Abstract
Background and Purpose
Prostanoids derived from COX‐2 and EP receptors are involved in vascular remodelling in different cardiovascular pathologies. This study evaluates the contribution of COX‐2 and EP1 receptors to vascular remodelling and function in hypertension.
Experimental Approach
Spontaneously hypertensive rats (SHR) and angiotensin II (AngII)‐infused (1.44 mg·kg−1·day−1, 2 weeks) mice were treated with the COX‐2 inhibitor celecoxib (25 mg·kg−1·day−1 i.p) or with the EP1 receptor antagonist SC19220 (10 mg·kg−1·day−1 i.p.). COX‐2−/− mice with or without AngII infusion were also used.
Key Results
Celecoxib and SC19220 treatment did not modify the altered lumen diameter and wall : lumen ratio in mesenteric resistance arteries from SHR‐infused and/or AngII‐infused animals. However, both treatments and COX‐2 deficiency decreased the augmented vascular stiffness in vessels from hypertensive animals. This was accompanied by diminished vascular collagen deposition, normalization of altered elastin structure and decreased connective tissue growth factor and plasminogen activator inhibitor‐1 gene expression. COX‐2 deficiency and SC19220 treatment diminished the increased vasoconstrictor responses and endothelial dysfunction induced by AngII infusion. Hypertensive animals showed increased mPGES‐1 expression and PGE2 production in vascular tissue, normalized by celecoxib. Celecoxib treatment also decreased AngII‐induced macrophage infiltration and TNF‐α expression. Macrophage conditioned media (MCM) increased COX‐2 and collagen type I expression in vascular smooth muscle cells; the latter was reduced by celecoxib treatment.
Conclusions and Implications
COX‐2 and EP1 receptors participate in the increased extracellular matrix deposition and vascular stiffness, the impaired vascular function and inflammation in hypertension. Targeting PGE2 receptors might have benefits in hypertension‐associated vascular damage.
Abbreviations
- AngII
angiotensin II
- ECM
extracellular matrix
- MCM
macrophage conditioned media
- mPGES
microsomal PGE synthase
- MRA
mesenteric resistance arteries
- SHR
spontaneously hypertensive rats
- VSMC
vascular smooth muscle cells
- WKY
Wistar Kyoto rats
Tables of Links
| TARGETS | |
|---|---|
| GPCRs a | |
| EP1 receptors | |
| EP3 receptors | |
| Enzymes b | |
| COX‐1 | |
| COX‐2 | |
| mPGES1, PGE synthase‐1 |
| LIGANDS | |
|---|---|
| 16,16‐dimethyl PGE2 | L798106 |
| AngII, angiotensin II | Rofecoxib |
| CCL2 | SC19220 |
| Celecoxib | SC51322 |
| CTGF, connective tissue growth factor | TNF‐α |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b Alexander et al., 2015a, 2015b).
Introduction
Hypertension is characterized by vascular functional and structural alterations such as increased vasoconstrictor responses, endothelial dysfunction and increased wall : lumen ratio (Schiffrin, 2012), which have prognostic value for cardiovascular disease (Perticone et al., 2001; Rizzoni et al., 2003). Despite long‐term BP normalization, altered small artery structure identifies individuals still at increased cardiovascular risk (Buus et al., 2013). Increased extracellular matrix (ECM) deposition and consequent increase in vessel stiffness are additional features of hypertensive vascular disease and contribute to inward remodelling and end organ damage observed in this pathology (Briones et al., 2009; 2010; Safar et al., 2012; Schiffrin, 2012). Increase in BP and prolonged vasoconstriction are mechanisms responsible for inward remodelling in hypertension (Schiffrin, 2012). However, in the last years, vascular infiltration of immune inflammatory cells has emerged as an important contributor to vascular remodelling, endothelial dysfunction and stiffness in this pathology (Bush et al., 2000; De Ciuceis et al., 2005; Wu et al., 2014).
Hypertension and angiotensin II (AngII) exposure is strongly associated with the augmented expression of the inducible isoform of COX (COX‐2) and production of prostanoids, which have a role in the altered vascular function observed in this pathology (Álvarez et al., 2007; Beltrán et al., 2009; Virdis et al., 2009; Kane et al., 2010; Wong et al., 2011; Martínez‐Revelles et al. 2013; Virdis et al., 2013). Among the prostanoids synthesized from COX‐2, PGE2 is the main produced in inflammatory processes. PGE2 modulates vascular tone through the four receptor subtypes, namely, the EP receptors (EP1–4) with EP1/3 vasoconstrictor and EP2/4 being vasodilator receptors (Foudi et al., 2012). In addition, recent studies demonstrate that PGE2 is a key mediator of vascular remodelling in different pathological situations such as atherosclerosis, aneurysms and restenosis (Cipollone et al., 2004; Yang et al., 2004; King et al., 2006; Wang et al., 2008; Wang et al., 2011; Camacho et al., 2013; Zhang et al., 2013), although its role in vascular remodelling in hypertension is unknown. Importantly, these effects of PGE2 on vascular function and structure might have a role in the control of BP because the genetic absence of EP1 receptors blunts acute pressure responses to AngII and reduces hypertension induced by chronic treatment with AngII (Guan et al., 2007). Macrophages are sources of COX‐2, and PGE2 facilitates macrophages migration into inflamed sites in different models of inflammation (Kamei et al., 2004) and atherosclerosis (Chen et al., 2014), suggesting that PGE2 might be a key pro‐inflammatory mediator of vascular damage.
This study evaluates the contribution of the COX‐2 and the EP1 receptors in vascular remodelling and function in hypertension. Our results suggest that COX‐2‐derived prostanoids, probably PGE2 acting on EP1 receptors, impair vascular function and increase vessel stiffness by facilitating the deposition of different ECM proteins, probably due to increased macrophage infiltration. These effects are responsible, at least in part, for the elevated BP.
Methods
Animals and animal models
All animal care and experimental procedures complied with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85‐23, revised 1996) and the current Spanish and European laws (RD 223/88 MAPA and 609/86) and were approved by the Animal Care and Use Committee of our institution (CEI‐UAM 31–759), according to the institutional guidelines for ethical care of experimental animals of the European Community. Studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010) and the editorial on reporting animal studies (McGrath and Lilley, 2015). A total of 69 rats and 131 mice were used.
Four‐weeks and six‐month‐old male Wistar Kyoto (WKY) and spontaneously hypertensive (SHR) rats breeded at the Animal Care Facility of the Faculty of Medicine, Universidad Autónoma de Madrid (UAM) were used. Adult rats were randomly divided into two groups: (1) control and (2) treated with the COX‐2 inhibitor celecoxib (25 mg·kg−1·day−1 i.p., 3 weeks). Three‐month old male C57BL6 mice breeded at the Animal Care Facility of the Faculty of Medicine, UAM were randomly distributed into five groups: (1) infused with AngII (1.44 mg·kg−1·day−1, 2 weeks) with s.c. implanted Alzet osmotic minipumps (Alza Corp., Cupertino, CA, USA) under isofluorane anaesthesia; (2) infused with AngII and celecoxib (25 mg·kg−1·day−1 i.p.); (3) infused with AngII and rofecoxib (10 mg·kg−1·day−1 i.p.); (4) infused with AngII and the EP1 receptor antagonist SC19220 (10 mg·kg−1·day−1 i.p.); and (5) control without treatment. Treatment with celecoxib, rofecoxib or SC19220 was started 24 h before AngII infusion. In another set of experiments, mice were treated with celecoxib 7 days after AngII infusion. BP was measured by tail‐cuff plethysmography.
COX‐2‐wild‐type (COX‐2+/+) and COX‐2‐deficient (COX‐2−/−) mice of a hybrid C57BL6 × 129SV genetic background were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) (B6;129S‐Ptgs2tm1Jed/J strain). COX‐2−/− and COX‐2+/+ matched controls were produced by crossing the COX‐2 heterozygous mutant line. Alzet osmotic minipumps containing AngII were implanted as described above for 4 weeks.
Tissue preparation
Animals were killed by CO2 inhalation and exsanguination. First‐order and third‐order branches of the mesenteric artery were dissected free of fat and connective tissue and placed in cold (4°C) Krebs–Henseleit solution (KHS) (115 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 1.2 mM MgSO4.7H2O, 2.5 mM CaCl2, 1.2 mM KH2PO4, 11.1 mM glucose and 0.01 mM Na2EDTA) bubbled with a 95% O2–5% CO2 mixture. Analysis of vascular structure and function was carried out on the same day. For immunofluorescence and histology studies, tissues were fixed with 4% phosphate‐buffered paraformaldehyde (pH = 7.4) for 1 h and washed in three changes of PBS solution (pH = 7.4). After washing, arterial segments were placed in PBS containing 30% sucrose for 20–50 min, transferred to a cryomold containing Tissue‐Tek OCT embedding medium and frozen in liquid nitrogen. Tissues were kept at −80°C until immunohistochemical studies. Other vascular segments were immediately frozen in liquid nitrogen and kept at −80°C until RNA isolation. Pooled first‐order, second‐order and third‐order branches of the mesenteric artery were incubated in KHS (2 h, 37°C), and media were frozen in liquid nitrogen and kept at −80°C until analysis for prostanoids.
