ABSTRACT
Induced pluripotent stem cells (iPSCs) have been directly generated from fibroblast cultures though retrovirus- or lentivirus-mediated ectopic overexpression of only a few defined transcriptional factors. This remarkable achievement has greatly enhanced our ability to explore the causes of, and potential cures for, many genetic diseases, and strengthened the promise of regenerative medicine. In fact, to date, many kinds of somatic cells from different tissues have exhibited a capacity for reprogramming toward an embryonic stem cell-like state, but major bottlenecks in iPSC derivation and therapeutic use remain, including low reprogramming efficiencies and the tumorigenesis of the generated iPSC. Here, we successfully generated miR-302s-induced pluripotent stem cells (mirPS cells) from human embryonic kidney (HEK) 293 cells via transfection of the miR-302s expression vector. We also determined the optimal culture conditions to generate mirPS on feeder cells, which included the use of serum-free N2B27 medium. The mirPS cells generated by our improved conditions showed the expression of pluripotent marker genes such as OCT3/4, NANOG, and SOX2 under growth conditions via reverse transcription-PCR, whereas no expression of these genes was observed in HEK293 cells. On the other hand, under differentiation conditions, mirPS cells formed ball-shaped structures (embryoid bodies), and showed the ability to differentiate into three germ layers (ectoderm, mesoderm, and endoderm) in vitro. The results suggested that our generated mirPS cells are actually functional as a cell resource to apply to regenerative medicine, and mirPS cells are suitable materials to clarify the mechanism underlying the reprogramming from somatic cells.
Key words: Embryonic stem cells, Pluripotent stem cells, Reprogramming, MicroRNA, Regenerative medicine
INTRODUCTION
Induced pluripotent stem cells (iPSCs) have been directly generated from fibroblast cultures though retrovirus or lentivirus-mediated ectopic overexpression of only a few defined transcriptional factors, such as Oct4, Sox2, Klf4, and c-Myc.1,2) This remarkable achievement has greatly enhanced our ability to explore the causes of, and potential cures for, many genetic diseases, and strengthened the promise of regenerative medicine. Improvements in gene-delivery methodology have further facilitated iPSC generation by minimizing the requirement for genetic modification.3) However, a major bottleneck in iPSC derivation and therapeutic use is the low reprogramming efficiencies,1,4,5) typically from 0.01% to 0.2%, although many kinds of somatic cells from different tissues have been shown to undergo reprogramming.4,6-8)
In recent years, microRNAs (miRNAs) have been found to function in many important processes, such as expression of self-renewal genes in human embryonic stem (ES) cells.9) miRNAs are a class of short, conserved, non-coding RNAs that play important regulatory roles in post-transcriptional repression.10) These are fundamental gene regulators that control proliferation, differentiation, and apoptosis during human and animal development.11) It was recently reported that ES cell-specific miRNAs enhanced mouse iPSC derivation and replaced the function of c-Myc during reprogramming,12) and that hES cell-specific miR-302 could alleviate the senescence response due to the four factor expression in human fibroblasts.13)
The miR-302 family (miR-302s) consists of four highly homologous miRNA members, which are transcribed together as a non-coding RNA cluster containing miR-302a, miR-302b, miR-302c, and miR-302d.14) Suh et al. also showed that these miR-302s are expressed most abundantly in slow-growing human ES cells and immediately decrease after differentiation and proliferation of the cells.14) To date, miR-302s have been a promising key candidate zygotic inhibitor of premature cell differentiation during early embryonic development.15-17) However, these miR302s-induced reprogramming mechanisms from fibroblast cultures to an ES cell-like state are still largely unclarified.
Here, we aimed to generate miR-302s-induced pluripotent stem cells (mirPS cells) from human embryonic kidney (HEK) 293 cells via transfection of the expression vector of miR-302s. Regarding our successfully generated mirPS cells, we examined the expression of the pluripotent marker genes OCT3/4, NANOG, and SOX2 under growth conditions via RT-PCR. Further, under differentiation conditions, we also examined the expression of some differentiated marker proteins via immunocytochemical staining. Finally, we showed improved and optimal conditions for the generation and maintenance of mirPS cells on feeder cells.
MATERIALS AND METHODS
HEK293 cells
HEK293 cells (Japan Health Sciences Foundation, Osaka, Japan) were maintained in Dulbecco’s Modified Eagle Medium (DMEM; Invitrogen, Carlsbad, CA) with 10% heat-inactivated fetal bovine serum (FBS; MP Biomedicals, Solon, OH) at 37°C under 5% CO2. After trypsin treatment, the detached cells were used for the transfection of miR-302s via electroporation.
