ABSTRACT
A study was conducted to clarify the prognostic factors related to recurrence of craniopharyngioma and to improve the quality of life of patients by the treatment with intentional partial removal and gamma knife radiosurgery. One hundred cases of craniopharyngioma have been treated at Komaki City Hospital since 1991. In a mean follow-up period of 65.5 months, the tumor control rate was 79.5%. The 5- and 10-year actuarial survival rates were 94.1% and 91%, respectively. However, the recurrence-free survival rates were 73.6% at 5 years and 60.2% at 10 years. Nine factors thought to be related to the recurrence were selected from past references and previous studies, including gender, age, pediatric (≤17 years) or adult patient, partial removal or recurrence, mean tumor diameter, tumor type (solid or cyst), pathological types (squamous cell or adamantinoma), number of previous treatments, and radiation dose. Statistical analysis was performed to determine which factors had a significant prognostic impact. Multivariate analysis showed that mean tumor diameter and radiation dose were independent predictors of outcome. To maximize the prognostic power of these factors, cut-off levels were determined using ROC analysis. These levels were 19 mm for tumor diameter and 13.2 Gy for marginal dose. Significant prognostic factors related to recurrence of craniopharyngioma are tumor diameter and radiation dose. A tumor diameter of <19 mm and a marginal dose of ≥13.2 Gy are favorable prognostic factors for gamma knife radiosurgery.
Key Words: Prognostic factor, Craniopharyngioma, Recurrence, Gamma knife radiosurgery, Statistical analysis
INTRODUCTION
Methods for treatment of craniopharyngioma have been controversial for a long time, and no standard method is yet employed. Microsurgical total removal is ideal for a benign intracranial tumor,1,2) but access is not easy because the tumor arises from the hypothalamic-pituitary area. Associated problems involve postoperative complications (including death) or morbidity leading to deterioration of the quality of life (QOL) of the patients, and recurrence of the tumor. While fractionated radiotherapy after partial removal3-9) has been shown to be effective for tumor control and improved patient survival, there are still problems such as tumor regrowth and radiation injury to vital organs such as the optic nerves or the hypothalamic-pituitary axis. Currently, stereotactic radiosurgery is used for treatment of residual or recurrent tumors instead of fractionated radiotherapy.10-15) We have been performing gamma knife radiosurgery (GKR) for craniopharyngioma since 1991 at a single center and reported the results13-15) along with discussion of the treatment strategy.16,17)
To devise strategies for prevention of regrowth and to improve the QOL of patients, we studied factors related to recurrence of craniopharyngioma by statistical analysis.
MATERIALS & METHODS
One hundred seven cases of craniopharyngioma have been treated by GKR after biopsy, partial removal, or at the time of recurrence at Komaki City Hospital during the past 12 years. Of these cases, 100 were followed up for periods ranging from 6 to 148 months. The patients comprised 36 children (age at diagnosis ≤17 years) and 64 adults, and the male: female ratio was 54: 46. The type of tumor was classified as solid in 40 cases, cystic in 24 and mixed in 36. Treatments prior to GK were surgery, including subtotal removal in 24 cases, partial removal in 107 times, biopsy in 10 cases, placement of an Ommaya reservoir in 26, and V-P shunt in 5. Conventional focal irradiation and bleomycin sulfate chemotherapy were performed in 13 and 3 cases, respectively. The mean tumor diameter and volume were 18.8 mm and 3.5 ml, respectively. Sixty-two cases were treated for a residual tumor after intentional partial removal and 38 were treated for a recurrent tumor after a variety of treatments. These tumors were treated with GKR using a mean maximum dose of 21.8 Gy and a marginal dose of 11.5 (10~18) Gy employing a mean number of 4.5 isocenters. “Intentional partial removal” means extensive but limited microsurgical removal of the tumor to prevent severe hypothalamic damage.
Dose planning was made using Gamma Plan (Elekta Instruments AB, Stockholm, Sweden). Reduction of the tumor volume and marginal dose was attempted during the study to minimize any optic or hypothalamic neuropathy and to determine the optimal dose for craniopharyngioma. The mean marginal dose was gradually decreased to 10.7 Gy in the most recent 31 cases, whereas the dose was 12.7 Gy in the earliest 30 cases. The mean tumor volume was also smaller (3.1 ml) in the most recent cases than in the earliest cases (5.1 ml).
