Abstract
Case series
Patient: Male, 27 • Female, 46 • Male, 46
Final Diagnosis: —
Symptoms: Vague abdominal pain • severe nausea • vomiting • fever and diffuse abdominal tendernes
Medication: —
Clinical Procedure: —
Specialty: —
Objective:
Rare co-existance of disease or pathology
Background:
Porto-mesenteric venous thrombosis (PMVT) is an infrequent but severe surgical complication developing in patients who underwent laparoscopic bariatric surgery (sleeve gastrectomy). Herein, we describe the clinical presentation, management, and outcome of 3 rare cases of PMVT after laparoscopic sleeve gastrectomy (LSG), successfully treated at our center.
Case Report:
All patients developed PMVT post-LSG and presented with diffused abdominal pain, nausea, and vomiting. Computed tomography (CT) of the abdomen confirmed the diagnosis of portal vein thrombosis. Two patients were treated conservatively with anticoagulation and thrombolytic therapy and the third patient required operative intervention with bowel resection.
Conclusions:
PMVT is a rare presentation after LSG, which requires early diagnosis and management. Conservative management through anticoagulants and thrombolytic therapy is quite effective and, if indicated, should always be considered as the primary treatment option.
MeSH Keywords: Bariatric Surgery, Gastric Bypass, Mesenteric Veins
Background
Laparoscopic bariatric surgery (LBS) is a popular intervention for the treatment of morbidly obese patients; it helps in weight loss, minimizes obesity-related co-morbidities, and improves quality of life [1,2]. Surgical weight loss interventions include gastric restrictive procedures (e.g., adjustable gastric banding and sleeve gastrectomy), malabsorptive procedures (e.g., Roux-en Y gastric bypass [RYGB]), and biliopancreatic diversion, or a combination of these procedures [3]. Although obese patients who underwent various types of LBS have favorable results, these surgical procedures also have complications, such as fluid collection, bleeding, pulmonary embolism, or septis [4]. Moreover, severely obese patients are at increased risk of thromboembolic events and ischemia secondary to vessel wall damage, gradual blood flow, and hypercoagulability [3].
Portomesenteric venous thrombosis (PMVT) is an infrequent event associated with higher rates of morbidity (mesenteric ischemia in 5–15% of cases) and mortality (20–50%) [4,5]. It has been suggested that venous stasis secondary to enhanced intra-abdominal pressure, operative intervention in splanchnic vasculature, and hypercoagulable state are the possible causative factors for PMVT [3]. Moreover, the diagnosis of PMVT after laparoscopic sleeve gastrectomy (LSG) requires a high index of suspicion because patients usually present with vague signs and symptoms that are difficult to elicit early [6]. Herein, we describe the clinical presentation, diagnosis, and management at our center of 3 patients who developed PMVT after LSG performed abroad.
Case Reports
In our case series, 3 patients developed PMVT after LSG performed outside the country. These patients were coincidentally admitted to the Hamad General hospital (HGH) in Qatar between December 2013 to January 2014. Also, we observed from patients’ history that all the 3 LSGs were performed within the same center abroad. Therefore, most of the operative details, actual circumstances of the surgery, and coagulation profiles prior to intervention were lacking. However, we assumed that the pre-operative measures included thromboprophylaxis given according to the patient body weight in addition to administration of a prophylactic antibiotic regimen.
During surgery the patients were kept in reverse Trendelenburg position. A trocar was placed to achieve a pneumoperitoneum by CO2 insufflation set at 20 mmHg, through a Veress needle placed in the left mid-clavicle subcostal region, and 5-port technique was used. The surgery included mobilization of the greater curvature, posterior mobilization of the stomach and angle of His, ventral mobilization of angle of His, mobilization of the greater curvature by dissecting the remaining short gastric vessels, followed by stapling and removal of a gastric specimen.
Post-operatively, elastic compression stockings (for 2 weeks) and pneumatic dynamic leg compression sleeves (for 24 h) were used and subcutaneous low molecular weight heparin (LMWH) injection was administered until discharge from the hospital. Patient discharge summaries indicated that anti-DVT prophylaxis was given to all patients, which continued until 2 weeks post-operatively. However, none of the patients were on LMWH upon admission to our hospital. Unfortunately, the specific dosage given was not mentioned, which makes it difficult to rule-out the possibility of insufficient thromboprophylaxis.
