Abstract
Background and Purpose
KCNQ‐encoded voltage‐dependent potassium channels (Kv7) are involved in the regulation of vascular tone. In this study we evaluated the influence of Kv7 channel activation on smooth muscle relaxation in rat penile arteries and corpus cavernosum from normal and spontaneously hypertensive, heart failure‐prone (SHHF) rats – a rat model of human metabolic syndrome.
Experimental Approach
Quantitative PCR and immunohistochemistry were used to determine the expression of KCNQ isoforms in penile tissue. Isometric tension was measured in intracavernous arterial rings and corpus cavernosum strips isolated from normal and SHHF rats.
Key Results
Transcripts for KCNQ3, KCNQ4 and KCNQ5 were detected in penile arteries and corpus cavernosum. KCNQ1 was only found in corpus cavernosum. Immunofluorescence signals to Kv7.4 and Kv7.5 were found in penile arteries, penile veins and corpus cavernosum. The Kv7.2–7.5 activators, ML213 and BMS204352, relaxed pre‐contracted penile arteries and corpus cavernosum independently of nitric oxide synthase or endothelium‐derived hyperpolarization. Relaxations to sildenafil, a PDE5 inhibitor, and sodium nitroprusside (SNP), an nitric oxide donor, were reduced by blocking Kv7 channels with linopirdine in penile arteries and corpus cavernosum. In SHHF rat penile arteries and corpus cavernosum, relaxations to ML213 and BMS204352 were attenuated, and the blocking effect of linopirdine on sildenafil‐induced and SNP‐induced relaxations reduced. KCNQ3, KCNQ4 and KCNQ5 were down‐regulated, and KCNQ1 was up‐regulated in corpus cavernosum from SHHF rats. KCNQ1–5 transcripts remained unchanged in penile arteries from SHHF rats.
Conclusions and Implications
These data suggest that Kv7 channels play a role in erectile function and contribute to the pathophysiology of erectile dysfunction, an early indicator of cardiovascular disease.
Abbreviations
- BKCa
large‐conductance calcium‐activated potassium channel
- BMS204352
(3S)‐(+)‐(5‐chloro‐2‐methoxyphenyl)‐1,3‐dihydro‐3‐fluoro‐6‐(trifluoromethyl)‐2H‐indol‐2‐one
- ED
erectile dysfunction
- IKCa
intermediate‐conductance calcium‐activated potassium channel
- Kv
voltage‐gated potassium channel
- ML213
N‐mesitylbicyclo[2.2.1]heptane‐2‐carboxamide
- qPCR
quantitative PCR
- SKCa
small‐conductance calcium‐activated potassium channel
- SNP
sodium nitroprusside
- TRAM‐34
1‐[(2‐chlorophenyl)diphenylmethyl]‐1H‐pyrazole
Tables of Links
| TARGETS | |
|---|---|
| Voltage‐gated ion channels a | Enzymes b |
| BKCa channels | eNOS |
| Kv7.1 channel (KCNQ1) | Guanylate cyclase |
| Kv7.2 channel (KCNQ2) | nNOS |
| Kv7.3 channel (KCNQ3) | |
| Kv7.4 channel (KCNQ4) | |
| Kv7.5 channel (KCNQ5) | |
| SKCa channels |
| LIGANDS | |
|---|---|
| Apamin | ML213 |
| BMS204352 | Nitric oxide (NO) |
| cGMP | Phenylephrine |
| Carbachol | Sildenafil |
| Linopirdine | TRAM‐34 |
| L‐NAME |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015a, 2015b).
Introduction
Penile erection is the end result of a complex neurovascular process in which nerves, endothelium of sinusoids and blood vessels, and smooth muscle cells in the target organ are involved. Penile erection is achieved by dilatation of penile arteries and relaxation of the trabecular smooth muscle located in the corpus cavernosum combined with the associated compression of penile veins. The main mediator of this smooth muscle cell relaxation in the penis is nitric oxide (NO), which is synthesized by endothelial (eNOS) and neuronal (nNOS) NO synthase (NOS) in nonadrenergic, noncholinergic nerves, endothelial cells and cavernosal smooth muscle cells. The relative contribution of the different forms of NOS to erection has not been definitely established, but existing evidence points towards a model in which nNOS initiates the erectile response, which is then maintained and increased by eNOS activity (Musicki et al., 2009; Gratzke et al., 2010). The increased NO production induces activation of soluble guanylate cyclase, increased cGMP levels and activation of cGMP‐dependent protein kinase (Gratzke et al., 2010). The importance of this mechanism is underlined by the successful use of PDE5 inhibitors, such as sildenafil, which prevent the breakdown of cGMP, in the treatment of erectile dysfunction (ED). ED is the failure to gain or maintain penile erection and is also associated with a risk of diabetes and cardiovascular disease. In fact, ED often precedes the development of cardiovascular disease, particularly in patients with diabetes, and is now considered as a prognostic indicator for serious cardiovascular diseases (Ioakeimidis and Kostis, 2014). Despite the fact that PDE5 inhibitors have a high efficacy to overcome ED in the general population (Goldstein et al., 1998; Padma‐Nathan et al., 2001; Porst et al., 2001), some patient groups, such as men with diabetes, are either unresponsive to (Rendell et al., 1999; Sáenz de Tejada et al., 2002; Goldstein et al., 2003) or contraindicated for this treatment because of other cardiovascular complications (Reffelmann and Kloner, 2005). Thus, alternative pharmacological strategies are required to the improve treatment of ED.
The physiological stimuli that generate an erection have been thoroughly investigated (Gratzke et al., 2010). However, the exact cellular mechanisms that lead to arterial and trabecular relaxation are not well defined, and in particular, the pathological mechanisms underlying ED are unclear. ED is predominately a disease of vascular origin and correlates to the development of endothelial dysfunction (Aversa et al., 2009). Among other things, endothelial dysfunction leads to a decreased NO bioavailability, again highlighting the importance of NO in erectile function, but other factors such as increased release of vasoconstriction‐mediating transmitters (noradrenaline, TxA2, endothelin‐1 and angiotensin II) also contribute to ED (Gratzke et al., 2010; Andersson, 2011). A number of new therapeutic strategies for the treatment of ED have been suggested, which include the ability to reverse, regenerate and replace underlying diseased endothelial, neural and penile vascular smooth muscle cells (Chung and Brock, 2011; Decaluwé et al., 2014). Among others, different potassium (K+) channels, such as intermediate‐conductance (IKCa) and large‐conductance (BKCa) Ca2 +‐activated K+ channels, have been suggested as novel therapeutic targets for the treatment of ED (Werner et al., 2008, 2005; Kun et al., 2009; González‐Corrochano et al., 2013; Király et al., 2013).
KCNQ‐encoded voltage‐dependent K+ (Kv7) channels have been identified as key determinants of vascular and non‐vascular smooth muscle tone (Stott et al., 2014). Rodent and human arteries express Kv7.1, Kv7.4 and Kv7.5 channels, and much evidence has been generated to suggest that the latter two channels are the most relevant physiologically (Chadha et al., 2014; Brueggemann et al., 2014a, 2014b). Kv7 channels not only regulate basal tone but are also functional endpoints for Gs‐linked receptor agonists (Chadha et al., 2012; Khanamiri et al., 2013; Chadha et al., 2014; Stott et al., 2015a, 2015b). More pertinently for penile physiology, Kv7 blockers also impair arterial relaxation produced by atrial natriuretic peptide and sodium nitroprusside (SNP) that increase cellular cGMP (Stott et al., 2015a). Moreover, in arteries from hypertensive rats, Kv7 channel function is compromised, which correlated with a decrease in the Kv7.4 protein levels (Jepps et al., 2011; Chadha et al., 2012; Khanamiri et al., 2013; Li et al., 2013). Based on these previous findings, we hypothesized that Kv7 channels regulate the smooth muscle of the penile artery and corpus cavernosum and that in spontaneously hypertensive rats, prone to heart failure (SHHF), a model of ED, Kv7 channel function is compromised. Therefore, we investigated the Kv7 expression profile and localization of Kv7.4 and 7.5 channels, as well as Kv7 channel involvement in NO‐ and sildenafil‐induced relaxation of penile arteries and corpus cavernosum from normal and SHHF rats.
