Abstract
Background
Little is known regarding the neurocognitive impact of temporal lobe tumor resection.
Objective
To clarify subacute surgery-related changes in neurocognitive functioning (NCF) in patients with left (LTL) and right (RTL) temporal lobe glioma.
Methods
Patients with glioma in the LTL (n=45) or RTL (n=19) completed comprehensive pre- and postsurgical neuropsychological assessments. NCF was analyzed with two-way mixed design repeated measures analysis of variance, with hemisphere (LTL or RTL) as an independent between-subjects factor and pre- and postoperative NCF as a within-subjects factor.
Results
About 60% of patients with LTL glioma and 40% with RTL lesions exhibited significant worsening on at least one NCF test. Domains most commonly impacted included verbal memory and executive functioning. Patients with LTL tumor showed greater decline than patients with RTL tumor on verbal memory and confrontation naming tests. Nonetheless, over one-third of patients with RTL lesions also showed verbal memory decline.
Conclusion
In patients with temporal lobe glioma, NCF decline in the subacute postoperative period is common. As expected, patients with LTL tumor show more frequent and severe decline than patients with RTL tumor, particularly on verbally-mediated measures. However, a considerable proportion of patients with RTL tumor also exhibit decline across various domains, even those typically associated with left hemisphere structures, such as verbal memory. While patients with RTL lesions may show even greater decline in visuospatial memory, this domain was not assessed. Nonetheless, neuropsychological assessment can identify acquired deficits and help facilitate early intervention in patients with temporal lobe glioma.
Keywords: brain tumor, cognition, glioma, neurosurgery, temporal lobe
Introduction
The primary goals of surgical resection of glioma include reduction of mass effect, symptom relief, determination of pathological diagnosis, and facilitation of adjuvant therapies.1 These aims are carefully balanced with preservation of neurological function, especially in light of emerging evidence that surgically-acquired deficits are associated with reduced quality of life and survival.2 Modern neuromonitoring techniques (e.g., awake craniotomy and direct cortical stimulation) assist surgeons by delineating the boundaries of “eloquent regions” of tissue that subserve important neurocognitive and motor functions.3 By avoiding damage to these areas, acquired deficits may be reduced and quality of life better preserved. Numerous studies indicate that neurologic impairment is largely transient following modern surgical intervention for glioma.4-6 However, most studies focus on basic functions as assessed by clinical neurological examination or brief mental status screening, which are relatively insensitive to impairment of higher level neurocognitive functioning (NCF).7,8
Studies of NCF in patients with glioma have increasingly utilized a comprehensive neuropsychological approach,9-13 though few include both pre- and postoperative testing. Nonetheless, a recent study of NCF in patients with insular glioma showed that approximately 50% of patients exhibited postoperative decline, particularly with memory and executive functions.14 While a sizeable proportion of patients with glioma exhibit postsurgical NCF decline, improved or stable functioning has also been documented.15,16 Additionally, it is often assumed that patients with right hemisphere tumors are less likely to develop postoperative decline. Given relatively small studies in glioma, larger outcome studies with other focal neurological populations are useful when considering potential relationships between NCF and resection of temporal lobe glioma.
A convergence of research has shown that the left temporal lobe (LTL) facilitates verbal memory and other language functions, while the right temporal lobe (RTL) is primarily associated with visuospatial memory and nonverbal abilities, assuming left hemisphere language dominance.17 In patients with temporal lobe epilepsy, dominant LTL resections are associated with verbal memory decline and RTL resection with visuospatial memory decline, particularly when the hippocampal complex is involved.18 In a systematic review, Sherman and colleagues reported pooled estimates indicating that 44% of LTL epilepsy patients exhibited verbal memory decline following surgery, compared to 20% showing verbal memory decline after RTL surgery.19 Additionally, object naming declined in 34% of LTL patients, with greater worsening related to larger extent of resection. Conversely, relatively few patients showed decline in intelligence, executive functioning, verbal fluency, and attention following LTL or RTL surgery.
Despite similarities between populations, generalizing from epilepsy studies to glioma may be misleading. Patients with brain tumors tend to have less severe but more diffuse NCF impairment compared to other focal neurological populations, even when similar brain areas are involved.20 This may relate to pathophysiological processes unique to glioma, in which most tumors begin by displacing neuronal tissues without actually causing immediate damage. Gliomas also tend to be diffuse and infiltrative, and residual tumor cells may invade functional brain regions even after resection, impacting the integrity of distributed neural networks underlying numerous cognitive functions.8 The present study aims to clarify changes in NCF associated with surgical resection of glioma within the LTL and RTL, while accounting for relevant patient and treatment characteristics. By characterizing NCF outcomes with a comprehensive neuropsychological approach, surgeons may be better able to counsel patients on the potential NCF risks and benefits associated with resection.
Methods
Participants
Adult patients with LTL or RTL glioma were identified in The University of Texas M.D. Anderson Cancer Center (MDACC) Brain and Spine Center Neuropsychology and Neurosurgery databases. Cases were included if they had baseline presurgical and follow-up postsurgical neuropsychological evaluations within 3 months of microsurgical resection. Sixty-four clinically referred patients (LTL, n=45; RTL, n=19) completed pre- and postsurgical neuropsychological evaluations between 2001 and 2010 and were included in the study. The MDACC Institutional Review Board approved the study.
Data Collection and Coding
Tumor Location and Volume
Three distinct temporal lobe areas were defined to specify tumor location, including the lateral anterior, lateral posterior, and medial regions (see Figure 1).21 Tumors with extension into 2 or more areas were considered multi-region. Tumor location was classified as involving eloquent, near-eloquent, or non-eloquent regions.22 Tumors classified as eloquent or near-eloquent involved or approached structures associated with speech/language (i.e., Brodmann's areas 22, 39, 40, 44, 45) and/or sensorimotor functions (i.e., Brodmann's areas 1, 2, 3, 4). Volumetric analysis was performed on MRI scans with MedVision 1.41 software.23 Postoperative MRI was obtained within 72 hours of surgery. Extent of resection was determined with volumetric data from pre- and postoperative T1 sequences. For primarily gadolinium-enhancing tumors, T1 hyperintensity was used for resection calculations, while T1 hypointensity was used with non-enhancing lesions.
