ABSTRACT
HCO3–-rich fluid in the pancreatic juice (2~3 L/day) is secreted by epithelial cells lining the pancreatic duct tree, while digestive enzymes are secreted by acinar cells with a small amount of Cl–-rich fluid. Ductal HCO3– secretion is not only regulated by gastrointestinal hormones and cholinergic nerves but is also influenced by luminal factors: intraductal pressure, Ca2+ concentration, pathological activation of protease and bile reflux. The maximum HCO3– concentration of the juice under secretin stimulation reaches 140–150 mM. Thus pancreatic duct cells secrete HCO3– against a ~7-fold concentration gradient. HCO3– secretion critically depends on the activity of CFTR, a cAMP-dependent anion channel localized in the apical membrane of various epithelia. In the proximal part of pancreatic ducts close to acinar cells HCO3– secretion across the apical membrane is largely mediated by SLC26A6 Cl–-HCO3– exchanger. In distal ducts where the luminal HCO3– concentration is already high, most of the HCO3– secretion is mediated by HCO3– conductance of CFTR. CFTR is the causative gene for cystic fibrosis. Loss of function due to severe mutations in both alleles causes typical cystic fibrosis characterized by dehydrated, thick, and viscous luminal fluid/mucus in the respiratory and gastrointestinal tract, pancreatic duct, and vas deferens. A compound heterozygote of mutations/polymorphisms (causing a mild dysfunction of CFTR) involves a risk of developing CFTR-related diseases such as chronic pancreatitis. In cystic fibrosis and certain cases of chronic pancreatitis, the pancreatic duct epithelium secretes a small amount of fluid with neutral~acidic pH, which causes an obstruction of the duct lumen by a protein plug or viscous mucus.
Key Words: Isolated pancreatic duct, CFTR, Cystic fibrosis, Alcoholic pancreatitis
INTRODUCTION
Exocrine glands of the human pancreas produce 2~3 L/day of pancreatic juice, which is an alkaline isotonic fluid that contains various kinds of digestive enzymes and other proteins such as lactoferrin and pancreatic stone protein. Pancreatic acinar cells occupying ~90% of the exocrine glands secrete the digestive enzymes by exocytosis with a small amount of Cl–-rich fluid secretion. The large amounts of NaHCO3-rich fluid in the pancreatic juice are secreted by epithelial cells lining the small/proximal pancreatic duct, though the duct cells occupy only ~10% of the exocrine glands. Thus the pancreatic duct is not merely a route for delivering digestive enzymes to the duodenal lumen but also secretes NaHCO3-rich fluid to effectively flush out the digestive enzymes. In cystic fibrosis and certain cases of chronic pancreatitis, the pancreatic duct epithelium secretes a small amount of fluid with neutral pH (sometimes even acidic), which causes an obstruction of the duct lumen by precipitated proteins or viscous juice.1,2)
SITE OF BICARBONATE SECRETION IN THE PANCREATIC JUICE
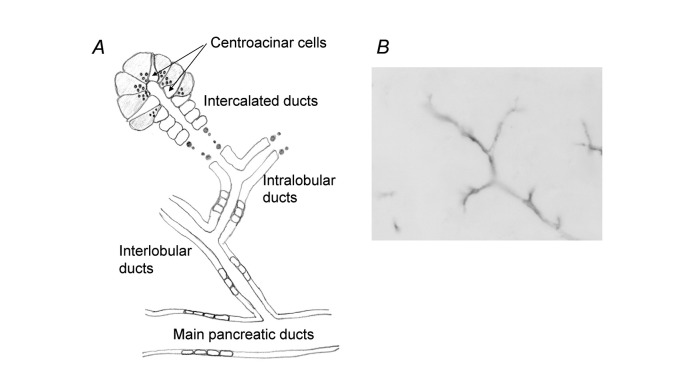
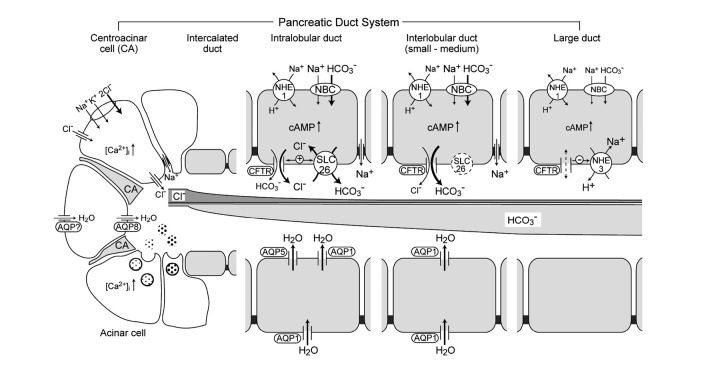
Figure 1A shows the schematic architecture of a pancreatic ductal tree. The main pancreatic duct branches into interlobular ducts and then into intralobular ducts that connect to the acinar lumen via intercalated ducts. Centroacinar cells located in the acinar lumen are connected to intercalated duct cells.3) The centroacinar cells are immunopositive to carbonic anhydrase IV4) and belong to the duct system. Figure 1B shows immunostaining of cystic fibrosis transmembrane conductance regulator (CFTR) in a guinea-pig pancreas. CFTR is the causative gene for cystic fibrosis.5) The CFTR protein functions as a cAMP-regulated anion channel in the apical membrane of epithelial cells,6) and HCO3– secretion by the pancreatic duct is totally dependent on CFTR activity.7) CFTR immunoreactivity has been detected in the apical membrane of centroacinar cells, intralobular ducts, small interlobular ducts in both a guinea-pig pancreas (Figure 1B) and a human pancreas.8) Aquaporin (AQP) 5 is co-localized with CFTR in a human pancreas.9)
Fig. 1.
Pancreatic duct system. (A) Schematic architecture of a pancreatic ductal tree. (B) Immunostaining of CFTR in guinea-pig pancreas.
When the exocrine pancreas is stimulated by intravenously-applied secretin (a gastrointestinal hormone), the volume and pH (HCO3– concentration) of the pancreatic juice increase10) As the HCO3– concentration of the juice increases, the Cl– concentration of the juice reciprocally decreases, keeping [HCO3–] + [Cl–] = constant at ~160 mM. The transepithelial transport of anion and water by the pancreatic duct is osmotically coupled, which suggests that the water permeability of the basolateral and apical membranes of pancreatic duct cells is sufficiently high.8) Micropuncture studies of in situ pancreas demonstrated that the luminal concentrations of HCO3– ([HCO3–]L) increased at the levels of small/proximal pancreatic ducts.11) Epithelium of the main duct allows exchange of luminal HCO3– with Cl– with no net flux of fluid.
These findings suggest that epithelial cells lining the small/proximal pancreatic ducts and centroacinar cells are the major sites of HCO3– and water secretion in the human pancreas. The presence of centroacinar cells in the acinar lumen may be important in flushing out the protein-rich content which is secreted by acinar cells. Significant differences of HCO3–-secreting capability have been found among various species.10) While the maximum HCO3– concentration in the pancreatic juice reaches 140–150 mM in humans, guinea-pigs, dogs, etc., the mice and rat pancreas secretes juice containing only ~50 and ~70 mM HCO3–, respectively. Although genetically-engineered mice are useful in studying the molecular mechanisms for HCO3– secretion, care must be taken in interpreting the physiological significance of the data. For this reason, guinea-pigs have been preferentially used in some laboratories including ours.
STUDIES OF BICARBONATE SECRETION USING ISOLATED PANCREATIC DUCT
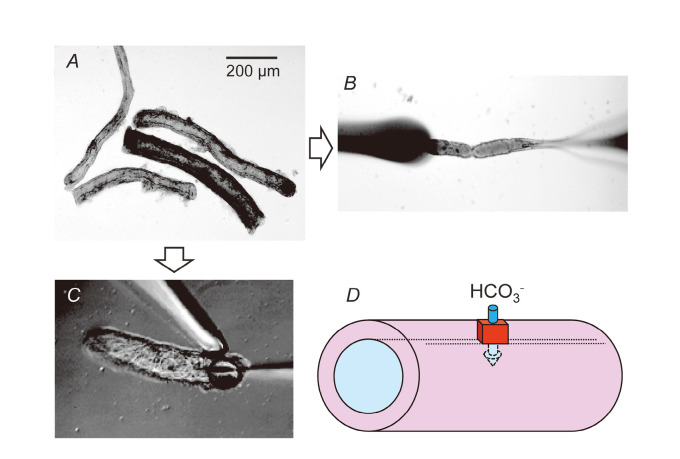
In vivo data suggest that the pancreatic duct cells of humans and guinea-pigs are able to secrete isotonic fluid containing very high concentrations of HCO3– when the intracellular cAMP-pathway is activated. To confirm this prediction and to study the cellular and molecular mechanisms of vectorial HCO3– transport, intralobular and interlobular pancreatic duct segments have been isolated and used for experiments.12) Figure 2A shows interlobular duct segments (diameter: ~100 μm) isolated from a guinea-pig pancreas. The isolated pancreatic duct retains cAMP-regulated vectorial HCO3– transport7,13) and provides useful experimental models for studying cellular mechanisms of HCO3– secretion by native polarized epithelia (Fig. 2D).