After treatment, patients were followed up using brain MRI, and checks for neurological and endocrinological changes and side effects every 3 to 6 months. The tumor findings on MRI were classified into four groups: CR (tumor disappearance), PR (≥25% decrease in tumor volume), NC (no change or <25% decrease in volume), and PG (increase in volume). In order to clarify the prognostic factors for recurrence of craniopharyngioma, nine factors were chosen from past studies and assessed by Cox analysis: gender, patient age, child or adult, partial removal, tumor diameter, tumor type, pathological type, number of previous treatments, and radiation dose. Recurrence-free survival was estimated by the Kaplan-Meier method.
RESULTS
The mean follow-up period in the 100 studied cases was 65.5 months, with a median of 63 months. Two patients were lost to follow-up because they moved out of the locality. The tumor response, assessed in the remaining 98 cases, was CR in 19 cases (19.4%), PR in 47 (48%), NC in 12 (12.2%), and PG in 20 (20.4%).
The CR group had a child: adult ratio of 4:15 with a mean age of 37.4 years. Solid tumors were found in 12 cases and cyst/mixed type in 7. The mean tumor diameter was less than 2 cm in 16 cases and greater than or equal to 2 cm in 3 cases. The mean number of previous treatments was 1.31 and no patient received radiotherapy prior to GKR. The MRI findings in the CR patients remained stable for a mean period of 75.1 months. Only one accidental death occurred at 9 years after the treatment.
The PG group had a child: adult ratio of 12:8 and a mean age of 27 years. The tumor was solid in 3 cases and cyst/mixed in 17. There was a mean of 2.47 previous treatments per person, and 5 patients received radiotherapy prior to GKR. The tumor diameter was more than 2 cm in 12 cases. The cause of tumor progression was cyst enlargement in 9 cases (45%), regrowth in 8 (40%) and development of new lesions in 3 (15%). The mean time to progression was 24.5 months. These tumors were treated by repeated surgery in 14 cases, and by a second GKR in one. The other 5 cases received no treatment. The outcome was excellent in one case, good in 4, fair in 3, poor in 2, and 8 patients died.
The overall results for the 98 cases treated by GKR were a tumor control and response rate of 79.5% and 67.4%, respectively, and a 5- and 10-year actuarial survival rate of 94.1% and 91.0%, respectively. However, the recurrence-free survival rate at 5 and 10 years was lower at 73.6% and 60.2%, respectively (Fig. 1).
Fig. 1.
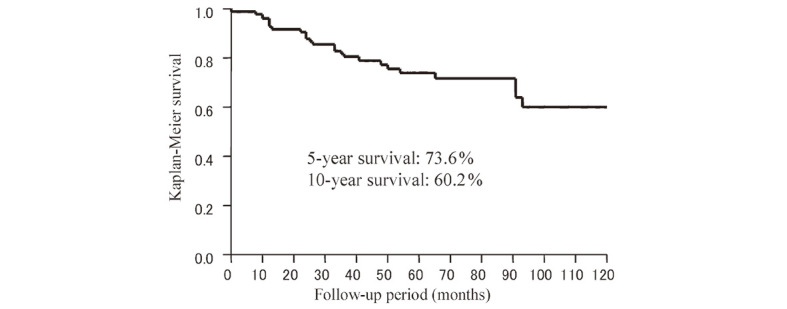
Kaplan-Meier estimates: Recurrence-free survival after GK therapy.
Univariate Cox analysis showed that tumor diameter hazard ratio (HR) 1.10, 95% confidence interval (CI) 1.04–1.15, p=0.0003, radiation dose (HR 0.64, 95% CI 0.44–0.92, p=0.015), solid tumor type (HR 0.39, 95% CI 0.14–0.99, p=0.046), number of previous treatments (HR.2.57, 95% CI 1.03–6.45, p=0.043) and pathological type (HR 0.32, 95% CI 0.11–0.93, p=0.036) were factors significantly related to outcome.