FIGURE 1.
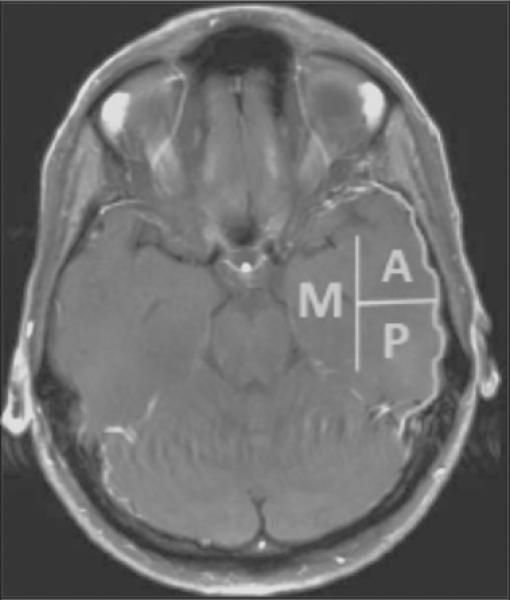
Temporal lobe segmentation. Abbreviations: A, lateral anterior; P, lateral posterior; M, medial.
Neurocognitive Testing
Table 1 lists the neuropsychological tests by domain that were routinely included in the assessment. Alternate forms were utilized for postsurgical evaluation to minimize practice effects on HVLT-R, Token, and COWA. The number of patients administered a given test differed by instrument, as the evaluations utilized a flexible approach and were performed for clinical purposes. Approximately half of the sample did not have data for HVLT-R DR and HVLT-R Rec, as clinic practices initially utilized an earlier version of the HVLT. Nonetheless, HVLT TR trials are identical between versions, and HVLT-R normative data were used for all HVLT variables as indicated in Table 1.
TABLE 1.
Neurocognitive Tests Grouped by Principal Domain
| Domain | Test | Abbreviation |
|---|---|---|
| Attention | WAIS-R/III Digit Span | Digit Span |
| Learning and Memory | HVLT-R Total Recall HVLT-R Delayed Recall HVLT-R Delayed Recognition |
HVLT-R TR HVLT-R DR HVLT-R Rec |
| Processing Speed | WAIS-R/III Digit Symbol Trail Making Test Part A |
Digit Symbol TMTA |
| Executive Function | Trail Making Test Part B WAIS-R/III Similarities MAE Controlled Oral Word Association |
TMTB Similarities COWA |
| Language | MAE Token Test MAE Visual Naming or Boston Naming Test |
Token Naming |
| Visuospatial Function | WAIS-R/III Block Design | Block Design |
| Motor Function | Grip Strength Difference Grooved Pegboard Test Difference |
Grip Peg |
| Clinical Trial Battery Composite | HVLT-R, TMT, COWA | CTB Comp |
Abbreviations: WAIS-R, Wechsler Adult Intelligence Scale–Revised; WAIS-III, Wechsler Adult Intelligence Scale–Third Edition; HVLT-R, Hopkins Verbal Learning Test–Revised; MAE, Multilingual Aphasia Examination.
NCF test scores were converted to demographically-adjusted z-scores (M=0.0, SD=1.0) from published normative data.24-31 Grip Strength Difference and Grooved Pegboard Difference scores were calculated as the difference between the z-scores of the hands contralateral and ipsilateral to the tumor. The Clinical Trial Battery Composite (CTB Comp), a measure of general cognitive functioning used in brain tumor clinical trials,32-34 was calculated as the mean of the z-scores for COWA, TMTA, TMTB, HVLT-R TR, HVLT-R DR, and HVLT-R Rec. Differences between pre- and postsurgical z-scores were described as follows: Improved (+1.00 or greater), Stable (−0.99 to +0.99), Mildly Declined (−1.00 to −1.99), Moderately Declined (−2.00 to −2.99), and Severely Declined (−3.00 or lower). The criterion for at least mild postsurgical decline (i.e., z-score change ≤ −1.0) is a commonly used heuristic for describing NCF change in clinical neuropsychological practice and neurosurgical research.35,36
Statistical Analysis
Independent-samples t-tests and chi-square goodness of fit tests were used to compare differences in demographic and clinical characteristics between LTL and RTL groups. Fisher's exact tests were used for categorical comparisons in which 20% or more cells had sample sizes less than 5. Results of NCF performances were evaluated with two-way mixed design repeated measures analyses of variance (ANOVA), with hemisphere (LTL or RTL) as an independent between-subjects factor and pre- and postoperative NCF as a within-subjects factor. Separate ANOVAs were performed for each NCF measure. Follow-up comparisons of NCF change scores were performed with independent-samples t-tests. Frequency of changes (i.e., Improved, Stable, Mildly Declined, etc.) was described with percentages. Due to sample size restrictions, classifications were collapsed into Declined (z-score change ≤ −1.0) or Stable/Improved groups (z-score change ≥ −0.99) for inferential analyses. Frequency of Decline versus Stable/Improved NCF was compared with chi-square goodness of fit or Fisher's exact tests. Spearman's rank-order correlations (ρ) were used to determine relationships between tumor and clinical characteristics with postsurgical NCF change for the LTL and RTL groups separately. Sensitivity analyses were conducted with independent-samples t-tests comparing NCF performances by handedness, seizure status, operative procedure (i.e., awake vs. asleep craniotomy), and adjuvant treatment received (chemotherapy and/or radiation vs. none) at the time of follow-up evaluation. NCF change scores were analyzed by temporal lobe tumor location (region) with one-way ANOVA. All statistical analyses were performed with SPSS 21.0 (IBM Corp).37 Given the exploratory nature of the investigation, two-sided tests were used with a significance level of P≤.05.
