ABSTRACT
Sixteen operative cases of parasagittal and falx meningioma were analyzed retrospectively. Parasagittal meningioma totaled 12 cases and falx meningioma numbered 4 cases. Preoperative symptoms were paresis of a lower extremity in 7 cases and disturbed consciousness or mentality in 6 cases. Paresis and/or consciousness deteriorated just after the operation in 11 cases. The deterioration was identified in paresis (6 cases), consciousness (3 cases), paresis and consciousness (2 cases). Motor function further deteriorated postoperatively when the patients had shown preoperative paresis. The cause of postoperative deterioration of motor function and/or consciousness level was intracerebral hematoma in 1 case, and newly-developed brain edema in 1 case. There was no obvious explanation for the symptomatic exacerbation in the other 9 cases. At discharge, 5 cases showed deterioration of motor function in comparison to their preoperative condition, and 3 cases showed an improvement. Eleven cases showed no change of consciousness in comparison to the preoperative condition, and 5 cases showed improvement at discharge. Surgical result was good for consciousness or mentality, but was relatively poor for motor function. It was considered that surgery should be performed carefully in patients with preoperative paresis.
Key Words: Parasagittal meningioma, Falx meningioma, Supplementary motor area, Cingulate cortex, Akinetic mutism
INTRODUCTION
The surgical outcome of parasagittal and falx meningioma is poor. Postoperative complications occur in 34.8% of patients.1) Patients frequently develop transient or permanent mutism and hemiparesis following injury to the supplementary motor cortex, anterior cingulated cortex and anterior part of the corpus callosum.2,3)
Sixteen cases of parasagittal and falx meningioma have been treated in this department. This report presents the surgical results and discusses the optimal surgical technique to avoid complications.
CASES
This study analyzed 12 cases of parasagittal meningioma and 4 cases of falx meningioma (Table 1). Fourteen tumors were located in the frontal region and 2 tumors in the parietal region. Preoperative symptoms were paresis of the lower extremity in 7 cases and disturbed consciousness or mentality in 6 cases. Brain edema around the tumor was identified in 8 cases.
Table 1.
Summary of cases. Abbreviations: M: male; F: female; L: left; R: right. In paresis column, + indicates the incomplete paresis and ++ represents the plegia.
| Case | Tumor | Operation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Age | Gender | Paresis (Upper limb/Lower limb) | Consciousness | Type | Laterality | Location | Brain edema | Simpson grade | Arachnoid membrane in tumor bed | ||
| Deteriorated group | ||||||||||||
| 1 | 64 | F | (–/–) | Acalculia | Parasagittal | L | Frontal | (–) | II | (+) | ||
| 2 | 51 | F | (–/–) | Alert | Parasagittal | L | Frontal | (–) | IV | (+) | ||
| 3 | 78 | F | (–/–) | Alert | Falx | R | Parietal | (–) | I | (+) | ||
| 4 | 73 | M | (–/–) | Alert | Parasagittal | L | Frontal | (+) | III | (–) | ||
| 5 | 56 | M | (–/–) | Alert | Parasagittal | L | Frontal | (–) | IV | (–) | ||
| 6 | 74 | F | (–/+) | Alert | Parasagittal | L | Frontal | (–) | II | (+) | ||
| 7 | 65 | M | (–/+) | Alert | Parasagittal | R | Parietal | (–) | I | (+) | ||
| 8 | 82 | F | (–/+) | Alert | Parasagittal | R | Frontal | (+) | II | (–) | ||
| 9 | 46 | F | (–/+) | Disorientation | Falx | R | Frontal | (–) | II | (–) | ||
| 10 | 80 | F | (–/+) | Alert | Parasagittal | R | Frontal | (–) | IV | (–) | ||
| 11 | 78 | F | (–/+) | Disorientation | Parasagittal | R | Frontal | (+) | II | (+) | ||
| No change or improved group | ||||||||||||
| 12 | 83 | F | (–/+) | Memory disturbance | Falx | R | Frontal | (+) | II | (+) | ||
| 13 | 71 | F | (–/–) | Alert | Parasagittal | L | Frontal | (+) | II | (–) | ||
| 14 | 62 | F | (–/–) | Alert | Parasagittal | R | Frontal | (+) | II | (+) | ||
| 15 | 73 | F | (–/–) | Dementia | Parasagittal | R | Frontal | (+) | II | (–) | ||
| 16 | 69 | F | (–/–) | Disorientation | Falx | R | Frontal | (+) | IV | (–) | ||
Table 1.