Methods
Animals
Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). Animal care and experimental procedures were performed according to the Principles of Laboratory Animal Care (National Institutes of Health, revised 1996) approved by the National Ethics Committee, Denmark (license number: 2014‐15‐2934‐0161). Adult male Wistar and SHHF rats (12–16 weeks old) were kept and cared for in standard cages under clean conditions in separate quarters in a 12–12 h light–dark cycle with free access to water and food pellets. Rats were killed by cervical dislocation and exsanguinated by decapitation. After cutting the crura corpora cavernosa at the point of adhesion to the lower pubic bone, the penis was removed and submerged in ice‐cold (4° C) physiological saline solution, and the corpus cavernosum and the intracavernous artery was microsurgically dissected free. The SHHF is a rat model that mimics the pathophysiology of human metabolic syndrome, defined as the simultaneous occurrence of at least three of the five risk factors, namely, obesity, hypertension, dyslipidaemia, type 2 diabetes and insulin resistance (Youcef et al., 2014), which is present in approximately 40% of the patients with ED (Kaya et al., 2015).
Quantitative PCR (qPCR)
The relative expression of the KCNQ1–5 isoforms was determined in the penile arteries and corpus cavernosum of the Wistar and SHHF rats by qPCR analysis, as described previously (Jepps et al., 2014). Briefly, RNA was extracted using the RNEasy Micro Extraction Kit, including a DNase treatment, (Qiagen, Copenhagen, Denmark) and reverse transcribed using the nanoScript 2 kit (PrimerDesign Ltd., Southampton, UK), as per the manufacturer's instructions. Quantitative analysis of the KCNQ genes within our cDNA samples (that had a concentration of 3 ng μL‐1) was determined using Precision PLUS‐iC SYBR mastermix (PrimerDesign Ltd., Southhampton, UK) in 20 μL samples containing 5 μL of cDNA and 300 nM primer, as per the manufacturer's instructions. Experiments were run on a CFX96 Real‐Time PCR Detection System (Bio‐Rad, Hertfordshire, UK). The following cycling conditions were used: initial activation at 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min; and data were collected during each cycling phase. Melt curve analysis, to ensure each primer set amplified a single, specific product, completed the protocol. RT samples and no‐template controls were run alongside all reactions to assess contamination. Quantification cycle (Cq) values were determined using Bio‐Rad cfx96 manager 3.0 software. The optimal reference genes in our samples were identified using the geNorm reference gene selection kit and the Biogazelle qbase plus software (PrimerDesign Ltd.; Vandesompele et al., 2002). Under our experimental conditions, the optimal reference genes were malate dehydrogenase 1 and ubiquitin C (UBC) for the corpus cavernosum and for the penile arteries beta‐2 microglobulin and UBC. The expression levels of the KCNQ isoforms were calculated relative to these reference genes in each artery to give a relative isoform expression profile (Livak and Schmittgen, 2001). The fold change in KCNQ gene expression between Wistar and SHHF rats was then calculated with 2−ΔΔCq. All reference genes in the rat geNorm reference gene selection kit and the KCNQ1–5 assays (Table 1) were designed and optimized by PrimerDesign Ltd. in accordance with the minimum information for publication of quantitative real‐time PCR experiments (MIQE) guidelines (Bustin et al., 2009) and had efficiencies of 90–100%, making them suitable for comparison according to Pfaffl (2001).
Table 1.
KCNQ1–5 primer assays
| Gene | Primer sequence (+) sense, (−) antisense | GenBank accession number | Region spanned |
|---|---|---|---|
| KCNQ1 | (+) 5'‐CCATCTTTGTTCATCCCCATCT‐3' | NM_032073 | 1797–1896 |
| (−) 5'‐CCAGTTGTGTCACCTTGTCTT‐3' | |||
| KCNQ2 | (+) 5'‐GGTGTCTCATTCTTCGCTCTT‐3' | NM_133322 | 1023–1122 |
| (−) 5'‐TCCGCCGTTTCTCAAAGTG‐3' | |||
| KCNQ3 | (+) 5'‐ATACACATTTATCTGCTCTTCCTTTTA‐3' | NM_031597 | 3299–3420 |
| (−) 5'‐TGCTCTCAGTTTATCCGAATCAA‐3' | |||
| KCNQ4 | (+) 5'‐GCTCATCTTCGCCTCTTTCC‐3' | XM_233477 | 861–972 |
| (−) 5'‐GCCAATGGTCGTCAGTGTAAT‐3' | |||
| KCNQ5 | (+) 5'‐CCTGGCGTACACGAGAGTAT‐3' | XM_001071249 | 2383–2462 |
| (−) 5'‐TTTGACTGGGCGAACTGAAC‐3' |
Immunohistochemistry
Approximately 2 mm segments of the penis were flash‐frozen in chilled isopentane, embedded in Tissue‐Tek optimal cutting temperature compound (Sakura Finetek, Zoeterwoude, the Netherlands), and frozen at −80°C. Sections (10 μm) were prepared and mounted onto poly‐l‐lysine‐coated slides prior to fixing in 4% paraformaldehyde for 20 min. Sections were washed in PBS‐TritonX (0.025%; PBS‐T) before being blocked for 1 h at room temperature in PBS‐T containing 0.1% BSA. Primary antibodies were applied to the sections for 18 h at 4°C. The following primary antibodies were used: Kv7.4 (1:200; 75–082, NeuroMab, Davis, CA, USA), Kv7.5 (1:200; ab19319, Abcam, Cambridge, UK) and smooth muscle actin antibodies (both 1:500; ab32575 and ab7817, Abcam, Cambridge, UK). Following washes in PBS‐T, secondary antibodies (Alexa‐Fluor 488 and 555, both at 1:200; Thermo Fisher Scientific, Waltham, MA, USA) were applied for 1 h at room temperature. Prolong Gold (Life Technologies, Nærum, Denmark) was applied before mounting the sections. Sections were visualized using a Confocal LSM 780 with zen software (Zeiss, Oberkochen, Germany).
Myography
Segments (2 mm) of intracavernous artery was dissected and mounted on two 40 μm stainless steel wires in a wire myograph (model 610; DMT, Aarhus, Denmark). The preparations were allowed to equilibrate in a Krebs solution of the following composition (in mM): 133 NaCl, 4.6 KCl, 2.5 CaCl2, 16.3 NaHCO3, 1.75 NaH2PO4, 0.6 MgSO4 and 10 glucose, equilibrated with 95% O2/5% CO2 to maintain pH at 7.4 at 37°C. In a relaxed vessel, the internal circumference, L100, was calculated corresponding to a transmural pressure of 100 mmHg. Subsequently, the internal circumference of the vessels was set to L1, where L1 = 0.9 × L100.
The corpus cavernosum was dissected, and segments (5 mm) were mounted with sutures in a tissue organ bath system (model 610; DMT Aarhus, Denmark) containing Krebs solution. During an equilibration period of 60 min, tension was adjusted until a mean stable tension of 1.2 mN was obtained, as previously described (Hedlund et al., 1999; Matsumoto et al., 2005).