Feeder cells
We used mouse embryonic fibroblasts (MEFs; GlobalStem, Rockville, MD) as feeder cells for the mirPS cells. The irradiated MEFs, which had been treated with 6,000 rads of gamma irradiation, were maintained in DMEM medium with 10% FBS at 37°C under 5% CO2. The irradiation treatment was performed to stop the proliferation of MEFs.
Electroporation and generation of mirPS cells
To generate the mirPS cells by miR-302s-induction from somatic cells, we transfected miR-302s expression vector via an electroporation method15) with modifications. As a representative line of somatic cells, we used HEK293. The miR-302s expression vector (pMir302-EGFP expression vector; Mello Biotechnology Inc., Santa Fe Springs, CA) was designed to induce miR-302a/b/c/d at the same time as described by Lin et al.15) The expression vector also harbored the EGFP reporter gene and a puromycin-selection system for choosing positive clones. HEK293 cells (40,000 cells), which were harvested from a 35-mm culture dish (50% confluency), were suspended in 200 μl of hypo-/iso-electroporation buffer (Mello Biotechnology Inc.) and incubated on ice for 10 minutes. The pMir302-EGFP expression vector or an empty vector (1 μg, respectively) was added to the HEK 293 suspension, and electroporation was performed using a NEON™ Transfection System (Invitrogen) at 1300 V for 10 ms (three pulses were serially added). The transfected cells were grown in serum-free N2B27-based medium.
The serum-free N2B27-based medium (500 ml scale) was prepared as follows. All of the media used for mixing and the additional reagents were from Invitrogen. (a) We mixed DMEM/F12 (240 ml) and Neurobasal medium (240 ml), and then added N2 supplement (5 ml) and B27 supplement (10 ml). (b) Recombinant human leukemia inhibitory factor (LIF), GlutaMAX, non-essential amino acids, β-mercaptoethanol, and Alubumax were added: final concentrations of the individual reagents were 20 ng/ml, 1 mM, 1%, 0.1 mM, and 5 mg/ml, respectively. (c) Finally, chemical compounds such as PD0325901, CHIR99021, and forskolin were added: final concentrations of the individual reagents were 1 μM, 3 μM, and 10 μM, respectively.
Next, the transfected cells were seeded onto the irradiated MEF cultures. Puromycin (70 μg/ml) was added and replaced with fresh medium with the antibiotic everyday (for approximately 5 days) until the EGFP was visible under fluorescence microscope observation (Olympus). The cells exhibiting green fluorescence were selected by flow cytometry (FACSAria™ III Cell sorter; Becton Dickinson, Franklin Lakes, NJ). The selected cells (mirPS cells) were further grown in serum-free N2B27 medium onto the irradiated MEFs. mirPS cells were detached every 5-7 days by treatment with collagenase type IV (Invitrogen), and reseeded onto the MEFs.
Differentiation assays
For the embryoid body (EB)-induced differentiation analysis, trypsinized mirPS cells were cultured on a non-adherent suspension culture plate (Sumitomo Bakelite Co., Ltd., Tokyo, Japan) in DMEM with 10% FBS for 8 days. Eight days later, the EBs were transferred to the gelatin-coated plate and cultured in the same medium for additional 8 days.
RNA isolation and RT-PCR analysis
Total RNA was isolated with an RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instruction. RT-PCR analysis was performed using an Omniscript RT kit (Qiagen) and a HotStarTaq PCR kit (Qiagen). PCR was performed with a Human Pluripotent Stem Cell Assessment Primer Pair Panel (R&D Systems, Minneapolis, MN).
Immunocytochemistry
All of the reactions were performed at room temperature. The cells were fixed with PBS (–) with 4% paraformaldehyde for 10 min. After washing with PBS (–), the cells were treated with PBS (–) with 0.1% Triton X-100 for 20 min. After an additional washing with PBS (–), the cells were blocked with PBS (–) with 3% bovine serum albumin for 2 hours. Primary antibodies against Tuj-1, also known as neuron-specific class III β-tubulin (1:500, Covance, Princeton, NJ), α-smooth muscle actin (α-SMA, 1:75; Abcam, Cambridge, UK), and α-fetoprotein (AFP, 1:200; R&D Systems), were used. Finally, the appropriate secondary antibody (1:400; Invitrogen), i.e., Alexa-568-conjugated goat anti-mouse IgG or Alexa-568-conjugated goat anti-rabbit IgG, was used to visualize the positive signals.
