantly closer to the lumen [80 μm (60–130) vs.130 μm (90–260), p = 0.015], and tended to be smaller, but did not show a significant difference [0.65 (0.26–1.3) mm3 vs. 1.2 (0.47–1.9) mm3, p = 0.153] compared to those of type I. In conclusion, OCT could visualize superficial coronary calcifications in detail and enable us to evaluate in vivo morphologic characterizations and quantify them.
Key Words: Coronary artery disease, Calcium, Angina pectoris
INTRODUCTION
Optical coherence tomography (OCT), a light-based technique, can provide detailed in vivo imaging of coronary vessel wall structure with a high resolution, as in the histological approach. The characterization of coronary artery plaques using this modality is well established. Notably, the histopathologic diagnostic accuracy of OCT for identifying coronary calcified lesions is over 90%.1-3) In OCT imaging, a calcified lesion is described as a sharply delineated signal-poor region. In addition to this important ability, the high-resolution of OCT (10 μm) allows detailed evaluation of the vessel structure accompanied with calcified lesions (e.g., depth from vessel lumen, influence on vessel lumen), and can quantify them precisely.4) A pathological study has reported that a coronary superficial calcified nodule intruding into the vessel lumen is a sign that the lesion is of vulnerable.5) We recognized that there are two types of coronary calcified nodules, one which intrudes the lumen, and the other which does not, from the daily use of OCT in vivo. Therefore, in the present study, we quantified superficial calcified nodules and compared the characteristics of each type using OCT. We also investigated the relationship between coronary superficial calcified nodules and clinical outcomes.
SUBJECTS AND METHODS
Study population
We enrolled a total of 94 consecutive stable angina pectoris (AP) patients with documented ischemia between August 2010 and March 2011 at Nagoya University Hospital. The study enrollment criterion was patients undergoing elective percutaneous coronary intervention (PCI). As for coronary lesions to be analyzed, inclusion criteria were lesions the previously untreated and those >10 mm away from the intervention site. Exclusion criteria consisted of patients with coronary plaques located in the left main trunk, just proximal to the left anterior descending artery or the left circumflex, and near right coronary artery ostium, because OCT imaging requires vessel occlusion. Written informed consent was given by all patients prior to procedures. We attempted to perform OCT imaging of treated vessels immediately after PCI in 94 patients. All patients underwent successful PCI. OCT was successfully performed in all patients. However, no superficial calcified nodule was detected in 55 patients. Thus, 39 patients with 70 superficial calcified nodules were eligible for this study. One-year follow-up data were obtained from hospital charts or telephone interviews with the patients.
OCT
Immediately after successful PCI, OCT imaging was performed in the angiographically determined proximal one-third of the treated vessel. An intracoronary infusion of 0.5 mg isosorbide dinitrate was performed immediately prior to OCT imaging. OCT imaging was conducted according to previously described method6) using an over-the-wire type occlusion balloon catheter, OCT imaging probe (Helios®, ImageWire®, St. Jude Medical, Inc., St. Paul, Minnesota, USA), and an auto-pullback system. Obtained images were evaluated using proprietary off-line software provided by LightLab Imaging Inc., according to previously defined criteria for plaque characterization.1,7) Coronary superficial calcified nodules were defined as well-delineated, signal-poor regions with sharp borders.1) Two independent observers who were blinded to patient characteristics analyzed the types of coronary calcifications. Type I was defined as a coronary superficial calcified nodule without intrusion of the lumen, while type II was with an intrusion. If the height from the estimated normal luminal surface to the actual luminal surface was ≥ 100 µm, the lesion was considered as intruding (Fig. 1). Cross-sectional areas of calcified nodules were measured at all frames. Its total volume (mm3) was calculated as 0.05 x (sum of each cross-sectional area of the calcification). Its depth (µm) was defined as the shortest distance measurable between the leading edge of the calcified nodule to the lumen boundary.
Fig. 1.
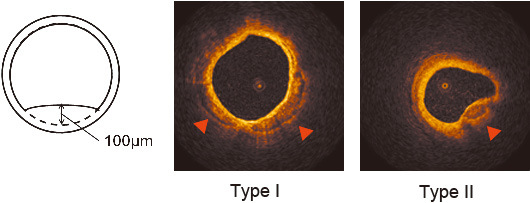
Representative images of optical coherence tomography (OCT).
If the height from estimated normal luminal surface to actual luminal surface was ≥ 100 µm, the lesion was considered as intruding. Calcifications are well-visualized using OCT (red arrowheads). Type I was defined as coronary calcification without intrusion of lumen, while type II was defined as lumen-intrusive.