(continued)
| Case | Complication after operation | Deterioration just after operation |
Neurology at discharge (Change compared with preoperation) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number | Brain edema | Intracerebral hemorrhage | Convulsion | Paresis | Consciousness | Paresis (Upper limb/Lower limb) | Consciousness | |||
| Deteriorated group | ||||||||||
| 1 | (–) | (–) | (–) | (–) | (+) | (–/–) (No change) | Alert (Improved calculation) | |||
| 2 | (–) | (–) | (–) | (–) | (+) | (–/–) (No change) | Alert (No change) | |||
| 3 | (–) | (–) | (–) | (–) | (+) | (–/–) (No change) | Alert (No change) | |||
| 4 | (+) | (–) | (–) | (+) | (–) | (–/–) (No change) | Alert (No change) | |||
| 5 | (+) | (–) | (–) | (+) | (–) | (–/+) (Deteriorate) | Alert (No change) | |||
| 6 | (–) | (–) | (+) | (+) | (–) | (–/+) (Improve) | Alert (No change) | |||
| 7 | (–) | (–) | (–) | (+) | (+) | (–/–) (Improve) | Alert (No change) | |||
| 8 | (+) | (–) | (–) | (+) | (–) | (+/+) (Deteriorate) | Alert (No change) | |||
| 9 | (–) | (–) | (+) | (+) | (+) | (+/+) (Deteriorate) | Alert (Improve) | |||
| 10 | (–) | (+) | (–) | (+) | (–) | (++/++) (Deteriorate) | Alert (No change) | |||
| 11 | (+) | (–) | (+) | (+) | (–) | (+/+) (Deteriorate) | Alert (Improve) | |||
| No change or improved group | ||||||||||
| 12 | (+) | (–) | (–) | (–) | (–) | (–/–) (Improve) | Memory disturbance (Improved memory function) | |||
| 13 | (+) | (–) | (–) | (–) | (–) | (–/–) (No change) | Alert (No change) | |||
| 14 | (+) | (–) | (–) | (–) | (–) | (–/–) (No change) | Alert (No change) | |||
| 15 | (+) | (–) | (–) | (–) | (–) | (–/–) (No change) | Alert (Improve) | |||
| 16 | (+) | (–) | (–) | (–) | (–) | (–/–) (No change) | Disorientation (No change) | |||
All cases were treated surgically. The degree of the excision was Simpson’s grade I in 2 cases, grade II in 9 cases, III in 1 case, and IV in 4 cases. Almost all of the arachnoid membrane remained around the tumor in 8 cases, but the membrane had a large defect in 8 cases. Surgery preserved the arachnoid membrane as much as possible.
RESULTS
Paresis and/or consciousness deteriorated just after the operation in 11 cases (deteriorated group) (Table 2). The deterioration was identified in paresis (6 cases; case 4, 5, 6, 8, 10, 11), consciousness (3 cases; case 1, 2, 3), and paresis and consciousness (2 cases; case 7, 9). It showed no change in 3 cases (case 13, 14, 16) and improved in 2 cases (case 12, 15) (no change or improved group).
Table 2.
Number of cases with deteriorated motor function and/or consciousness just after operation.
| Motor function | ||
|---|---|---|
| Deteriorated | No change or improved | |
| Consciousness | ||
| Deteriorated | 2 | 3 |
| No change or improved | 6 | 5 |
Deterioration of motor function occurred just after the operation in 8 cases (cases 4–11) (Table 1). Six cases showed hemiparesis (complete hemiplegia in 5 cases (case 5, 7, 9–11) and incomplete hemiparesis in 1 case (case 8)) and 2 showed monoparesis of the lower extremities (case 4, 6) (Figs. 1, 2). Convulsions occurred in 3 cases (case 6, 9, 11). Six of the 7 cases with preoperative paresis showed deterioration (cases 6–11) (Fig. 1). Two of the 9 cases that evidenced no preoperative paresis, showed deterioration of paresis (case 4, 5) (Fig. 2). The cases with preoperative paresis exhibited a significant deterioration of motor function just after the operation (Chi-square test, p = 0.012; Table 3).
Fig. 1.
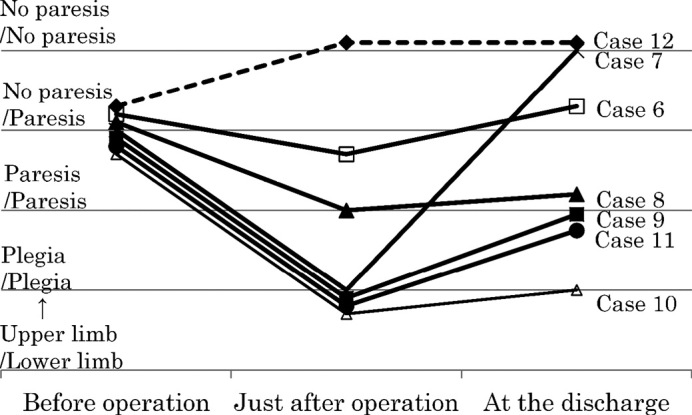
Change of motor function before and just after operation, and at discharge in cases with preoperative paresis.