PBMC isolation from human blood
According to institutional guidelines, subjects were aware of the research nature of the study and agreed to participate by giving informed consent. The study was performed in accordance with the Declaration of Helsinki, and the Ethical Committee of the Hospital La Paz approved the protocol (HULP‐31‐759). Data of normotensive and hypertensive patients are included in Table 1.
Table 1.
Systolic and diastolic BP (SBP, DBP in mmHg), age and gender ratio in normotensive and untreated hypertensive patients
| SBP | DBP | Age | Gender ratio | |
|---|---|---|---|---|
| Normotensive | 120 ± 4 | 77 ± 2.5 | 48 ± 4 | 2M/3F |
| Hypertensive | 137.2 ± 1 * | 90.2 ± 2.6 * | 46 ± 7 | 3M/2F |
Data are expressed as mean ± SEM. M = male, F = female. n = 5 in each group.
P < 0.05, significantly different from normotensive.
The study was performed in 10 unrelated white age‐matched subjects who came to our institution for a routine medical work‐up after a 12‐h overnight fast. Subjects were considered hypertensive (n = 5) if they presented systolic BP (SBP) ≥130 mmHg and diastolic BP (DBP) ≥80 mmHg, measured by 24‐h ambulatory BP monitoring as stated in reference guidelines (Mancia et al., 2013). Patients had appropriate clinical, laboratory and radiological evaluations to exclude secondary hypertension and chronic kidney disease. Normotensive subjects (n = 5) presented ambulatory BP monitoring measurements of SBP and DBP below 130 and 80 mmHg respectively. Hypertensive patients were untreated for hypertension.
Peripheral blood mononuclear cells (PBMC) were isolated from venous blood samples, taken from normotensive and hypertensive subjects, with Histopaque (Sigma‐Aldrich). Cell populations were determined by flow cytometry. Cells were seeded at 1 × 106 cells·mL−1 in a six‐well plate and harvested 24 h later.
Cell culture and conditioned medium
Primary cultures of aortic vascular smooth muscle cells (VSMC) were obtained from cleaned rat aortas and grown in DMEM‐F12 medium supplemented with 10% FBS containing 100 U·mL−1 of penicillin and 100 μg·mL−1 of streptomycin (all from Sigma‐Aldrich), as previously reported (Aguado et al., 2013). Cell cultures were used between passages 2 and 4. Cells were identified as smooth muscle cells by α‐actin positive immunostaining. RAW 264.7 cells (American Type Culture Collection, Manassas, VA, USA) were grown in DMEM with 10% FBS, 100 U·mL−1 of penicillin and 100 μg·mL−1 of streptomycin. Macrophage conditioned medium (MCM) was obtained from confluent RAW 264.7 cells that were serum‐deprived for 24 h. After that, medium was collected, centrifuged for 10 min at 2000× g and supernatants frozen at −80°C. VSMCs were starved in serum‐free media for 24 h. Then, VSMCs were stimulated with MCM. Growth medium not exposed to cells was used as control. In another set of experiments, VSMCs were incubated with AngII (0.1 μM).
Pressure myography
The structural and mechanical properties of mesenteric resistance arteries (MRA) were studied with a pressure myograph (Danish Myo Tech, Model P100; J.P. Trading I/S, Aarhus, Denmark). Vessels were placed on two glass microcannulae and secured with surgical nylon suture. After any small branches were tied off, vessel length was adjusted so that the vessel walls were parallel without stretch. Intraluminal pressure was then raised to 120 mmHg in mice and 140 mmHg in rats, and the artery was unbuckled by adjusting the cannulae. The segment was then set to a pressure of 45 mmHg in mice and 70 mmHg in rats and allowed to equilibrate for 60 min at 37°C in calcium‐free KHS (0Ca2 +; omitting calcium and adding 1 mM EGTA) intravascular and extravascular perfused, gassed with a mixture of 95% O2 and 5% CO2. Intraluminal pressure was reduced to 3 mmHg. A pressure–diameter curve was obtained by increasing intraluminal pressure in 20 mmHg steps from 3 to 120 mmHg in mice and from 3 to 140 mmHg in rats. Internal and external diameters were continuously measured under passive conditions (Di0Ca, De0Ca) for 3 min at each intraluminal pressure. The final value used was the mean of the measurements taken during the last 30 s when the measurements reached a steady state. Finally, the artery was set to apressure of 45 mmHg in mice and 70 mmHg in rats in 0Ca2 +‐KHS and then pressure fixed with 4% paraformaldehyde in 0.2 M phosphate buffer, pH 7.2–7.4, at 37°C for 60 min and kept in 4% paraformaldehyde at 4°C for confocal microscopy studies. Calculation of passive structural and mechanical parameters was performed as previously described (Briones et al., 2003).
Organization of internal elastic lamina
The elastin organization within the internal elastic lamina was studied in segments of MRA, using fluorescence confocal microscopy based on the autofluorescent properties of elastin (Ex 488 nm and Em 500–560 nm), as previously described (Briones et al., 2003). Briefly, the experiments were performed in intact pressure‐fixed segments with a Leica TCS SP2 confocal system (Leica Microsystems, Wetzlar, Germany). Serial optical sections from the adventitia to the lumen (z step = 0.5 μm) were captured with an X63 oil objective (Zoom 4 in mice, and Zoom 2 in rats), using the 488 nm line of the confocal microscope. A minimum of two stacks of images of different regions were captured in each arterial segment. Quantitative analysis of the internal elastic lamina was performed with metamorph image analysis software, as described (Briones et al., 2003). From each stack of serial images, individual projections of the internal elastic lamina were reconstructed, and total fenestrae number and mean fenestrae area were measured.
Collagen determination
Tissue Tek OCT embedded tissues were cut into 5 μm sections using a cryostat. Collagen was quantified in sections stained with Picro‐Sirius Red [0.1% (wt·vol−1) Sirius Red 3FB in saturated aqueous picric acid for 30 min with gentle agitation]. Three to four sections for each animal were analysed with a 40× objective lens under microscopy transmitted light (Leica DM 2000; Leica Microsystems) using an image system analysis (Leica LAS Image Analysis; Leica Microsystems). The area of collagen in the media layer was identified after excluding perivascular fibrosis of the vessel as the ratio of collagen deposition to the total media area.
qRT‐PCR assay
mRNA levels were determined in rat or mice aortic or mesenteric tissues and in human PBMC. Total RNA was obtained by using TRI Reagent (Sigma‐Aldrich). One microgram of total RNA was reverse transcribed using TaqMan® Reverse Transcription Reagents (Applied Biosystems, Foster City, CA, USA) in a final volume of 10 μL. All qPCRs were performed in duplicate. qPCR for rat or mice connective tissue growth factor (CTGF), plasminogen activator inhibitor (PAI‐1), vimentin and human mPGES‐1 were performed using TaqMan Gene Expression Assays (Applied Biosystems): CTGF: Rn00573960_m1; PAI‐1: Rn00578319_m1; vimentin: Rn00579738_ml in rats; CTGF: Mm01192932_g1; PAI‐1: Mm00435860_m1; vimentin: Mm01333430_m1 in mice; mPGES‐1: Hs00610420_m1. qPCR for the chemokine CCL2, the macrophage membrane marker Mac‐3 and TNF‐α in mice, mPGES‐1 and EP1 receptors in rats and mice, and COX‐2 in human were performed using the fluorescent dye SyBRGreen (iTaq FAST SyBRGreen Supermix with ROX; Bio‐Rad, Hercules, CA, USA) using specific primers (Supporting Information Table S1). Cyclophilin in rats, β2‐microglobulin in mice and β‐actin in humans were used as internal controls. Quantification was performed on a 7500 Fast thermal cycler (Applied Biosystems). PCR cycles proceeded as follows: 30 s 95°C and 40 cycles: 5 s 95°C, 30 s 60°C. At the end of the PCR, a melting curve analysis was performed to show PCR product specificity. To calculate the relative index of gene expression, we employed the 2−ΔΔCt method using untreated samples as calibrator.
Immunofluorescence
Mac‐3 and mPGES‐1 immunolocalization was performed in aortic sections. Briefly, frozen transverse sections (14 μm) were cut on to gelatin‐coated slides and air‐dried for at least 60 min. After blockade, sections were incubated with a polyclonal antibody against Mac‐3 (1:75; Santa Cruz Biotechnology, Dallas, TX, USA) or mPGES‐1 (1:100; Cayman Chemical, Ann Arbor, MI, USA) in PBS containing 2% BSA for 1 h at 37°C in a humidified chamber. After washing, rings were incubated with an anti‐rat IgG conjugated to Alexa488 or an anti‐rabbit IgG conjugated to Cy™3 (1:200) (Jackson Immunoresearch Laboratories, Inc., West Grove, PA, USA) for 1 h at 37°C. Immunofluorescent signals were viewed using an inverted Leica TCS SP2 confocal laser scanning microscope with oil immersion lens (×40). The specificity of the immunostaining was evaluated by omission of the primary antibody and processed as described above. Under these conditions, no staining was observed in the vessel wall.