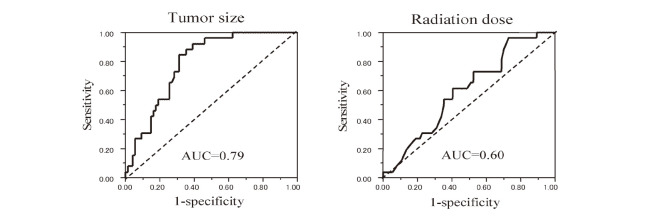
Multivariate analysis showed that tumor diameter (HR 1.08, 95% CI 1.03–1.14, p=0.0041) and radiation dose (HR 0.74, 95% CI 0.53–0.99, p=0.036) were independent predictive factors (Table 1). Furthermore, to maximize the prognostic power of tumor diameter and radiation dose, the cut-off levels were determined using receiver operating characteristic (ROC) analysis (AUC=0.79 for tumor diameter and 0.60 for radiation dose). These levels were 19 mm for tumor diameter and 13.2 Gy for marginal dose (Fig. 2). On this basis, the 10-year recurrence-free survival rate was significantly lower in the group with a tumor diameter of ≥19 mm than in the group with a tumor diameter of <19 mm (38.0% vs. 89.4%, HR 6.89, 95% CI 2.36–20.0, p<0.0001) (Fig. 3). A marginal dose of >13.2 Gy was also a favorable prognostic factor (92.9% vs. 46.7%, HR 0.12, 95% CI 0.02–0.88, p=0.012) (Fig. 4).
Table 1.
Predictive value of clinical characteristics for tumor recurrence by Cox Analysis
| Non-adjusted | Adjusted | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender (male) | 0.83 (0.38–1.81) | 0.63 | ||
| Age | 0.99 (0.97–1.01) | 0.23 | ||
| Children<17 years | 1.07 (0.48–2.37) | 0.87 | ||
| Partial removal with GKR | 0.59 (0.27–1.29) | 0.19 | ||
| Tumor size (mm) | 1.10 (1.04–1.15) | 0.0003 | 1.07 (1.01–1.14) | 0.017 |
| Tumor type (Solid) | 0.39 (0.14–0.99) | 0.046 | 0.62 (0.22–1.75) | 0.37 |
| Pathological type (Squam.) | 0.32 (0.11–0.93) | 0.036 | 0.73 (0.06–2.26) | 0.79 |
| Previous treatment ≥ 2 | 2.57 (1.03–6.45) | 0.043 | 1.32 (0.48–3.61) | 0.58 |
| Radiation dose (Gy) | 0.64 (0.44–0.92) | 0.015 | 0.76 (0.53–0.99) | 0.047 |
Multivariate model includes variable with p<0.05 by univariate analysis
Squam.=squamous cell type, GKR=gamma knife radiosurgery, Gy=grey
Fig. 2.
Receiver operating characteristic (ROC) curves showing the relationship of recurrence to tumor diameter (left column) and radiation dose (right column): Area under the ROC curve (AUC) was 0.79 for tumor size and 0.60 for radiation dose. Dotted line represents the no-discrimination line.
Fig. 3.
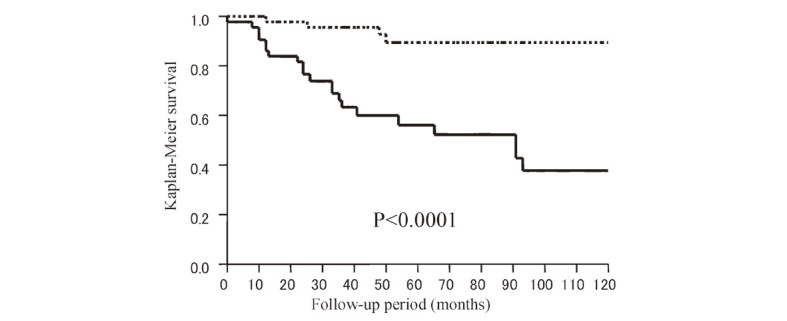
Kaplan-Meier estimates: Recurrence-free survival after GK therapy between patients with a tumor diameter of ≥19 mm (solid line, n=46), which was determined as the cut-off level using ROC analysis, and patients with a tumor diameter of <19 mm (dotted line, n=54).
Fig. 4.