Fig. 2.
Isolated pancreatic interlobular duct. (A) Interlobular duct segments isolated from guinea-pig pancreas. (B) Microperfusion of the lumen. (C) Micropuncture of the lumen. (D) Vectorial HCO3 – transport by pancreatic duct cells.
Fluxes of ions mediated by channels, transporters, and pumps depend on intra- and extracellular concentrations of the transported ions and membrane potential as well as the activity and density of the transporters. To study the mechanisms for HCO3– transport across the apical and basolateral membranes, the duct lumen can be “microperfused” to modify [HCO3–]L (Fig. 2B). Fluorescent indicators of intracellular pH, Cl–, Ca2+, Na+ and microelectrodes can be applied to the isolated pancreatic duct.
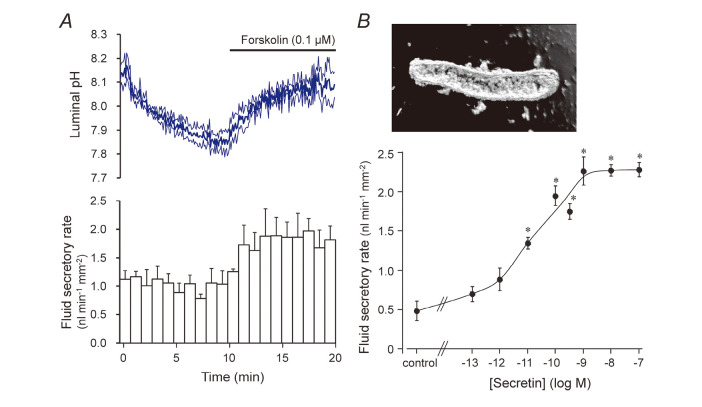
When isolated segments of interlobular ducts are cultured for a few hours~overnight, both ends of the ducts seal spontaneously, thus isolating the lumen from the bath (Fig. 3B). The rate of fluid secretion into the lumen can be estimated from the swelling rate of the lumen and expressed per unit area of epithelium. When the sealed ducts were superfused with the standard HCO3–-buffered solution at 37oC, a bath application of secretin increased the rate of fluid secretion in a concentration-dependent manner14) (Fig. 3B). The isolated ducts from guinea-pig pancreas responded to physiological concentrations (1–10 μM) of secretin. That response was critically dependent on the temperature of the bathing solution. To examine the changes in the luminal pH/[HCO3–]L associated with fluid secretion, BCECF-dextran (a membrane-impermeable pH indicator) can be applied to the lumen using the “micropuncture” technique15) (Fig. 2C). The accumulated fluid in the lumen is discarded and the luminal space is filled with an experimental solution of a known ion composition containing BCECF-dextran. Figure 3A shows the concurrent changes in luminal pH and the fluid secretory rate in the ducts of which the lumen was filled with the high-HCO3– (125 mM HCO3–-24 mM Cl–, pH ~8.2) solution. Under the basal condition, luminal pH slowly decreased, which was presumably accompanied by a Cl–-rich fluid secretion. Upon stimulation with forskolin (a stimulator of adenylate cyclase), luminal pH started to rise and the fluid secretory rate increased by ~70%. This confirms that pancreatic duct epithelium secretes HCO3–-rich fluid when stimulated by cAMP.
Fig. 3.
Measurement of HCO3 – and fluid secretion by isolated interlobular pancreatic ducts. (A) Concurrent measurements of HCO3 – and fluid secretion in ducts filled with high-HCO3 – solution. Time course changes in luminal pH and fluid secretory rates are shown (mean ± S.E.M., n = 4). (B) Effects of secretin concentration on the rate of fluid secretion into the lumen of sealed pancreatic ducts. Mean ± S.E.M. of 4–5 experiments. Asterisks denote significant (p < 0.05) difference from the control.
For details of our methods, please refer to our recent article in THE PANCREAPEDIA http://www.lib.umich.edu/spo/panc/.16)
CELLULAR MECHANISMS OF BICARBONATE SECRETION BY PANCREATIC DUCT
Previous studies by whole-cell and single-channel recordings of isolated single pancreatic duct cells revealed the presence of CFTR anion channel17) and maxi-K+ channel.18) Measurements of the transepithelial and intracellular potential of microperfused pancreatic ducts have revealed the presence of cAMP-activated Cl– channel, Cl–-HCO3– exchanger in the apical membrane19) and Na+-H+ exchanger, Na+,K+-ATPase, K+ conductance in the basolateral membrane.20) The classical model of HCO3– secretion by pancreatic duct cells10) is based on these findings as well as on the abundant expression of carbonic anhydrase21) and the low HCO3– permeability of CFTR.22) The combination of H+ extrusion via Na+-H+ exchange across the basolateral membrane and the hydration of CO2 in the cells results in the accumulation of HCO3– in the cells. The intracellular HCO3– is secreted into the lumen in exchange for luminal Cl–, and CFTR recycles intracellular Cl– into the lumen. When the Cl– recycling is accelerated by the activation of CFTR by cAMP, the apical Cl–-HCO3– exchanger operates faster and HCO3– secretion increases.
To re-examine the cellular mechanisms for HCO3– secretion by pancreatic duct epithelium, we have measured intracellular pH/HCO3– ([HCO3–]i),23) Cl– concentration ([Cl–]i),24) and membrane potential (Vm)25) in guinea-pig interlobular pancreatic ducts, the lumen of which was microperfused with either high-Cl– (25 mM HCO3–-124 mM Cl–) or high-HCO3– (125 mM HCO3–-24 mM Cl–) solution.
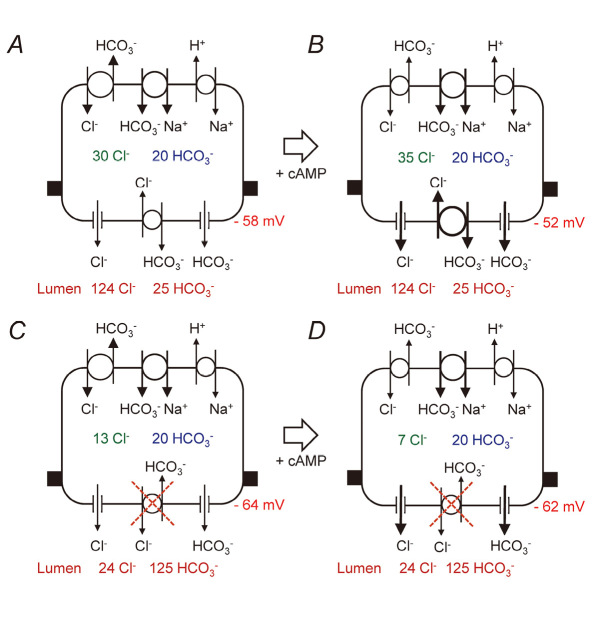
With 25 mM HCO3–-124 mM Cl– in the lumen, [Cl–]i and [HCO3–]i were estimated to be ~30 and ~20 mM, and Vm was estimated to be ~-58 mV in the unstimulated condition (Fig. 4A). Stimulation with cAMP resulted in the elevation of [Cl–]i to ~35 mM, and membrane depolarization to ~-52 mV (Figure 4B). Those data are consistent with the activation of CFTR anion channel and more fluxes of Cl–-HCO3– exchange across the apical membrane. HCO3– secretion is thus largely mediated by Cl–-HCO3– exchange, which is essentially similar to the classic model except for the presence of Na+-HCO3– cotransporter in the basolateral membrane. It is generally thought that Na+-HCO3– cotransport mediated by pNBC1 contributes more to HCO3– accumulation than Na+-H+ exchange (NHE1).26)
Fig. 4.
Implications of measured values of intracellular Cl– and HCO3 – concentrations and intracellular potential for HCO3 – transport in ducts luminally-microperfused with high-Cl– (25 mM HCO3 –-124 mM Cl–) solution (A and B) or high-HCO3 – (125 mM HCO3 –-24 mM Cl–) solution (C and D). Adapted from reference 25.