RESULTS
mirPS cells were generated by the transfection of miR-302s
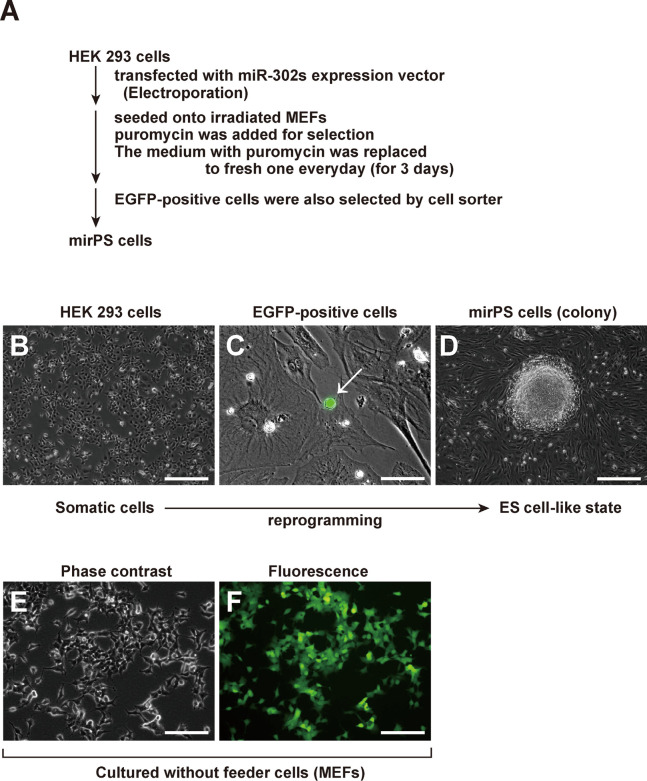
HEK293 cells were used as a resource cell to generate mirPS cells (Fig. 1). In brief, the procedure used to generate human mirPS cells was as follows (Fig. 1A). HEK293 cells (4×104 cells) were transfected with the miR-302s expression vector (1 μg) or an empty vector (1 μg) by electroporation (NEON™ Transfection System; Invitrogen). The electroporation was conducted at a voltage of 1,300 V with 3 pulses (pulse on, 10 ms). The transfected cells were seeded onto irradiated MEFs, and selected with puromycin (70 μg/ml). Finally, the mirPS cells were selected by a cell sorter according to the fluorescence of EGFP (Fig. 1C). The empty vector-transfected HEK293 cells were not reprogrammed to mirPS cells at all (data not shown). Real-time qPCR analysis revealed that our mirPS cells showed extremely high expressions of miR-302a, miR-302b, miR-302c, and miR-302d compared with HEK 293 cells, suggesting successful induction of the desired miRNAs (unpublished results). Finally, we successfully obtained mirPS cells forming a packed dome colony on MEF feeder cells (Fig. 1D).
Fig. 1.
Generation of mirPS cells from HEK293 cells via transfection of the miR-302s expression vector. (A) Flow chart of the generation of mirPS cells. (B) Morphology of HEK293 cells. Scale bar, 250 μm. (C) EGFP expression was detected in the transfected HEK293 cells (white arrow). Scale bar, 50 μm. (D) Expanded EGFP-positive cells exhibited the packed-dome colony formation. Scale bar, 250 μm. (E) Morphology of HEK293 cells transfected with the miR-302s expression vector onto type IV collagen-coated plates without any feeder cells (MEFs). Scale bar, 100 μm. (F) EGFP-positive cells were not able to form the colony without any feeder cells (MEFs). Scale bar, 100 μm.
In the absence of feeder cells (MEFs), the generation of mirPS cells ended in failure
We next attempted to generate mirPS cells without using feeder cells (MEFs). However, we failed to obtain mirPS cells under this condition (Fig. 1E, F), although many EGFP-positive cells were observed (Fig. 1F). This failure suggested that the presence of MEFs or other feeder cells was crucial for generating mirPS cells.