Results
Demographic and Clinical Characteristics
Sample characteristics are presented in Table 2. The sample was predominantly white (89%) and right-handed (86%). The majority of patients were diagnosed with glioblastoma (55%), astrocytoma (25%), or oligodendroglioma (11%), and 71% of all tumors were anaplastic. Tumors were most commonly located in the medial (41%) temporal lobe region, with 95% of lesions involving eloquent or near-eloquent tissue. Most patients had gross or near total resections (% tumor resected; M = 94.8, SD = 10.8). The average intervals between NCF assessments and surgery were slightly more than 1 month for both preoperative and postoperative evaluations. Demographic and clinical characteristics did not significantly differ between the LTL and RTL groups across all demographic and clinical characteristics, with the exception of surgical procedure in which patients with LTL tumors had significantly more frequent awake craniotomies than the RTL group [64% vs. 5%, χ2(1,N=64)=18.79, P<.001].
TABLE 2.
Demographic and Clinical Characteristics
| LTL (n = 45) | RTL (n = 19) | p-value | |
|---|---|---|---|
| Age in years | |||
| Mean (SD) | 50.3 (15.2) | 53.2 (8.6) | .429 |
| Range | 18 – 78 | 36 – 65 | |
| Male, n (%) | 21 (47) | 11 (58) | .412 |
| White, n (%) | 39 (87) | 18 (95) | .664 |
| Right hand dominant, n (%) | 38 (84) | 17 (90) | .713 |
| Education, years | |||
| Mean (SD) | 14.7 (2.5) | 14.2 (2.0) | .450 |
| Range | 7 – 20 | 11 – 19 | |
| Histology, n (%) | |||
| Glioblastoma | 22 (49) | 13 (68) | .914 |
| Astrocytoma | 12 (27) | 4 (21) | |
| Oligodendroglioma | 5 (11) | 2 (11) | |
| Other | 6 (13) | 0 (0) | |
| WHO tumor gradea | |||
| High grade, n (%) | 36 (80) | 14 (74) | .202 |
| Temporal Lobe Region, n (%) | |||
| Anterior | 10 (22) | 4 (21) | .603 |
| Posterior | 12 (26) | 4 (21) | |
| Medial | 16 (36) | 10 (53) | |
| Multi | 7 (16) | 1 (5) | |
| Functional Region, n (%) | |||
| Eloquent | 16 (36) | 7 (37) | .999 |
| Near-eloquent | 27 (60) | 11 (58) | |
| Non-eloquent | 2 (4) | 1 (5) | |
| Tumor Volume,b cm3 | |||
| Mean (SD) | 23.0 (26.5) | 33.4 (29.1) | .173 |
| Range | 0.4 – 142.4 | 3.3 – 115.8 | |
| Awake Craniotomy, n (%) | 29 (64) | 1 (5) | .013* |
| Extent of Resection, % removed | 94.9 (11.6) | 94.7 (9.20) | .947 |
| Postoperative FLAIR Volume,c cm3 | |||
| Mean (SD) | 14.7 (21.8) | 21.3 (20.9) | .276 |
| Range | 0.00 – 121.3 | 0.0 – 70.3 | |
| Seizure History, n (%) yes | 19 (42) | 7 (37) | .689 |
| Therapy at Follow-up, n (%) | |||
| Chemotherapy | 2 (4) | 0 (0) | .564 |
| Radiation | 11 (24) | 4 (21) | |
| Both | 2 (4) | 0 (0) | |
| Prior Biopsy, n (%) yes | 11 (24) | 1 (5) | .090 |
| NCF Testing Interval, days | |||
| Baseline to surgery, Mean (SD) | 39.2 (28.3) | 31.0 (28.5) | .295 |
| Surgery to follow-up, Mean (SD) | 34.0 (28.3) | 28.9 (28.9) | .517 |
High Grade = WHO Grade III or IV.
LTL, n = 44; RTL, n = 19. T1 hyperintense volume for enhancing tumors; T1 hypointense volume for nonenhancing lesions
LTL, n = 45; RTL, n = 18.
Significant difference, P ≤ .05.
Neurocognitive Functioning
The results of two-way mixed design repeated measures ANOVA are displayed in Table 3. Mean NCF performances significantly decreased following tumor resection on indices of attention [Digit Span: F(1,62)=8.08, P=.006, partial η2=.12], verbal recognition memory [HVLTR Rec: F(1,29)=4.44, P=.044, partial η2=.13], processing speed [TMTA: F(1,59)=4.65, P=.035, partial η2=.07], executive function [TMTB: F(1,57)=11.89, P=.001, partial η2=.17; COWA: F(1,58)=7.88, P=.007, partial η2=.12], and CTB Comp [F(1,27)=17.61, P<.001, partial η2=.40]. Surgery and hemisphere showed a significant interaction demonstrating greater postoperative decline in patients with LTL compared to RTL tumor on verbal learning and memory [HVLT-R TR: F(1,59)=5.15, P=.027, partial η2=.08; HVLT-R DR: F(1,29)=4.89, P=.035, partial η2=.14] and object naming [Naming: F(1,57)=5.98, P=.018, partial η2=.10].
TABLE 3.
