Abstract
The unique outer ear of crocodylians consists of a large meatal chamber (MC) concealed by a pair of muscular earlids that shape a large part of the animal's head. This chamber is limited medially by the enlarged tympanic membrane. Yet, the anatomy of this distinctive and complex region is underexplored and its evolutionary history untraced. The osteology and soft tissues of the MC in extant crocodylians was analysed to describe it and establish osteological correlates within this region. A broad survey of the osteological correlates was conducted in major clades of fossil crocodyliforms to estimate evolutionary trends of the MC. The reorganization of the MC at the origin of crocodyliforms includes characters also present in more basal taxa such as ‘sphenosuchians’ as well as unique traits of crocodyliforms. Three major patterns are recognized in the MC of basal mesoeucrocodylians. The distinct ‘thalattosuchian pattern’ indicates that extensive modifications occurred in this clade of aquatic fossil crocodyliforms, even when multiple alternative phylogenetic positions are taken into account. Some traits already established in putative closely related clades are absent or modified in this group. The ‘basal notosuchian/sebecian pattern’ is widespread among basal metasuchians, and establishes for the first time characters maintained later in neosuchians and extant forms. The ‘advanced notosuchian pattern’ includes modifications of the MC possibly related to a terrestrial lifestyle and potentially a structure analogous to the mammalian pinna. The main variation in the MC of neosuchians is associated with the homoplastic secondary opening of the cranioquadrate passage. The inferred phylogenetic trends in the crocodyliform MC suggest the great anatomical disparity in this region followed a complex evolutionary pattern, and tympanic hearing played an important role in the origin and diversification of Crocodyliformes.
Keywords: Crocodyliformes, outer‐ear, tympanic membrane
Introduction
Impedance‐matching middle ears have evolved multiple times in tetrapods and are correlated with major radiations (Lombard & Bolt, 1979; Bolt & Lombard, 1985; Clack, 1997, 2002a,b; Müller & Tsuji, 2007; Christensen‐Dalsgaard & Carr, 2008). This type of hearing is achieved by a tympanic membrane (TM) transmitting airborne sound waves to the inner ear via a single or multiple small middle ear bones suspended within an air‐filled middle ear chamber (Wever, 1978; Müller & Tsuji, 2007; Nummela & Thewissen, 2008; Mason & Farr, 2013; Dufeau & Witmer, 2015). The basic mechanism remains superficially the same in all tetrapods, although there is considerable anatomical variation in the structural details of the components involved in tympanic hearing (Bolt & Lombard, 1985; Christensen‐Dalsgaard, 2005; Christensen‐Dalsgaard & Carr, 2008; Mason & Farr, 2013; Bierman et al. 2014). Such variation has been proposed to be related to differences in functional requirements and evolutionary contingencies (Clack, 1997; Christensen‐Dalsgaard & Carr, 2008; Christensen‐Dalsgaard et al. 2011, 2012). The impedance‐matching ears of Archosauromorpha may be convergent with that of other independent origins in frog, mammal, turtle and lepidosaur antecedents (Christensen‐Dalsgaard & Carr, 2008). Each extant lineage of Archosauria, crocodiles and birds went different ways evolving inner, middle and outer ear structures. This study focuses on the evolution of the crocodylian outer ear.
Within the pseudosuchian lineage of Archosauria, anatomical changes in the outer ear are mostly derived in Crocodyliformes, the clade that includes extant crocodiles. This clade has extensive modifications to their outer ear that are unique among vertebrates (Thewissen & Nummela, 2008; Vergne et al. 2009; Bierman et al. 2014). In spite of their divergent ear evolution, the basic anatomy of the crocodylian hearing apparatus and its ecological and evolutionary implications have been oddly neglected (Thewissen & Nummela, 2008; Vergne et al. 2009; Bierman et al. 2014).
The crocodylian ear is divided into an outer, middle and inner region as in all other amniotes with impedance‐matching middle ears (Shute & Bellairs, 1955; Wever, 1978; Saunders et al. 2000; Vergne et al. 2009; Dufeau & Witmer, 2015). In crocodylians, the outer ear consists of a large meatal chamber (MC). The MC is concealed superficially by the muscular upper and lower earlids that occupy most of the posterior portion of crocodylian head behind the eyes (Wever, 1978; Saunders et al. 2000). The TM is located within the medial surface of the MC (Wever, 1978; Saunders et al. 2000). From the inside surface of the TM, the crocodylian middle ear expands internally into a series of pneumatic diverticula forming the enlarged paratympanic sinus system characteristic of the group (Owen, 1850; Thewissen & Nummela, 2008; Witmer et al. 2008; Tahara & Larsson, 2011; Bierman et al. 2014; Dufeau & Witmer, 2015).