Experimental procedure
To test contractility of the rat erectile tissue preparations, cumulative concentration–response curves for phenylephrine (10 nM–100 μM) were created. For cumulative concentration–response curves for the Kv7.2–7.5 activators, ML213 (0.1–3 μM) and BMS204352 (0.1–3 μM) (Jepps et al., 2014), the NO donor, SNP (0.1 nM–100 μM), and the PDE5 inhibitor, sildenafil (0.1 nM–10 μM), arteries and corpus cavernosum strips were contracted with phenylephrine corresponding to approximately 80% of maximum contraction. For those experiments investigating the involvement of Kv7.1–7.5 channels in tone regulation, the Kv7.1–7.5 channel inhibitor, linopirdine (10 μM) (Jepps et al., 2014), was added 15 min prior to contraction with phenylephrine. For those experiments investigating the involvement of NO and endothelium‐derived hyperpolarization in Kv7 channel‐evoked relaxations, the NOS inhibitor, L‐NAME (100 μM) (Graves et al., 2000), or the IKCa and small‐conductance calcium‐activated K+ channel (SKCa) channel blockers, TRAM‐34 (1 μM) (Krøigaard et al., 2012) and apamin (0.3 μM) (Sonkusare et al., 2012), were added 15 min prior to contraction with phenylephrine. For confirmation of a functional endothelium, only those arteries and strips that showed dilations to 1 μM of carbachol greater than 50% and 20%, respectively, were included (Sadeghipour et al., 2007; Prieto et al., 2010).
Data analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). PowerLab4/25‐Chart7 acquisition systems (ADInstruments Ltd., Oxford, UK) were used for data recording. The mechanical responses of the vessels were measured as force and expressed as active wall tension, ΔT, which is the increase in measured force, ΔF, divided by twice the segment length. The magnitude of relaxant responses is given as percentage of the contraction level just prior to the addition of the drug.
Results are expressed as mean ± SEM, and n denotes the number of preparations. Data were analysed using GraphPad prism6 software (GraphPad, La Jolla, CA, USA). For those experimental series where all experiments reached a relaxation greater than 50%, individual concentration–response curves were fitted to a non‐linear regression curve, and EC50 and Emax values were calculated. Differences between EC50 and Emax values were then analysed using either Student's unpaired t‐test or a one‐way ANOVA with a Bonferroni post hoc test (Curtis et al., 2015). For those experimental series where all experiments did not reach a relaxation greater than 50%, differences in concentration–response relationships between treatments were analysed using a two‐way ANOVA with a Bonferroni post hoc test. A Bonferroni post hoc test was only applied if P < 0.05, and there was no significant variance in homogeneity (Curtis et al., 2015). Differences in qPCR data were analysed using Student's unpaired t‐test. Differences at the P < 0.05 level were considered significant.
Materials
The following drugs were used: phenylephrine, SNP, sildenafil and TRAM‐34 (Sigma‐Aldrich, Copenhagen, Denmark) and ML213, BMS204352 and apamin (Tocris, Bristol, UK). ML213, BMS204352, sildenafil and TRAM‐34 were dissolved in DMSO in a stock concentration of 10 mM, before further dilutions in distilled water. The maximal DMSO concentration applied in vitro did not modulate smooth muscle tone in control experiments. All other drugs were dissolved in distilled water.
Results
Relative expression of KCNQ isoforms in penile arteries and corpus cavernosum
In penile arteries and corpus cavernosum, expression of KCNQ5 predominated, but KCNQ4 and KCNQ3 were also detected. KCNQ1 was only found in corpus cavernosum, and KCNQ2 was not detected in either the penile arteries or the corpus cavernosum (Figure 1A). To determine if the mRNA expression of the KCNQ isoforms translated to protein expression, we performed immunohistochemistry on transverse slices of whole rat penis. Antibodies specific for Kv7.4 and Kv7.5 resulted in strong immunofluorescence signals in the penile artery, penile vein and around the vascular channels (cavernosum blood spaces) of the corpus cavernosum. Kv7.4 staining was also particularly high in the corpus spongiosum surrounding the urethra, whereas Kv7.5 staining was more localized to the urethra than to the corpus spongiosum (Figure 1B).
Figure 1.
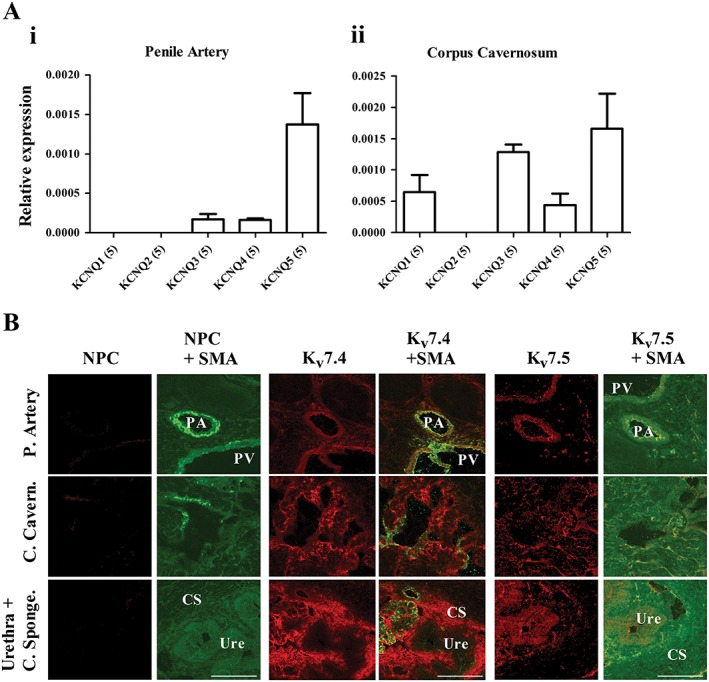
(A) qPCR analysis of relative abundance of KCNQ genes in rat penile artery (i) and corpus cavernosum (ii) normalized to the mean of two reference genes. The relative abundance of each gene was calculated using the 2−ΔCq method. Data are mean ± SEM, and n is indicated in parentheses after each experimental group. (B) Representative fluorescence images from transverse sections (10 μm) of the penis, using primary antibodies against smooth muscle actin, Kv7.4, and Kv7.5 taken at ×10 magnification. Presence of protein was identified by the specific red staining in the case of the Kv7 antibodies and specific green staining for α‐smooth muscle actin (SMA) above respective controls. For the Kv7 antibodies, no primary controls (NPC) were performed. Scale bar represents 200 μm. CS, corpus spongiosum; PA, penile artery; PV, penile vein; Ure, Urethra.
Kv7 channel activation relaxes penile arteries and corpus cavernosum
The functional role of Kv7 channels in the penile arteries and corpus cavernosum was investigated using different pharmacological tools. Application of the Kv7.2–7.5 activators, ML213 (0.1–3 μM) and BMS204352 (0.1–3 μM), relaxed precontracted penile artery segments (Figure 2A and 2C) and corpus cavernosum strips (Figure 2B and 2D) contracted with phenylepinephrine. ML213 and BMS204352 were more potent in the penile arteries compared with corpus cavernosum strips, with maximum relaxations of 87 ± 2% and 82 ± 5% versus 39 ± 4% and 35 ± 5%, n = 5, respectively. In the presence of the Kv7 channel inhibitor, linopirdine (lino, 10 μM), relaxations to ML213 and BMS204352 were attenuated in both the penile arteries and corpus cavernosum strips (Figure 2). Furthermore, to investigate the involvement of NO and endothelium‐derived hyperpolarization in Kv7 channel‐evoked relaxations, ML213‐ and BMS204352‐induced relaxations were performed in the presence of the NOS inhibitor, L‐NAME (100 μM), or the IKCa and SKCa channel blockers, TRAM‐34 (1 μM) and apamin (0.3 μM). Neither L‐NAME nor TRAM‐34/apamin changed Kv7 channel‐evoked relaxations (Figure 3).
Figure 2.