Western blot analysis
To obtain whole‐cell lysates, cells were harvested and mixed gently in RIPA buffer containing 50 mM Tris pH 7.5, 150 mM NaCl, 1 mM MgCl2, 1 mM EDTA, 1% Nonidet‐P40, 0.5% sodium deoxycholate, 1% SDS, a protease inhibitor cocktail (Roche Applied Science, Barcelona, Spain) and a mix of phosphatase inhibitors (1 mM orthovanadate, 20 mM β‐glycerophosphate, 10 mM NaF; from Sigma‐Aldrich). Samples were centrifuged for 10 min at 15700 g, and protein content in the supernatants was determined with Lowry (Bio‐Rad), using BSA (Sigma‐Aldrich) as standard. Total protein equivalents of each sample (30 μg) were separated on a 7.5% SDS‐PAGE and electrophoretically transferred to polyvinylidene difluoride (Amersham, GE Healthcare, Buckinghamshire, UK) in Tris‐glycine transfer buffer with 20% methanol in a Bio‐Rad Trans‐Blot Cell (Bio‐Rad Laboratories). Membranes were blocked with 4% skim milk in TBS‐Tween for 30 min at room temperature before being incubated with antibodies for collagen I (1:1000; Calbiochem Darmstadt, Germany), CTGF (1:1000), COX‐2 (1:200) or β‐actin (1:50,000; all from Sigma‐Aldrich) overnight at 4° C. Membranes were thoroughly washed and incubated with HRP‐coupled anti‐rabbit IgG antibodies (1:2,000; Bio‐Rad) for 1 h at room temperature. Bands were detected using the Luminata Forte (Millipore Corporation, Billerica, MA, USA) detection system. Signals on the immunoblot were quantified using a computer program (NIH imagej software). β‐actin expression was used as loading control.
Measurement of PGE2 production
The levels of PGE2 were determined in the incubation medium of mesenteric arteries using an enzyme immunoassay commercial kit (R&D Systems, Minneapolis, MN, USA) following the manufacturer's instructions.
Responses of vascular tissues
Contractile responses of mouse aorta was studied in a wire myograph. After a 30 min equilibration period in oxygenated KHS, arterial segments were stretched to their optimal lumen diameter for active tension development. Contractility of segments was then tested by an initial exposure to a high‐K+ solution (K+‐KHS, 120 mM). The presence of endothelium was determined by the ability of 10 μM ACh to relax arteries precontracted with phenylephrine at approximately 50% K+‐KHS contraction. Afterwards, concentration–response curves to phenylephrine, ACh and diethylamine NONOate (DEA‐NO) were constructed. A single concentration‐dependent curve was performed in each segment.
In another set of experiments, mouse aortic segments were preincubated in vitro with AngII (1 μM, 1 h) in the absence or presence of SC51322 (EP1 receptor antagonist) or L798106 (EP3 receptor antagonist) before determining concentration–response curves to phenylephrine or 16,16‐dimethyl PGE2. Segments without preincubation with AngII were used as controls. Drugs were added 30 min before AngII.
Vasoconstrictor responses were expressed as a percentage of the tone generated by K+‐KHS. Vasodilator responses were expressed as a percentage of the previous tone generated by phenylephrine.
Data analysis and statistics
This study follows the editorial on experimental design and analysis in pharmacology (Curtis et al., 2015). All data are expressed as mean ± SEM of the number of animals used in each experiment or independent cell culture‐based experiments. Results were analysed by using paired or unpaired Student's t‐test or one‐way or two‐way ANOVA followed by Bonferroni's post hoc test using the graphpad prism 5 software (GraphPad Software, Inc., San Diego, CA, USA). P < 0.05 was considered significant.
Materials
The compounds used were supplied as follows: 16,16‐dimethyl PGE2, ACh, AngII, diethylamine NONOate, L798106, phenylephrine, SC19220 and SC51322 were from Sigma‐Aldrich Co. (St Louis, MO, USA). Rofecoxib was from LKT Laboratories (St. Paul, MN, USA) and celecoxib was generously provided by Pfizer Inc. (Groton, CT, USA).
Results
Role of COX‐2 in vascular remodelling and mechanical changes in hypertension
As described previously (Martínez‐Revelles et al., 2013), AngII‐infused mice showed increased SBP that was partly prevented by celecoxib treatment (Table 2). Another COX‐2 inhibitor, rofecoxib, also partly prevented AngII‐induced hypertension (Supporting Information Fig. S1A).
Table 2.
Systolic BP (in mmHg) in angiotensin II (AngII)‐infused mice with or without celecoxib or SC19220
| Untreated | AngII | AngII + celecoxib | AngII + celecoxib (started 7 days after AngII infusion) | AngII + SC19220 | |
|---|---|---|---|---|---|
| C57BL6 | 102 ± 2.5 | 144 ± 3.9 * | 123 ± 4.5 * , # | 130 ± 3.2 * , # | 119 ± 3.2 * , # |
| COX‐2+/+ | 101 ± 2 | 163 ± 3.5 * | |||
| COX‐2−/− | 103 ± 1.9 | 178 ± 4.6 * , # |
Data are expressed as mean ± SEM; n = 5–9.
P < 0.05 significantly different from untreated.
P < 0.05 significantly different from AngII or COX‐2+/+.
In MRA of AngII‐infused mice, lumen and vessel diameters were decreased and the wall : lumen ratio was increased. These effects were unaffected by celecoxib treatment (Figure 1A). AngII administration also reduced incremental distensibility and increased vessel stiffness, which were prevented by celecoxib (Figure 1B). Rofecoxib treatment did not modify the effects of AngII in vessel or lumen diameters or incremental distensibility (Supporting Information Fig. S1B,C) but decreased the augmented vessel stiffness induced by AngII (Supporting Information Fig. S1C).
Figure 1.
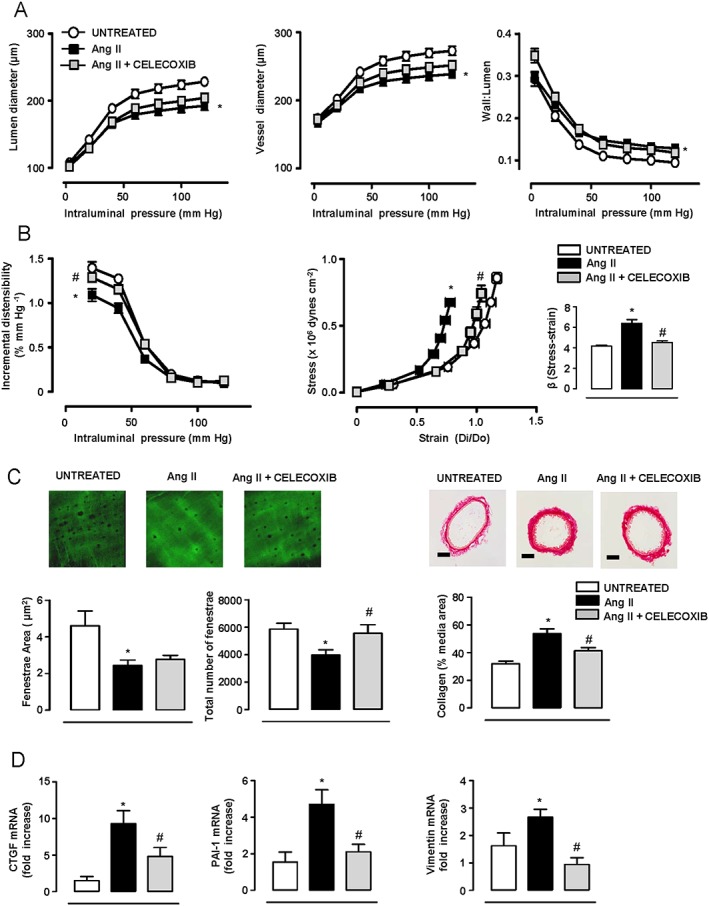
COX‐2 inhibition prevents angiotensin II (AngII)‐induced increased vascular stiffness and changes in the ECM. Structural (A) and mechanical parameters (B) in mesenteric resistance arteries (MRA) from mice untreated (n = 18) or treated with AngII (n = 15) or AngII plus celecoxib (n = 16). (C) Quantification of internal elastic lamina structure and collagen content in the media layer of MRA (n = 6–9). Elastin image size: 59.5 × 59.5 μm. Scale bar: 50 μm. (D) Gene expression of connective tissue growth factor (CTGF), plasminogen activator inhibitor (PAI‐1) and vimentin in aortic homogenates (n = 7–11). Data represent mean ± SEM. Gene expression data are expressed as fold increase of the untreated group mean value. *P < 0.05, significantly different from untreated, # P < 0.05, significantly different from AngII.
Differences in elastin organization are a central element in small artery remodelling and stiffness in hypertension (Briones et al., 2003). AngII decreased the mean fenestrae area and the total number of fenestrae, the latter being abolished by celecoxib (Figure 1C). AngII also increased collagen content and the expression of aortic CTGF, PAI‐1 and vimentin genes, which were reduced by celecoxib (Figure 1C,D).