Perhaps not by coincidence, the other extant lineage of archosaurs, birds, also have a complex expansion of their middle ears throughout a great portion of their heads (Hogg, 1990; Witmer, 1990, 1997, 1999; Baumel & Witmer, 1993; Tahara & Larsson, 2011). Their paratympanic sinuses are so elaborated that large portions extend external to the head skeleton and are either pressed on the bone surface or entirely bounded by soft tissue. This degree of extraskeletal expansion makes interpreting the fossil record of bird antecedents more difficult, although advances have been made in interpreting many interskeletal paratympanic sinuses (Witmer, 1990, 1997, 1999; Kundrat & Janacek, 2007; Sampson & Witmer, 2007; Witmer & Ridgely, 2008, 2009;. Tahara & Larsson, 2011; Gold et al. 2013).
Crocodylians, on the other hand, have nearly all of their paratympanic sinuses bounded within their skulls. Crocodylians also come from a long lineage of archosaurs called pseudosuchians that are represented by a rich fossil record spanning the entire Mesozoic and Cenozoic eras. The bony encasement of their paratympanic sinuses and extensive fossil record provide an outstanding potential to gain insights into the evolution of the hearing apparatus of its long lineage as may offer evolutionary patterns to test for in other archosaurs with highly developed paratympanic sinuses – theropod dinosaurs and pterosaurs.
All extant crocodylians share a similar biology and rely on acoustic communication for a range of vital behaviours (Thewissen & Nummela, 2008; Vergne et al. 2009; Walsh et al. 2009). Their acoustic repertoire approaches that of birds and may be facilitated by their elaborate paratympanic sinuses. Bierman et al. (2014) presented evidence that the paratympanic sinuses of crocodyliforms function as a pressure difference receiver. The authors argue that this device is the primary factor responsible for the sound detection and amplification with virtually no participation of the outer ear in this process. However, the fossil record of Crocodyliformes (Fig. 1) is much more diverse, and reveals broad morphological and ecological disparity encompassing fully marine metriorhynchids to fully terrestrial notosuchians (Gasparini et al. 2006; O'Connor et al. 2010; Young et al. 2010; Montefeltro et al. 2011; Stubbs et al. 2013; Tolijagic’ & Butler, 2013). Herein, this study investigates and provides evidence for osteological correlates of structures within the MC in extant and fossil crocodyliforms to discuss the evolutionary history and ecological implications of such features in the outer portion of the hearing apparatus. The evolution of these structures has direct implications on the evolution of crocodylian hearing.
Figure 1.
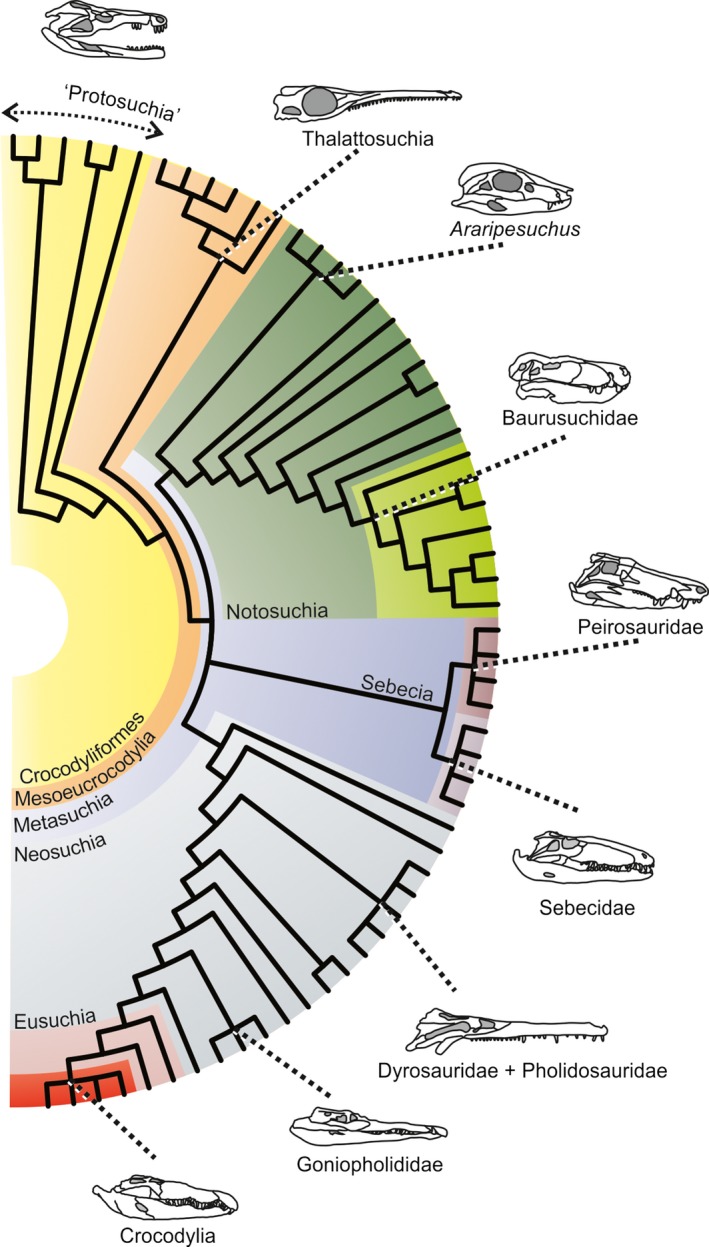
Phylogenetic framework of Crocodyliformes with major clades discussed in the text.