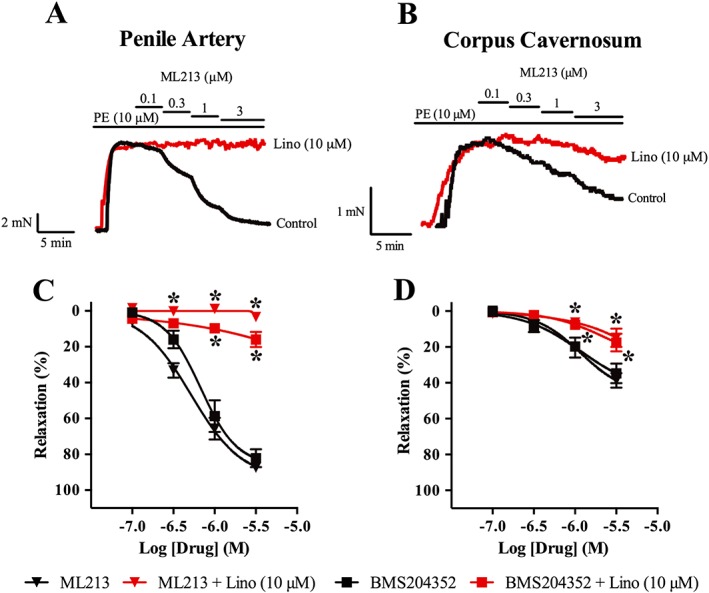
ML213 and BMS204352, activators of Kv7.2–7.5 channels, induce relaxation of both penile arteries and corpus cavernosum. (A) Representative trace of concentration‐dependent relaxation in response to ML213 (0.1–3 μM) in phenylephrine‐contracted penile. (B) Representative trace of concentration‐dependent relaxation in response to ML213 (0.1–3 μM) in phenylephrine‐contracted corpus cavernosum strips. (C,D) Summary of results showing relaxations to ML213 and BMS204352 in both penile arteries (C) and corpus cavernosum strips (D) sensitive to linopirdine (lino, 10 μM) in 3‐month‐old Wistar rats. Data are mean ± SEM. n = 5 for each experimental group. Two‐way ANOVA with Bonferroni's post hoc test. *P < 0.05 from control.
Figure 3.
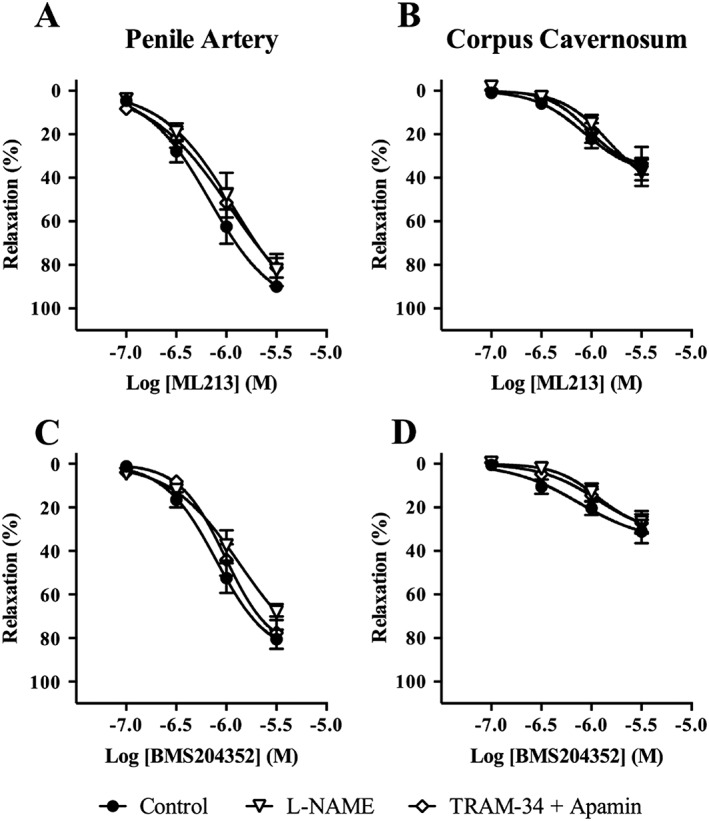
Relaxations induced by the Kv7 activators, ML213 and BMS204352, do not involve NOS activity or endothelium‐derived hyperpolarizations through IKCa/SKCa channels in penile arteries and corpus cavernosum. Concentration‐dependent relaxations for ML213 in penile arteries (A) and corpus cavernosum strips (B), and for BMS204352 in penile arteries (C) and corpus cavernosum strips (D) from 3‐month‐old Wistar rats in the absence and presence of the NOS inhibitor, L‐NAME (100 μM), or the IKCa and SKCa channel blockers, TRAM‐34 (1 μM) and apamin (0.3 μM). Data are mean ± SEM. n = 5. Two‐way ANOVA with Bonferroni's post hoc test. *P < 0.05 from control.
Inhibition of Kv7 channels reduces relaxations to NO and PDE5 inhibition
We investigated whether Kv7 channel activation was involved in NO‐mediated relaxation in penile arteries and corpus cavernosum. Both SNP (0.1 nM–100 μM) and sildenafil (0.1 nM–10 μM) induced concentration‐dependent relaxations in penile arteries and corpus cavernosum strips contracted with phenylepinephrine (Figure 4). In the presence of the Kv7 channel inhibitor, linopirdine (lino, 10 μM), SNP‐ and sildenafil‐induced relaxations were attenuated in both penile arteries and corpus cavernosum strips (Figure 4).
Figure 4.
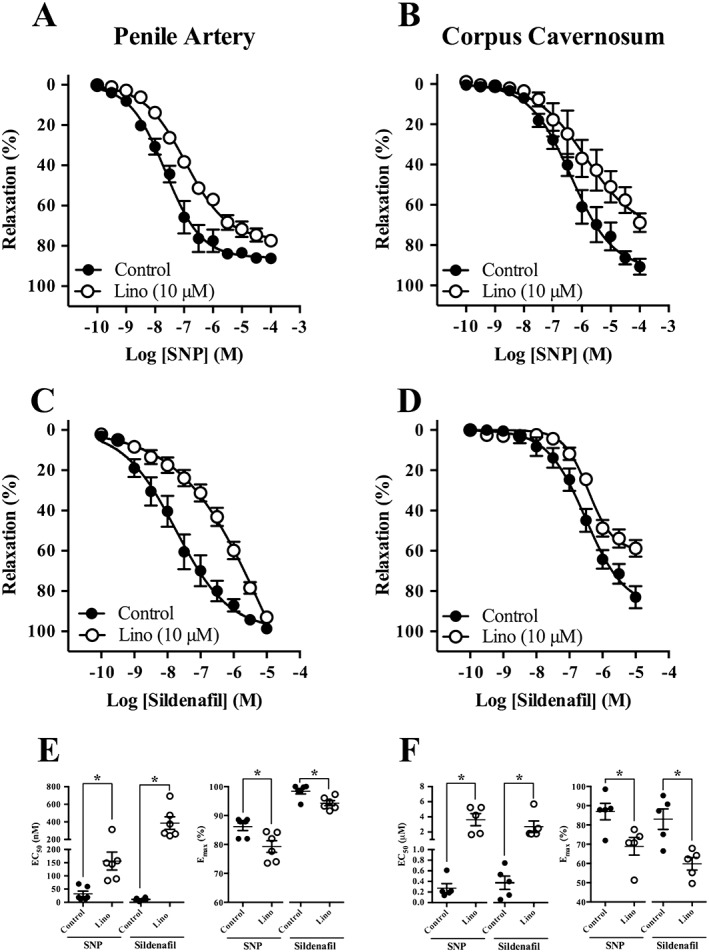
Kv7 channel inhibition reduces relaxations to the NO donor, SNP, and the PDE5 inhibitor, sildenafil, in penile arteries and corpus cavernosum. Concentration‐dependent relaxations for SNP (A) and sildenafil (C) in the absence and presence of linopirdine (lino, 10 μM) in penile arteries from 3 month‐old Wistar rats. Concentration‐dependent relaxations for SNP (B) and sildenafil (D) in the absence and presence of linopirdine (lino, 10 μM) in corpus cavernosum strips from 3‐month‐old Wistar rats. EC50 and Emax values for SNP‐induced and sildenafil‐induced relaxations are represented for penile arteries in (E) and for corpus cavernosum strips in (F). Data are mean ± SEM. n = 6 and n = 5 for each experimental group of penile arteries and corpus cavernosum respectively. Student's unpaired t‐test. *P < 0.05 from control.