The expression of pluripotent marker genes was observed in our mirPS cells
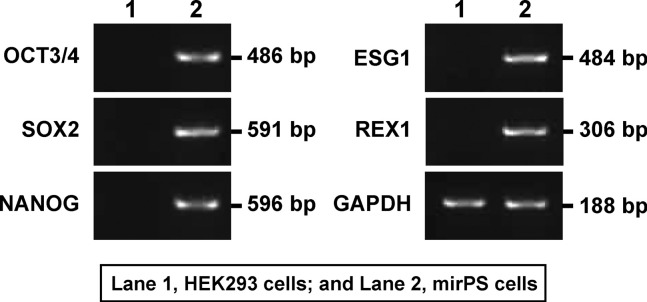
Our successfully generated mirPS cells showed the expression of some typical undifferentiated ES cell-marker genes (pluripotent marker genes) under growth conditions via RT-PCR analysis (Fig. 2). Thus, clear expressions of OCT3/4 (POU domain, class 5, transcription factor 1; Pou5f1), SOX2 (SRY-related HMG box-2), NANOG, REX1 (reduced expression 1), and ESG1 (embryonic cell-specific gene 1) were observed in mirPS cells, whereas no expression of these genes was observed in HEK293 cells (Fig. 2).
Fig. 2.
Expression profiles of mirPS cells generated from HEK293 cells. Lane 1, HEK293 cells; and lane 2, mirPS cells. mirPS cells showed the expression of some typical undifferentiated ES cell-marker genes, such as octamer-binding transcription factor 4 (OCT3/4), also known as POU domain, class 5, transcription factor 1 (POU5F1), sex determining region Y (SRY)-box2 (SOX2), NANOG, embryonic cell-specific gene 1 (ESG1), and reduced expression 1 (REX1). The predicted size of each PCR product is shown.
mirPS cells formed embryoid bodies (EBs) and differentiated into three germ layers
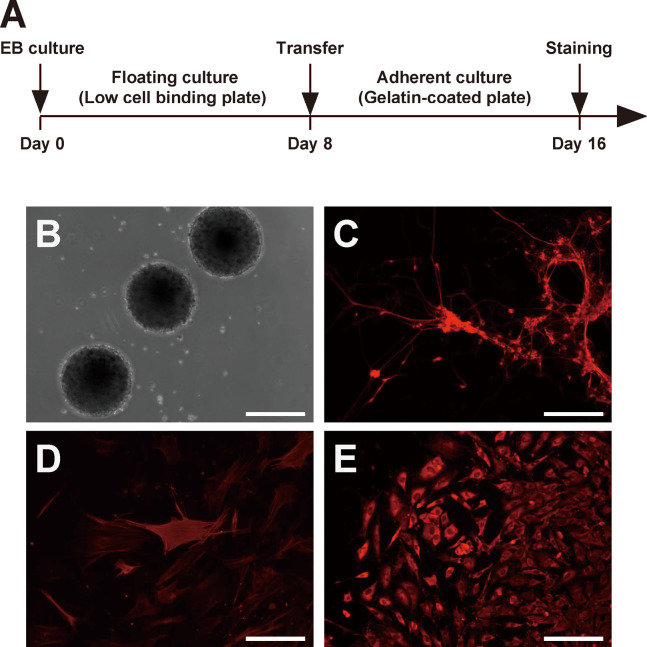
Finally, we determined the differentiation ability of mirPS cells in vitro by performing a floating culture experiment18) to allow the mirPS cells to form EBs (Fig. 3A). After 8 days of floating culture, our mirPS cells formed EBs just like a ball-shape (Fig. 3B). The formed EBs were transferred onto gelatin-coated plates, and cultured for an additional 8 days. Immunocytochemical analysis detected the cells, which showed positive staining for neuron-specific class III β-tubulin (Tuj1, a marker of ectoderm; Fig. 3C), α-smooth muscle actin (α-SMA, a marker of mesoderm; Fig. 3D), or α-fetoprotein (AFP, a marker of endoderm; Fig. 3E). These results suggested that our mirPS cells were able to differentiate into three germ layers in vitro.
Fig. 3.
In vitro differentiation of mirPS cells through EB formation. (A) Time schedule of the differentiation experiment in vitro. (B) mirPS cells formed EB-like spheroids under a floating culture condition at day 8. Scale bar, 250 µm. (C-E) Images of differentiated cells at day 16. Immunocytochemical analysis of Tuj-1 (C), α-smooth muscle actin (D), and α-fetoprotein (E) was performed. Scale bars, 100 μm.
DISCUSSION
Recent advances in nuclear reprogramming technology have allowed the transformation of terminally differentiated, adult cells into induced pluripotent stem cells (iPSCs) whose phenotype is indistinguishable from that of ES cells.19) The ES cell-specific miRNAs have previously been shown to enhance the efficiency of transcription-factor-based reprogramming.14-17) However, whether reprogramming could be achieved entirely by miRNAs remained unclear. A recent report showed that the expression of the miR-302 cluster of miRNAs can directly reprogram somatic cells without the use of any transcription factors.15,20) This new method raises interesting questions about the mechanisms of reprogramming and is likely to facilitate the generation of iPSCs for potential future clinical use.