Pre- and Postoperative Neurocognitive Performances by LTL and RTL Glioma Group
| Pre-Operative | Postoperative | Surgery | Hemisphere | Surg*Hem | |||||
|---|---|---|---|---|---|---|---|---|---|
| Domain and Test | n | z-score M (SD) | z-score M (SD) | P | η 2 | P | η 2 | P | η 2 |
| Attention | |||||||||
| Digit Span | |||||||||
| LTL | 45 | −0.48 (0.74) | −0.64 (0.97) | ||||||
| RTL | 19 | −0.11 (1.17) | −0.49 (0.97) | .006* | .12 | .271 | .02 | .229 | .02 |
| Learning and Memory | |||||||||
| HVLT-R TR | |||||||||
| LTL | 42 | −1.19 (1.56) | −2.74 (1.63) | ||||||
| RTL | 19 | −0.75 (1.30) | −1.32 (1.70) | <.001* | .29 | .016* | .10 | .027* | .08 |
| HVLT-R DR | |||||||||
| LTL | 24 | −1.02 (1.76) | −3.01 (1.92) | ||||||
| RTL | 7 | −1.42 (2.35) | −1.76 (2.65) | .004* | .25 | .589 | .01 | .035* | .14 |
| HVLT-R Recog | |||||||||
| LTL | 24 | −0.78 (1.70) | −2.20 (2.10) | ||||||
| RTL | 7 | −1.76 (2.73) | −1.92 (2.69) | .044* | .13 | .680 | .01 | .103 | .09 |
| Processing Speed | |||||||||
| Digit Symbol | |||||||||
| LTL | 44 | 0.01 (0.81) | −0.17 (0.98) | ||||||
| RTL | 19 | −0.25 (1.18) | −0.37 (0.97) | .116 | .04 | .348 | .01 | .760 | .00 |
| TMTA | |||||||||
| LTL | 43 | −0.38 (2.55) | −0.83 (2.86) | ||||||
| RTL | 18 | −0.10 (0.96) | −1.83 (5.06) | .035* | .07 | .593 | .01 | .210 | .03 |
| Executive Function | |||||||||
| TMTB | |||||||||
| LTL | 41 | −0.59 (1.43) | −2.61 (4.74) | ||||||
| RTL | 18 | −1.21 (1.91) | −2.84 (3.97) | .001* | .17 | .596 | .01 | .720 | .00 |
| Similarities | |||||||||
| LTL | 42 | −0.24 (0.81) | −0.42 (1.03) | ||||||
| RTL | 17 | −0.06 (0.83) | 0.31 (1.02) | .750 | .00 | .037* | .07 | .059 | .06 |
| COWA | |||||||||
| LTL | 41 | −0.57 (1.16) | −0.87 (1.35) | ||||||
| RTL | 19 | −0.30 (1.11) | −0.79 (1.36) | .007* | .12 | .585 | .01 | .487 | .01 |
| Language | |||||||||
| Token | |||||||||
| LTL | 34 | −0.19 (1.19) | −0.40 (1.28) | ||||||
| RTL | 16 | 0.32 (1.00) | 0.21 (0.99) | .222 | .03 | .097 | .06 | .656 | .00 |
| Naming | |||||||||
| LTL | 41 | −0.91 (1.23) | −1.64 (1.45) | ||||||
| RTL | 18 | 0.02 (1.16) | 0.07 (1.29) | .037* | .07 | .001* | .22 | .018* | .10 |
| Visuospatial Function | |||||||||
| Block Design | |||||||||
| LTL | 45 | −0.09 (0.84) | −0.07 (0.96) | ||||||
| RTL | 18 | −0.00 (0.82) | −0.31 (0.88) | .113 | .04 | .731 | .00 | .068 | .05 |
| Motor Function | |||||||||
| Grip Difference | |||||||||
| LTL | 39 | −0.14 (0.94) | −0.12 (0.86) | ||||||
| RTL | 15 | −0.01 (1.34) | 0.35 (1.01) | .114 | .04 | .277 | .02 | .204 | .03 |
| Peg Difference | |||||||||
| LTL | 43 | 0.14 (1.10) | −0.14 (0.96) | ||||||
| RTL | 15 | 0.14 (1.55) | −0.30 (1.27) | .053 | .07 | .771 | .00 | .663 | .00 |
| CTB Comp | |||||||||
| LTL | 22 | −0.50 (0.86) | −1.91 (1.67) | ||||||
| RTL | 7 | −1.12 (1.33) | −1.94 (1.81) | <.001* | .40 | .554 | .01 | .273 | .04 |
Note: ν2 refers to partial eta squared.
See Table 1 for abbreviations.
Significant difference, P ≤ .05.
Follow-up analyses were conducted for measures with significant surgery by hemisphere interaction effects. Mean z-score changes are depicted in Figure 2. Postsurgical worsening was significantly greater for the LTL compared to RTL group in verbal learning [HVLT-R TR; LTL M=−1.55, SD=1.75 vs. RTL M=−0.57, SD=1.01; t(59)=−2.27, P=.008] and memory [HVLT-R DR; LTL M=−1.99, SD=1.84 vs. RTL: M=−0.35, SD=1.25; t(29)=−2.21, P=.035], and Naming [LTL: M=−0.73, SD=1.30 vs. RTL: M=0.05, SD=0.56; t(57)=−2.45, P=.002].
FIGURE 2.
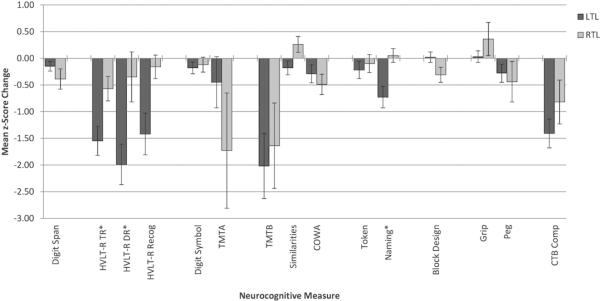
Pre- to postoperative neurocognitive change by patient group. See Table 3 for sample size descriptions. Error bars represent standard error of the mean. *Significant difference between LTL and RTL groups, P≤.05.