SBP was similar in untreated COX‐2+/+ and COX‐2−/− mice, and AngII infusion increased SBP more in COX‐2−/− mice (Table 2). MRA from COX‐2−/− mice showed a greater wall : lumen ratio and increased stiffness, compared with those in COX‐2+/+ mice (Figure 2A,B). AngII infusion increased the wall : lumen ratio and vascular stiffness in COX‐2+/+ mice but not in COX‐2−/− (Figure 2A,B). Fenestrae area of the internal elastic lamina and collagen deposition were similar in MRA from both genotypes, although the numbers of fenestrae were smaller in COX‐2−/− mice (Figure 2C). AngII infusion decreased fenestrae area and number and increased collagen deposition in MRA from COX‐2+/+ mice, but not in those from COX‐2−/− mice (Figure 2C).
Figure 2.
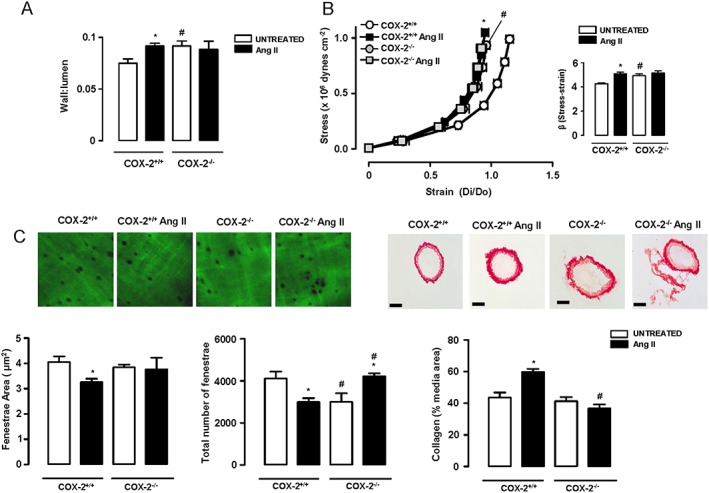
COX‐2 deletion prevents angiotensin II (AngII)‐induced vascular remodelling, stiffness and extracellular matrix alterations. Wall : lumen at 60 mmHg (A) and stress–strain relationship and β values (B) in mesenteric resistance arteries (MRA) from untreated and AngII‐treated COX‐2+/+ (n = 8–9) and COX‐2−/− (n = 6 per group) mice. (C) Quantification of internal elastic lamina structure and collagen content in the media layer of MRA (n = 5–9). Elastin image size: 59.5 × 59.5 μm. Scale bar: 50 μm. Data represent mean ± SEM. *P < 0.05, significantly different from untreated, # P < 0.05, significantly different from COX‐2+/+.
We next evaluated whether COX‐2 blockade might also reverse vascular remodelling and/or mechanical alterations in established hypertension. Thus, we analysed the effects of celecoxib when administered 7 days after the beginning of AngII infusion as well as its effects in SHR, a model of established hypertension. As reported (Martínez‐Revelles, et al., 2013), celecoxib treatment decreased SBP in SHR (235 ± 3.1 vs. 208 ± 6.3 mmHg, n = 8, P < 0.05) or when administered 7 days after AngII in mice (Table 2). This celecoxib treatment did not modify the altered vascular structure, but it increased the diminished distensibility, decreased the augmented vessel stiffness and normalized the altered elastin structure and the increased collagen deposition observed in both hypertension models (Figures 3A–C and 4). Moreover, the augmented aortic CTGF, PAI‐1 and vimentin gene expression in the SHR was normalized by celecoxib (Figure 3D). Celecoxib or rofecoxib treatments did not affect SBP, vascular structure or mechanical properties in control mice or in WKY rats (data not shown).
Figure 3.
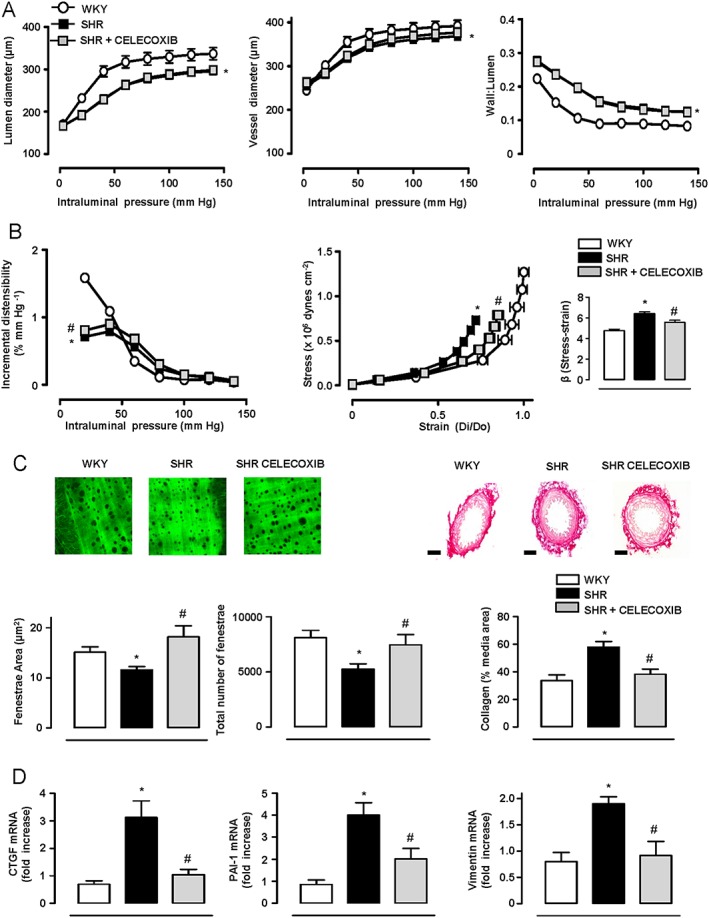
COX‐2 inhibition reduces the increased vascular stiffness and changes in the ECM in SHR. Structural (A) and mechanical parameters (B) in mesenteric resistance arteries (MRA) from WKY (n = 14), SHR (n = 16) and SHR treated with celecoxib (n = 16). (C) Quantification of internal elastic lamina structure and collagen content in the media layer of MRA (n = 5–9). Elastin image size: 119 × 119 μm. Scale bar: 50 μm. (D) Gene expression of connective tissue growth factor (CTGF), plasminogen activator inhibitor (PAI‐1) and vimentin in aortic homogenates (n = 5–13). Data represent mean ± SEM. Gene expression data are expressed as fold increase of the WKY group mean value. *P < 0.05, significantly different from WKY, # P < 0.05, significantly different from SHR.
Figure 4.

COX‐2 inhibition reduces the increased vascular stiffness and changes in the ECM in established hypertension. Structural (A) and mechanical parameters (B) in mesenteric resistance arteries (MRA) from mice untreated (n = 5) or treated with AngII (n = 5) or AngII plus celecoxib (n = 7) (administered 7 days after AngII infusion). (C) Quantification of internal elastic lamina structure and collagen content in the media layer of MRA (n = 5–7). Elastin image size: 59.5 × 59.5 μm. Scale bar: 50 μm. Data represent mean ± SEM. *P < 0.05, significantly different from untreated, # P < 0.05, significantly different from AngII.
Altogether, these data demonstrate that COX‐2 has a role in ECM deposition in hypertension that in turn determines vascular stiffness.
Role of COX‐2‐derived PGE2 in vascular remodelling in hypertension
AngII infusion increased expression of mPGES‐1, which was present mainly in the media layer, and PGE2 production (Figure 5A–C). Adult SHR aorta also showed increased mPGES‐1 expression compared with aorta samples from WKY (Figure 5D), a difference not observed in prehypertensive (4 weeks old) animals (relative mPGES‐1 gene expression WKY: 1.01 ± 0.06, n = 7; SHR: 1.05 ± 0.09, n = 6, P > 0.05). Celecoxib treatment normalized the increased PGE2 production observed in the AngII model (Figure 5C).
Figure 5.

EP1 receptor antagonism prevents AngII‐induced vascular stiffness and changes in the ECM. Representative fluorescent confocal photomicrographs of mPGES‐1 immunolocalization (A) (n = 5) and mRNA levels of mPGES‐1 (B) in aortic segments from mice with or without AngII treatment (n = 6). Image size 238 × 238 μm. (C) PGE2 production by mesenteric arteries from mice with or without AngII treatment (n = 9) or AngII plus celecoxib (n = 10). (D) mRNA levels of mPGES‐1 in aortic homogenates from WKY (n = 10) and SHR (n = 11). Structural (E) and mechanical parameters (F) in mesenteric resistance arteries (MRA) from mice with or without AngII treatment (n = 5) or AngII plus SC19220 (n = 5). (G) Quantification of internal elastic lamina structure and collagen content in the media layer of MRA (n = 5). Elastin image size: 59.5 × 59.5 μm. Scale bar: 50 μm. Data represent mean ± SEM. Gene expression data are expressed as fold increase of the untreated or WKY groups mean value. *P < 0.05, significantly different from untreated. # P < 0.05, significantly different from AngII.