Kv7 channel expression and function in SHHF rat penile arteries and corpus cavernosum
In SHHF rats, we investigated the ability of SNP (0.1 nM–100 μM) and sildenafil (0.1 nM–10 μM) to relax segments of penile artery and corpus cavernosum strips. In both tissues, SNP‐ and sildenafil‐induced relaxations were reduced in SHHF rats compared with normal rats. Moreover, the relaxations to SNP and sildenafil in penile arteries and corpus cavernosum from SHHF rats were not impaired by pre‐incubation with 10 μM linopirdine (Figure 5). There was also an attenuation in the penile artery and corpus cavernosum of the SHHF rats to relaxations mediated by ML213 and BMS204352 (Figure 6).
Figure 5.
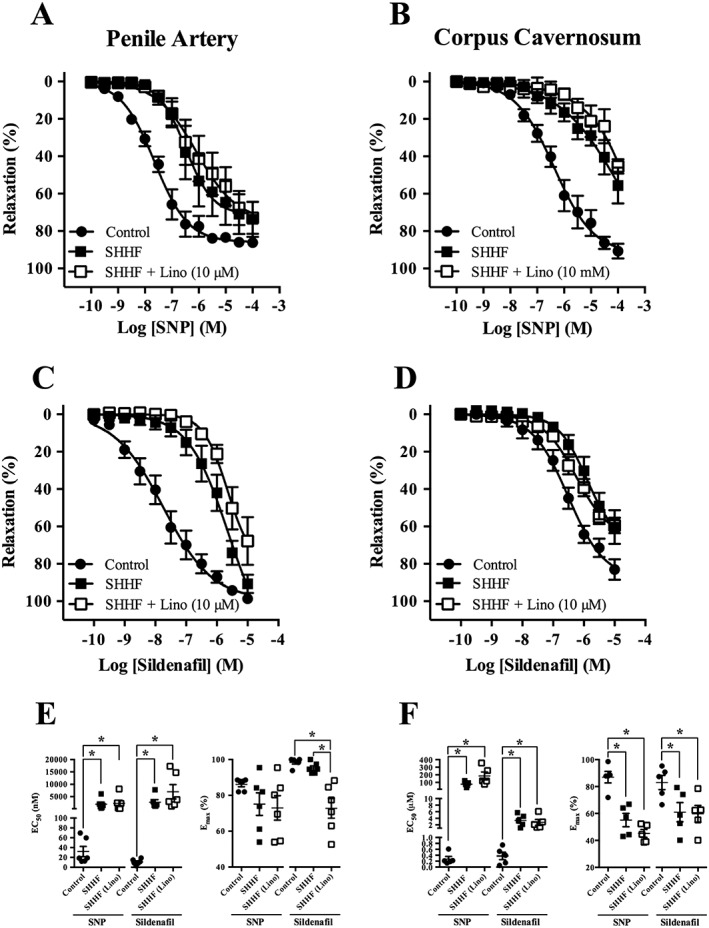
Relaxations to the NO donor, SNP, and the PDE5 inhibitor, sildenafil, as well as the effect of Kv7 channel inhibition are reduced in penile arteries and corpus cavernosum. Concentration‐dependent relaxations of SNP (A) and sildenafil (C) in penile arteries from 3‐month‐old Wistar and SHHF rats in the absence and presence of linopirdine (lino, 10 μM). Concentration‐dependent relaxations of SNP (B) and sildenafil (D) in corpus cavernosum strips from 3‐month‐old Wistar and SHHF rats in the absence and presence of linopirdine (lino, 10 μM). EC50 and Emax values for SNP‐induced and sildenafil‐induced relaxations are represented for penile arteries in (E) and for corpus cavernosum strips in (F). Data are mean ± SEM. n = 6 and n = 5 for each experimental group of penile arteries and corpus cavernosum respectively. One‐way ANOVA with Bonferroni's post hoc test. *P < 0.05.
Figure 6.
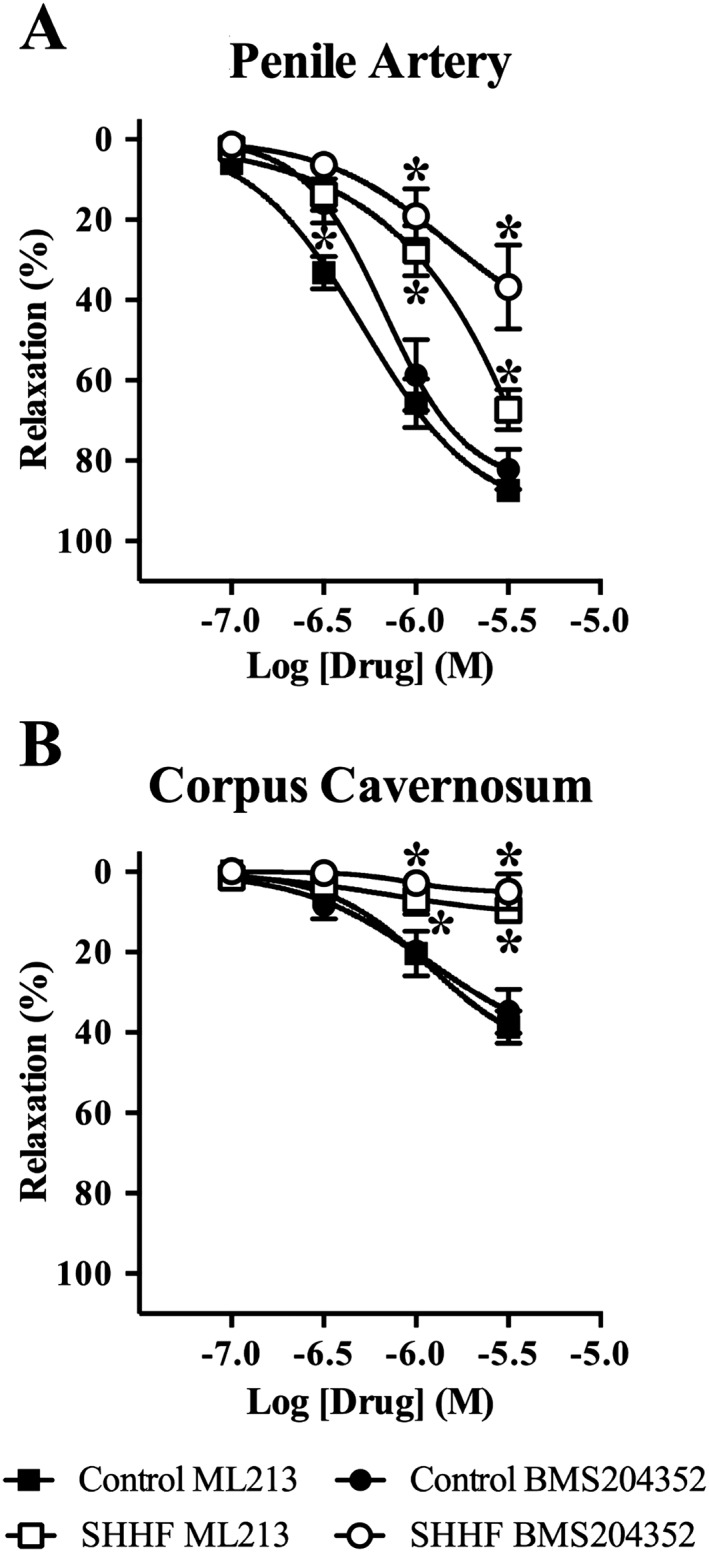
Relaxations to ML213 and BMS204352, activators of Kv7 channels, are reduced in penile arteries (A) and absent in corpus cavernosum strips (B) from 3‐month‐old SHHF rats. Data are mean ± SEM. n = 5 for each experimental group. Two‐way ANOVA with Bonferroni's post hoc test. *P < 0.05 from control.