Statistical Analysis
SPSS ver. 18 (SPSS, Chicago, IL, USA) was used for all statistical analyses. Continuous variables were presented as mean ± standard deviation or median (range), and categorical variables were presented as numbers (%). Differences between the 2 groups were evaluated by the Mann-Whitney U-test, or the chi-square test. A two-tailed p value of <0.05 was considered significant.
RESULTS
The characteristics of the enrolled patients are shown in Table 1. Morphologic classification and quantification of coronary superficial calcified nodules are described in Table 2. The average number of calcified nodules in each coronary artery was: left anterior descending, 1.8; left circumflex, 1.5; right coronary, 1.3. The median volume and depth from the lumen were 0.79 (0.38–1.8) mm3 and 100 (70–170) μm, respectively. The superficial calcified nodules of type II tended to be smaller than those of type I, but there was no significant difference [0.65 (0.26–1.3) mm3 vs. 1.2 (0.47–1.9) mm3, p = 0.153]. Superficial calcified nodules of type II were located significantly closer to the lumen than those of type I [80 (60–130) μm vs. 130 (90–260) μm, p = 0.015] (Table 2). No clinical event related to the coronary superficial calcified nodules analyzed was obtained during a 1-year follow-up.
Table 1.
Patient characteristics
| N = 39 | |
| Age (years) | 68 ± 10 |
| Male | 33 (85) |
| Body mass index (kg/m2) | 23.4 ± 3.4 |
| Clinical history | |
| Hypertension | 29 (74) |
| Dyslipidemia | 30 (77) |
| Diabetes | 19 (49) |
| Current smoker | 9 (23) |
| Previous revascularization | 17 (44) |
| Previous myocardial infarction | 9 (23) |
| Multiple vessel disease | 25 (64) |
| Familial history of CHD | 8 (21) |
| Total cholesterol (mg/dl) | 175.1 ± 35.4 |
| LDL cholesterol (mg/dl) | 97.6 ± 29.1 |
| HDL cholesterol (mg/dl) | 48.5 ± 14.0 |
| LDL to HDL cholesterol ratio | 2.1 ± 0.79 |
| Triglyceride (mg/dl) | 141.5 ± 67.0 |
| HemoglobinA1c (%) | 6.2 ± 0.97 |
| High-sensitivity CRP (mg/l) | 1.7 ± 2.5 |
| Analyzed vessel | |
| Left anterior descending | 19 (43) |
| Left circumflex | 13 (30) |
| Right coronary | 12 (27) |
Values are mean ± SD or number (%).
CHD = coronary heart disease; LDL = low-density lipoprotein; HDL = high-density lipoprotein; CRP = C-reactive protein.
Table 2.
Morphologic classification and quantification of coronary calcifications
| All | Type I | Type II | p | |
|---|---|---|---|---|
| Number | 70 | 39 (56) | 31 (44) | – |
| Location | 0.513 | |||
| Left anterior descending | 35 (50) | 22 (63) | 13 (37) | |
| Left circumflex | 20 (29) | 10 (50) | 10 (50) | |
| Right coronary | 15 (21) | 7 (47) | 8 (53) | |
| *Average calcification number | – | |||
| Left anterior descending | 1.8 | 1.2 | 0.7 | |
| Left circumflex | 1.5 | 0.8 | 0.8 | |
| Right coronary | 1.3 | 0.6 | 0.7 | |
| Volume (mm3) | 0.79 (0.38–1.8) | 1.2 (0.47–1.9) | 0.65 (0.26–1.3) | 0.153 |
| Depth from lumen (μm) | 100 (70–170) | 130 (90–260) | 80 (60–130) | 0.015 |
Values are number (%) or median (range).
*Forty–four vessels were analyzed (left anterior descending, n = 19; left circumflex, n = 13; right coronary, n = 12).