Expression of EP1 receptors in VSMC was not modified by incubation with AngII (Supporting Information Fig. S2A) or by AngII infusion in mesenteric arteries (Supporting Information Fig. S2B). However, the EP1 receptor antagonist SC19220 partly prevented the increased SBP induced by AngII infusion (Table 2), but it did not modify the altered wall : lumen ratio in MRA (Figure 5E). Importantly, SC19220 treatment normalized the altered mechanical properties, elastin structure and collagen content (Figure 5F,G) in AngII‐infused mice. These results indicate that PGE2 probably derived from COX‐2 and mPGES‐1 in response to AngII acts on EP1 receptors to induce changes in vascular stiffness and collagen and elastin deposition.
Role of COX‐2 and EP1 receptors in the altered vascular function in AngII‐induced hypertension
Responses to phenylephrine were similar in aorta from COX‐2+/+ and COX‐2−/− mice (Emax and pD2, data not shown). However, endothelium‐dependent relaxation to ACh was slightly impaired in COX‐2−/− mice (Emax COX‐2+/+: 81 ± 4%; Emax COX‐2−/−: 69 ± 4%, P < 0.05). AngII infusion increased phenylephrine vasoconstrictor responses and diminished ACh vasodilator responses in aorta from COX‐2+/+ but not from COX‐2−/− mice (Supporting Information Fig. S3A,B). Treatment of AngII‐infused mice with SC19220 not only reduced the increased aortic contractile responses but also normalized the impaired endothelium‐dependent relaxations (Supporting Information Fig. S4A,B). DEA‐NO‐induced relaxation was not modified by AngII infusion, by COX‐2 gene deletion or by SC19220 treatment (Supporting Information Figs. S3C and S4C).
Incubation of mouse aorta with AngII in vitro, a model that closely resembles vascular alterations induced by AngII in vivo (Martínez‐Revelles et al., 2013), increased phenylephrine responses; this potentiation was inhibited by another EP1 receptor antagonist (SC51322) but not by an EP3 receptor antagonist (L798106) (Supporting Information Fig. S4D). The EP1 receptor antagonist, but not the EP3 receptor antagonist, also decreased contractile responses to 16,16‐dimethyl PGE2 in aorta and mesenteric arteries (data not shown).
Role of COX‐2 in vascular inflammation in hypertension: participation in ECM deposition
Medial macrophage infiltration, expression of the chemokine CCL2 and of TNF‐α were increased in arteries from AngII‐infused mice (Figure 6A–D). Celecoxib treatment normalized the expression of the macrophage marker Mac‐3 and of TNF‐α expression, but it did not modify AngII‐induced CCL2 expression (Figure 6A–D). To ascertain whether mononuclear cells might be a source of prostanoids in hypertension, we analysed COX‐2 and mPGES‐1 expression in human PBMC. As shown in Figure 6E, expression of COX‐2 and mPGES‐1 were higher in PBMC obtained from untreated hypertensive patients than in normotensive individuals.
Figure 6.
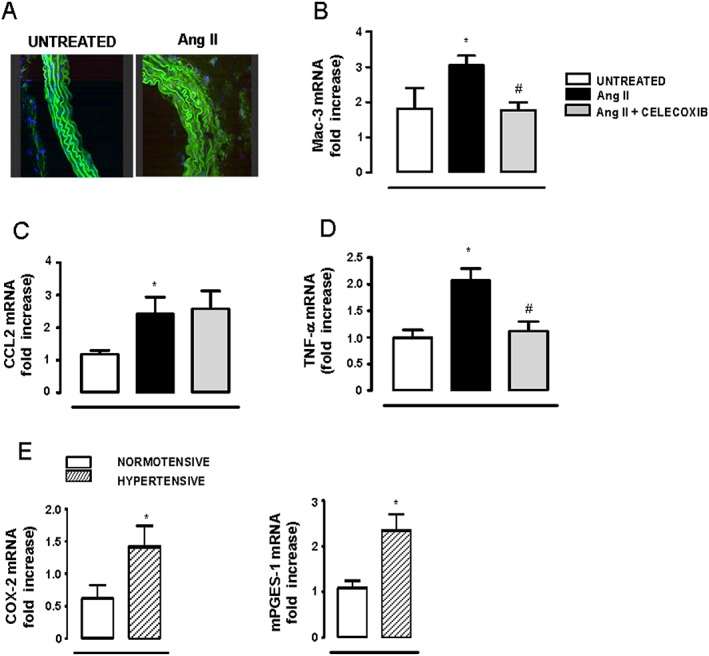
COX‐2 participates in vascular inflammation in hypertension. (A) Representative fluorescent confocal photomicrographs of Mac‐3 immunolocalization in aortic segments (n = 5 per group). Image size 238 × 238 μm. mRNA levels of Mac‐3 (B), CCL2 (C) and TNF‐α (D) in aortic homogenates from mice without (n = 5–7) or with AngII treatment (n = 6–10) or AngII plus celecoxib (n = 8–11). (E) mRNA levels of COX‐2 and mPGES‐1 in PBMC isolated from normotensive (n = 5) or hypertensive (n = 5) patients. Data represent mean ± SEM. Gene expression data are expressed as fold increase of the untreated or normotensive groups mean value. *P < 0.05, significantly different from untreated or normotensive. # P < 0.05, significantly different from AngII.
We then assessed the contribution of macrophage infiltration to ECM deposition. Exposure of VSMC to MCM induced an early decrease in collagen type I and CTGF expression that was later increased. Thus, MCM increased collagen type I but not CTGF expression in VSMC after 24 h (Figure 7). Pretreatment of VSMC with celecoxib prevented the MCM‐induced collagen type I expression (Figure 7). In agreement, COX‐2 expression was also increased in VSMC exposed to MCM (Figure 7).
Figure 7.
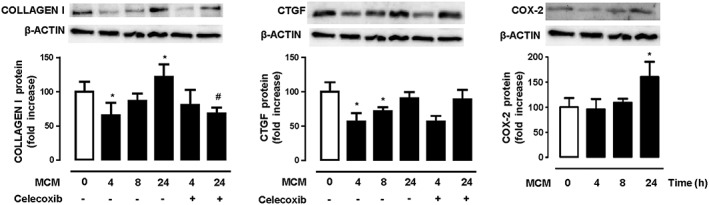
Effect of macrophage conditioned media (MCM) in extracellular matrix deposition in vascular smooth muscle cells. Representative blots and quantification of collagen type I, CTGF and COX‐2 protein expression showing the effects of MCM or MCM plus celecoxib on vascular smooth muscle cells. Data represent mean ± SEM. Protein expression data are expressed as fold increase of the time 0 groups mean value. *P < 0.05, significantly different from untreated. # P < 0.05, significantly different from absence of celecoxib. n = 5.
Discussion
The major novel finding of this study is that COX‐2‐derived PGE2 acting on EP1 receptors contributed to the main signs of vascular damage in hypertension, namely, increased vessel stiffness and ECM deposition, increased vasoconstrictor responses, endothelial dysfunction and vascular inflammation. By modulating these processes, PGE2 might have an important role in hypertension.
COX‐2 expression and prostanoids production are induced by AngII in different vessels and vascular cell types (Álvarez et al., 2007; Beltrán et al., 2009; Wong et al., 2011; Martínez‐Revelles et al., 2013), and high levels of COX‐2 are expressed in vessels from hypertensive patients or animal models, where it participates in vascular dysfunction (Álvarez et al., 2007; Tian et al., 2012; Martínez‐Revelles et al., 2013; Virdis et al., 2013). A role for COX‐2 in vascular remodelling has been demonstrated in pathological situations associated with enhanced proliferation and/or migration of VSMCs such as atherosclerosis, aneurysms and restenosis (Cipollone et al., 2004; Yang et al., 2004; King et al., 2006; Gitlin et al., 2007; Zhang et al., 2013). However, MRA from AngII‐infused mice or SHR showed eutrophic remodelling (data not shown), and we did not observe improved vascular structure after celecoxib treatment. Interestingly, in a recent study, we demonstrated that celecoxib treatment prevented the hypertrophic remodelling induced by AngII in aorta (Aguado et al., 2015), suggesting that COX‐2 blockade might be more efficient in vascular remodelling associated with proliferation and/or migration of vascular cells. Importantly, our results clearly demonstrate that COX‐2‐derived prostanoids are responsible for the mechanical vascular alterations observed in hypertension by modulating ECM deposition. This is because prophylactic and therapeutic administration of celecoxib strongly decreased the greater vascular stiffness and collagen deposition, the altered elastin structure and the increased expression of profibrotic factors observed in the hypertensive vasculature of SHR and AngII‐infused mice, thus leading to improved vascular distensibility. Moreover, another selective COX‐2 inhibitor, rofecoxib, also improved the mechanical alterations, and COX‐2−/− mice were resistant to AngII‐induced vascular stiffness and ECM alterations. Our results are in agreement with previous data demonstrating that COX‐2‐derived products participate in the increased collagen levels observed in different tissues such as the infarcted (LaPointe et al., 2004) or the diabetic (Kellogg et al., 2009) heart. To our knowledge, only one study (Virdis et al., 2012) has demonstrated a relationship between COX‐derived prostanoids and mechanical alterations in hypertension but here it appeared to involve the COX‐1 isoform. We do not have a clear explanation for this discrepancy. However, neither the dose of AngII nor the COX‐2 inhibitor was the same as those used in the present study. An interesting result of our study was the finding that, in the absence of AngII, arteries from COX‐2−/− mice showed increased wall : lumen ratio, vascular stiffness and altered elastin structure when compared with wild‐type mice, effects not observed in normotensive animals treated with celecoxib. This is consistent with the idea of a protective role of COX‐2‐derived prostanoids in physiological conditions because long‐term inhibition as achieved by genetic deletion of COX‐2 results in vascular damage. However, as mentioned, COX‐2 knockout mice were protected against the deleterious effects of AngII in the vascular wall, suggesting that, in inflammatory conditions, up‐regulation of COX‐2 is a key promoter of vascular damage.