Case 1
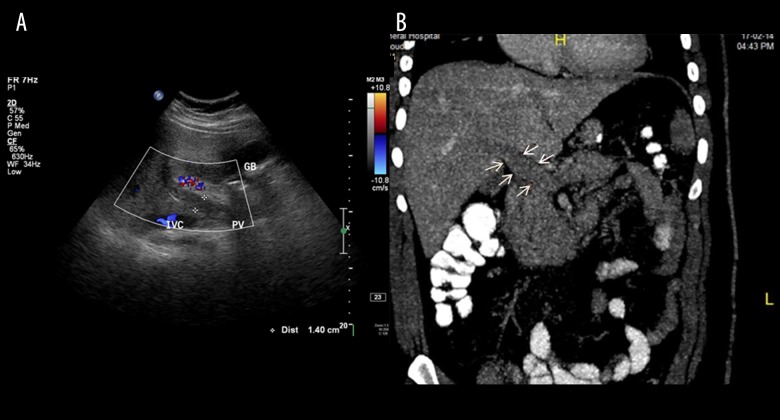
A 27-year-old man presented as an emergency with diffuse abdominal pain, nausea, and vomiting. The body mass index (BMI) of the patient was 41 kg/m2. He had undergone LSG 1 week prior to the index admission. The laboratory findings were insignificant, with only an increase in white cell count. Doppler ultrasound and computerized tomography (CT) of the abdomen confirmed the diagnosis of portal vein thrombosis (Figure 1A, 1B). Therefore, the patient was treated conservatively with anticoagulation therapy, initially started with heparin and thereafter shifted to warfarin, which was continued after discharge.
Figure 1.
(A, B) Diagnosis of portal vein thrombosis confirmed by Doppler ultrasound and computerized tomography (CT) of the abdomen (Case 1).
Case 2
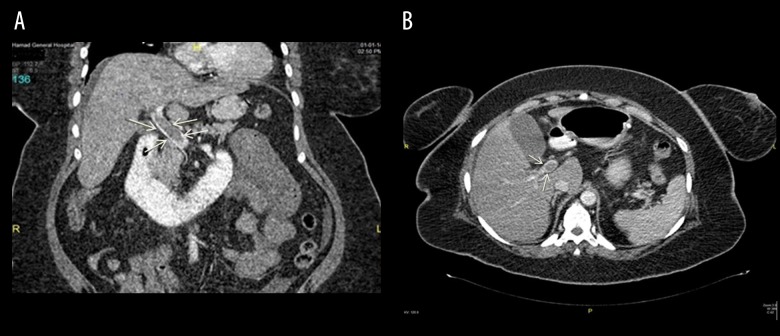
A 46-year-old obese woman (BMI=38 kg/m2) presented with diffuse abdominal pain (more prominent at the epigastrium), nausea, and constipation 14 days post-LSG. The CT scan findings revealed PMVT (Figure 2A, 2B) and thickened proximal jejunal bowel loops. Retrograde thrombolytic therapy was given to the patient under ultrasonography guidance using a transhepatic approach. A loading dose of tissue plasminogen activator (tPA) followed by maintenance doses were injected through the transhepatic tube. Follow-up angiogram study showed re-canalization of the portal vein and improvement in blood flow (Figure 3). Thereafter, the patient continued on subcutaneous heparin overlapped by warfarin, and she was discharged from the hospital with good recovery.
Figure 2.
(A) Coronal section showing superior mesenteric vein thrombosis (Case 2). (B) CT scan sagittal section showing portal vein thrombosis (Case 2).
Figure 3.