In the present article, we described improved optimal culture conditions of the mirPS cells reprogrammed from HEK293 cells via transfection of the miR-302s expression vector. In brief, the conventional method15) used feeder cell-free culture system, and they used the medium with FBS, bFGF, and FGF-4. In contrast, our improved method adopted the culture condition with feeder cells (irradiated MEFs), and we used N2B27 medium without FBS. Thus, our culture method led to a high efficiency of generation of mirPS cells, compared with the previously described conventional method.15) According to our raw data, our method gave the colony number of mirPS cells (1022±19 colonies from 40,000 initial HEK293 cells: n=3 dishes). In contrast, the conventional method gave the colony number of mirPS cells (132±9 colonies from 40,000 initial HEK293 cells: n=3 dishes). Our conditions also contributed to the packed-dome colony formation of mirPS cells. Further, under our culture conditions, the feeder cells (MEFs) were indispensable for the generation of mirPS cells and maintained the pluripotency of the cells. Indeed, we failed to obtain any mirPS cells at all without the feeder cells. Under feeder-free conditions, we set the just-transfected HEK293 cells onto a type-I collagen or type-IV collagen-coated plate instead of MEFs, but this effort was in vain. The MEF feeder cells produce multiple proteins and soluble factors, including activin A, TGF-β, bFGF, Wnt ligands, and BMP4, which are important for maintaining ES cell proliferation and pluripotency.21-23) Although it is not clear whether or not the induced reprogramming process is actually improved by the factors secreted by the MEF feeder cells, in fact, our generation and maintenance of mirPS cells obviously required MEF feeder cells. Much work remains to be performed before feeder-free conditions can be applied to the generation and maintenance of mirPS cells.
The identification of miRNAs and their varied effects on ES cells has provided a far better understanding of the molecular mechanisms that fine tune the complex gene regulatory networks which control the proliferation and the differentiation of iPSCs.24) Specific miRNAs, both ES cell- and tissue-specific, have been shown to regulate the expression of critical transcription factors, cell cycle proteins, epigenetic modifiers, and other regulatory proteins, to confer either ES cell or differentiated cell phenotypes. Recent works have demonstrated that iPSCs and ES cells can be distinguished by gene expression signatures, including expression of miRNAs.25,26) These findings suggest that iPSCs are very similar to ES cells, but these are important differences between them. From this point of view, further investigation into mirPS cells, including global gene expression analyses, would clarify the similarity and difference between mirPS cells, iPSCs, and ES cells.
In conclusion, we established and determined improved optimal culture conditions for mirPS cells generated from HEK293 cells via transfection of miR-302s expression vector. The successfully generated mirPS cells showed the expression of pluripotent marker genes under growth conditions. Under differentiation conditions, mirPS cells formed EBs, and showed the ability to differentiate into three germ layers. These results suggested that our generated mirPS cells are actually functional as a cell resource to apply to regenerative medicine, and mirPS cells are suitable materials to clarify the mechanism underlying reprogramming from somatic cells.
ACKNOWLEDGEMENTS
We thank Ms. Tae Hayashi for her excellent technical assistance. This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (17016030) and from the Japan Society for the Promotion of Science (17790185, 19590273, and 21590305).