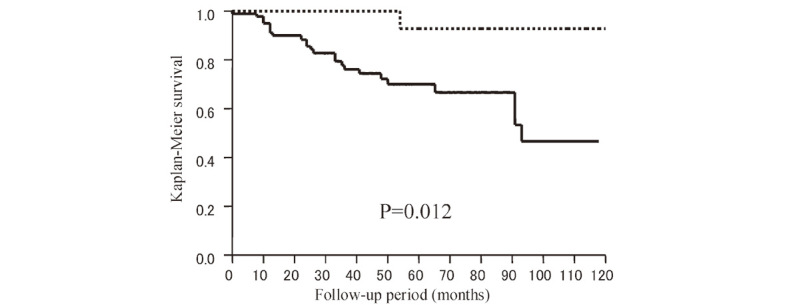
Kaplan-Meier estimates: Recurrence-free survival after GK therapy between patients receiving a radiation dose of <13.2 Gy (solid line, n=84), which was determined as the cut-off level using ROC analysis, and patients receiving a radiation dose of >13.2 Gy (dotted line, n=16).
DISCUSSION
The treatment of craniopharyngioma is still controversial. Total removal is the goal for benign tumors, but this is often difficult without damaging the surrounding critical organs such as the optic nerve, hypothalamus and pituitary gland. Moreover, the rate of recurrence is not inconsiderable.1,2) Partial removal with conventional radiotherapy is another option. Better tumor control and higher survival rates have been achieved,3,6-9) but complications of radiotherapy remain problematic; optic neuropathy,18) hypopituitarism and mental19) or cognitive disturbance20) have been reported. Maximum dose (55~60 Gy), a fractional dose of 1.8 Gy/day, patient age <50 years,3,7,8) tumor size (<5 cm)4) and early adjuvant radiotherapy5) have been pointed out as factors indicative of good outcome.
After the invention of GKR in 1967, Leksell and Backlund et al. advocated the use of radiosurgery for craniopharyngioma21). However, it took two decades to apply modern imaging techniques such as CT or MRI for dose planning. A new treatment strategy for craniopharyngioma was developed in this study using intentional partial removal followed by GKR as the initial treatment. The results of combined microsurgery and GKR for craniopharyngioma have revealed high response and survival rates with lower morbidity in previous studies.11,12,14,15) A variety of factors predictive of a favorable outcome after treatment of residual or recurrent tumors by GKR have been reported. These include patient age, the nature of the tumor, tumor size, marginal dose, and the number of surgical treatments before radiosurgery.14) Others have advocated histological subtype,22) intratumoral homogeneous irradiation using multiple isocenters,10) distance from the tumor to the optic nerves, and tumor radiosensitivity16) as prognostic factors.
The present statistical analysis verified that the most significant factors were marginal dose and tumor diameter. With regard to these two factors, we previously studied the effects of dose and size reduction to optimize the effects of radiosurgery.15) Upon comparison with our initial 33 cases,14) we found that the control rate decreased from 84.8% to 79.4% and the progression rate increased 15.2% to 20.4%, whereas the rate of complications, such as hypopituitarism and visual deterioration, decreased from 15.1% to 6%.15)
From a study of 19 CR cases, we speculated that the factors indicative of a favorable outcome were higher patient age (mean, 37.4 years), solid tumor, smaller tumor diameter (mean, 15.9 mm), fewer previous treatments, and a marginal dose of 12.1 Gy, which was thought to be an effective dose for CR.
A current ROC study showed for the first time that a tumor diameter of less than 19 mm and a marginal dose exceeding 13.2 Gy were factors significantly predictive of a favorable outcome after radiosurgical treatment of craniopharyngioma. The higher the marginal dose, the better the anti-tumor effects will be, but the greater the incidence of complications to critical organs in the tumor vicinity. Chiou et al.11) used a higher mean marginal dose of 16.4 Gy and obtained a CR rate of 40% and a control rate of 80%, but noted that cyst enlargement occurred in 30% and visual deterioration in 20%. Ulfarsson et al.23) used an even lower mean dose of 7 (2.5~15) Gy and obtained a lower control of 36%, a response rate of 23% and a high recurrence rate of 63.6%, with an even higher rate of visual deterioration (42.1%).
On the other hand, the optimal radiation dose for craniopharyngioma is still unknown. It may be possible to regard it as 13.2 Gy, which will deliver a maximum anti-tumor effect but might be hazardous to the surrounding brain, especially to the optic nerves.