Frequency of Neurocognitive Change
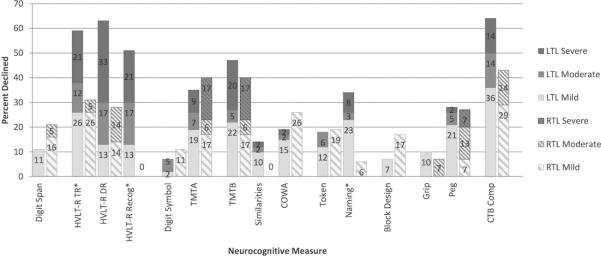
Frequency and severity of NCF decline across LTL and RTL groups are depicted in Figure 3. For the LTL group, Decline (z-score change ≤ −1.00) was most frequent on measures of verbal learning and memory (HVLT-R: TR, 59%; DR, 63%; Rec, 51%), executive functioning (TMTB, 47%), object naming (Naming, 34%), upper extremity manual dexterity (Peg, 28%), and CTB Comp (64%). Of those who declined on these measures, 7% to 33% exhibited Severe Decline (z-score change ≤ −3.00). Rates of Improvement (z-score change > 1.00) were less than 15% across all measures. For the RTL group, decline was most frequent on measures of processing speed (TMTA, 40%), executive functioning (TMTB, 40%), verbal learning and memory (HVLT-R: TR, 31%; DR, 28%), and CTB Comp (43%). Of those who declined, Severe Decline was only noted on measures of processing speed and executive functioning (17%). For patients with RTL glioma, Improvement was noted on measures of motor functioning (Grip, 27%; Peg, 20%) and a verbally-based measure of executive function (Similarities, 18%). Comparisons of rates of change (Declined versus Stable/Improved) revealed that the LTL group declined significantly more frequently than RTL patients in verbal learning [HVLT-R TR: 59% vs. 31%, χ2(1,N=61)=4.09, P=.043], verbal recognition memory [HVLT-R Rec: 28% vs. 0%, P=.026, Fisher's exact test], and object naming [Naming: 34% vs. 6%, P=.044, Fisher's exact test].
FIGURE 3.
Rates of postoperative neurocognitive decline by patient group across measures Severe Decline, z-score change < −3.00; Moderate Decline, z-score change −2.99 to −2.00; Mild Decline, z-score change −1.99 to −1.00.
*Significant difference in total decline between LTL and RTL groups, P≤.05.
Clinical Characteristics and Neurocognitive Change
In light of the potential impact of residual lesion and resection characteristics, correlational analyses were performed associating NCF change scores with extent of resection and postoperative FLAIR volume. FLAIR volume was not associated with any change scores for the LTL or RTL group. Change scores did not differ by temporal lobe region involved for either group. For the LTL group, extent of resection had significant inverse associations with verbal memory [HVLT-R Rec: ρ(34)=0.35, P=.038] and upper extremity strength change scores [Grip: ρ(32)=−0.40, P=0.020], indicating that as more tissue is removed, verbal memory and contralateral weakness worsen.
Given the finding that patients with LTL glioma were more likely to have awake craniotomies than the RTL group, NCF performances and change scores were compared by operative procedure (i.e., awake vs. asleep). As previously noted, these analyses were only conducted for the LTL group since only a single patient with RTL tumor had the awake procedure. For LTL patients, those with awake craniotomies exhibited significantly greater postoperative decline than those with asleep craniotomies across indices of attention [Digit Span; Asleep M=0.13, SD=0.56 vs. Asleep M=−0.32, SD=0.63; t(42)=−2.36, P=.023], verbal learning [HVLT-R TR; Asleep M=−0.58, SD=1.67 vs. Awake M=−2.14, SD=1.58; t(39)=−2.99, P=.005] and memory [HVLT-R DR; Asleep M=−0.91, SD=1.55 vs. Awake: M=−2.64, SD=1.73; t(22)=−2.47, P=.022], and Naming [Asleep: M=−0.03, SD=1.07 vs. Awake: M=−1.09, SD=1.31; t(38)=−2.60, P=.013]. Extent of resection, preoperative tumor volume, and postoperative FLAIR volume did not differ between awake and asleep procedure groups. However, a trend was observed in which LTL patients who underwent awake craniotomy had somewhat better pre-operative neurocognitive performances across most measures compared to those who underwent asleep procedures, though these differences were not significant.
Extent of resection was not associated with any change scores for the RTL group. For the LTL group, time from resection to postsurgical evaluation was significantly associated with improved upper extremity strength [Grip: ρ(32)=0.43, P=.010]. For patients with RTL tumor, time between resection and postsurgical evaluation was associated with improved verbal memory [HVLT-R DR: ρ(5)=0.89, P=.007]. Left-handed patients did not exhibit significantly different change scores than right-handed patients for either LTL or RTL tumor groups. Seizure history and treatment with chemotherapy and/or radiation therapy was not associated with differences in NCF change scores for either LTL or RTL group.