DISCUSSION
Acute coronary syndrome (ACS), which is thought to be the result of sudden luminal thrombosis, is a major cause of life-threatening events.8,9) The most common type of vulnerable plaque causing ACS is thin-cap fibroatheroma, which has a large necrotic core with a thin fibrous cap.5,10) Many reports using various modalities have demonstrated detailed in vivo analyses of this type of vulnerable plaque.11-15) Also, calcified nodules are associated with luminal thrombus in cases of ACS without plaque rupture.5) Studies using computed tomography coronary angiography (CTCA) or intravascular ultrasound (IVUS) demonstrated that spotty calcifications are more frequently detected in ACS patients than in stable AP patients.16,17) According to the American Heart Association classification10), a coronary superficial calcified nodule protruding into the vessel lumen is one type of vulnerable plaque based on pathologic study, but its prevalence is low.5,10) However, to our knowledge, there has been no report which sheds light on morphologic features, because of the limited resolution of the above mentioned modalities. This study showed for the first time that superficial calcified nodules intruding of the lumen certainly exist in vivo. Furthermore, we measured the volume and depth of each coronary superficial calcified nodule from the lumen using OCT. There are some technical concerns when measuring of calcifications using CTCA or IVUS, such as the partial volume effects or the acoustic shadowing they cause. Although we could not show the clinical impact of this type of coronary superficial calcified nodule, we speculated that a coronary superficial calcified nodule classified as type II, especially a shallow one, might be vulnerable.
We used the term “calcified nodule” to refer to calcifications we analyzed. However, more accurately, a calcified nodule is considered to be pathologically different from a fibrocalcified lesion. A calcified nodule which might appear to be associated with healed plaque is very close to the lumen. Besides, fibrocalcified lesions which might be the end result of plaque rupture and/or erosion healing and calcification are relatively large and have thick overlying fibrous caps in the intima close to the media.5) Therefore, a type I superficial calcified nodule might be a fibrocalcified lesion, and type II might be a calcified nodule. However, the methodology of how to distinguish a calcified nodule from a fibrocalcified lesion using OCT has not been well established. Furthermore, their origin, as well as their atherosclerosis process, are not well clarified. We considered that detailed in vivo visualization of coronary plaque using the OCT technique might serve to resolve these issues.
PCI is one of the established treatments for patients with AP. The reported frequency of angiographic coronary calcification in patients undergoing PCI is ranges from 17% to 35%.18,19) Enhanced lesion rigidity due to coronary calcification complicates PCI procedures, such as device delivery and deployment. Moussa et al. have reported that technical failure because of an inability to deliver stents took place more frequently in patients with calcified lesions than in those without.19) Type II coronary superficial calcified nodules might complicate to the delivery of various devices, such as a balloon or stent, to the target coronary lesion. Balloon dilatation of calcified lesions is associated with an increased risk of coronary dissection and perforation. Furthermore, coronary calcification is also associated with vessel dissection caused by balloon dilatation and/or underexpansion of coronary stents, which are considered as risk factors for restenosis, stent thrombosis, and target-lesion revascularization following PCI.18,19) However, the question of which type of coronary calcification is associated with procedural and/or future complications of PCI is still under consideration. Detailed observation of coronary calcifications might be helpful for resolving these clinical issues. Since only morphologic features of non-culprit calcified lesions were evaluated in this study, the coronary calcification of target lesions, including clinical outcomes, should be undertaken in future studies.
Limitations of this study need to be discussed. We failed to show the association between morphologic features of superficial calcified nodules with future cardiovascular events, due to our limited number of patients and short duration of follow-up. Long-term follow-up studies with a larger population are warranted to confirm the clinical significance of our findings obtained from OCT examinations.
In summary, OCT could visualize coronary superficial calcified nodules in detail, and enabled us to make in vivo morphologic characterizations and quantifications of them. In the current study, coronary superficial calcified nodules were classified into two groups, depending on whether or not they intruded the lumen. Quantitative analyses revealed that coronary superficial calcified nodules which intruded the lumen existed significantly closer to the lumen, and tended to have a smaller volume than the non-intrusive nodules.