With 125 mM HCO3–-24 mM Cl– in the lumen, [Cl–]i and [HCO3–]i were estimated to be ~13 and ~20 mM and Vm was estimated to be ~–64 mV in the unstimulated condition (Fig. 4C). Stimulation with cAMP resulted in the decrease of [Cl–]i to ~7 mM, while Vm remained at ~-62 mV (Fig. 4D). Apical Cl–-HCO3– exchange can no longer work for HCO3– secretion/Cl– absorption, and may even operate in reverse to absorb luminal HCO3–. Activation of CFTR and the diminished activity of apical and basolateral Cl–-HCO3– exchange results in the decrease of [Cl–]i. CFTR functions as a HCO3– channel27) under this condition that [Cl–]i is low and the electrochemical gradient of Cl– across the apical membrane is small. The apical Cl–-HCO3– exchange is probably mediated by SLC26A6 anion transporter.28-30) If the apical anion exchanger mediates 1 Cl– : 1 HCO3– exchange, the activity needs to be reduced to prevent HCO3– absorption.31) If the stochiometry is 1 Cl– to 2 HCO3– as reported elsewhere,32,33) it does not need to be inhibited because the predicted equilibrium value of the luminal HCO3– concentration ([HCO3–]L) is ~130 mM.33)
Figure 5 shows an integrated model of HCO3– secretion by pancreatic duct system.33,34) A relatively small volume of Cl–-rich fluid is secreted by the acinar cells. In the proximal ducts (near acini), HCO3– secretion across the apical membrane is largely mediated by SLC26A6 Cl–-HCO3– exchanger. Due to HCO3– secretion and Cl– absorption, [Cl–]L falls and [HCO3–]L rises as the secreted fluid flows through the duct system. In distal ducts (far from acini) most of the HCO3– secretion is mediated by CFTR HCO3– conductance as the Cl–-HCO3– exchanger approaches equilibrium.
Fig. 5.
An integrated model for HCO3 – secretion in the pancreatic duct system (adapted from references 34 and 35).
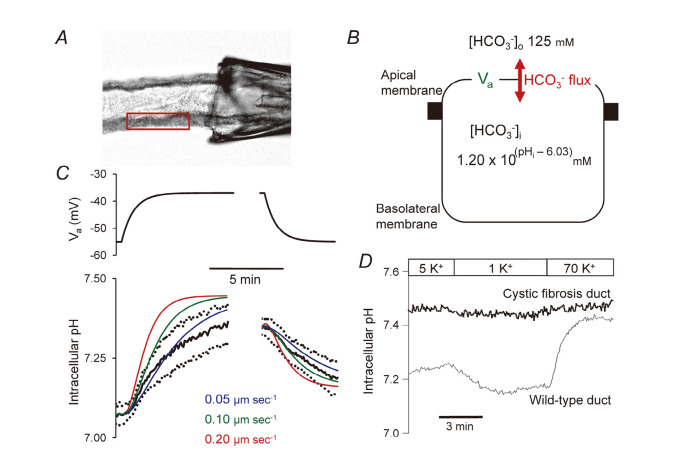
To check whether the HCO3– permeability (PHCO3) of the apical membrane is large enough to mediate the observed HCO3– secretion, we have measured the voltage-driven fluxes of HCO3– through conductive pathways in the apical membrane (Fig. 6).27) The conductive HCO3– flux was largely dependent on CFTR activity (Fig. 6D), and the estimated value of PHCO3 (~0.1 μm s–1), Fig. 6C) is close to the value required for the observed rates of HCO3– secretion (~0.5 nmol sec–1) cm–2).25) This is the first study to estimate absolute HCO3– permeability in native epithelium.35)
Fig. 6.
Estimation of the HCO3 – permeability of the apical membrane. (A) The lumen was microperfused, and cells were loaded with BCECF, pH-sensitive dye. A small region of the duct epithelium was selected for measurements of intracellular pH (pHi). (B) Since the pancreatic duct is a leaky epithelium (transepithelial potential is small), the potential difference of the apical membrane (Va) could be manipulated by changing the bath K+ concentration. HCO3 – flux across the basolateral membrane was inhibited by the anion transport inhibitor, H2DIDS (dihydro-4,4’-diisothiocyanatostilbene-2,2’-disulphonic acid). Voltage-driven fluxes of HCO3 – across the apical membrane were detected as changes in pHi. (C) Changes in pHi resulting from changes in Va. Theoretical curves based on 3 alternative apical HCO3 – permeability values were superimposed on averaged data (mean ± SEM). (D) HCO3 – fluxes across the apical membrane were abolished in pancreatic ducts isolated from a cystic fibrosis mouse model (∆F mouse) lacking in CFTR activity. Adapted from reference 27.
A recent work has demonstrated the presence of [Cl–]i-sensitive kinase, with-no-lysine (WNK1) kinase and its downstream kinases, oxidative stress-responsive kinase (OSR1), and sterile 20/SPS1-related proline/alanine-rich kinase (SPAK) in pancreatic duct cells.36) Low [Cl–]i induced the phosphorylation of those kinases, which (1) increased the relative permeability of HCO3– over Cl– of CFTR anion channel and (2) inhibited the activity of Cl–-HCO3– exchanger in the apical membrane. These modulations would assist in the fluid secretion of >140 mM HCO3– concentration by the pancreatic duct system.31)
Aquaporin (AQP) water channels are included in the pancreatic duct model (Fig. 5). The osmotic water permeability (Pf) of isolated pancreatic duct epithelium from rat was estimated to be 160–230 μm sec–1,37) and its value was comparable with the values of the distal airway and bile duct. AQP5 is thought to be a major AQP in the apical membrane of secretory epithelia such as the salivary gland. In Aqp5-/- mice, the rate of salivary flow was decreased, the saliva was hypertonic,38) and the fluid secretion by tracheal submucosal glands was reduced.39) In the human pancreas AQP5 is localized in the apical membrane of intercalated ducts.9) AQP1 (an ubiquitous type of AQP) is localized in both the basolateral and apical membranes of the rat37) and the human9) pancreatic duct. AQP1 immunoreactivity was also found in the caveolae and intracellular vesicles.40)
REGULATION OF BICARBONATE SECRETION BY PANCREATIC DUCT CELLS
Food intake activates both the acinar and duct cells of the pancreas to stimulate enzyme and fluid secretion, which is mediated by various gastrointestinal hormones and cholinergic nerves. Secretin, vasoactive intestinal peptide (VIP), bombesin, and acetylcholine stimulate HCO3– and fluid secretion by isolated pancreatic ducts, while 5-hydroxytryptamine (5-HT, serotonin), substance P (SP), arginine vasopressin (AVP), and ATP (when applied to the basolateral side) inhibit secretion.41) SP and ATP probably function as neurotransmitters. SP is contained in the capsaicin-sensitive nerve endings of sensory neurons in the pancreas, and its local release induces neurogenic inflammation during pancreatitis.42) The potent inhibitory effects of SP on fluid and HCO3– secretion by pancreatic duct cells43) may reduce intraductal pressure, and thus may be advantageous for the conservation of pancreatic tissue during the course of acute painful pancreatitis. AVP inhibits pancreatic fluid secretion by directly acting on pancreatic duct cells as well as by inducing vasoconstriction of the pancreatic artery.44) When fluid intake is restricted or fluid loss is increased, pancreatic fluid secretion may be inhibited via the elevation of plasma osmolality and the subsequent release of AVP.45)
The long-term or slow regulation of HCO3– and fluid secretion by pancreatic duct cells is probably governed by such neurohormonal control and is mediated by receptors localized in the basolateral membranes. However, the ion composition and pH of the luminal fluid as well as intraductal pressure should be precisely and quickly controlled to avoid mechanical injury to the pancreatic duct, the autoactivation of trypsin, and the precipitation of pancreatic stone protein and CaCO3 in the lumen. Activation of Ca2+-sensing receptor in the apical membrane enhanced HCO3– secretion, which involved the elevation of intracellular Ca2+ concentration.46) This suggests that pancreatic duct cells sense the elevation of [Ca2+]L and respond by sufficiently increasing fluid secretion to dilute [Ca2+]L. Multiple types of purinergic receptors (P2Y2, P2Y4, P2Y11, P2X4 and P2X7) have been found in the pancreatic duct.47) ATP or UTP injected into the lumen of isolated pancreatic ducts enhanced HCO3– and fluid secretion.48) Those nucleotides are probably released from acinar cells together with the exocytotic release of digestive enzymes, and they activate HCO3– and fluid secretion by the pancreatic duct to effectively flush out the enzymes.