With regard to the optic nerve dose in gamma knife radiosurgery, the safe dose has been believed to be less than 10 Gy.18) A marginal dose of 13.2 Gy may be high enough to give rise to optic neuropathy when the size of tumor is large and/or the distance to the optic nerves is short in cases of craniopharyngioma. There will be some radiobiological solutions to this dilemma. The first is to increase the distance from the optic nerve to the tumor.16) Second, the volume of the tumor can be reduced by surgery in order to decrease the burden on the surrounding normal brain tissue.24) Third, multiple, small isocenters can be used in dose planning to decrease the burden on the surrounding normal brain tissue.25)
Finally, the application of stereotactic radiotherapy for craniopharyngioma26-28) will be indicated in cases where a large tumor involving the optic nerve or hypothalamus makes surgical intervention difficult or impossible, although a large number of cases and longer follow-up are necessary to establish the feasibility of this approach.
CONCLUSIONS
From a statistical analysis of 100 cases of craniopharyngioma treated with microsurgery and gamma knife radiosurgery, it was found that the factors significantly indicative of a favorable outcome were radiation dose and tumor diameter. A tumor diameter of less than 19 mm and a marginal dose of more than 13.2 Gy are factors favoring a good outcome after stereotactic radiosurgery for craniopharyngioma.
The outcome is dependent on a balance between the optimal dose for achieving a maximum anti-tumor effect and minimizing damage to the optic pathway or the hypothalamic-pituitary axis.
REFERENCES
- 1).Hoffman HJ, De Silva M, Humphreys RP, Drake JM, Smith ML, Blaser SI. Aggressive surgical management of craniopharyngiomas in children. J Neurosurg, 1992; 76: 47–52. [DOI] [PubMed]
- 2).Yasargil MG, Curcic M, Kis M, Siegenthaler G, Teddy PJ, Roth P: Total removal of craniopharyngiomas: approaches and long-term results in 144 patient. J Neurosurg, 1990; 73: 3–11. [DOI] [PubMed]
- 3).Habrand JL, Ganry O, Couanet D, Rowe IV, Levy-Piedbois C, Pierre-Kahn A, Kalifa C. The role of radiation therapy in the management of craniopharyngioma: a 25-year experience and review of the literature. Int J Radiat Oncol Biol Phys, 1999; 44: 255–263. [DOI] [PubMed]
- 4).Hetelekidis S, Barnes PD, Tao ML, Fischer EG, Schneider L, Scott RM, Tarbell NJ. 20-year experience in childhood craniopharyngioma. Int J Radiat Oncol Biol Phys, 1993; 27: 189–195. [DOI] [PubMed]
- 5).Moon SH, Kim IH, Park SW, Kim I, Hong S, Park CI, Wang KC, Cho BK. Early adjuvant radiotherapy toward long-term survival and better quality of life for craniopharyngiomas-a study in a single institute. Childs Nerv Syst, 2005; 21: 799–807. [DOI] [PubMed]
- 6).Pemberton LS, Dougal M, Magee B, Gattamaneni RH. Experience of external beam radiotherapy given adjuvantly or at relapse following surgery for craniopharyngioma. Radiother Oncol, 2005; 77: 99–104. [DOI] [PubMed]
- 7).Rajan B, Ashley S, Gorman C, Jose CC, Horwich A, Bloom HJ, Marsh H, Brada M. Craniopharyngioma – a long-term result following limited surgery and radiotherapy. Radiother Oncol, 1993; 26: 1–10. [DOI] [PubMed]
- 8).Regine WF, Mohiuddin M, Kramer S. Long-term results of pediatric and adult craniopharyngiomas treated with combined surgery and radiation. Radiother Oncol, 1993; 27: 13–21. [DOI] [PubMed]
- 9).Varlotto JM, Flickinger JC, Kondziolka D, Lunsford LD, Deutsch M. External beam irradiation of craniopharyngiomas: long-term analysis of tumor control and morbidity. Int J Radiat Oncol Biol Phys, 2002; 54: 492–499. [DOI] [PubMed]
- 10).Amendola BE, Wolf A, Coy SR, Amendola MA: Role of radiosurgery in craniopharyngiomas: a preliminary report. Med Pediatr Oncol, 2003; 41: 123–127. [DOI] [PubMed]
- 11).Chiou SM, Lunsford LD, Niranjan A, Kondziolka D, Flickinger JC. Stereotactic radiosurgery of residual or recurrent craniopharyngioma after surgery, with or without radiation therapy. Neuro Oncol, 2001, 3: 159–166. [DOI] [PMC free article] [PubMed]
- 12).Chung WY, Pan DHC, Shiau CY, Guo WY, Wang LW. Gamma knife radiosurgery for craniopharyngiomas. J Neurosurg, 2000, 93 (Suppl): 47–56. [DOI] [PubMed]
- 13).Kobayashi T, Tanaka T, Kida Y. Stereotactic gamma radiosurgery of craniopharyngiomas. Pediatr Neurosurg, 1994; 21: 69–74. [DOI] [PubMed]
- 14).Kobayashi T, Kida Y, Mori Y. Effects and prognostic factors in the treatment of craniopharyngioma by gamma knife. Radiosurgery, vol 3, pp192–204, 1999, Karger, Basel.