Discussion
To our knowledge, this represents the first study to comprehensively characterize changes in NCF in the subacute postoperative period following resection of glioma within the temporal lobes. Postsurgical decline was common, with over 60% of patients with LTL glioma and 40% with RTL lesions exhibiting at least mild worsening on one or more measures. However, the pattern and severity of decline differed by hemisphere. For LTL patients, decline was diffuse but most frequent in verbal learning, memory, executive functioning, object naming, and manual dexterity. Postsurgical worsening was relatively less frequent in patients with RTL lesions but still common on measures of processing speed, executive functioning, and verbal learning and memory. It is notable that both LTL and RTL patients exhibited decline in verbal learning and memory, but the frequency and magnitude of decline tended to be greater in LTL patients. For both groups, postsurgical decline was unrelated to seizure status or adjuvant treatment, in line with prior investigations.7,14
Patients with LTL tumors were more likely to have awake craniotomies than those with RTL glioma, which is unsurprising given the known importance of LTL structures to language functions that are frequently examined during awake procedures. Such procedures permit intraoperative mapping of language abilities and presumably allow for greater surgical avoidance of eloquent tissues important to speech. Interestingly, LTL patients who underwent awake craniotomy had greater postoperative decline than those under general anesthesia across measures of attention, memory, and naming abilities. Perhaps the most intuitive explanations of this finding include the possibility that awake mappings were performed on those with greatest involvement of eloquent tissues, and/or the awake procedure may afford the surgeon greater extent of resection, both of which may involve increased risk of adverse impact upon NCF. However, the observed differences by operative procedure appeared unrelated to temporal lobe region involved, eloquence, preoperative tumor volume, extent of resection, or postoperative FLAIR volume, making such potential explanations unsatisfactory. Nonetheless, a trend was observed in which those who underwent the awake procedure had somewhat better pre-operative NCF than those who underwent asleep procedures, though these differences were not significant. As such, it is possible that those with greater pre-operative NCF are at increased risk of decline following surgery, as they have more NCF capacity to lose following any acquired injury, whether from surgery or otherwise. Indeed, these trends are consistent with the temporal lobe epilepsy literature indicating that those with greater pre-operative levels of NCF show the greatest decline following surgery.38 Future studies are needed to better understand these and other risk factors associated with postoperative decline.
Also consistent with known NCF outcomes following neurosurgery for temporal lobe epilepsy19,39-41 is the finding of more prominent verbal memory and object naming decline in patients with LTL tumor. While slightly different criteria were utilized to define decline, Sherman and colleagues reported that 34% of patients with epilepsy who underwent LTL surgery exhibited worsening in object naming—the same proportion of patients with LTL glioma exhibiting decline after resection in the present study.19 However, decline in verbal memory was considerably more common in patients with glioma post-resection compared to patients with temporal lobe epilepsy post-surgery, for both LTL (63% vs. 44%) and RTL (31% vs. 20%) surgeries. While this difference may be due in part to method variance, it is also possible that surgical differences play a role. In the present study, tumors were most commonly located within the medial temporal lobe (41%), which includes the hippocampal formation important to memory.42 Given that most patients had gross total resections of their lesions, it is likely that a large proportion of tumor resections involved at least partial removal of the hippocampal formation with resulting decline in memory performances. This is in contrast to temporal lobe epilepsy surgeries, which often involve hippocampal sparing techniques that may result in greater preservation of memory. Despite this possibility, no relationship was discernible between NCF change and temporal lobe region involved. However, these analyses were limited by the relatively small sample size, particularly for the RTL group. This question should be investigated in future larger scale studies to better determine which structures are most critical to maintaining postsurgical NCF in patients with glioma, and for which cognitive domains.
While no significant relationships between temporal lobe region and neurocognitive profile were observed, the results remain generally consistent with known brain-behavior relationships. Specifically, the prominent declines in verbal memory and naming following LTL glioma resection are expected given the role of LTL cortical association areas in the perception and semantic activation of verbal material,42 LTL white matter tracts in language processing,43 and LTL medial structures in verbal learning and memory.42,44 In contrast, the RTL is often associated with visuospatial rather than verbal functions.40,45 Surprisingly, verbal learning and memory decline of at least mild magnitude was observed in 31% and 28% of patients with RTL lesions, respectively. Further, this finding cannot be attributed to the possibility of mixed or crossed language dominance, as few patients (n = 2) with RTL tumors were left-handed, and change scores did not differ by handedness. This adds to mounting evidence suggesting that the temporal lobes do not process material in a wholly dichotomous, material-specific manner.46,47 That is, broader bilateral networks appear to support important cognitive functions previously presumed to be strictly lateralized in nature. Accordingly, neurosurgical intervention for RTL tumor poses risk for executive and processing speed decline, in addition to significant worsening in domains not typically associated with RTL structures, such as verbal memory.
Although decline was frequent in all patients, about 34% of patients with LTL glioma and 57% of patients with RTL tumors were stable or slightly improved on the global composite measure. These findings are similar to those of Talachhi and colleagues, who reported stable postoperative functioning in 38%-55% of glioma patients.15 Additionally, relatively few patients exhibited postoperative worsening of a moderate or severe degree (i.e., z-score change ≤ −2.0; LTL, 28%; RTL, 14%). It should be noted that follow-up NCF evaluation was conducted in the subacute postsurgical period, and it is possible that additional patients improved or returned to baseline over a longer interval. Indeed, positive change in delayed memory was associated with greater time since resection in patients with RTL tumors, and improved strength was associated with time since surgery for patients with LTL glioma. Future longitudinal investigations with longer follow-up intervals would help clarify the postsurgical recovery trajectories in temporal lobe glioma.
Limitations
While it is possible that the relatively modest sample size may limit the robustness of the findings, our sample is of comparable or even favorable size with previous studies regarding relationships between glioma and NCF. Importantly, the findings remained similar when the data were analyzed as discrete (i.e., impairment frequency) or continuous variables (i.e., z-score change). Further, the results dovetail with the more established literature discussed above regarding NCF outcomes following neurosurgery for temporal lobe epilepsy, lending additional support to the present results. Another potential limitation involves selection bias, as the data represent a retrospective investigation of clinically referred patients. However, our sample is of similar composition to glioma epidemiological data regarding distribution of histology, tumor location, and age at diagnosis,48 supporting the representativeness of the sample. It is also notable that the study sample was slightly more highly educated than the general population. Given the potential impact of such demographic factors on NCF, performances were adjusted for age and education on appropriate measures. Additionally, no clinical or demographic factors significantly differed between LTL and RTL groups. Accordingly, such concerns cannot account for differences in NCF outcomes between the LTL and RTL groups. Nonetheless, large-scale prospective studies are needed to replicate and validate these findings. Such investigations would additionally benefit from standardization of neuroimaging protocols, more detailed description of operative approach, and employment of more stringent thresholds for significance not afforded by this relatively small exploratory study.