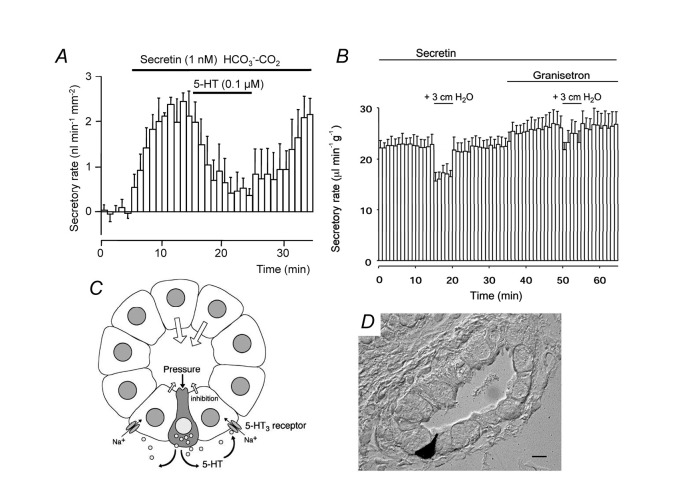
5-hydroxytryptamine (5-HT, serotonin)-immunoreactive cells with the morphological characteristics of enterochromaffin cells (EC cells) were found scattered in the pancreatic duct epithelium (Fig. 7D).49) These cells may sense the elevation of intraductal pressure (as EC cells do in the intestine), and the released 5-HT may inhibit fluid secretion by pancreatic duct (Figure 7C). In fact, basolateral application of 5-HT at relatively low concentrations (IC50: ~30 nM) inhibited fluid secretion strongly (by ~75%) by isolated pancreatic ducts (Fig. 7A). A small elevation (+ 3 cm H2O) of pressure reversibly reduced pancreatic fluid secretion in vivo (anesthetized guinea-pig), and the effect was attenuated by intravenous granisetron, a 5-HT3 receptor antagonist (Fig. 7B). The intraductal pressure of the human pancreas increases after feeding by as much as ~20 cm of H2O.50) That pressure is elevated in some patients with chronic pancreatitis. Thus 5-HT may regulate pancreatic fluid secretion under physiological and pathological conditions.
Fig. 7.
5-hydroxytryptamine regulates fluid secretion by the pancreatic duct. (A) Effects of 5-hydroxytryptamine (5-HT, serotonin) on secretin-stimulated fluid secretion by isolated guinea-pig pancreatic ducts. Mean ± S.E.M. of 4 experiments. (B) Effects of small elevations of intraductal pressure and 5-HT3 receptor antagonist (granisetron) on in vivo pancreatic fluid secretion under secretin stimulation (guinea-pig). Mean ± S.E.M. of 4 experiments. (C) A hypothetical mechanism. EC cell-like cells in the duct epithelium sense the elevation of intraductal pressure and release 5-HT into the interstitium. The released 5-HT binds to 5-HT3 receptor (a ligand-gated non-selective cation channel) in the basolateral membrane of neighboring duct cells, which facilitates Na+ entry into the cell. That reduces the inward gradient of Na+, which is necessary for intracellular HCO3 – accumulation via basolateral Na+-HCO3 – cotransport. (D) 5-HT-immunoreactive cells are embedded in the pancreatic duct epithelium (guinea-pig). Adapted from reference 49.
The volume and anion composition of the pancreatic juice (secretory function of pancreatic ductal epithelium) during the course of human acute pancreatitis is not well understood. In experimental acute pancreatitis in rats (edematous pancreatitis by repetitive subcutaneous injections of cerulein and hemorrhagic pancreatitis by retrograde injections of sodium taurocholate into the pancreatic duct), pancreatic fluid secretion was greatly increased by 4–6 times of the control, and the secreted fluid was relatively Cl–-rich.51,52) An inhibition of pancreatic exocrine secretion (and so a reduction of intraductal pressure) has been generally considered to be a clinical principle in the treatment of acute pancreatitis. However, stimulation of HCO3–-rich fluid secretion could flush out luminal noxious agents such as bile acids, and prevent the intraductal activation of trypsin, thus affording protection against pancreatitis. It is now recognized that the direct effects of bile and protease on pancreatic duct cells may be involved in the changes in pancreatic fluid secretion during the course of acute pancreatitis. The application of low concentrations (0.1 mM) of bile acids (chenodeoxycholate) into the lumen of isolated guinea-pig pancreatic duct stimulated HCO3– secretion, while a higher dose (1 mM) inhibited it.53) The increase of HCO3– secretion was mediated by the activation of large-conductance Ca2+-activated K+ channels in the apical membrane.54)
Protease-activated receptor 2 (PAR2) is a member of the PAR family of G protein-coupled receptors that are activated by proteolytic cleavage within their extracellular N terminus. PAR2 is a receptor for trypsin and mast cell tryptase and is ubiquitously expressed in human tissues. In the pancreas, PAR2 is expressed in acinar cells,55) duct cells,56) satellite cells,57) nerve endings,58) and endothelial cells. PAR2 is thought to mediate a variety of biological responses (inflammation, enzyme secretion, ion transport, fibrosis, pain) associated with pancreatitis. Although PAR2 is a potential target for the therapy of acute pancreatitis, experimental data are confusing, e.g, in PAR2 -/- mice, experimental pancreatitis induced by supramaximal cerulein stimulation was more severe,59) whereas another model of experimental pancreatitis by retrograde injection of bile salts into the pancreatic duct was more mild.60) Applications of PAR2 agonist induced systemic vasodilation (and a decrease in blood pressure), while exerting local protection in experimental pancreatitis (supramaximal cerulein stimulation) in rats.61) In human and rat pancreatic duct cells, basolateral (but not luminal) applications of trypsin or PAR2 agonist induced intracellular Ca2+ mobilization and HCO3– secretion.56,61) However, a recent study using guinea-pig pancreatic duct cells revealed that luminal applications of trypsin inhibit HCO3– secretion.62)
ETHANOL AUGMENTS FLUID SECRETION BY PANCREATIC DUCT
Ethanol abuse is the leading cause of acute and chronic pancreatitis. To investigate how ethanol induces pancreatic injury, the effects of ethanol and its metabolites on isolated pancreatic acinar cells have been examined. Acinar cells catabolize ethanol and produce acetaldehyde and fatty acid ethyl esters, both of which are cytotoxic.63) Ethanol enhanced the caerulein-induced activation of trypsin inside the acinar cells,64) which is a critical event in acute pancreatitis. However, the effective doses of ethanol in the studies were over 30 mM. This concentration of ethanol in the blood could be found when 5 pints of beer were ingested in 1.5 hours,65) which may not reflect normal drinking conditions.66) Thus, the direct toxic/metabolic effect of high doses of ethanol on pancreatic acinar cells may underlie the pathogenesis of alcoholic acute pancreatitis. However, that effect may not be related to chronic pancreatitis, because there is no known threshold dose of ethanol that induces chronic pancreatitis.67) Patients drinking small quantities of ethanol (1–20 g per day) run an even higher risk of developing chronic pancreatitis. A recent study revealed that ethanol doses as low as 10 mM induced Ca2+ release from intracellular stores in acinar cells, while the elevation of intracellular Ca2+ concentrations ([Ca2+]i) was prevented by calmodulin in intact cells.68)
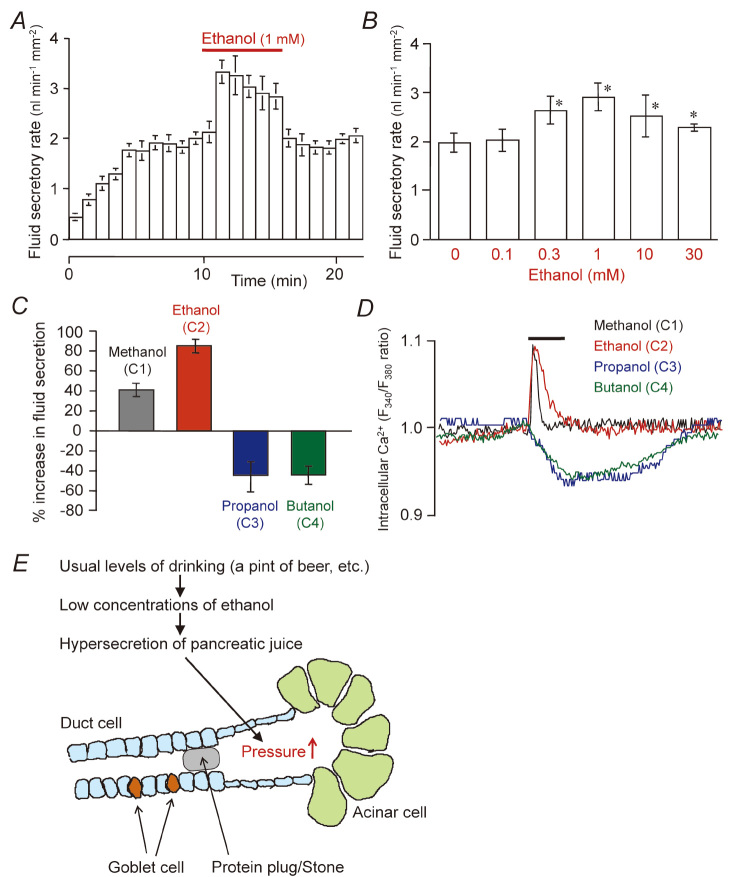
The objective effects of ethanol on the central nervous system (CNS) appear at ~5 mM of the blood ethanol level. Those effects are attributed to the modulation of several neurotransmitter-gated ion channels in CNS neurons69) and are characterized by the cutoff effect (potency increases with an increasing alkyl chain length of n-alcohol up to a point but then disappears with a further increase in chain length).70) In our previous study, we examined the direct effects of ethanol on a concentration range (0.1–30 mM) relevant to normal drinking conditions upon fluid secretion by interlobular ducts isolated from guinea-pig pancreas (Fig. 8A and B).14) Ethanol reversibly and strongly augmented fluid secretion stimulated with physiological concentrations of secretin, which involved a transient increase of [Ca2+]i. We discovered a cutoff effect by which methanol (C1) and ethanol (C2) augmented fluid secretion and induced a transient increase of [Ca2+]i, while propanol (C3) and butanol (C4) inhibited fluid secretion and reduced [Ca2+]i (Fig. 8C and D).71) The presence of such a cutoff effect suggests that ethanol directly modified the activity of some ion channels/transporters in pancreatic duct cells. Their target would be plasma membrane a Ca2+ channel, but some other ion channels/transporters may be affected. Concerning the pathogenesis of alcoholic chronic pancreatitis, the ductal fluid hypersecretion by ethanol may raise the intraductal pressure when the flow of pancreatic juice is blocked by the presence of highly viscous juice or protein plugs (Fig. 8E).