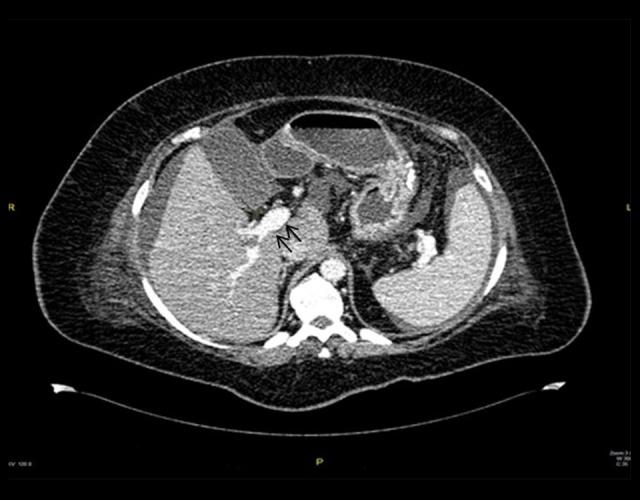
Re-canalization of the portal vein after tissue plasminogen activator injection (Case 2).
Case 3
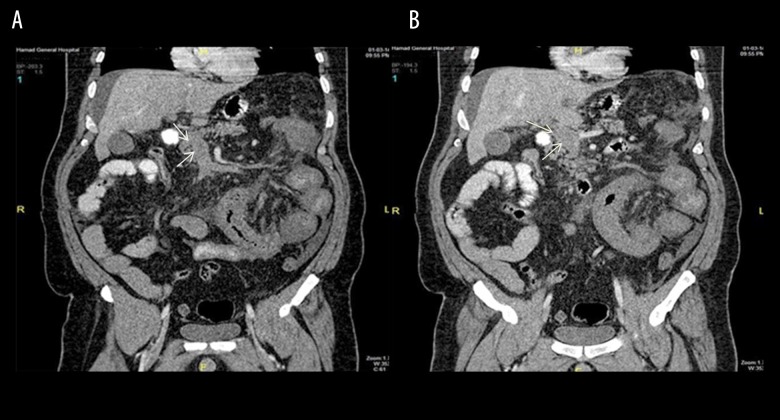
A 46-year-old male patient presented with fever, diffuse abdominal pain and tenderness, nausea, and vomiting. He was known to have diabetes mellitus, hypertension, dyslipidemia, and obesity (BMI=44 kg/m2). He underwent LSG 2 weeks before the index hospitalization. The CT scan showed extensive PMVT, partial splenic vein thrombosis (Figure 4A, 4B), and small bowel ischemia. Laparoscopic exploration revealed a gangrenous small bowel loop about 25 cm from the DJ junction. The surgery was converted to open laparotomy and 75 cm of small bowel was resected. The rest of the small bowel was congested. The skin was closed using a surgical skin stapler for reexamination after 48 h, at that time 10 cm of the bowel was resected with end-to-end anastomosis. He was started on heparin infusion overlapped by oral warfarin. A follow-up CT scan showed intact small bowel and patent splenic vein with established venous cavernoma. He underwent successful bowel resection along with thrombolytic therapy and was successfully discharged from the hospital with good recovery.
Figure 4.
(A) Coronal section for superior mesenteric vein thrombosis (SMV). (Case 3). (B) Coronal section for portal vein thrombosis (Case 3).
Briefly, the 3 patients were successfully managed and survived. Two patients were treated conservatively: 1 treated by IV heparin infusion (anticoagulation therapy) and the other patient was treated with tissue plasminogen activator thrombolytic therapy. However, the third patient underwent surgical bowel resection. Unfortunately, detailed information on the prophylactic pharmacologic and mechanical approaches used before, during, and after initial surgery were lacking.
Discussion
Portomesenteric venous thrombosis is an uncommon surgical complication secondary to LBS. In 20% to 35% of cases, LSG remains the primary cause of PMVT [6]. The other possible etiologies of PMVT include hypercoagulable state, intra-abdominal Inflammation, portal hypertension, and neoplastic diseases [7]. The clinical diagnosis of PMVT is difficult, and is usually confirmed by contrast CT abdomen, with a sensitivity of 90%. All the 3 cases of our series were diagnosed with the help of abdominal CT scan.
Table 1 shows a review of the literature for the possible causative factors, management and outcome of mesenteric venous thrombosis (MVT) patients [4,7–17]. There are many case reports and series have described the clinical presentation and management of MVT after bariatric surgery. However, this is the first case series from Qatar to present PMVT, which is an unusual clinical entity at our center. It is important to report such cases because the clinical signs and symptoms may differ widely, leading to delayed diagnosis. Notably, patients who sustain PMVT usually present with non-specific abdominal pain and, less commonly, with nausea, vomiting, diarrhea, or gastrointestinal tract bleeding [4]. The clinical findings in our cases were consistently difficult to interpret. The delay from the day of LSG to the onset of symptoms ranged from 7 to 14 days in our series.