REFERENCES
- 1).Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by difined factors. Cell, 2006; 126: 663−676. [DOI] [PubMed]
- 2).Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda H, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by difined factors. Cell, 2007; 131: 861−872. [DOI] [PubMed]
- 3).Feng B, Ng JH, Heng JC, Ng HH. Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell, 2009; 4: 301−312. [DOI] [PubMed]
- 4).Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science, 2008; 321: 699−702. [DOI] [PubMed]
- 5).Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol, 2008; 26: 101−106. [DOI] [PubMed]
- 6).Eminli S, Utikal J, Arnold K, Jaenisch R, Hochedlinger K. Reprogramming of neural progenitor cells into induced pluripotent stem cells in the absence of exogenous Sox2 expression. Stem Cells, 2008; 26: 2467−2474. [DOI] [PubMed]
- 7).Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C,Wernig M, Creyghton MP, Steine EJ, Cassady JP, Foreman R, Lengner CJ, Dausman JA, Jaenisch R. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell, 2008; 133: 250−264. [DOI] [PMC free article] [PubMed]
- 8).Giorgetti A, Montserrat N, Aasen T, Gonzalez F, Rodriguez-Piza I, Vassena R, Raya A, Boue S, Barrero MJ, Corbella BA, Torrabadella M, Veiga A, Izpisua Belmonte JC. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell, 2009; 5: 353−357. [DOI] [PMC free article] [PubMed]
- 9).Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell, 2009; 137: 647−658. [DOI] [PubMed]
- 10).Ambros V. The functions of animal microRNAs. Nature, 2004; 431: 350−355. [DOI] [PubMed]
- 11).Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, 2004; 116: 281−297. [DOI] [PubMed]
- 12).Judson RL, Babiarz JE, Venere M, Blelloch R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol, 2009; 27: 459−461. [DOI] [PMC free article] [PubMed]
- 13).Banito A, Rashid ST, Acosta JC, Li S, Pereira CF, Geti I, Pinho S, Silva JC, Azuara V, Walsh M, Vallier L, Gil J. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev, 2009; 23: 2134−2139. [DOI] [PMC free article] [PubMed]
- 14).Suh MR, Lee Y, Kim JY, Kim SK, Moon SH, Lee JY, Cha KY, Chung HM, Yoon HS, Moon SY, Kim VN, Kim KS. Human embryonic stem cells express a unique set of microRNAs. Dev Biol, 2004; 270: 488−498. [DOI] [PubMed]
- 15).Lin SL, Chang DC, Chang-Lin S, Lin CH, Wu DT, Chen DT, Ying SY. Mir-302 reprograms human skin cancer cells into a pluripotent ES-cell-like state. RNA, 2008; 14: 2115−2124. [DOI] [PMC free article] [PubMed]
- 16).Lin SL, Chang DC, Lin CH, Ying SY, Leu D, Wu DT. Regulation of somatic cell reprogramming through inducible mir-302 expression. Nucleic Acids Res, 2011; 39: 1054−1065. [DOI] [PMC free article] [PubMed]
- 17).Miyoshi N, Ishii H, Nagano H, Haraguchi N, Dewi DL, Kano Y, Nishikawa S, Tanemura M, Mimori K, Tanaka F, Saito T, Nishimura J, Takemasa I, Mizushima T, Ikeda M, Yamamoto H, Sekimoto M, Doki Y, Mori M. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell, 2011; 8: 633−638. [DOI] [PubMed]
- 18).Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med, 2000; 6: 88−95. [PMC free article] [PubMed]
- 19).Lau F, Ahfeldt T, Osafune K, Akustsu H, Cowan CA. Induced pluripotent stem (iPS) cells: an up-to-the-minute review. F1000 Biol Rep, 2009; 84: B1−84. [DOI] [PMC free article] [PubMed]
- 20).Chang HM, Gregory RI. MicroRNAs and reprogramming. Nat Biotechnol, 2011; 29: 499−500. [DOI] [PubMed]
- 21).Eiselleova L, Peterkova I, Neradil J, Slaninova I, Hampl A, Dvorak P. Comparative study of mouse and human feeder cells for human embryonic stem cells. Int J Dev Biol, 2008; 52: 353−363. [DOI] [PubMed]
- 22).Lim JW, Bodnar A. Proteome analysis of conditioned medium from mouse embryonic fibroblast feeder layers which support the growth of human embryonic stem cells. Proteomics, 2002; 2: 1187−1203. [DOI] [PubMed]
- 23).Soh BS, Song CM, Vallier L, Li P, Choong C, Yeo BH, Lim EH, Pedersen RA, Yang HH, Rao M, Lim B. Pleiotrophin enhances clonal growth and long-term expansion of human embryonic stem cells. Stem Cells, 2007; 25: 3029−3037. [DOI] [PubMed]
- 24).Mallanna SK, Rizzino A. Emerging roles of microRNAs in the control of embryonic stem cells and the generation of induced pluripotent stem cells. Dev Biol, 2010; 344: 16−25. [DOI] [PMC free article] [PubMed]
- 25).Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, Aimiuwu O, Richter L, Zhang J, Khvorostov I, Ott V, Grunstein M, Lavon N, Benvenisty N, Croce CM, Clark AT, Baxter T, Pyle AD, Teitell MA, Pelegrini M, Plath K, Lowry WE. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell, 2009; 1: 111−123. [DOI] [PMC free article] [PubMed]
- 26).Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, Kono T, Shioda T, Hochedlinger K. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature, 2010; 465: 175−181. [DOI] [PMC free article] [PubMed]