To determine if the functional impairment of Kv7 channels correlated with a down‐regulation of KCNQ transcripts in SHHF rats, we performed qPCR analysis of SHHF penile arteries and corpus cavernosum. No change in any KCNQ isoform was observed at a transcript level in the penile arteries from SHHF rats (Figure 7A), whereas analysis of the SHHF corpus cavernosum revealed a down‐regulation of KCNQ4 and KCNQ5 and up‐regulation of KCNQ1 transcripts compared with the normal Wistar rat tissue (Figure 7B).
Figure 7.
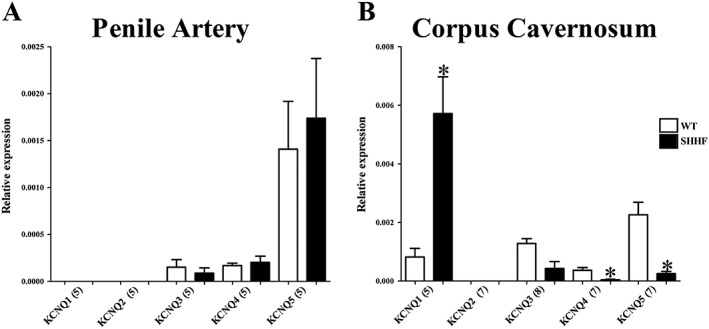
qPCR analysis comparing the relative abundance of KCNQ genes in penile arteries (A) and corpus cavernosum (B) of Wistar (WT) and SHHF rats. The relative abundance of each gene was calculated using the 2−ΔCq method. Data represent the mean ± SEM, and n is indicated in parentheses after each experimental group. Student's unpaired t‐test. *P < 0.05 from Wistar (WT).
Discussion
In this study, we have made the following novel findings: (1) KCNQ isoforms are expressed in the penile artery and corpus cavernosum. (2) Kv7.4 and Kv7.5 are readily detected in vascular smooth muscle layers of penile arteries and corpus cavernosum. (3) Kv7 channels have a functional role in the regulation of smooth muscle tone in penile arteries and corpus cavernosum. (4) Finally, SHHF rats, a model for metabolic syndrome, show a striking down‐regulation and functional impairment of these channels.
Previous studies identified different KCNQ isoforms in various vascular and non‐vascular smooth muscles (Stott et al., 2014). In the vasculature, KCNQ expression profiles have been characterized in a wide range of arteries including pulmonary, mesenteric, cerebral and renal arteries, where a Kv7.4/Kv7.5 heteromer has been postulated to be the predominant channel subtype (Joshi et al., 2009; Chadha et al., 2014; Jepps et al., 2014; Brueggemann et al., 2014a). The present study represents the first extensive characterization of KCNQ expression in penile arteries and corpus cavernosum, where KCNQ3–5 were identified in penile arteries and KCNQ1,3–5 were identified in corpus cavernosum. Moreover, the Kv7.4 and Kv7.5 proteins were identified in both the penile arteries and corpus cavernosum, as well as being identified in the corpus spongiosum and urethra, suggesting that the translated proteins form functional channels that can regulate penile artery and corpus cavernosum smooth muscle tone. A functional role for Kv7 channels in the regulation of penile artery and corpus cavernosum smooth muscle tone was determined using various pharmacological tools to either block or enhance the activity of Kv7 channels. In line with findings from other arteries, where Kv7 channel activity has been found to be a major determinant of smooth muscle tone (Jepps et al., 2014, 2011; Brueggemann et al., 2014a), we found that the penile artery as well as the corpus cavernosum was sensitive to ML213 and BMS204352, two structurally different Kv7.2–7.5 channel activators. The relaxations elicited by Kv7 channel enhancement were independent of NOS or endothelium‐derived hyperpolarization. A previous study has proposed that KV channels are involved in the regulation of basal tone of rat penile arteries by using the non‐specific Kv channel blocker, 4‐aminopyridine, although a role for Kv1 and Kv11 channels was excluded by using the subtype specific blockers α‐dendrotoxin and E‐4031 respectively (Kun et al., 2003). Similarly, in human corpus cavernosum strips, Kv channel activation has been implicated in testosterone‐induced relaxations, although the molecular species was undefined (Yildiz et al., 2009). Our present data suggest that the unidentified Kv channels are likely to be Kv7 channels.
We further investigated the physiological role of the Kv7 channels in mediating the NO–cGMP‐dependent relaxations in both penile arteries and corpus cavernosum. Blockade of the Kv7 channels by linopirdine inhibited the relaxations mediated by a NO donor (SNP) and a PDE5 inhibitor (sildenafil). These data suggest that Kv7 channels contribute to NO–cGMP‐dependent vasorelaxations in these tissues. These observations follow a recent study showing that Kv7 channels were involved in mediating cGMP‐dependent relaxations in the rat vasculature and that the role of Kv7 channels in cGMP‐mediated relaxations was different between arteries, with responses to SNP, atrial and C‐type natriuretic peptide being linopirdine‐sensitive in the aorta, but in the renal artery, only relaxations to the former were sensitive to linopirdine (Stott et al., 2015a).
It is important to note that although this study clearly demonstrates a key role for Kv7 channels in the penile smooth muscles, it does not negate the role of other K+ channels, such as the IKCa or BKCa channel, which have been implicated in cGMP‐mediated responses and penile erectile function (Werner et al., 2008, 2005; Kun et al., 2009; González‐Corrochano et al., 2013; Király et al., 2013). Future studies will elaborate on the relative importance and specific contributions of the different K+ channels to NO–cGMP‐dependent and NO–cGMP‐independent relaxations and overall penile function.
It has recently been shown that men with severe ED were more likely to develop ischaemic heart disease, heart failure, peripheral vascular disease and other types of cardiovascular disease compared with men without ED (Banks et al., 2013), thereby making ED a prognostic indicator of cardiovascular disease (Ioakeimidis and Kostis, 2014). Additionally, ED is closely associated with metabolic syndrome, with the prevalence of ED being approximately double in patients with metabolic syndrome compared with those without (Kaya et al., 2015). Because some of these patient groups are either unresponsive to (Rendell et al., 1999; Sáenz de Tejada et al., 2002; Goldstein et al., 2003) or contraindicated for treatment with PDE5 inhibitors because of other cardiovascular complications (Reffelmann and Kloner, 2005), alternative pharmacological strategies are required to improve treatment of ED. Therefore, we investigated the expression and function of Kv7 channels in penile arteries and corpus cavernosum of the SHHF rat model that mimics the pathophysiology of human metabolic syndrome. This model has been characterized as having severe obesity associated with dyslipidaemia, hypertension and impaired renal function by 3 months of age (the age used in this study); cardiac complications and heart failure then develop from 6 months of age (Youcef et al., 2014). We found that the SHHF rats, at 3 months of age, had attenuated responses to NO and PDE5 inhibition in penile arteries and corpus cavernosum, which was associated with a reduced effectiveness of linopirdine to inhibit SNP‐ and sildenafil‐mediated relaxations. We also found an attenuated response of the Kv7.2–7.5 activators in both the penile arteries and corpus cavernosum and a down‐regulation of KCNQ3, KCNQ4 and KCNQ5 isoforms in corpus cavernosum. Interestingly, KCNQ1 expression was markedly increased in the SHHF rat corpus cavernosum. However, the data presented in this study suggest that the up‐regulation of KCNQ1 is not adequate to compensate for the impaired cGMP‐dependent relaxations in the corpus cavernosum, which are more likely to be mediated through Kv7.4 and Kv7.5 channels. Future studies will determine the significance of KCNQ1 up‐regulation, which might prove to be an interesting therapeutic target for ED. In the penile artery, no change in the expression of any KCNQ isoform was observed. However, the results from the functional experiments suggest that Kv7.4 and Kv7.5 channel function is attenuated downstream of transcription, which is consistent with a number of studies, showing decreased Kv7.4 protein expression, but not transcription in several arteries from hypertensive animals (Jepps et al., 2011; Chadha et al., 2012; Khanamiri et al., 2013). The reasons behind these post‐transcriptional modifications are unknown, but there is strong evidence that diseases associated with vascular dysfunction are accompanied by down‐regulation of vascular Kv7.4 channels. Nevertheless, these data provide evidence that ED is associated with decreased Kv7 channel function.