Table 1.
Review of literature for causative factors, management and outcome of mesenteric venous thrombosis.
| Author | Year | No. of cases | Procedures | Management | Thrombosed vein | Delay period post-OP (days) | Hypercoagulation | Outcome |
|---|---|---|---|---|---|---|---|---|
| Goitein et al. [4] | 2010 | 17 | 16 LSG 1 LAGB |
Anticoagulation (n=15), Trans-hepatic thrombolysis (n=1), Laparscopic spleenectomy (n=1), Laparotomy with bowel resection (n=1) | PV, SMV | 10 (median) | Factor V Leiden deficiency (n=1) Protein C and S deficiency (n=1) | All survived |
| Swartz and Felix [9] | 2004 | 3 | LRYGB | 2 Bowel resection & 1 conservative | SMV | 8,9 & 18 | – | All survived |
| Pineda et al. [10] | 2013 | 1 | LSG | Conservative anticoagulation | SMV | 30 | – | Survived |
| Denne and Kowalski [11] | 2005 | 1 | LRYGB | Conservative anticoagulation | PV | 15 | – | Survived |
| Rosenberg et al. [7] | 2012 | 1 | LSG | Conservative anticoagulation | PV | 10 | – | Survived |
| Bellanger et al. [12] | 2010 | 3 | LSG | – | PV, SMV, SV | No | – | – |
| Berthet et al. [13] | 2009 | 1 | LSG | Conservative anticoagulation | PV, SMV, SV | 14 | – | Survived |
| Gandhi et al. [8] | 2010 | 1 | LRYGB | Conservative anticoagulation | SMV | 8 | – | Survived |
| James et al. [14] | 2009 | 7 | LRYGB | Anticoagulation (n=2) Anticoagulation + thrombolytic (n= 1) Anticoagulation + Laprotomy (n=3) Laparotomy (n=1) | SMV=3, PVT=1, PV + SMV=1, PVT + SV + IMV=1, PVT + SV + IMV + SMV=1 | 14 (mean) | – | 1 Died |
| Singh et al. [15] | 2010 | 1 | LSG | anticoagulation | SVT | 21 | – | Survived |
| Sonpal et al. [16] | 2004 | 1 | Open RYGB | Conservative | SMV | 10 | VIII anti-hemophilic globulin positive | Survived |
| Salinas et al. [17] | 2014 | 17 | LSG | Conservative | PV, SMV, SV | 15 | Protein C (n=1), Protein S (n=1), Protein C+S (n=1), Protein C+20210a mutation (n=1), Protein S +20210a mutation (n=1) | All Survived |
| Present cases | 2015 | 3 | LSG | 2 conservatively (anticoagulation & thrombolytic therapy) and one operated with bowel resection. | PMV | 7, 14, 14 | – | All Survived |
LRYGB – laparoscopic Roux-en-Y gastric bypass; LSG – laparoscopic sleeve gastrectomy; LAGB – laparoscopic adjustable gastric banding; PV – portal vein; SMV – superior mesenteric vein; SV – splenic vein; MSV – mesenteric vein; IMV – inferior mesenteric vein.
One of the proposed causative factors for PMVT is the development of splanchnic vessels thrombosis, but its underlying mechanism remains unclear [11,18]. Kotzampassi et al. [19] suggested that a sustained pressure of 14 mmHg of carbon dioxide could affect the mucosal blood flow in an experimental animal model. An earlier study demonstrated an inverse relationship between intraperitoneal pressure and the portal blood flow because an increase in intra-abdominal pressure beyond 14 mmHg is enough to reduce the portal venous flow by 50% [8]. It is not the direct pressure alone that might pre-dispose to thrombus formation; carbon dioxide causes sympathetic vasoconstriction through the release of vasopressors, which eventually reduces venous blood flow and increases the risk of thrombosis [11,18]. In addition, the prolonged reverse-Trendelenburg position is considered as another possible explanation for the development of portal vein thrombosis [20]. An earlier study of 35 MVT cases from Qatar identified protein C and S deficiency, homocysteinemia, and prior abdominal surgery as the major etiological factors [21]. However, the 3 patients in our series underwent LSG outside the country, so the potential identifiable cause of complications remains difficult to establish, but could possibly be attributed to the surgical intervention.