Fig. 2.
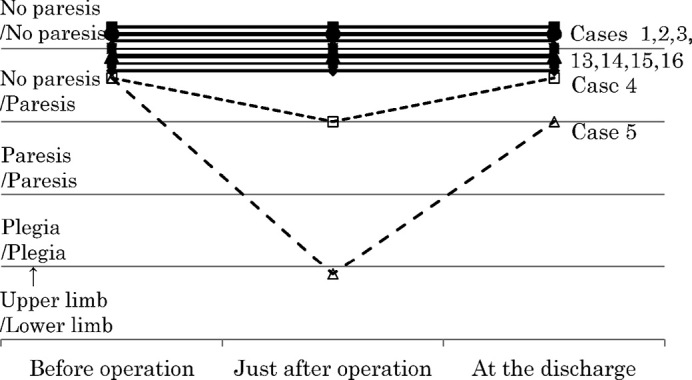
Change of motor function before and just after operation, and at discharge in cases with no preoperative paresis.
Table 3.
Relation between preoperative paresis and deterioration in motor function just after the operation. Cases with preoperative paresis showed significant deterioration of motor function just after operation (Chi-square test, p = 0.012).
| Deterioration of motor function just after operation | ||
|---|---|---|
| (+) | (–) | |
| Paresis before operation (+) | 6 | 1 |
| Paresis before operation (–) | 2 | 7 |
Ultimately, 5 cases (cases 5, 8–11) showed deterioration of motor function in comparison to the preoperative condition, whereas 3 cases (case 6, 7, 12) evidenced an improvement upon discharge (Figs. 1, 2). In 8 patients (cases 1–4, 13–16) that had no preoperative paresis, motor function did not change.
Deterioration of consciousness occurred after surgery in 5 cases (Table 1). This deterioration included drowsiness in 2 cases (case 1, 2), disorientation in 1 case (case 3), and mutism in 2 cases (case 7, 9) just after the operation (Fig. 3). All patients who exhibited the deterioration of consciousness just after the operation, showed a complete recovery thereafter.
Fig. 3.
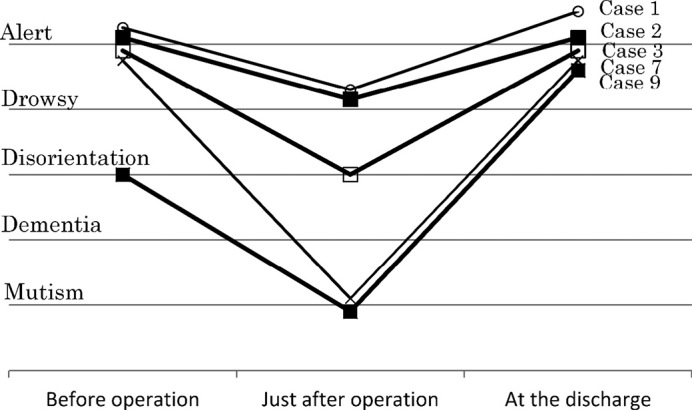
Change of consciousness before and just after operation, and at discharge in cases whose consciousness or mentality deteriorated just after operation.
Eventually, 11 cases showed no change of consciousness in comparison to the preoperative condition, and 5 cases (case 1, 9, 11, 12, 15) displayed improvement at discharge (Figs. 3, 4). Five of the 6 cases with preoperative disturbed consciousness or mentality, showed improvement. No cases showed deterioration at discharge, in comparison with the preoperative condition.
Fig. 4.
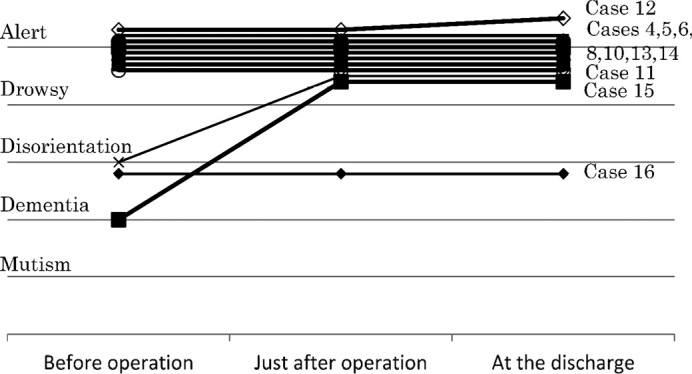
Change of consciousness before and just after operation, and at discharge in cases whose consciousness or mentality improved or showed no change just after operation.