AngII can enhance PGE2 generation as a consequence of inducing mPGES‐1 (Cipollone et al., 2004; Wang et al., 2008; Aguado et al., 2015). In agreement, we found increased mPGES‐1 expression in vessels from adult hypertensive animals but not in prehypertensive rats, suggesting that the increased mPGES‐1 expression might be a consequence of high BP. We also observed that AngII‐induced PGE2 production was blocked by celecoxib treatment, which would be consistent with the hypothesis that mPGES‐1 and COX‐2 could be co‐regulated and that stimulated PGE2 synthesis may depend on up‐regulation of both enzymes (Cipollone et al., 2008). PGE2 has important actions in the cardiovascular system such as control of vascular tone and cell adhesion/migration via EP receptor subtypes (Foudi et al., 2012; Aguado et al., 2015). Recently, a role for EP3 receptors has been demonstrated in neointima hyperplasia (Zhang et al., 2013), and EP4 receptor activation increases fibrosis in the kidney (Mohamed et al., 2013). Herein, we report for the first time that the EP1 receptor antagonist SC19220 normalized the altered mechanical properties, collagen deposition and altered elastin structure induced by AngII infusion in MRA, without changing expression of EP1 receptors, suggesting that increased PGE2 production and binding to available EP1 receptors is likely to be responsible for the observed effects. Nevertheless, SC19220 did not modify structural parameters. Interestingly, a role for TxA2 receptors (TP) in AngII‐induced vascular remodelling has been described (Virdis et al., 2012; Sparks et al., 2013). Altogether, these results suggest that the role of prostanoids in vascular remodelling and stiffness in hypertension is a complex issue probably involving more than one prostanoid and receptor. To build on this complexity, it is well known that the selectivity of prostanoids for their respective receptors is not absolute and binding to other receptors, particularly in pathological conditions, can be found for PGI2, PGE2 and TXA2 (Ungrin et al., 2001; Félétou et al., 2011).
Vasoconstrictor prostanoids from COX‐2 are increased in different animal models or in patients with hypertension and are partly responsible for the endothelial dysfunction or the increased vasoconstrictor responses observed in this pathology (Widlansky et al., 2003; Álvarez et al., 2007; Virdis et al., 2009; Wong et al., 2011; Tian et al., 2012; Martínez‐Revelles et al., 2013; Virdis et al., 2013). Here, we confirmed our results that AngII induced COX‐2‐dependent production of contractile prostanoids, that impaired vascular function (Martínez‐Revelles et al., 2013) and describe now that PGE2 acting on EP1 receptors also played a key role. Thus, COX‐2−/− mice and SC19220‐treated WT mice were partly protected from the deleterious effect of AngII in vascular contraction and endothelium‐dependent relaxation. In agreement, both EP1 (Guan et al., 2007) and EP3 receptors (Chen et al., 2012) were involved in acute vasoconstrictor responses induced by AngII, although none of these studies evaluated the role of EP receptors in vascular function in chronic AngII‐dependent hypertension. Of note, in the absence of AngII, COX‐2−/− mice showed some degree of endothelial dysfunction, suggesting that COX‐2‐derived prostanoids maintain physiological vessel function and it is only after inflammatory challenge, that COX‐2 deletion protects against vascular damage.
It is now evident that leukocytes have a role in vascular remodelling, endothelial dysfunction and stiffness in response to AngII (De Ciuceis et al., 2005; Wenzel et al., 2011; Wu et al., 2014). We demonstrate that COX‐2 pathway is central to explain the AngII‐induced macrophage recruitment and the expression of TNF‐α in the vascular wall, because its blockade decreased macrophage infiltration and expression of pro‐inflammatory cytokines in the vascular wall. We also demonstrate that macrophages might have a role in ECM deposition in adjacent VSMC. Thus, after an initial decrease of collagen type I expression probably driven by macrophage‐derived MMPs, prolonged exposure to MCM induced a significant increase in collagen deposition and COX‐2 expression. Importantly, COX‐2 inhibition in VSMC prevented the profibrotic effects of MCM, suggesting that products of macrophages diffuse to the adjacent VSMC to induce COX‐2 activation and ECM production. In support of the crucial role of prostanoids derived from macrophages in vascular damage, recent studies have demonstrated that mice lacking mPGES‐1 in their myeloid cells, show reduced macrophage infiltration and TNF‐α expression in a model of murine atherosclerosis (Chen et al., 2014). Furthermore, these mice are protected against exaggerated neointimal hyperplastic response to wire injury (Chen et al., 2013) and against atherogenesis (Chen et al., 2014). Of note, we observed that circulating PBMC from untreated hypertensive patients exhibited increased COX‐2 and mPGES‐1 expression compared with normotensive individuals, changes that might facilitate vascular damage in the hypertensive vasculature.
Pharmacological blockade of COX‐2 partly prevented and also reversed high BP in the SHR and/or in the AngII mice, which is in agreement with previous reports (Tian et al., 2012; Martínez‐Revelles et al., 2013). However, we observed that COX‐2−/− mice were not protected against AngII‐induced hypertension, probably reflecting adaptive responses to gene deletion or differences attributable to genetic backgrounds (Yang et al., 2004). Interestingly, we observed that blockade of EP1 receptors prevented the increase in BP induced by AngII, which is in agreement with previous reports (Guan et al., 2007). In addition, other authors found that deletion of EP3 (Chen et al., 2012) or TP receptors (Francois et al., 2004), but not pharmacological blockade of TP receptors (Virdis et al., 2012), partly prevented AngII‐induced hypertension. Together, these results suggest that different prostanoid receptors might contribute to BP regulation both in physiological and pathological conditions.
In conclusion, we propose that COX‐2‐derived and presumably mPGES‐1‐derived PGE2 acting through EP1 receptors is a key pathway involved in hypertension‐associated vascular damage by inducing increased vessel stiffness, ECM deposition, vascular dysfunction and inflammation. Specific COX‐2 inhibitors increase the risk of cardiovascular disease (Amer et al., 2010) probably due to suppression of PGI2 biosynthesis without concomitant inhibition of platelet COX‐1‐derived TxA2 production (Cheng et al., 2006). Our data provide mechanistic information of the effects of prostanoids in the vasculature in the setting of hypertension. By highlighting these effects, we provide novel information that might help to identify more selective components of the prostanoid pathway that might be more suitable and safer targets for inhibition than the COX‐2 enzyme. Therefore, targeting PGE2 production by mPGES‐1 and/or the EP receptors might have potential benefit for the treatment of hypertension and hypertension‐associated vascular damage.
Author contributions
M.S.A., S.M.‐R., A.A., M.R.S., M.G.‐A., R.P. and M.V.C. performed the experiments. D.V.V., L.V., M.J.A., M.S. and A.M.B. designed the research study. P.G.‐L., J.G.‐P. and L.B. contributed essential reagents or tools. M.S.A., S.M.‐R., A.A., A.M.B. analysed the data. M.J.A., A.M.B. and M.S. wrote the manuscript. M.S.A., S.M.‐R., A.A., M.R.S., M.G.‐A., R.P., P.G.‐L., D.V.V., L.V., J.G.‐P., L.B., M.J.A., M.V.C., M.S. and A.M.B. approved the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organizations engaged with supporting research.
Supporting information
Figure S1 COX‐2 inhibition prevents angiotensin II‐induced hypertension and increased vascular stiffness. Systolic BP (SBP) (A) and structural (B) and mechanical parameters (C) in mesenteric resistance arteries from mice, with or without AngII treatment (n = 6) or AngII plus rofecoxib (n = 6). Data represent mean ± SEM. *P < 0.05, significantly different from untreated, # P < 0.05, significantly different from AngII.
Figure S2 Angiotensin II does not modify EP1 receptor expression. Levels of mRNA for EP1 receptors in (A) rat aortic vascular smooth muscle cells stimulated with AngII (0.1 μM) (n = 5) or (B) mesenteric arteries from mice, with or without AngII treatment (n = 6). Data represent mean ± SEM. Gene expression data are shown as fold increase of the time 0 or untreated groups mean value.