Given the clinical nature of the NCF evaluations, a flexible neuropsychological approach was utilized, and patients were administered both a core set of common tests as well as individually prescribed tests based on particular clinical questions and patient performance. These factors limited the sample sizes across a number of tests, particularly for the smaller RTL group, reducing the power and robustness of some analyses. The study would have also benefitted from inclusion of measures of visuospatial memory and other nonverbal abilities with greater sensitivity to processes believed to be more dependent on RTL structures.42,45 Given this limitation, it is possible that rates of postoperative impairment or decline are underestimated for the RTL group, which would further highlight the importance of RTL structures to NCF. Nonetheless, the findings provide valuable information regarding NCF change related to surgical intervention for glioma of the temporal lobes, supporting the importance of both LTL and RTL structures in the maintenance of postoperative NCF.
Conclusion
NCF decline following resection of temporal lobe glioma is common, particularly in the domains of verbal memory and executive functioning, regardless of hemisphere involved. However, patients with LTL lesions exhibit the greatest risk of decline in both of these domains, as well as expressive language. While at least mild decline was frequent in both LTL and RTL patients, severe decline was relatively infrequent. As such, maximal resection of temporal lobe glioma appears feasible with acceptable risk of large postoperative NCF worsening. However, patients are likely to experience some degradation in NCF in the subacute postsurgical period, including verbal memory decline, even for those with tumors within the RTL. This study provides information to guide discussions with patients regarding the risks involved with surgical resection. Further, neuropsychological assessment can identify acquired deficits in the early postoperative period and facilitate early intervention, both pharmacological (e.g., psychostimulants) and neurobehavioral (e.g., cognitive rehabilitation and speech therapy), which may help maximize recovery and patient well-being.
Acknowledgments
Funding: Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number R01NR014195 (J.S.Wefel). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure: The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Yong RL, Lonser RR. Surgery for glioblastoma multiforme: striking a balance. World Neurosurg. 2011;76:528–530. doi: 10.1016/j.wneu.2011.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGirt MJ, Mukherjee D, Chaichana KL, et al. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65:463–469. doi: 10.1227/01.NEU.0000349763.42238.E9. [DOI] [PubMed] [Google Scholar]
- 3.Duffau H, Capelle L, Denvil D, et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg. 2003;98:764–778. doi: 10.3171/jns.2003.98.4.0764. [DOI] [PubMed] [Google Scholar]
- 4.Duffau H. A personal consecutive series of surgically treated 51 cases of insular WHO Grade II glioma: advances and limitations. J Neurosurg. 2009;110:696–708. doi: 10.3171/2008.8.JNS08741. [DOI] [PubMed] [Google Scholar]
- 5.Ilmberger J, Ruge M, Kreth FW, Briegel J, Reulen HJ, Tonn JC. Intraoperative mapping of language functions: a longitudinal neurolinguistic analysis. J Neurosurg. 2008;109:583–592. doi: 10.3171/JNS/2008/109/10/0583. [DOI] [PubMed] [Google Scholar]
- 6.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358:18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- 7.Satoer D, Vork J, Visch-Brink E, Smits M, Dirven C, Vincent A. Cognitive functioning early after surgery of gliomas in eloquent areas. J Neurosurg. 2012;117:831–838. doi: 10.3171/2012.7.JNS12263. [DOI] [PubMed] [Google Scholar]
- 8.Klein M, Duffau H, Hamer PCDW. Cognition and resective surgery for diffuse infiltrative glioma: an overview. J Neurooncol. 2012;108:309–318. doi: 10.1007/s11060-012-0811-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheibel RS, Meyers CA, Levin VA. Cognitive dysfunction following surgery for intracerebral glioma: influence of histopathology, lesion location, and treatment. Neuro Oncol. 1996;30:61–69. doi: 10.1007/BF00177444. [DOI] [PubMed] [Google Scholar]
- 10.Henriksson R, Asklund T, Poulsen HS. Impact of therapy on quality of life, neurocognitive function and their correlates in glioblastoma multiforme: a review. J Neurooncol. 2011;104:639–646. doi: 10.1007/s11060-011-0565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wefel JS, Cloughesy T, Zazzali JL, et al. Neurocognitive function in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13:660–668. doi: 10.1093/neuonc/nor024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumors. Lancet Neurol. 2004;3:159–168. doi: 10.1016/S1474-4422(04)00680-5. [DOI] [PubMed] [Google Scholar]
- 13.Tucha O, Smely C, Preier M, Lange KW. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47:324–333. doi: 10.1097/00006123-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115:1115–1125. doi: 10.3171/2011.8.JNS11488. [DOI] [PubMed] [Google Scholar]
- 15.Talacchi A, Santini B, Savazzi S, Gerosa M. Cognitive effects of tumour and surgical treatment in glioma patients. J Neurooncol. 2011;103:541–549. doi: 10.1007/s11060-010-0417-0. [DOI] [PubMed] [Google Scholar]
- 16.Friedman MA, Meyers CA, Sawaya R. Neuropsychological effects of third ventricle tumor surgery. Neurosurgery. 2003;52:791–798. doi: 10.1227/01.neu.0000053367.94965.6b. [DOI] [PubMed] [Google Scholar]
- 17.Golby AJ, Poldrack RA, Brewer JB, et al. Material-specific lateralization in the medial temporal lobe and prefrontral cortex during memory encoding. Brain. 2001;124:1841–1854. doi: 10.1093/brain/124.9.1841. [DOI] [PubMed] [Google Scholar]