REFERENCES
- 1).Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Kang DH, Halpern EF, Tearney GJ. Characterization of human atherosclerosis by optical coherence tomography. Circulation, 2002; 106: 1640–1645. [DOI] [PubMed]
- 2).Jang IK, Tearney GJ, MacNeill B, Takano M, Moselewski F, Iftima N, Shishkov M, Houser S, Aretz HT, Halpern EF, Bouma BE. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation, 2005; 111: 1551–1555. [DOI] [PMC free article] [PubMed]
- 3).Kawasaki M, Bouma BE, Bressner J, Houser SL, Nadkarni SK, MacNeill BD, Jang IK, Fujiwara H, Tearney GJ. Diagnostic accuracy of optical coherence tomography and integrated backscatter intravascular ultrasound images for tissue characterization of human coronary plaques. J Am Coll Cardiol, 2006; 48: 81–88. [DOI] [PubMed]
- 4).Kume T, Okura H, Kawamoto T, Yamada R, Miyamoto Y, Hayashida A, Watanabe N, Neishi Y, Sadahira Y, Akasaka T, Yoshida K. Assessment of the coronary calcification by optical coherence tomography. EuroIntervention, 2011; 6: 768–772. [DOI] [PubMed]
- 5).Kume T, Akasaka T, Kawamoto T, Watanabe N, Toyota E, Neishi Y, Sukmawan R, Sadahira Y, Yoshida K. Assessment of coronary arterial plaque by optical coherence tomography. Am J Cardiol, 2006; 97: 1172–1175. [DOI] [PubMed]
- 6).Yoshikawa D, Ishii H, Kurebayashi N, Sato B, Hayakawa S, Ando H, Hayashi M, Isobe S, Okumura T, Hirashiki A, Takeshita K, Amano T, Uetani T, Yamada S, Murohara T. Association of cardiorespiratory fitness with characteristics of coronary plaque: Assessment using integrated backscatter intravascular ultrasound and optical coherence tomography. Int J Cardio,l 2011 (Epub ahead of print) [DOI] [PubMed]
- 7).Mintz GS, Pichard AD, Popma JJ, Kent KM, Satler LF, Bucher TA, Leon MB. Determinants and correlates of target lesion calcium in coronary artery disease: a clinical, angiographic and intravascular ultrasound study. J Am Coll Cardiol, 1997; 29: 268–274. [DOI] [PubMed]
- 8).Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med, 1997; 336: 1276–1282. [DOI] [PubMed]
- 9).Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol, 2000; 20: 1262–1275. [DOI] [PubMed]
- 10).Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation, 2003; 108: 1664–1672. [DOI] [PubMed]
- 11).Amano T, Matsubara T, Uetani T, Nanki M, Marui N, Kato M, Arai K, Yokoi K, Ando H, Ishii H, Izawa H, Murohara T. Impact of metabolic syndrome on tissue characteristics of angiographically mild to moderate coronary lesions integrated backscatter intravascular ultrasound study. J Am Coll Cardiol, 2007; 49: 1149–1156. [DOI] [PubMed]
- 12).Miyagi M, Ishii H, Murakami R, Isobe S, Hayashi M, Amano T, Arai K, Yoshikawa D, Ohashi T, Uetani T, Yasuda Y, Matsuo S, Matsubara T, Murohara T. Impact of renal function on coronary plaque composition. Nephrol Dial Transplant, 2010; 25: 175–181. [DOI] [PubMed]
- 13).Harada K, Amano T, Uetani T, Funahashi H, Arai K, Okada K, Hirashiki A, Hayashi M, Oshima S, Ishii H, Izawa H, Matsubara T, Murohara T. Accuracy of 64–slice multidetector computed tomography for classification and quantitation of coronary plaque: Comparison with integrated backscatter intravascular ultrasound. Int J Cardiol, 2011; 149: 95–101. [DOI] [PubMed]
- 14).Ino Y, Kubo T, Tanaka A, Kuroi A, Tsujioka H, Ikejima H, Okouchi K, Kashiwagi M, Takarada S, Kitabata H, Tanimoto T, Komukai K, Ishibashi K, Kimura K, Hirata K. Difference of culprit lesion morphologies between ST-segment elevation myocardial infarction and non-ST-segment elevation acute coronary syndrome: an optical coherence tomography study. JACC Cardiovasc Interv, 2011; 4: 76–82. [DOI] [PubMed]
- 15).Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation, 1995; 92: 657–671. [DOI] [PubMed]
- 16).Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, Inoue K, Okumura M, Ishii J, Anno H, Virmani R, Ozaki Y, Hishida H, Narula J. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol, 2007; 50: 319–326. [DOI] [PubMed]
- 17).Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, Nakamura Y, Yamashita H, Yamagishi H, Takeuchi K, Naruko T, Haze K, Becker AE, Yoshikawa J, Ueda M. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation, 2004; 110:3424–3429. [DOI] [PubMed]
- 18).Kawaguchi R, Tsurugaya H, Hoshizaki H, Toyama T, Oshima S, Taniguchi K. Impact of lesion calcification on clinical and angiographic outcome after sirolimus-eluting stent implantation in real-world patients. Cardiovasc Revasc Med, 2008; 9: 2–8. [DOI] [PubMed]
- 19).Moussa I, Ellis SG, Jones M, Kereiakes DJ, McMartin D, Rutherford B, Mehran R, Collins M, Leon MB, Popma JJ, Russell ME, Stone GW. Impact of coronary culprit lesion calcium in patients undergoing paclitaxel-eluting stent implantation (a TAXUS-IV sub study). Am J Cardiol, 2005; 96: 1242–1247. [DOI] [PubMed]