Fig. 8.
Effects of low concentrations of ethanol and other n-alcohols on fluid secretion and intracellular Ca2+ response in isolated pancreatic ducts. (A) Time course of fluid secretion in isolated ducts stimulated by secretin (0.3 nM). Ethanol (1 mM) was added to the bath solution as indicated. Mean ± S.E.M. of 6 experiments. (B) Effects of various concentrations of ethanol (0.1–30 mM) on secretin (1 nM)-stimulated fluid secretion. Mean ± S.E.M. of 4–6 experiments. (C) Effects of n-alcohols (C1 methanol, C2 ethanol, C3 propanol, C4 butanol) on secretin (0.3 nM)-stimulated fluid secretion. Mean ± S.E.M. of 5 experiments. (D) Effects of n-alcohols (C1-C4) on intracellular Ca2+ under secretin stimulation. Each trace is representative of 4 experiments. (E) A hypothesis of ethanol-induced pancreatic injury. Adapted from references 14 and 71.
GLUCOSE TRANSPORT BY PANCREATIC DUCTS
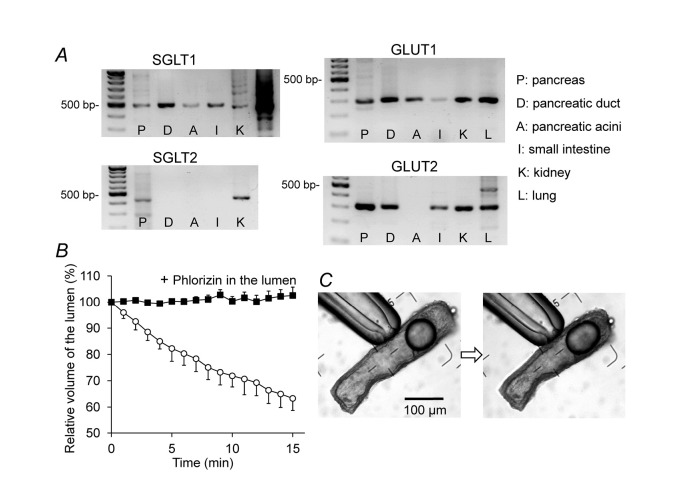
The glucose concentration in human pancreatic juice (0.5–1 mM) is much lower than in plasma.72) We have recently shown that rat interlobular pancreatic ducts express Na+-glucose cotransporter (SGLT1) and glucose transporter (GLUT1 and GLUT2) (Fig. 9A), and absorb luminal glucose iso-osmotically (Fig. 9B and C).73) The absorption of luminal glucose was abolished by phlorizin, an inhibitor of SGLT1. Under a physiological condition, the pancreatic duct epithelium probably absorbs luminal glucose via apical SGLT1 to maintain the glucose concentration at a low level in the pancreatic juice.
Fig. 9.
Expression of glucose transporters and transepithelial glucose transport by isolated pancreatic ducts. (A) mRNA expression of SGLT1, SGLT2, GLUT1, and GLUT2 in the entire pancreas (P), isolated interlobular pancreatic ducts (D), isolated pancreatic acini (A), small intestine (I), kidney (K), and lung (L) of the rat. (B) Absorption of luminal glucose. The lumen of the sealed ducts was micropunctured, and the luminal fluid was replaced with HEPES-buffered solution containing 44.4 mM glucose with or without phlorizin (0.5 mM). The bath was perfused with HEPES-buffered solution containing 44.4 mM glucose. Means ± S.E.M. (n = 4) of the time course changes in luminal volume. (C) Images of a duct at the beginning and end of a representative experiment.
Pancreatic exocrine dysfunction is frequently found in patients with type-I (insulin-dependent) and type-II (noninsulin-dependent) diabetes mellitus (DM). Both enzyme secretion from acinar cells and fluid/HCO3– secretion by duct cells are impaired.74) It was reported that high glucose activates the polyol metabolism in Capan-1 pancreatic duct cells, which results in a decrease in the Na+,K+-ATPase.75) The resultant elevation of [Na+]i would attenuate the Na+ gradient across the basolateral membrane, which reduces HCO3– uptake via Na+-coupled transporters at the basolateral membrane, i.e., Na+-HCO3– cotransporter and Na+-H+ exchanger. SGLT1 activity in the apical membrane may also be involved in the high glucose-induced decrease of ductal secretion.73) The active absorption of luminal Na+ and glucose via SGLT1 increases [Na+]i and depolarizes the apical membrane. [Na+]i elevation reduces HCO3– uptake. Apical depolarization reduces the driving force for HCO3– secretion via CFTR at the apical membrane.
CYSTIC FIBROSIS AND CFTR-RELATED DISEASE OF THE PANCREAS
Cystic fibrosis (CF) is an autosomal recessive hereditary disease caused by the mutations in CFTR. More than 1800 different mutations have been reported to the Cystic Fibrosis Mutation Database (www.genet.sickkids.on.ca/cftr). The incidence of CF and the spectrum of CFTR mutations vary considerably among ethnic groups. CF is the most common hereditary disease in Caucasians (one in 2,500–3,500 newborns), while it is very rare in Asian countries with the incidence in Japan being ~3 per million.76,77) The major mutation F508del (found in 70% of CF chromosomes) has rarely been found among Japanese.
CFTR functions as a cyclic AMP-regulated anion channel in the apical membranes of various epithelia. The loss of function due to severe mutations in both alleles accounts for the typical/classic CF characterized by dehydrated, thick, viscous luminal fluid/mucus in the respiratory and gastrointestinal tract, pancreatic duct, and vas deferens. Such lesions are also called “mucovisidosis.” “Cystic fibrosis” derives its name from the pathology of pancreatic lesions: intra- and interlobular fibrosis, obstruction of small ducts with viscid secretions and cellular debris, and multiple cysts. CFTR mediates Cl– absorption via sweat ducts and the elevated sweat Cl– concentration has become the gold standard for CF diagnosis.