Whether Kv7 channels offer a new therapeutic target for the treatment of ED is yet to be determined. There are, however, several new therapeutic targets that have recently been proposed for the treatment of ED. They include targets associated with vasorelaxation induced by the NO–cGMP pathway, such as increasing cGMP production through release of carbon monoxide (CO) (Decaluwé et al., 2012, 2011) or activation of Ca2 +‐activated K+ channels (Werner et al., 2005; Werner et al., 2008; Kun et al., 2009; González‐Corrochano et al., 2013; Király et al., 2013). They also include targets independent of NO–cGMP, such as release of hydrogen sulfide (Srilatha et al., 2007), inhibiting the RhoA/Rho‐kinase (Decaluwé et al., 2011), and the angiotensin II signalling pathway (Jin, 2009), increasing prostaglandin E1 (Bratus et al., 2007), and blocking endothelin receptors (Ritchie and Sullivan, 2011). Alternatively, anti‐inflammatory and anti‐fibrotic therapies as well as regenerative medicine are being developed (Decaluwé et al., 2014).
In conclusion, the present study provides novel evidence for an important role for Kv7 channels in penile physiology, which is compromised in rats with metabolic syndrome, before the onset of cardiac complications. These data suggest that Kv7 channels have a pivotal role in penile erection and might be a novel therapeutic target for treatment of ED.
Author contributions
T.D. performed experiments, analysed data and drafted the manuscript. T.J. performed experiments, analysed data and contributed to manuscript writing. S.P.O. provided project supervision and research funding environment. I.A.G. contributed to manuscript writing, project supervision and funding provision.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organizations engaged with supporting research.
Acknowledgements
We would like to thank Jeppe E. Kirchhoff and associate professor Thomas Jespersen at the Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, for the provision of SHHF tissue. We would like to thank the Core Facility for Integrated Microscopy at the University of Copenhagen for their support. T.A.J. received funding from the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme (FP7/2007–2013) under REA grant agreement n° 608765 and a grant from the Lundbeck Foundation. T.D. received funding from the Novo Nordisk Foundation awarded to I.A.G. (107118). I.A.G. also received support from the Medical Research Council (MR/K019074/1) and British Heart Foundation (PG/12/63/29824).
Jepps, T. A. , Olesen, S. P. , Greenwood, I. A. , and Dalsgaard, T. (2016) Molecular and functional characterization of Kv7 channels in penile arteries and corpus cavernosum of healthy and metabolic syndrome rats. British Journal of Pharmacology, 173: 1478–1490. doi: 10.1111/bph.13444.
References
- Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE, et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson KE (2011). Mechanisms of penile erection and basis for pharmacological treatment of erectile dysfunction. Pharmacol Rev 63: 811–859. [DOI] [PubMed] [Google Scholar]
- Aversa A, Bruzziches R, Francomano D, Natali M, Gareri P, Spera G (2009). Endothelial dysfunction and erectile dysfunction in the aging man. Int J Urol 17: 38–47. [DOI] [PubMed] [Google Scholar]
- Banks E, Joshy G, Abhayaratna WP, Kritharides L, Macdonald PS, Korda RJ, et al. (2013). Erectile dysfunction severity as a risk marker for cardiovascular disease hospitalisation and all‐cause mortality: a prospective cohort study. PLoS Med 10: e1001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratus D, Hlebic G, Hajdinjak T (2007). Relation between intracavernosal dose of prostaglandin Pge 1 and mean duration of erection in men with different underlying causes of erectile dysfunction. Croat Med J 48: 76–80. [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Haick JM, Cribbs LL, Byron KL (2014a). Differential activation of vascular smooth muscle Kv7.4, Kv7.5, and Kv7.4/7.5 channels by ML213 and ICA‐069673. Mol Pharmacol 86: 330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Mackie AR, Cribbs LL, Freda J, Tripathi A, Majetschak M, et al. (2014b). Differential protein kinase C‐dependent modulation of Kv7.4 and Kv7.5 subunits of vascular Kv7 channels. J Biol Chem 289: 2099–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. (2009). The MIQE guidelines: minimum information for publication of quantitative real‐time PCR experiments. Clin Chem 55: 611–622. [DOI] [PubMed] [Google Scholar]
- Chadha PS, Jepps TA, Carr G, Stott JB, Zhu HL, Cole WC, et al. (2014). Contribution of kv7.4/kv7.5 heteromers to intrinsic and calcitonin gene‐related peptide‐induced cerebral reactivity. Arterioscler Thromb Vasc Biol 34: 887–893. [DOI] [PubMed] [Google Scholar]
- Chadha PS, Zunke F, Zhu HL, Davis AJ, Jepps TA, Olesen SP, et al. (2012). Reduced KCNQ4‐encoded voltage‐dependent potassium channel activity underlies impaired β‐adrenoceptor‐mediated relaxation of renal arteries in hypertension. Hypertension 59: 877–884. [DOI] [PubMed] [Google Scholar]
- Chung E, Brock GB (2011). Emerging and novel therapeutic approaches in the treatment of male erectile dysfunction. Curr Urol Rep 12: 432–443. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPA, Giembycz MA, et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaluwé K, Pauwels B, Boydens C, Van de Voorde J (2012). Divergent molecular mechanisms underlay CO‐ and CORM‐2‐induced relaxation of corpora cavernosa. J Sex Med 9: 2284–2292. [DOI] [PubMed] [Google Scholar]
- Decaluwé K, Pauwels B, Boydens C, Van de Voorde J (2014). Treatment of erectile dysfunction: new targets and strategies from recent research. Pharmacol Biochem Behav 121: 146–157. [DOI] [PubMed] [Google Scholar]
- Decaluwé K, Pauwels B, Verpoest S, Van de Voorde J (2011). New therapeutic targets for the treatment of erectile dysfunction. J Sex Med 8: 3271–3290. [DOI] [PubMed] [Google Scholar]
- Goldstein I, Lue TF, Padma‐Nathan H, Rosen RC, Steers WD, Wicker PA (1998). Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. New Engl J Med 338: 1397–1404. [DOI] [PubMed] [Google Scholar]
- Goldstein I, Young JM, Fischer J, Bangerter K, Segerson T, Taylor T, et al. (2003). Vardenafil, a new phosphodiesterase type 5 inhibitor, in the treatment of erectile dysfunction in men with diabetes: a multicenter double‐blind placebo‐controlled fixed‐dose study. Diabetes Care 26: 777–783. [DOI] [PubMed] [Google Scholar]
- González‐Corrochano R, La Fuente JM, Cuevas P, Fernández A, Chen MX, de Is T, et al. (2013). Ca2+‐activated K+ channel (KCa) stimulation improves relaxant capacity of PDE5 inhibitors in human penile arteries and recovers the reduced efficacy of PDE5 inhibition in diabetic erectile dysfunction. Br J Pharmacol 169: 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzke C, Angulo J, Chitaley K, Dai Y‐T, Kim NN, Paick J‐S, et al. (2010). Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med 7: 445–475. [DOI] [PubMed] [Google Scholar]
- Graves JE, Greenwood IA, Large WA (2000). Tonic regulation of vascular tone by nitric oxide and chloride ions in rat isolated small coronary arteries. Am J Physiol Heart Circ Physiol 279: H2604–H2611. [DOI] [PubMed] [Google Scholar]
- Hedlund P, Alm P, Andersson K‐E (1999). NO synthase in cholinergic nerves and NO‐induced relaxation in the rat isolated corpus cavernosum. Br J Pharmacol 127: 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioakeimidis N, Kostis JB (2014). Pharmacologic therapy for erectile dysfunction and its interaction with the cardiovascular system. J Cardiovasc Pharmacol Ther 19: 53–64. [DOI] [PubMed] [Google Scholar]