Figure S3 COX‐2‐derived prostanoids have a role in vascular dysfunction in hypertension. Concentration–response curves to phenylephrine (Phe) (A), ACh (B) and diethylamine NONOate (DEA‐NO) (C) in aorta from COX‐2+/+ mice with or without AngII treatment (n = 8 per group) and from COX‐2−/− mice with or without AngII treatment (n = 4–9). Data represent mean ± SEM. *P < 0.05, significantly different from untreated.
Figure S4 EP1 receptors participate in vascular dysfunction in hypertension. Concentration–response curves to phenylephrine (Phe) (A), ACh (B) and diethylamine NONOate (DEA‐NO) (C) in aorta from mice with or without AngII treatment (n = 5) or AngII plus SC19220 (n = 6). (D) Concentration–response curves to Phe in aortic segments incubated in vitro with AngII (1 μM, 1 h) alone or in combination with the EP1 receptor antagonist SC51322 (1 μM) or the EP3 receptor antagonist L798106 (1 μM) (n = 6–8). Control tissues were not incubated with AngII. Data represent mean ± SEM. *P < 0.05, significantly different from untreated or control, # P < 0.05, significantly different from AngII.
Table S1 Sequences of the specific primers used for qPCR assays
Supporting info item
Acknowledgements
The authors thank the Animal Care Facility (Faculty of Medicine, UAM), Laura García‐Redondo for her technical assistance, Aránzazu Sánchez for her help with human blood extraction and Dr Miguel Angel Iñiguez for his critical reading of the manuscript.
This work was supported by Ministerio de Economía y Competitividad (SAF2012‐36400), Instituto de Salud Carlos III (ISCIII)–Fondo Europeo de Desarrollo Regional (FEDER) (PI13/01488 and Red de Investigación Cardiovascular, RD12/0042/0024, RD12/0042/0033, RD12/0042/0051), COST‐ADMIRE BM1301, L'Oréal‐FWIS‐Spain, UAM‐Grupo Santander and the Ramón y Cajal Program (AMB, RyC‐2010‐06473).
Avendaño, M. S. , Martínez‐Revelles, S. , Aguado, A. , Simões, M. R. , González‐Amor, M. , Palacios, R. , Guillem‐Llobat, P. , Vassallo, D. V. , Vila, L. , García‐Puig, J. , Beltrán, L. M. , Alonso, M. J. , Cachofeiro, M. V. , Salaices, M. , and Briones, A. M. (2016) Role of COX‐2‐derived PGE2 on vascular stiffness and function in hypertension. British Journal of Pharmacology, 173: 1541–1555. doi: 10.1111/bph.13457.
References
- Aguado A, Galán M, Zhenyukh O, Wiggers GA, Roque FR, Redondo S, et al. (2013). Mercury induces proliferation and reduces cell size in vascular smooth muscle cells through MAPK, oxidative stress and cyclooxygenase‐2 pathways. Toxicol Appl Pharmacol 268: 188–200. [DOI] [PubMed] [Google Scholar]
- Aguado A, Rodríguez C, Martínez‐Revelles S, Avendaño MS, Zhenyukh O, Orriols M, et al. (2015). HuR mediates the synergistic effects of angiotensin II and IL‐1β on vascular COX‐2 expression and cell migration. Br J Pharmacol 172: 3028–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G Protein‐Coupled Receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez Y, Pérez‐Girón JV, Hernanz R, Briones AM, García‐Redondo A, Beltrán A, et al. (2007). Losartan reduces the increased participation of cyclooxygenase‐2‐derived products in vascular responses of hypertensive rats. J Pharmacol Exp Ther 321: 381–388. [DOI] [PubMed] [Google Scholar]
- Amer M, Bead VR, Bathon J, Blumenthal RS, Edwards DN (2010). Use of nonsteroidal anti‐inflammatory drugs in patients with cardiovascular disease: a cautionary tale. Cardiol Rev 18: 204–212. [DOI] [PubMed] [Google Scholar]
- Beltrán AE, Briones AM, García‐Redondo AB, Rodríguez C, Miguel M, Alvarez Y, et al. (2009). p38 MAPK contributes to angiotensin II‐induced COX‐2 expression in aortic fibroblasts from normotensive and hypertensive rats. J Hypertens 27: 142–154. [DOI] [PubMed] [Google Scholar]
- Briones AM, Arribas SM, Salaices M (2010). Role of extracellular matrix in vascular remodeling of hypertension. Curr Opin Nephrol Hypertens 19: 187–194. [DOI] [PubMed] [Google Scholar]
- Briones AM, González JM, Somoza B, Giraldo J, Daly CJ, Vila E, et al. (2003). Role of elastin in spontaneously hypertensive rat small mesenteric artery remodelling. J Physiol 552: 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones AM, Rodríguez‐Criado N, Hernanz R, García‐Redondo AB, Rodrigues‐Díez RR, Alonso MJ, et al. (2009). Atorvastatin prevents angiotensin II‐induced vascular remodeling and oxidative stress. Hypertension 54: 142–149. [DOI] [PubMed] [Google Scholar]
- Bush E, Maeda N, Kuziel WA, Dawson TC, Wilcox JN, DeLeon H, et al. (2000). CC chemokine receptor 2 is required for macrophage infiltration and vascular hypertrophy in angiotensin II‐induced hypertension. Hypertension 36: 360–363. [DOI] [PubMed] [Google Scholar]
- Buus NH, Mathiassen ON, Fenger‐Grøn M, Præstholm MN, Sihm I, Thybo NK, et al. (2013). Small artery structure during antihypertensive therapy is an independent predictor of cardiovascular events in essential hypertension. J Hypertens 31: 791–797. [DOI] [PubMed] [Google Scholar]
- Camacho M, Dilmé J, Solà‐Villà D, Rodríguez C, Bellmunt S, Siguero L, et al. (2013). Microvascular COX‐2/mPGES‐1/EP‐4 axis in human abdominal aortic aneurysm. J Lipid Res 54: 3506–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Miao Y, Zhang Y, Dou D, Liu L, Tian X, et al. (2012). Inactivation of the E‐prostanoid 3 receptor attenuates the angiotensin II pressor response via decreasing arterial contractility. Arterioscler Thromb Vasc Biol 32: 3024–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yang G, Xu X, Grant G, Lawson JA, Bohlooly‐Y M, et al. (2013). Cell selective cardiovascular biology of microsomal prostaglandin E synthase‐1. Circulation 127: 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yang G, Monslow J, Todd L, Cormode DP, Tang J, et al. (2014). Myeloid cell microsomal prostaglandin E synthase‐1 fosters atherogenesis in mice. Proc Natl Acad Sci U S A 111: 6828–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Wang M, Yu Y, Lawson J, Funk CD, Fitzgerald GA (2006). Cyclooxygenases, microsomal prostaglandin E synthase‐1, and cardiovascular function. J Clin Invest 116: 1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipollone F, Cicolini G, Bucci M (2008). Cyclooxygenase and prostaglandin synthases in atherosclerosis: recent insights and future perspectives. Pharmacol Ther 118: 161–180. [DOI] [PubMed] [Google Scholar]
- Cipollone F, Fazia M, Iezzi A, Pini B, Cuccurullo C, Zucchelli M, et al. (2004). Blockade of the angiotensin II type 1 receptor stabilizes atherosclerotic plaques in humans by inhibiting prostaglandin E2‐dependent matrix metalloproteinase activity. Circulation 109: 1482–1488. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPH, Giembycz MA, et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP . Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL (2005). Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II‐infused macrophage colony‐stimulating factor‐deficient mice: evidence for a role in inflammation in angiotensin‐induced vascular injury. Arterioscler Thromb Vasc Biol 25: 2106–2113. [DOI] [PubMed] [Google Scholar]
- Félétou M, Huang Y, Vanhoutte PM (2011). Endothelium‐mediated control of vascular tone: COX‐1 and COX‐2 products. Br J Pharmacol 164: 894–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foudi N, Gomez I, Benyahia C, Longrois D, Norel X (2012). Prostaglandin E2 receptor subtypes in human blood and vascular cells. Eur J Pharmacol 695: 1–6. [DOI] [PubMed] [Google Scholar]
- Francois H, Athirakul K, Mao L, Rockman H, Coffman TM (2004). Role for thromboxane receptors in angiotensin‐II‐induced hypertension. Hypertension 43: 364–369. [DOI] [PubMed] [Google Scholar]
- Gitlin JM, Trivedi DB, Langenbach R, Loftin CD (2007). Genetic deficiency of cyclooxygenase‐2 attenuates abdominal aortic aneurysm formation in mice. Cardiovasc Res 73: 227–236. [DOI] [PubMed] [Google Scholar]
- Guan Y, Zhang Y, Wu J, Qi Z, Yang G, Dou D, et al. (2007). Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J Clin Invest 117: 2496–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei D, Yamakawa K, Takegoshi Y, Mikami‐Nakanishi M, Nakatani Y, Oh‐Ishi S, et al. (2004). Reduced pain hypersensitivity and inflammation in mice lacking microsomal prostaglandin E synthase‐1. J Biol Chem 279: 33684–33695. [DOI] [PubMed] [Google Scholar]