A compound heterozygote of severe and mild mutations (5~50% of CFTR function remained) poses a risk for developing atypical/non-classic CF, which involves the dysfunction of a single organ such as idiopathic chronic pancreatitis, male infertility due to congenital bilateral absence of the vas deferens, bronchiectasis, and nasal polyposis. They are also called CFTR-related diseases.78) Although CF is rare among the Japanese, certain cases of chronic pancreatitis in Japanese patients showed high levels (≥ 60 mM) of sweat Cl–, suggesting a dysfunction of CFTR.79,80) Two haplotypes, M470V-Q1352H and M470V-R1453W, were associated with idiopathic chronic pancreatitis.81)
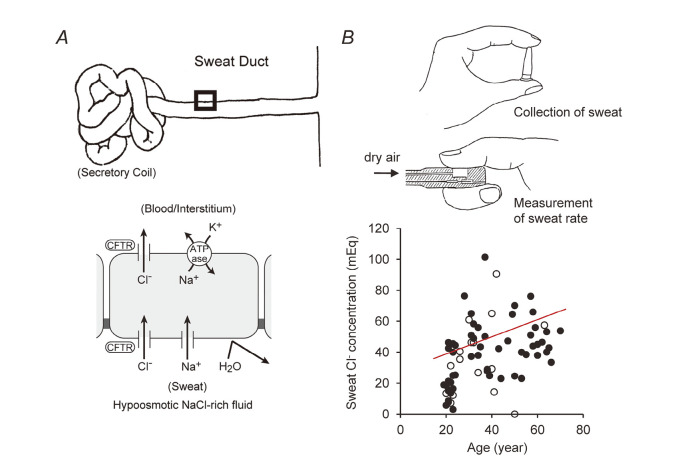
Primary sweat (containing ~150 mM NaCl) is produced by the secretory coil with adrenergic stimulation. Cl– is re-absorbed via CFTR, while the primary sweat flows through the sweat ducts. Because the water permeability of the sweat duct epithelium is very low, the final excreted sweat is hypo-osmotic (containing 20–30 mM NaCl in healthy subjects) (Fig. 10).82) Measurements of Cl– concentration in the sweat excreted from forearm skin after stimulation with pilocarpine iontophoresis is the standard method for estimating sweat Cl– levels. Though pilocarpine iontophoresis is not readily available in Japan, it can be replaced by the “finger sweat chloride test”.80) This method is based on the observation that sweat rates of the right and left fingers were almost identical.83) Sweat electrolytes are collected from one thumb while the sweat rate of the other thumb is measured by a perspiration meter (Fig. 10B). A significant correlation was found between sweat Cl– concentrations and age in healthy subjects.84)
Fig. 10.
Sweat Cl– concentration is a measure of CFTR function in humans. (A) Role of the CFTR anion channel in Cl– reabsorption by sweat ducts. (B) Finger sweat Cl– test. Correlation between age and sweat Cl– concentration in healthy subjects (n = 73, p < 0.01). Closed circles: males; open circles: females. Adapted from references 80 and 84.
CONCLUSION AND REMARKS
Transepithelial HCO3– transport is not only important for pancreatic juice secretion and the digestion of nutrients but also plays a key role in maintaining the proper mucosal defense (immune system and ciliary movement) in the gastrointestinal and respiratory tracts. The active transport of HCO3– across epithelia induces water movement, which maintains the water content and neutral pH of the mucus layer. The isolated interlobular pancreatic duct retains cAMP-regulated vectorial HCO3– transport, thus providing useful experimental models for studying the cellular mechanisms of HCO3– secretion by native polarized epithelia.
ACKNOWLEDGMENTS
This work was supported by grants from the Japanese Society for the Promotion of Science and the Research Committee of Intractable Pancreatic Diseases (principal investigator: Tooru Shimosegawa) provided by the Ministry of Health, Labour, and Welfare of Japan.
REFERENCES
- 1).Kopelman H, Corey M, Gaskin K, Durie P, Weizman Z, Forstner G. Impaired chloride secretion, as well as bicarbonate secretion, underlies the fluid secretory defect in the cystic fibrosis pancreas. Gastroenterology, 1988; 95: 349–55. [DOI] [PubMed]
- 2).Freedman SD. New concepts in understanding the pathophysiology of chronic pancreatitis. Int J Pancreatol, 1998; 24: 1–8. [DOI] [PubMed]
- 3).Ashizawa N, Sakai T, Yoneyama T, Naora H, Kinoshita Y. Three-dimensional structure of peripheral exocrine gland in rat pancreas: reconstruction using transmission electron microscopic examination of serial sections. Pancreas, 2005; 31: 401–404. [DOI] [PubMed]
- 4).Fanjul M, Alvarez L, Salvador C, Gmyr V, Kerr-Conte J, Pattou F, Carter N, Hollande E. Evidence for a membrane carbonic anhydrase IV anchored by its C-terminal peptide in normal human pancreatic ductal cells. Histochem Cell Biol, 2004; 121: 91–99. [DOI] [PubMed]
- 5).Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, Drumm ML, Iannuzzi MC, Collins FS, Tsui LC. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science, 1989; 245: 1066–1073. [DOI] [PubMed]
- 6).Quinton PM. Chloride impermeability in cystic fibrosis. Nature, 1983; 301: 421–422. [DOI] [PubMed]
- 7).Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol, 2005; 67: 377–409. [DOI] [PubMed]
- 8).Burghardt B, Nielsen S, Steward MC. The role of aquaporin water channels in fluid secretion by the exocrine pancreas. J Membr Biol, 2006; 210: 143–153. [DOI] [PubMed]
- 9).Burghardt B, Elkaer ML, Kwon TH, Rácz GZ, Varga G, Steward MC, Nielsen S. Distribution of aquaporin water channels AQP1 and AQP5 in the ductal system of the human pancreas. Gut, 2003; 52: 1008–1016. [DOI] [PMC free article] [PubMed]
- 10).Case RM, Argent BE. Pancreatic duct cell secretion: control and mechanisms of transport. In: The Pancreas: Biology, Pathobiology and Disease, 2nd edition, edited by Go VLW, DiMagno EP, Gardner JD, Labenthal E, Reher HA, and Scheele GA. pp. 301–350, 1993, Raven, New York.
- 11).Lightwood R, Reber HA. Micropuncture study of pancreatic secretion in the cat. Gastroenterology, 1977; 72: 61–66. [PubMed]
- 12).Arkle S, Lee CM, Cullen MJ, Argent BE. Isolation of ducts from the pancreas of copper-deficient rats. Q J Exp Physiol, 1986; 71: 249–265. [DOI] [PubMed]
- 13).Argent BE, Arkle S, Cullen MJ, Green R. Morphological, biochemical and secretory studies on rat pancreatic ducts maintained in tissue culture. Q J Exp Physiol, 1986; 71: 633–648. [DOI] [PubMed]
- 14).Yamamoto A, Ishiguro H, Ko SBH, Suzuki A, Wang Y, Hamada H, Mizuno N, Kitagawa M, Hayakawa T, and Naruse S. Ethanol induces fluid hypersecretion in guinea-pig pancreatic duct cells. J Physiol, 2003; 551: 917–926. [DOI] [PMC free article] [PubMed]
- 15).Ishiguro H, Naruse S, Steward MC, Kitagawa M, Ko SB, Hayakawa T, Case RM. Fluid secretion in interlobular ducts isolated from guinea-pig pancreas. J Physiol, 1998; 511: 407–422. [DOI] [PMC free article] [PubMed]
- 16).Ishiguro H, Steward MC, Yamamoto A. Microperfusion and micropuncture analysis of ductal secretion. In; THE PANCREAPEDIA (http://www.lib.umich.edu/spo/panc/), University of Michigan Library, 2011; DOI: 10.3998/panc.2011.16
- 17).Gray MA, Plant S, Argent BE. cAMP-regulated whole cell chloride currents in pancreatic duct cells. Am J Physiol, 1993; 264: C591–602. [DOI] [PubMed]
- 18).Gray MA, Greenwell JR, Garton AJ, Argent BE. Regulation of maxi-K+ channels on pancreatic duct cells by cyclic AMP-dependent phosphorylation. J Membr Biol, 1990; 115: 203–215. [DOI] [PubMed]