- Jepps TA, Bentzen BH, Stott JB, Povstyan OV, Sivaloganathan K, Dalby‐Brown W, et al. (2014). Vasorelaxant effects of novel Kv 7.4 channel enhancers ML213 and NS15370. Br J Pharmacol 171: 4413–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepps TA, Chadha PS, Davis AJ, Harhun MI, Cockerill GW, Olesen SP, et al. (2011). Downregulation of Kv7.4 channel activity in primary and secondary hypertension. Circulation 124: 602–611. [DOI] [PubMed] [Google Scholar]
- Jin LM (2009). Angiotensin II signaling and its implication in erectile dysfunction. J Sex Med 6: 302–310. [DOI] [PubMed] [Google Scholar]
- Joshi S, Sedivy V, Hodyc D, Herget J, Gurney AM (2009). KCNQ modulators reveal a key role for KCNQ potassium channels in regulating the tone of rat pulmonary artery smooth muscle. J Pharmacol Exp Ther 329: 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya E, Sikka SC, Gur S (2015). A comprehensive review of metabolic syndrome affecting erectile dysfunction. J Sex Med 12: 856–875. [DOI] [PubMed] [Google Scholar]
- Khanamiri S, Soltysinska E, Jepps TA, Bentzen BH, Chadha PS, Schmitt N, et al. (2013). Contribution of Kv7 channels to basal coronary flow and active response to ischemia. Hypertension 62: 1090–1097. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). NC3Rs Reporting Guidelines Working Group. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Király I, Pataricza J, Bajory Z, Simonsen U, Varro A, Papp JG, et al. (2013). Involvement of large‐conductance Ca(2+)‐activated K(+) channels in both nitric oxide and endothelium‐derived hyperpolarization‐type relaxation in human penile small arteries. Basic Clin Pharmacol Toxicol 113: 19–24. [DOI] [PubMed] [Google Scholar]
- Krøigaard C, Dalsgaard T, Nielsen G, Laursen BE, Pilegaard H, Köhler R, et al. (2012). Activation of endothelial and epithelial K(Ca) 2.3 calcium‐activated potassium channels by NS309 relaxes human small pulmonary arteries and bronchioles. Br J Pharmacol 167: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kun A, Martinez AC, Tankó LB, Pataricza J, Papp JG, Simonsen U (2003). Ca2+‐activated K+ channels in the endothelial cell layer involved in modulation of neurogenic contractions in rat penile arteries. Eur J Pharmacol 474: 103–115. [DOI] [PubMed] [Google Scholar]
- Kun A, Matchkov VV, Stankevicius E, Nardi A, Hughes AD, Kirkeby HJ, et al. (2009). NS11021, a novel opener of large‐conductance Ca2+‐activated K+ channels, enhances erectile responses in rats. Br J Pharmacol 158: 1465–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Andersen I, Aleke J, Golubinskaya V, Gustafsson H, Nilsson H (2013). Reduced anti‐contractile effect of perivascular adipose tissue on mesenteric small arteries from spontaneously hypertensive rats: role of Kv7 channels. Eur J Pharmacol 698: 310–315. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real‐time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Yoshida M, Andersson K‐E, Hedlund P (2005). Effects in vitro and in vivo by apomorphine in the rat corpus cavernosum. Br J Pharmacol 146: 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musicki B, Ross AE, Champion HC, Burnett AL, Bivalacqua TJ (2009). Posttranslational modification of constitutive nitric oxide synthase in the penis. J Androl 30: 352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padma‐Nathan H, McMurray JG, Pullman WE, Whitaker JS, Saoud JB, Ferguson KM, et al. (2001). On‐demand IC351 (Cialis) enhances erectile function in patients with erectile dysfunction. Int J Impot Res 13: 2–9. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. (2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucleic Acids Res 42: D1098-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW (2001). A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porst H, Rosen R, Padma‐Nathan H, Goldstein I, Giuliano F, Ulbrich E, et al. (2001). The efficacy and tolerability of vardenafil, a new, oral, selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction: the first at‐home clinical trial. Int J Impot Res 13: 192–199. [DOI] [PubMed] [Google Scholar]
- Prieto D, Kaminski PM, Bagi Z, Ahmad M, Wolin MS (2010). Hypoxic relaxation of penile arteries: involvement of endothelial nitric oxide and modulation by reactive oxygen species. Am J Physiol Heart Circ Physiol 299: H915–H924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reffelmann T, Kloner RA (2005). Pharmacotherapy of erectile dysfunction: focus on cardiovascular safety. Expert Opin Drug Saf 4: 531–540. [DOI] [PubMed] [Google Scholar]
- Rendell MS, Rajfer J, Wicker PA, Smith MD (1999). Sildenafil for treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. Sildenafil Diabetes Study Group. JAMA 281: 421–426. [DOI] [PubMed] [Google Scholar]
- Ritchie R, Sullivan M (2011). Endothelins & erectile dysfunction. Pharmacol Res 63: 496–501. [DOI] [PubMed] [Google Scholar]
- Sadeghipour H, Ghasemi M, Ebrahimi F, Dehpour AR (2007). Effect of lithium on endothelium‐dependent and neurogenic relaxation of rat corpus cavernosum: role of nitric oxide pathway. Nitric Oxide 16: 54–63. [DOI] [PubMed] [Google Scholar]
- Sáenz de Tejada I, Anglin G, Knight JR, Emmick JT (2002). Effects of tadalafil on erectile dysfunction in men with diabetes. Diabetes Care 25: 2159–2164. [DOI] [PubMed] [Google Scholar]
- Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, et al. (2012). Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srilatha B, Adaikan PG, Li L, Moore PK (2007). Hydrogen sulphide: a novel endogenous gasotransmitter facilitates erectile function. J Sex Med 4: 1304–1311. [DOI] [PubMed] [Google Scholar]
- Stott JB, Barrese V, Jepps TA, Leighton EV, Greenwood IA (2015a). Contribution of Kv7 channels to natriuretic peptide mediated vasodilation in normal and hypertensive rats. Hypertension 65: 676–682. [DOI] [PubMed] [Google Scholar]
- Stott JB, Jepps TA, Greenwood IA (2014). K(V)7 potassium channels: a new therapeutic target in smooth muscle disorders. Drug Discov Today 19: 413–424. [DOI] [PubMed] [Google Scholar]
- Stott JB, Povstyan OV, Carr G, Barrese V, Greenwood IA (2015b). G‐protein βγ subunits are positive regulators of Kv7.4 and native vascular Kv7 channel activity. Proc Natl Acad Sci U S A 112: 6497–6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. (2002). Accurate normalization of real‐time quantitative RT‐PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ME, Meredith AL, Aldrich RW, Nelson MT (2008). Hypercontractility and impaired sildenafil relaxations in the BKCa channel deletion model of erectile dysfunction. Am J Physiol Regul Integr Comp Physiol 295: R181–R188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ME, Zvara P, Meredith AL, Aldrich RW, Nelson MT (2005). Erectile dysfunction in mice lacking the large‐conductance calcium‐activated potassium (BK) channel. J Physiol 567: 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz O, Seyrek M, Irkilata HC, Yildirim I, Tahmaz L, Dayanc M (2009). Testosterone might cause relaxation of human corpus cavernosum by potassium channel opening action. Urology 74: 229–232. [DOI] [PubMed] [Google Scholar]
- Youcef G, Olivier A, L'Huillier CPJ, Labat C, Fay R, Tabcheh L, et al. (2014). Simultaneous characterization of metabolic, cardiac, vascular and renal phenotypes of lean and obese SHHF rats. PLoS One 9: e96452. [DOI] [PMC free article] [PubMed] [Google Scholar]