- Kane MO, Etienne‐Selloum N, Madeira SV, Sarr M, Walter A, Dal‐Ros S, et al. (2010). Endothelium‐derived contracting factors mediate the AngII‐induced endothelial dysfunction in the rat aorta: preventive effect of red wine polyphenols. Pflugers Arch 459: 671–679. [DOI] [PubMed] [Google Scholar]
- Kellogg AP, Converso K, Wiggin T, Stevens M, Pop‐Busui R (2009). Effects of cyclooxygenase‐2 gene inactivation on cardiac autonomic and left ventricular function in experimental diabetes. Am J Physiol Heart Circ Physiol 296: H453–H461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King VL, Trivedi DB, Gitlin JM, Loftin CD (2006). Selective cyclooxygenase‐2 inhibition with celecoxib decreases angiotensin II‐induced abdominal aortic aneurysm formation in mice. Arterioscler Thromb Vasc Biol 26: 1137–1143. [DOI] [PubMed] [Google Scholar]
- LaPointe MC, Mendez M, Leung A, Tao Z, Yang XP (2004). Inhibition of cyclooxygenase‐2 improves cardiac function after myocardial infarction in the mouse. Am J Physiol Heart Circ Physiol 286: H1416–H1424. [DOI] [PubMed] [Google Scholar]
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. (2013). 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 34: 2159–2219. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez‐Revelles S, Avendaño MS, García‐Redondo AB, Alvarez Y, Aguado A, Pérez‐Girón JV, et al. (2013). Reciprocal relationship between reactive oxygen species and cyclooxygenase‐2 and vascular dysfunction in hypertension. Antioxid Redox Signal 18: 51–65. [DOI] [PubMed] [Google Scholar]
- Mohamed R, Jayakumar C, Ramesh G (2013). Chronic administration of EP4‐selective agonist exacerbates albuminuria and fibrosis of the kidney in streptozotocin‐induced diabetic mice through IL‐6. Lab Invest 93: 933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SPH, Buneman OP, et al. , NC‐IUPHAR(2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucl Acids Res 42 (Database Issue): D1098–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, et al. (2001). Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 104: 191–196. [DOI] [PubMed] [Google Scholar]
- Rizzoni D, Porteri E, Boari GE, de Ciuceis C, Sleiman I, Muiesan ML, et al. (2003). Prognostic significance of small‐artery structure in hypertension. Circulation 108: 2230–2235. [DOI] [PubMed] [Google Scholar]
- Safar ME, Nilsson PM, Blacher J, Mimran A (2012). Pulse pressure, arterial stiffness, and end‐organ damage. Curr Hypertens Rep 14: 339–344. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL (2012). Vascular remodeling in hypertension: mechanisms and treatment. Hypertension 59: 367–374. [DOI] [PubMed] [Google Scholar]
- Sparks MA, Makhanova NA, Griffiths RC, Snouwaert JN, Koller BH, Coffman TM (2013). Thromboxane receptors in smooth muscle promote hypertension, vascular remodeling, and sudden death. Hypertension 61: 166–173. [DOI] [PubMed] [Google Scholar]
- Tian XY, Wong WT, Leung FP, Zhang Y, Wang YX, Lee HK, et al. (2012). Oxidative stress‐dependent cyclooxygenase‐2‐derived prostaglandin f(2α) impairs endothelial function in renovascular hypertensive rats. Antioxid Redox Signal 16: 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungrin MD, Carrière MC, Denis D, Lamontagne S, Sawyer N, Stocco R, et al. (2001). Key structural features of prostaglandin E(2) and prostanoid analogs involved in binding and activation of the human EP(1) prostanoid receptor. Mol Pharmacol 59: 1446–1456. [DOI] [PubMed] [Google Scholar]
- Virdis A, Bacca A, Colucci R, Duranti E, Fornai M, Materazzi G, et al. (2013). Endothelial dysfunction in small arteries of essential hypertensive patients: role of cyclooxygenase‐2 in oxidative stress generation. Hypertension 62: 337–344. [DOI] [PubMed] [Google Scholar]
- Virdis A, Colucci R, Neves MF, Rugani I, Aydinoglu F, Fornai M, et al. (2012). Resistance artery mechanics and composition in angiotensin II‐infused mice: effects of cyclooxygenase‐1 inhibition. Eur Heart J 33: 2225–2234. [DOI] [PubMed] [Google Scholar]
- Virdis A, Colucci R, Versari D, Ghisu N, Fornai M, Antonioli L, et al. (2009). Atorvastatin prevents endothelial dysfunction in mesenteric arteries from spontaneously hypertensive rats: role of cyclooxygenase 2‐derived contracting prostanoids. Hypertension 53: 1008–1016. [DOI] [PubMed] [Google Scholar]
- Wang M, Ihida‐Stansbury K, Kothapalli D, Tamby MC, Yu Z, Chen L, et al. (2011). Microsomal prostaglandin E2 synthase‐1 modulates the response to vascular injury. Circulation 123: 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Lee E, Song W, Ricciotti E, Rader DJ, Lawson JA, et al. (2008). Microsomal prostaglandin E synthase‐1 deletion suppresses oxidative stress and angiotensin II‐induced abdominal aortic aneurysm formation. Circulation 117: 1302–1309. [DOI] [PubMed] [Google Scholar]
- Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, et al. (2011). Lysozyme M‐positive monocytes mediate angiotensin II‐induced arterial hypertension and vascular dysfunction. Circulation 124: 1370–1381. [DOI] [PubMed] [Google Scholar]
- Widlansky ME, Price DT, Gokce N, Eberhardt RT, Duffy SJ, Holbrook M, et al. (2003). Short‐ and long‐term COX‐2 inhibition reverses endothelial dysfunction in patients with hypertension. Hypertension 42: 310–315. [DOI] [PubMed] [Google Scholar]
- Wong SL, Lau CW, Wong WT, Xu A, Au CL, Ng CF, et al. (2011). Pivotal role of protein kinase Cdelta in angiotensin II‐induced endothelial cyclooxygenase‐2 expression: a link to vascular inflammation. Arterioscler Thromb Vasc Biol 31: 1169–1176. [DOI] [PubMed] [Google Scholar]
- Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, et al. (2014). Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen‐activated protein kinase. Circ Res 114: 616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HM, Kim HS, Park KW, You HJ, Jeon SI, Youn SW, et al. (2004). Celecoxib, a cyclooxygenase‐2 inhibitor, reduces neointimal hyperplasia through inhibition of Akt signaling. Circulation 110: 301–308. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zou F, Tang J, Zhang Q, Gong Y, Wang Q, et al. (2013). Cyclooxygenase‐2‐derived prostaglandin E2 promotes injury‐induced vascular neointimal hyperplasia through the E‐prostanoid 3 receptor. Circ Res 113: 104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 COX‐2 inhibition prevents angiotensin II‐induced hypertension and increased vascular stiffness. Systolic BP (SBP) (A) and structural (B) and mechanical parameters (C) in mesenteric resistance arteries from mice, with or without AngII treatment (n = 6) or AngII plus rofecoxib (n = 6). Data represent mean ± SEM. *P < 0.05, significantly different from untreated, # P < 0.05, significantly different from AngII.
Figure S2 Angiotensin II does not modify EP1 receptor expression. Levels of mRNA for EP1 receptors in (A) rat aortic vascular smooth muscle cells stimulated with AngII (0.1 μM) (n = 5) or (B) mesenteric arteries from mice, with or without AngII treatment (n = 6). Data represent mean ± SEM. Gene expression data are shown as fold increase of the time 0 or untreated groups mean value.
Figure S3 COX‐2‐derived prostanoids have a role in vascular dysfunction in hypertension. Concentration–response curves to phenylephrine (Phe) (A), ACh (B) and diethylamine NONOate (DEA‐NO) (C) in aorta from COX‐2+/+ mice with or without AngII treatment (n = 8 per group) and from COX‐2−/− mice with or without AngII treatment (n = 4–9). Data represent mean ± SEM. *P < 0.05, significantly different from untreated.
Figure S4 EP1 receptors participate in vascular dysfunction in hypertension. Concentration–response curves to phenylephrine (Phe) (A), ACh (B) and diethylamine NONOate (DEA‐NO) (C) in aorta from mice with or without AngII treatment (n = 5) or AngII plus SC19220 (n = 6). (D) Concentration–response curves to Phe in aortic segments incubated in vitro with AngII (1 μM, 1 h) alone or in combination with the EP1 receptor antagonist SC51322 (1 μM) or the EP3 receptor antagonist L798106 (1 μM) (n = 6–8). Control tissues were not incubated with AngII. Data represent mean ± SEM. *P < 0.05, significantly different from untreated or control, # P < 0.05, significantly different from AngII.
Table S1 Sequences of the specific primers used for qPCR assays
Supporting info item