- 18.Bell B, Lin JJ, Seidenberg M, Hermann B. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat Rev Neurol. 2011;7:154–164. doi: 10.1038/nrneurol.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherman E, Wiebe S, Fay-McClymont, et al. Neuropsychological outcomes after epilepsy surgery: systematic review and pooled estimates. Epilepsia. 2011;52:857–869. doi: 10.1111/j.1528-1167.2011.03022.x. [DOI] [PubMed] [Google Scholar]
- 20.Anderson SW, Damasio H, Tranel D. Neuropsychological impairments associated with lesions caused by tumor or stroke. Arch Neurol. 1990;47:397–405. doi: 10.1001/archneur.1990.00530040039017. [DOI] [PubMed] [Google Scholar]
- 21.Noll KR, Sullaway C, Ziu M, Weinberg JS, Wefel JS. Relationships between tumor grade and neurocognitive functioning in patients with glioma of the left temporal lobe prior to surgical resection. Neuro Oncol. doi: 10.1093/neuonc/nou233. Advance Access published September 16, 2014, doi:10.1093/neuonc/nou233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42(5):1044–1055. doi: 10.1097/00006123-199805000-00054. [DOI] [PubMed] [Google Scholar]
- 23.Shi WM, Wildrick DM, Sawaya R. Volumetric measurement of brain tumors from MR imaging. J Neurooncol. 1998;37(1):87–93. doi: 10.1023/a:1005944724470. [DOI] [PubMed] [Google Scholar]
- 24.Wechsler D. Wechsler Adult Intelligence Scale–Revised. The Psychological Corporation; San Antonio: 1981. [Google Scholar]
- 25.Wechsler D. Wechsler Adult Intelligence Scale–III. The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- 26.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12(1):43–55. [Google Scholar]
- 27.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 28.Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329–338. [PubMed] [Google Scholar]
- 29.Benton A, Hamsher K, Sivan A. Multilingual Aphasia Examination Manual. AJA Associates Inc.; Iowa City, IA: 2000. [Google Scholar]
- 30.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norm for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Psychological Assessment Resources, Inc.; Lutz, FL: 2004. [Google Scholar]
- 31.Trites, Ronald L. Neuropsychological Test Manual. Royal Ottawa Hospital; Ottawa, Ontario, Canada: 1977. [Google Scholar]
- 32.Armstrong TR, Wefel JS, Wang M, et al. Net clinical benefit analysis of Radiation Therapy Oncology Group 0525: a phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol. 2013;31:4076–4084. doi: 10.1200/JCO.2013.49.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. J Neuroncol. 2013;15(10):1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meekes J, Braams OB, Braun KP, et al. Visual memory after epilepsy surgery in children: A standardized regression-based analysis of group and individual outcomes. Epilepsy Behav. 2014;36:57–67. doi: 10.1016/j.yebeh.2014.04.016. (2014) [DOI] [PubMed] [Google Scholar]
- 36.Maekawa K, Baba T, Otomo S, Morishita S, Tamura N. Low pre-existing gray matter volume in the medial temporal lobe and white matter lesions are associated with postoperative cognitive dysfunction after cardiac surgery. PloS one. 2014;9:e87375. doi: 10.1371/journal.pone.0087375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.IBM Corp. IBM SPSS Statistics for Windows, Version 21.0. IBM Corp.; Armonk, NY: Released 2012. [Google Scholar]
- 38.Chelune GJ, Naugle RI, Lüders H, Awad IA. Prediction of cognitive change as a function of preoperative ability status among temporal lobectomy patients seen at 6-month follow-up. Neurology. 1991;41:399–399. doi: 10.1212/wnl.41.3.399. [DOI] [PubMed] [Google Scholar]
- 39.Dulay MF, Levin HS, York MK, Li X, et al. Changes in individual and group spatial and verbal learning characteristics after anterior temporal lobectomy. Epilepsia. 2009;50:1385–1395. doi: 10.1111/j.1528-1167.2008.01730.x. [DOI] [PubMed] [Google Scholar]
- 40.Martin RC, Sawrie SM, Roth DL, et al. Individual memory change after anterior temporal lobectomy: a base rate analysis using regression-based outcome methodology. Epilepsia. 1998;39:1075–1082. doi: 10.1111/j.1528-1157.1998.tb01293.x. [DOI] [PubMed] [Google Scholar]
- 41.Schwarz M, Pauli E, Stefan H. Model based prognosis of postoperative object naming in left temporal lobe epilepsy. Seizure. 2005;14:562–568. doi: 10.1016/j.seizure.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed. Oxford; New York: 2004. [Google Scholar]
- 43.Griffiths JD, Marslen-Wilson WD, Stamatakis EA, Tyler LK. Functional organization of the neural language system: dorsal and ventral pathways are critical for syntax. Cereb Cortex. 2013;23:139–147. doi: 10.1093/cercor/bhr386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lillywhite LM, Saling MM, Briellmann RS, Weintrob DL, Pell GS, Jackson GD. Differential contributions of the hippocampus and rhinal cortices to verbal memory in epilepsy. Epilepsy Behav. 2007;10:553–559. doi: 10.1016/j.yebeh.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Amlerova J, Laczo J, Vlcek K, Javurkova A, Andel R, Marusic P. Risk factors for spatial memory impairment in patients with temporal lobe epilepsy. Epilepsy Behav. 2013;26:57–60. doi: 10.1016/j.yebeh.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 46.Jones-Gotman M, Zatorre RJ, Olivier A, et al. Learning and retention of words and designs following excision from medial or lateral temporal-lobe structures. Neuropsychologia. 1997;35:963–973. doi: 10.1016/s0028-3932(97)00024-9. [DOI] [PubMed] [Google Scholar]
- 47.Kennepohl S, Sziklas V, Garver KE, Wagner DD, Jones-Gotman M. Memory and the medial temporal lobe: hemispheric specialization reconsidered. Neuroimage. 2007;36:969–978. doi: 10.1016/j.neuroimage.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 48.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(suppl 4):iv1–iv63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]