The cause of postoperative deterioration in motor function and/or consciousness level was intracerebral hematoma in 1 case (case 10), and newly-developed brain edema in 1 case (case 5) (Table 1). There was no obvious explanation for the symptomatic exacerbation in the other 9 cases.
DISCUSSION
Our study showed the unexpectedly poor surgical results of parasagittal and falx meningioma. The motor area, supplementary motor area and cingulate cortex of the brain are affected by the tumor or the operation. The affected motor area contributes to motor function, especially in the lower extremities. The supplementary motor area has a role in motor planning and initiation of movement.4) Transient or permanent akinetic mutism, hemiparesis (especially paresis of the lower extremity) and gaze preference can occur following surgery around the supplementary motor area.5-7)
The anterior cingulate cortex is involved in conditioned emotional learning, vocalization associated with expressing internal states, assessment of motivational content and assigning emotional valence to internal and external stimuli.8) Therefore, reduced cingulate activity following surgery can contribute to behavioral disorders including akinetic mutism, diminished self-awareness and depression, motor neglect, impaired motor initiation, reduced responses to pain, and aberrant social behavior.5,8,9) The posterior cingulated cortex is associated with motor programmed disorders affecting more complex motor behavior.4) Motor weakness, marked reduced speech output, ataxia, dysmetria and postural instability may develop due to a lesion in the posterior cingulate cortex.4,10)
In the present study, the deterioration of motor function and consciousness was suspected to be caused by injury of the motor area, supplementary motor area and/or cingulate cortex of the brain. However, the lesion that caused the deterioration could not be identified precisely. Surgical results were good in terms of consciousness, but relatively poor for motor function. The reason the surgical results differed between motor function and consciousness could not be clarified. However, it was evident in our cases that motor function further deteriorated after the operation when the patients had shown preoperative paresis. So it was considered that the surgery should be undertaken very carefully in patients with preoperative paresis.
The cause of postoperative deterioration of motor function and/or consciousness level was intracerebral hematoma in 1 case, and newly-developed brain edema in 1 case. However, no lesion was found in postoperative MRI and CT to account for the symptomatic exacerbation in the other 9 cases. The symptomatic deterioration may have been caused by the injury of an artery or vein on the cerebral cortex in one case of newly-developed brain edema and a considerable portion of the postoperative deteriorated cases. There was no injury to the large cortical artery and ascending cerebral vein in any of the cases. However, small arteries and veins over the cortex, which were compressed by the tumor, might be damaged during surgery. Disruption of the small cortical arteries and veins can cause ischemia. Accordingly, it is crucial that the surgeon precisely dissects the arachnoid plane under a microscope to preserve the arachnoid membrane and the microvessels on the cortical surface.
REFERENCES
- 1).Skudas G, Tamasauskas A. Prognosis of the surgical treatment of parasagittal meningioma. Medicina, 2002; 38: 1089–1096. [Article in Lithuanian] [PubMed]
- 2).Crutchfield JS, Sawaya R, Meyers CA, Moore BD 3rd. Postoperative mutism in neurosurgery. Report of two cases. J Neurosurg, 1994; 81: 115–121. [DOI] [PubMed]
- 3).Tahta K, Cirak B, Pakdemirli E, Suzer T, Tahta F. Postoperative mutism after removal of an anterior falcine meningioma. J Clin Neurosci, 2007; 14: 793–796. Epub 2007 May 9. [DOI] [PubMed]
- 4).Freund HJ. Differential effects of cortical lesions in humans. Ciba Found Symp, 1987; 132: 269–281. [DOI] [PubMed]
- 5).Mochizuki H, Saito H. Mesial frontal lobe syndromes: correlations between neurological deficits and radiological localizations. Tohoku J Exp Med, 1990; 161 Suppl: 231–239. [PubMed]
- 6).Schell G, Hodge CJ Jr, Cacayorin E. Transient neurological deficit after therapeutic embolization of the arteries supplying the medial wall of the hemisphere, including the supplementary motor area. Neurosurgery, 1986; 18: 353–356. [DOI] [PubMed]
- 7).Zentner J, Hufnagel A, Pechstein U, Wolf HK, Schramm J. Functional results after resective procedures involving the supplementary motor area. J Neurosurg, 1996; 85: 542–549. [DOI] [PubMed]
- 8).Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain, 1995; 118 (Pt 1): 279–306. [DOI] [PubMed]
- 9).Kunishio K, Matsumoto K, Asari S, Ohmoto T. Transient mutism after resection of left frontal lobe astrocytoma in adult: case report. No Shinkei Geka, 1997; 25: 61–65. [Article in Japanese] [PubMed]
- 10).Nagaratnam N, Nagaratnam K, Ng K, Diu P. Akinetic mutism following stroke. J Clin Neurosci, 2004; 11: 25–30. [DOI] [PubMed]