- 19).Novak I, Greger R. Properties of the luminal membrane of isolated perfused rat pancreatic ducts; effect of cyclic AMP and blockers of chloride transport. Pflügers Arch, 1988; 411: 546–553. [DOI] [PubMed]
- 20).Novak I, Greger R. Electrophysiological study of transport systems in isolated perfused pancreatic ducts: properties of the basolateral membrane. Pflügers Arch, 1988; 411: 58–68. [DOI] [PubMed]
- 21).Kumpulainen T, Jalovaara P. Immunohistochemical localization of carbonic anhydrase isoenzymes in the human pancreas. Gastroenterology, 1981; 80: 796–799. [PubMed]
- 22).Gray MA, Pollard CE, Harris A, Coleman L, Greenwell JR, Argent BE. Anion selectivity and block of the small-conductance chloride channel on pancreatic duct cells. Am J Physiol, 1990; 259: C752–761. [DOI] [PubMed]
- 23).Ishiguro H, Naruse S, Kitagawa M, Suzuki A, Yamamoto A, Hayakawa T, Case RM, Steward MC. CO2 permeability and bicarbonate transport in microperfused interlobular ducts isolated from guinea-pig pancreas. J Physiol, 2000; 528: 305–315. [DOI] [PMC free article] [PubMed]
- 24).Ishiguro H, Naruse S, Kitagawa M, Mabuchi T, Kondo T, Hayakawa T, Case RM, Steward MC. Chloride transport in microperfused interlobular ducts isolated from guinea-pig pancreas. J Physiol, 2002; 539: 175–189. [DOI] [PMC free article] [PubMed]
- 25).Ishiguro H, Steward MC, Sohma Y, Kubota T, Kitagawa M, Kondo T, Case RM, Hayakawa T, Naruse S. Membrane potential and bicarbonate secretion in isolated interlobular ducts from guinea-pig pancreas. J Gen Physiol, 2002; 120: 617–628. [DOI] [PMC free article] [PubMed]
- 26).Steward MC, Ishiguro H. Molecular and cellular regulation of pancreatic duct cell function. Curr Opin Gastroenterol, 2009; 25: 447–453. [DOI] [PubMed]
- 27).Ishiguro H, Steward MC, Naruse S, Ko SB, Goto H, Case RM, Kondo T, Yamamoto A. CFTR functions as a bicarbonate channel in pancreatic duct cells. J Gen Physiol, 2009; 133: 315–326. [DOI] [PMC free article] [PubMed]
- 28).Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, Thomas PJ, Muallem S. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3– secretion: relevance to cystic fibrosis. EMBO J, 2006; 25: 5049–5057. [DOI] [PMC free article] [PubMed]
- 29).Ishiguro H, Namkung W, Yamamoto A, Wang Z, Worrell RT, Xu J, Lee MG, Soleimani M. Effect of Slc26a6 deletion on apical Cl–/HCO3– exchanger activity and cAMP-stimulated bicarbonate secretion in pancreatic duct. Am J Physiol Gastrointest Liver Physiol, 2007; 292: G447–455. [DOI] [PubMed]
- 30).Ohana E, Yang D, Shcheynikov N, Muallem S. Diverse transport modes by the solute carrier 26 family of anion transporters. J Physiol, 2009; 587: 2179–2185. [DOI] [PMC free article] [PubMed]
- 31).Sohma Y, Gray MA, Imai Y, Argent BE. HCO3– transport in a mathematical model of the pancreatic ductal epithelium. J Membr Biol, 2000; 176: 77–100. [DOI] [PubMed]
- 32).Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO3– transport in cystic fibrosis. EMBO J, 2002; 21: 5662–5672. [DOI] [PMC free article] [PubMed]
- 33).Ishiguro H, Steward M, Naruse S. Cystic fibrosis transmembrane conductance regulator and SLC26 transporters in HCO3– secretion by pancreatic duct cells. Sheng Li Xue Bao, 2007; 59: 465–476. [PubMed]
- 34).Ishiguro H, Naruse S, Kondo T, Yamamoto A. Dysfunction of pancreatic HCO3– secretion and pathogenesis of cystic fibrosis/chronic pancreatitis. Journal of the Japan Pancreas Society (in Japanese), 2006; 21: 13–25.
- 35).Highlights from the literature. Physiology 2009; 24: 77.
- 36).Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, Lee MG. Dynamic regulation of CFTR bicarbonate permeability by [Cl–]i and its role in pancreatic bicarbonate secretion. Gastroenterology, 2010; 139: 620–631. [DOI] [PubMed]
- 37).Ko SBH, Naruse S, Kitagawa M, Ishiguro H, Furuya S, Mizuno N, Wang Y, Yoshikawa T, Suzuki A, Shimano S, Hayakawa T. Aquaporins in rat pancreatic interlobular ducts. Am J Physiol Gastrointest Liver Physiol, 2002; 282: G324–G331. [DOI] [PubMed]
- 38).Krane CM, Melvin JE, Nguyen HV, Richardson L, Towne JE, Doetschman T, Menon AG. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J Biol Chem, 2001; 276: 23413–23420. [DOI] [PubMed]
- 39).Verkman AS. Role of aquaporins in lung liquid physiology. Respir Physiol Neurobiol, 2007; 159: 324–330. [DOI] [PMC free article] [PubMed]
- 40).Furuya S, Naruse S, Ko SBH, Ishiguro H, Yoshikawa T, Hayakawa T. Distribution of aquaporin 1 in the rat pancreatic duct system examined with light and electron microscopic immunohistochemistry. Cell Tissue Res, 2002; 308: 75–86. [DOI] [PubMed]
- 41).Argent B, Gray M, Steward M, Case M. Cell Physiology of Pancreatic Ducts. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR, Barrett KE, Ghishan FK, Merchant JL, Said HM, Wood JD. pp. 1371–1396. 2006, Elsevier Academic Press.
- 42).Nathan JD, Patel AA, McVey DC, Thomas JE, Prpic V, Vigna SR, Liddle RA. Capsaicin vanilloid receptor-1 mediates substance P release in experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol, 2001; 281: G1322–1328. [DOI] [PubMed]
- 43).Hegyi P, Gray MA, Argent BE. Substance P inhibits bicarbonate secretion from guinea pig pancreatic ducts by modulating an anion exchanger. Am J Physiol Cell Physiol, 2003; 285: C268–276. [DOI] [PMC free article] [PubMed]
- 44).Ko SB, Naruse S, Kitagawa M, Ishiguro H, Murakami M, Hayakawa T. Arginine vasopressin inhibits fluid secretion in guinea pig pancreatic duct cells. Am J Physiol, 1999; 277: G48–54. [DOI] [PubMed]
- 45).Kitagawa M, Hayakawa T, Kondo T, Shibata T, Oiso Y. Plasma osmolality and exocrine pancreatic secretion. Int J Pancreatol, 1990; 6: 25–32. [DOI] [PubMed]
- 46).Bruce JI, Yang X, Ferguson CJ, Elliott AC, Steward MC, Case RM, Riccardi D. Molecular and functional identification of a Ca2+ (polyvalent cation)-sensing receptor in rat pancreas. J Biol Chem, 1999; 274: 20561–20568. [DOI] [PubMed]
- 47).Novak I. Purinergic receptors in the endocrine and exocrine pancreas. Purinergic Signal, 2008; 4: 237–253. [DOI] [PMC free article] [PubMed]
- 48).Ishiguro H, Naruse S, Kitagawa M, Hayakawa T, Case RM, Steward MC. Luminal ATP stimulates fluid and HCO3– secretion in guinea-pig pancreatic duct. J Physiol, 1999; 519: 551–558. [DOI] [PMC free article] [PubMed]
- 49).Suzuki A, Naruse S, Kitagawa M, Ishiguro H, Yoshikawa T, Ko SB, Yamamoto A, Hamada H, Hayakawa T. 5-hydroxytryptamine strongly inhibits fluid secretion in guinea pig pancreatic duct cells. J Clin Invest, 2001; 108: 749–756. [DOI] [PMC free article] [PubMed]
- 50).Hallenbeck GA. Biliary and pancreatic intraductal pressures. In: Handbook of Physiology. Alimentary Canal. Secretion. pp.1007–1025. 1967, American Physiological Society, Washington D.C.