Bariatric surgery patients are particularly susceptible to thrombotic events due to the potential hypercoagulable state. Therefore, the hypercoagulability is attributed to metabolic syndrome, which is associated with increased release of fibrinogen and coagulation factors [22]. Some investigators suggested that the mechanical or thermal effect of laparoscopic surgeries could be associated with PMVT, but this has not been established. Therefore, consistent with recent studies, we believe that the involvement of several predisposing factors is responsible for the development of PMVT in our patients [6,17].
PMVT can be managed successfully with a conservative approach if there is no evidence of bowel infarction. Most patients with mild PVT can be successfully treated with low molecular weight heparin alone. It has been recommended that subjects with acute PMVT should be treated with anticoagulation therapy as early as possible, which enhances the recanalization of the portal venous system and reduces the risk of further thrombotic events [23,24]. One of our patients was treated with anticoagulation therapy with good outcome. However, the optimal duration of treatment with systemic anticoagulation is not well defined. Ghandi et al. [8] recommended 3–6 months of anticoagulation, which can be extended further if the signs and symptoms persist. Another study suggested a longer duration of anticoagulation therapy, ranging from 6 to 12 months [25]. However, patients with a systemic etiology are required to be on lifelong anticoagulation therapy [26]. Supportive measures that could also be used to supplement anticoagulation therapy include bowel rest, fluid resuscitation, and nasogastric suction for conservative management [25].
To the best of our knowledge, no protocols or guidelines are available that are specific for prevention of PMVT in patients who underwent bariatric surgery, due to the infrequent development [27] or reporting of such complications. Therefore, the general standard prophylactic measures that apply to obese and post-laparoscopic patients in general surgery are currently applied for bariatric patients. As bariatric surgery grows in popularity, patients should receive more attention, screening, and management preoperatively [28]. Early mobility and pneumatic compression are the non-pharmacological measures applied in current practice. Low molecular weight heparin (LMWH) and unfractionated heparin (UFH) are helpful to prevent PMVT, however, no clear guidelines about the optimal dose, duration, and type of heparin are currently available. A recent study demonstrated that protocol-based, opti mized, post-operative care facilitates quick recovery and minimizes the rate of perioperative complications [29]. To decrease the length of hospital stay without increasing the rate of complications after bariatric surgery, the feasibility and effectiveness of a postoperative protocol was studied in a high-volume laparoscopic center [29]. In this regard, the Enhanced Recovery After Surgery (ERAS®)-inspired protocol for bariatric patients, showed favorable outcomes.
Despite the evolving role of invasive therapies for acute PVT, there is still a lack of consistency regarding the utility of thrombolytics because most of the findings are based on either case reports or case series, which needs solid evidence- based management from prospective studies and clinical trials. The current literature describes the indications, utility, and possible complications associated with thrombolytic therapy. Several investigators supported either percutaneous or transhepatic thrombolytic therapy when anticoagulation is not showing progressive response in severe presentations, or is clinically and/or radiologically compatible with bowel ischemia, or otherwise when a non-operative approach is indicated [4,14,30]. In 1 of our PMVT cases, transhepatic thrombolysis was effective in terms of portal vein re-canalization. Therefore, we believe that thrombolysis could be an appropriate treatment option in selected cases with no evidence of bowel ischemia.
The implications of laparoscopic exploration for the diagnosis of PMVT remain controversial. Cho et al. [31] supported diagnostic laparoscopy by all means, irrespective of the clinical conditions or laboratory findings. On the other hand, Kumar et al. [25] opposed laparoscopic exploration for the diagnosis of PMVT because it may compromise the mesenteric bowel flow. However, Swartz et al. [9] suggested that the extent of bowel ischemia could not be accurately determined by CT scan alone. Therefore, to determine the exact magnitude of bowel ischemia, a combination approach should be used, which involves both radiological investigation and laparoscopic exploration. We agree that the decision for diagnostic laparoscopy should be based on the clinical course of the patient and CT findings, as corroborated in an earlier report by Ghandi et al. [8]. However, if CT findings are not conclusive or if the patient shows signs of deterioration, laparoscopic exploration is highly indicated. Our third case showed small bowel ischemia on CT examination and therefore underwent open laparotomy with bowel resection.