- 15).Kobayashi T, Kida Y, Mori Y, Hasegawa T. Long-term results of gamma knife surgery for the treatment of craniopharyngioma in 98 consecutive cases. J Neurosurg (6 Suppl Pediatrics), 2005; 103: 482–488. [DOI] [PubMed]
- 16).Kobayashi T. A strategy of treating sellar-parasellar tumors based on long-term results of microsurgery and gamma knife. Radiosurgery, vol 5, pp1–12, 2004, Karger, Basel.
- 17).Kobayashi T, Mori Y, Uchiyama Y, Hayashi N, Kida Y, Hasegawa T. New treatment strategy for craniopharyngioma using gamma knife radiosurgery. Radiosurgery, vol 6, pp 152–163, 2006, Karger, Basel.
- 18).Leber KA, Bergloff J, Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg, 1998; 88: 43–50. [DOI] [PubMed]
- 19).Thomsett MJ, Conte FA, Kaplan SL, Grumbach MM. Endocrine and neurologic outcome in childhood craniopharyngioma: review of effect of treatment in 42 patients. J Pediatr, 1980; 97: 728–735. [DOI] [PubMed]
- 20).Glauser TA, Packer RJ. Cognitive deficits in long-term survivors of childhood brain tumors. Childs Nerv Syst, 1991; 7: 2–12. [DOI] [PubMed]
- 21).Leksell L, Backlund EO, Johansson L. Treatment of craniopharyngioma. Acta Chir Scand, 1967; 133: 345–350. [PubMed]
- 22).Inoue HK, Kohga H, Kagekawa T, Ono N, Hirato M, Nakamura H, Ohye C, Shibazaki T, Andou Y, Tamada J. Radiosensitive craniopharyngiomas; the role of radiosurgery. Acta Neurochir Suppl, 1994; 62: 43–46. [DOI] [PubMed]
- 23).Ulfarsson E, Lindquist C, Roberts M, Grunbech MM. Gamma knife radiosurgery for craniopharyngiomas: long-term results in the first Swedish patients. J Neurosurg, 2002; 97 (Suppl 5): 613–622. [DOI] [PubMed]
- 24).Marks LB, Spencer DP. The influence of volume on the tolelance of the brain to radiosurgery. J Neurosurg, 1991; 75: 177–180. [DOI] [PubMed]
- 25).Larson DA, Flickinger JC, Loeffler JS. The radiobiology of radiosurgery. Int J Radiat Oncol Biol Phys, 1993; 25: 557–561. [DOI] [PubMed]
- 26).Minniti G, Esposito V, Amichetti M, Enrici RM. The role of fractionated radiotherapy and radiosurgery in the management of patients with craniopharyngioma. Neurosurg Rev, 2009; 32: 125–132. [DOI] [PubMed]
- 27).Selch MT, De Salles AA, Wade M, Lee SP, Solberg TD, Wallace RE, Ford JM, Rubino G, Cabatan-Awang C, Withers HR. Initial clinical results of stereotactic radiotherapy for the treatment of craniopharyngioma. Technol Cancer Res Treat, 2002; 1: 51–59. [DOI] [PubMed]
- 28).Hashizume C, Mori Y, Kobayashi T, Shibamoto Y, Nagai A, Hayashi N. Stereotactic radiotherapy using Novalis for craniopharyngioma adjacent to optic pathways. J Neurooncol, 2010; 98: 239–247. [DOI] [PubMed]