- 51).Czakó L, Yamamoto M, Otsuki M. Pancreatic fluid hypersecretion in rats after acute pancreatitis. Dig Dis Sci, 1997; 42: 265–272. [DOI] [PubMed]
- 52).Czakó L, Yamamoto M, Otsuki M. Exocrine pancreatic function in rats after acute pancreatitis. Pancreas, 1997; 15: 83–90. [DOI] [PubMed]
- 53).Venglovecz V, Rakonczay Z Jr, Ozsvári B, Takács T, Lonovics J, Varró A, Gray MA, Argent BE, Hegyi P. Effects of bile acids on pancreatic ductal bicarbonate secretion in guinea pig. Gut, 2008; 57: 1102–1112. [DOI] [PubMed]
- 54).Venglovecz V, Hegyi P, Rakonczay Z Jr, Tiszlavicz L, Nardi A, Grunnet M, Gray MA. Pathophysiological relevance of apical large-conductance Ca2+-activated potassium channels in pancreatic duct epithelial cells. Gut, 2011; 60: 361–369. [DOI] [PubMed]
- 55).Kawabata A, Kuroda R, Nishida M, Nagata N, Sakaguchi Y, Kawao N, Nishikawa H, Arizono N, Kawai K. Protease-activated receptor-2 (PAR-2) in the pancreas and parotid gland: immunolocalization and involvement of nitric oxide in the evoked amylase secretion. Life Sci, 2002; 71: 2435–2446. [DOI] [PubMed]
- 56).Nguyen TD, Moody MW, Steinhoff M, Okolo C, Koh DS, Bunnett NW. Trypsin activates pancreatic duct epithelial cell ion channels through proteinase-activated receptor-2. J Clin Invest, 1999; 103: 261–269. [DOI] [PMC free article] [PubMed]
- 57).Masamune A, Kikuta K, Satoh M, Suzuki N, Shimosegawa T. Protease-activated receptor-2-mediated proliferation and collagen production of rat pancreatic stellate cells. J Pharmacol Exp Ther, 2005; 312: 651–658. [DOI] [PubMed]
- 58).Hoogerwerf WA, Shenoy M, Winston JH, Xiao SY, He Z, Pasricha PJ. Trypsin mediates nociception via the proteinase-activated receptor 2: a potentially novel role in pancreatic pain. Gastroenterology, 2004; 127: 883–891. [DOI] [PubMed]
- 59).Sharma A, Tao X, Gopal A, Ligon B, Andrade-Gordon P, Steer ML, Perides G. Protection against acute pancreatitis by activation of protease-activated receptor-2. Am J Physiol Gastrointest Liver Physiol, 2005; 288: G388–395. [DOI] [PubMed]
- 60).Laukkarinen JM, Weiss ER, van Acker GJ, Steer ML, Perides G. Protease-activated receptor-2 exerts contrasting model-specific effects on acute experimental pancreatitis. J Biol Chem, 2008; 283: 20703–20712. [DOI] [PMC free article] [PubMed]
- 61).Namkung W, Han W, Luo X, Muallem S, Cho KH, Kim KH, Lee MG. Protease-activated receptor 2 exerts local protection and mediates some systemic complications in acute pancreatitis. Gastroenterology 2004; 126: 1844–1859. [DOI] [PubMed]
- 62).Pallagi P, Venglovecz V, Rakonczay Z Jr, Borka K, Korompay A, Ozsvári B, Judák L, Sahin-Tóth M, Geisz A, Schnúr A, Maléth J, Takács T, Gray MA, Argent BE, Mayerle J, Lerch MM, Wittmann T, Hegyi P. Trypsin reduces pancreatic ductal bicarbonate secretion by inhibiting CFTR Cl– channels and luminal anion exchangers. Gastroenterology, 2011; 40: 2228–2239. [DOI] [PMC free article] [PubMed]
- 63).Gukovskaya AS, Mouria M, Gukovsky I, Reyes CN, Kasho VN, Faller LD, Pandol SJ. Ethanol metabolism and transcription factor activation in pancreatic acinar cells in rats. Gastroenterology, 2002; 122: 106–118. [DOI] [PubMed]
- 64).Lu Z, Karne S, Kolodecik T, Gorelick FS. Alcohols enhance caerulein-induced zymogen activation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol, 2002; 282: G501–507. [DOI] [PMC free article] [PubMed]
- 65).Laurence DR, Bennett PN. Central nervous system. Non-medical use of drags. Ethyl alcohol (ethanol). In: Clinical Pharmacology. pp. 411–422, 1987, Churchill Livingstone.
- 66).Deitrich RA, Harris RA. How much alcohol should I use in my experiments? Alcohol Clin Exp Res, 1996; 20: 1–2. [DOI] [PubMed]
- 67).Sarles H. Alcoholic pancreatitis. In: Disorders of the Pancreas. pp. 273–282, 1992, McGraw-Hill, New York.
- 68).Gerasimenko JV, Lur G, Ferdek P, Sherwood MW, Ebisui E, Tepikin AV, Mikoshiba K, Petersen OH, Gerasimenko OV. Calmodulin protects against alcohol-induced pancreatic trypsinogen activation elicited via Ca2+ release through IP3 receptors. Proc Natl Acad Sci U S A, 2011; 108: 5873–5878. [DOI] [PMC free article] [PubMed]
- 69).Little HJ. The contribution of electrophysiology to knowledge of the acute and chronic effects of ethanol. Pharmacol Ther, 1999; 84: 333–353. [DOI] [PubMed]
- 70).Korpi ER, Mäkelä R, Uusi-Oukari M. Ethanol: Novel actions on nerve cell physiology explain impaired functions. News Physiol Sci, 1998; 13: 164–170. [DOI] [PubMed]
- 71).Hamada H, Ishiguro H, Yamamoto A, Shimano-Futakuchi S, Ko SB, Yoshikawa T, Goto H, Kitagawa M, Hayakawa T, Seo Y, Naruse S. Dual effects of n-alcohols on fluid secretion from guinea pig pancreatic ducts. Am J Physiol Cell Physiol, 2005; 288: C1431–1439. [DOI] [PubMed]
- 72).Benjamin K, Matzner MJ, Sobel AE. A study of external pancreatic secretion in man. J Clin Invest, 1936; 15: 393–396. [DOI] [PMC free article] [PubMed]
- 73).Futakuchi S, Ishiguro H, Naruse S, Ko SB, Fujiki K, Yamamoto A, Nakakuki M, Song Y, Steward MC, Kondo T, Goto H. High glucose inhibits HCO3– and fluid secretion in rat pancreatic ducts. Pflügers Arch, 2009; 459: 215–226. [DOI] [PubMed]
- 74).Creutzfeldt W, Gleichmann D, Otto J, Stöckmann F, Maisonneuve P, Lankisch PG. Follow-up of exocrine pancreatic function in type-1 diabetes mellitus. Digestion, 2005; 72: 71–75. [DOI] [PubMed]
- 75).Busik JV, Hootman SR, Greenidge CA, Henry DN. Glucose-specific regulation of aldose reductase in capan-1 human pancreatic duct cells in vitro. J Clin Invest, 1997; 100: 1685–1692. [DOI] [PMC free article] [PubMed]
- 76).Imaizumi Y. Incidence and mortality rates of cystic fibrosis in Japan, 1969–1992. Am J Med Genet, 1995; 58: 161–168. [DOI] [PubMed]
- 77).Yamashiro Y, Shimizu T, Oguchi S, Shioya T, Nagata S, Ohtsuka Y. The estimated incidence of cystic fibrosis in Japan. J Pediatr Gastroenterol Nutr, 1997; 24: 544–547. [DOI] [PubMed]
- 78).Naruse S, Kitagawa M, Ishiguro H, Fujiki K, Hayakawa T. Cystic fibrosis and related diseases of the pancreas. Best Pract Res Clin Gastroenterol, 2002; 16: 511–526. [DOI] [PubMed]
- 79).Hanawa M, Takebe T, Takahashi S, Koizumi M, Endo K. The significance of the sweat test in chronic pancreatitis. Tohoku J Exp Med, 1978; 125: 59–69. [DOI] [PubMed]
- 80).Naruse S, Ishiguro H, Suzuki Y, Fujiki K, Ko SB, Mizuno N, Takemura T, Yamamoto A, Yoshikawa T, Jin C, Suzuki R, Kitagawa M, Tsuda T, Kondo T, Hayakawa T. A finger sweat chloride test for the detection of a high-risk group of chronic pancreatitis. Pancreas, 2004; 28: e80–85. [DOI] [PubMed]
- 81).Fujiki K, Ishiguro H, Ko SB, Mizuno N, Suzuki Y, Takemura T, Yamamoto A, Yoshikawa T, Kitagawa M, Hayakawa T, Sakai Y, Takayama T, Saito M, Kondo T, Naruse S. Genetic evidence for CFTR dysfunction in Japanese: background for chronic pancreatitis. J Med Genet, 2004; 41: e55. [DOI] [PMC free article] [PubMed]
- 82).Quinton PM. The sweat gland. In: Cystic fibrosis in adults, edited by Yankaskas JR and Knowles MR. pp. 419–437, 1999, Lippincott-Raven, Philadelphia.
- 83).Tsuda T, Noda S, Kitagawa S, Morishita T. Proposal of sampling process for collecting human sweat and determination of caffeine concentration in it by using GC/MS. Biomed Chromatogr, 2000; 14: 505–510. [DOI] [PubMed]
- 84).Nakakuki M, Ishiguro H, Shirota K, Yamamoto A, Ko SB, Goto H, Fujiki K, Kondo T, Naruse S. Measurement of chloride concentrations of insensible sweat collected from thumb. J Jpn Pancreas Soc (in Japanese), 2008; 23: 486–493.