Conclusions
In conclusion, portomesenteric vein thrombosis is a rare presentation after LSG. However, it is a serious complication that needs a high index of suspicion. Moreover, familiarity with this entity facilitates early diagnosis and management. Conservative management through anticoagulants and thrombolytics is quite effective, and, if indicated, should always be considered as the primary treatment option. Therefore, we recommend that bariatric surgery patients should be maintained on anticoagulants for at least 2 weeks.
Acknowledgments
The authors thank all the general surgery staff for their cooperation.
Footnotes
Statement
The authors have no conflict of interests and no financial issues to disclose. All authors read and approved this case report, which was approved by the Medical Research Center (IRB # 14295/14), Hamad Medical Corporation, Doha, Qatar.
References:
- 1.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 2.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: Systematic review and meta-analysis. Am J Med. 2009;122:248–56.e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 3.Ochner CN, Gibson C, Shanik M, et al. Changes in neurohormonal gut peptides following bariatric surgery. Int J Obes. 2011;35:153–66. doi: 10.1038/ijo.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goitein D, Matter I, Raziel A, et al. Portomesenteric thrombosis following laparoscopic bariatric surgery: Incidence, patterns of clinical presentation, and etiology in a bariatric patient population. JAMA Surg. 2013;148:340–46. doi: 10.1001/jamasurg.2013.1053. [DOI] [PubMed] [Google Scholar]
- 5.Robbins MR, Comerota AJ, Pigott JP. Mesenteric venous thrombosis. Vasc Med. 2005;10:121–22. doi: 10.1191/1358863x05vm612xx. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med. 2001;345:1683–88. doi: 10.1056/NEJMra010076. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg JM, Tedesco M, Yao DC, Eisenberg D. Portal vein thrombosis following laparoscopic sleeve gastrectomy for morbid obesity. JSLS. 2012;16:639–43. doi: 10.4293/108680812X13517013316636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi K, Singh P, Sharma M, et al. Mesenteric vein thrombosis after laparoscopic gastric sleeve procedure. J Thromb Thrombolysis. 2010;30:179–83. doi: 10.1007/s11239-009-0434-z. [DOI] [PubMed] [Google Scholar]
- 9.Swartz DE, Felix EL. Acute mesenteric venous thrombosis following laparoscopic Roux-en-Y gastric bypass. JSLS. 2004;8:165–69. [PMC free article] [PubMed] [Google Scholar]
- 10.Pineda L, Sarhan M, Ahmed L. Superior mesenteric vein thrombosis after laparoscopic sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech. 2013;23:e162–63. doi: 10.1097/SLE.0b013e31828b7fdb. [DOI] [PubMed] [Google Scholar]
- 11.Denne J, Kowalski C. Portal vein thrombosis after Laparoscopic gastric bypass. Obes Surg. 2005;15:886–89. doi: 10.1381/0960892054222650. [DOI] [PubMed] [Google Scholar]
- 12.Bellanger DE, Hargroder AG, Greenway FL. Mesenteric venous thrombosis after laparoscopic sleeve gastrectomy. Surg Obesity Related Dis. 2010;6:109–11. doi: 10.1016/j.soard.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Berthet B, Bollon E, Valero R, et al. Portal vein thrombosis due to factor 2 leiden in the post-operative course of a laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg. 2009;19:1464–67. doi: 10.1007/s11695-009-9910-y. [DOI] [PubMed] [Google Scholar]
- 14.James AW, Rabl C, Westphalen AC, et al. Portomesenteric venous thrombosis after laparoscopic surgery: A systematic literature review. Arch Surg. 2009;144:520–26. doi: 10.1001/archsurg.2009.81. [DOI] [PubMed] [Google Scholar]
- 15.Singh P, Sharma M, Gandhi K, et al. Acute mesenteric vein thrombosis after laparoscopic gastric sleeve surgery for morbid obesity. Surg Obesity Related Dis. 2010;6:107–8. doi: 10.1016/j.soard.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Sonpal IM, Patterson L, Schreiber H, Benmeir A. Mesenteric venous thrombosis after gastric bypass. Obes Surg. 2004;14:419–21. doi: 10.1381/096089204322917972. [DOI] [PubMed] [Google Scholar]
- 17.Salinas J, Barros D, Salgado N, et al. Portomesenteric vein thrombosis after laparoscopic sleeve gastrectomy. Surg Endosc. 2014;28:1083–89. doi: 10.1007/s00464-013-3055-8. [DOI] [PubMed] [Google Scholar]
- 18.Poultsides GA, Lewis WC, Feld R, et al. Portal vein thrombosis after laparoscopic colectomy: thrombolytic therapy via the superior mesenteric vein. Am Surg. 2005;71:856–60. [PubMed] [Google Scholar]
- 19.Kotzampassi K, Kapanidis N, Kazamias P, Eleftheriadis E. Hemodynamic events in the peritoneal environment during pneumoperitoneum in dogs. Surg Endosc. 1993;7:494–99. doi: 10.1007/BF00316688. [DOI] [PubMed] [Google Scholar]
- 20.Denninger MH, Chaït Y, Casadevall N, et al. Cause of portal or hepatic venous thrombosis in adults: The role of multiple concurrent factors. Hepatology. 2000;31:587–91. doi: 10.1002/hep.510310307. [DOI] [PubMed] [Google Scholar]
- 21.Al-Thani H, El-Mabrok J, El-Menyar A, et al. Clinical presentation and outcome of mesenteric vein thrombosis: A single-center experience. Angiology. 2015;66:249–56. doi: 10.1177/0003319714531480. [DOI] [PubMed] [Google Scholar]
- 22.Ay C, Tengler T, Vormittag R, et al. Venous thromboembolism – a manifestation of the metabolic syndrome. Haematologica. 2007;92:374–80. doi: 10.3324/haematol.10828. [DOI] [PubMed] [Google Scholar]
- 23.Sarin SK, Sollano JD, Chawla YK, et al. Consensus on extra-hepatic portal vein bstruction. Liver Int. 2006;26:512–19. doi: 10.1111/j.1478-3231.2006.01269.x. [DOI] [PubMed] [Google Scholar]
- 24.Condat B, Pessione F, Hillaire S, et al. Current outcome of portal vein thrombosis in adults: risk and benefit of anticoagulant therapy. Gastroenterol. 2001;120:490–97. doi: 10.1053/gast.2001.21209. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med. 2001;345:1683–88. doi: 10.1056/NEJMra010076. [DOI] [PubMed] [Google Scholar]
- 26.de Franchis R. Evolving consensus in portal hypertension report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167–76. doi: 10.1016/j.jhep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Bartlett MA, Mauck KF, Daniels PR. Prevention of venous thromboembolism in patients undergoing bariatric surgery. Vasc Health Risk Manag. 2015;11:461–77. doi: 10.2147/VHRM.S73799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatia P, John SJ, Kalhan S, Bindal V. Portomesenteric venous thrombosis after laparoscopic sleeve gastrectomy: A case report and a call for prevention. J Minim Access Surg. 2015;11:276–78. doi: 10.4103/0972-9941.152101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matłok M, Pędziwiatr M, Major P, et al. One hundred seventy-nine consecutive bariatric operations after introduction of protocol inspired by the principles of enhanced recovery after surgery (ERAS®) in bariatric surgery. Med Sci Monit. 2015;21:791–97. doi: 10.12659/MSM.893297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozkan U, Oğuzkurt L, Tercan F, Tokmak N. Percutaneous transhepatic thrombolysis in the treatment of acute portal venous thrombosis. Diagn Interv Radiol. 2006;12:105–7. [PubMed] [Google Scholar]
- 31.Cho YP, Jung SM, Han MS, et al. Role of diagnostic laparoscopy in managing acute mesenteric venous thrombosis. Surg Laparosc Endosc Percutan Tech. 2003;13:215–17. doi: 10.1097/00129689-200306000-00015. [DOI] [PubMed] [Google Scholar]