Institutional abbreviations
AMNH, American Museum of Natural History, New York, USA; BPI, Bernard Price Institute, Johannesburg, South Africa; DG‐CTG‐UFPE, Centro de Tecnologia e Geociências da UFPE, Recife, Brazil; HLMD, Hessisches Landesmuseum, Darmstadt, Germany; IGM, Mongolian Institute of Geology, Ulaan Bataar, Mongolia; IRScNBr, Institut Royal des Sciences naturelles de Belgique, Brussels, Belgium; LACM, Los Angeles County Museum of Natural History, Los Angeles, USA; LPRP/USP, Laboratório de Paleontologia de Ribeirão Preto‐USP, Ribeirão Preto, Brazil; MLP, Museo de La Plata, La Plata, Argentina; MN, Museu Nacional, Rio de Janeiro, Brazil; MNN, Musée National du Niger, Niamey, Niger; MOZ, Museo Professor J. Olsacher, Zapala, Argentina; MPMA, Museu de Paleontologia ‘Prof. Antonio Celso de Arruda Campos’, Monte Alto, Brazil; MUCP, Museo de Geología y Paleontología, Universidad Nacional del Comahue, Neuquén, Argentina; NHMUK, Natural History Museum, London, UK; PVL, Instituto Miguel Lillo, Tucumán, Argentina; ROM, Royal Ontario Museum, Toronto, Canada; SAM, Iziko, South African Museum, Cape Town, South Africa; UFRJ, Coleção de Paleontologia de Vertebrados da Universidade Federal do Rio de Janeiro no Rio de Janeiro, Rio de Janeiro, Brazil.
Anatomical abbreviations
ADQ, anterodorsal process of the quadrate; BOA, bony otic aperture; DOI, dorsal otic incisure; EAM, external auditory meatus; IOC, incisure of the otic aperture of the cranioquadrate passage; MC, meatal chamber; OB, otic buttress; OI, otic incisure; POF; periotic fossa; TM, tympanic membrane.
Materials and methods
Crocodyliformes anatomy
A two‐step approach was taken to investigate the MC in Crocodyliformes. Osteological analysis and dissections of extant forms were performed to establish the relationships among the soft tissue structures of the MC and osteological correlates. These correlates were then investigated in fossil forms to reconstruct their evolutionary history.
The osteology of 10 extant taxa covering all three major lineages of crown‐group Crocodylia was analysed (sensu Brochu et al. 2009): Alligatoridae, Crocodylidae and Gavialidae (see Supporting Information for the full list of taxa and institutional abbreviations). Five specimens of captive‐raised young adult Caiman latirostris (Daudin, 1802) were dissected to reconstruct the soft tissue anatomy of the MC. These specimens were acquired from Criatório Caiman (Porto Feliz, São Paulo, Brazil), and had body lengths longer than 1200 mm and skull lengths (tip of the snout to the posterior edge of the skull table) ranging from 130 mm long to 165 mm long. The MC of one Caiman yacare (Daudin, 1802) and one Alligator mississippiensis (Daudin, 1802) was also examined. All the dissected Caiman latirostris specimens are housed in Departamento de Biologia e Zootecnia, Faculdade de Engenharia, Unesp, Ilha Solteira, Brazil. The Caiman yacare is deposited in Laboratório de Paleontologia de Ribeirão Preto – USP with a skull length of 185 mm; and the Alligator mississippiensis is deposited in Redpath Museum (Montréal, Canada) and has a skull length of 97 mm.
All the Caiman latirostris specimens and the Caiman yacare were obtained frozen and were fixed in 10% neutral‐buffered formalin, whereas the Alligator mississippiensis specimens were obtained unfrozen and fixed with the same solution parameters. The specimens were dissected by hand using scalpels and dissecting scissors. The entire process was documented by digital photography. To access the medial portions of the MC and the TM, the upper earlid was removed, as well as the associated musculature (m. depressor auricular superior and m. levator auricular superior; Shute & Bellairs, 1955; Wever, 1978). An electric drill was used to remove sections of squamosal and postorbital bones at the skull roof.
Forty tree fossil taxa (see SEM for full list of taxa and institutional abbreviations) were examined first‐hand to supplement published resources (acknowledged accordingly). Taxa were sampled based on their phylogenetic position to cover major points of interest along the evolutionary history of the group and the diversity of MC anatomy (see SEM for the full list of taxa and its taxonomic affinities).
Crocodyliformes phylogenetic framework
Crocodyliform phylogeny is a topic of recurrent debate in vertebrate palaeontology (Pol & Larsson, 2011; Bronzati et al. 2012, 2015). A general pattern is recognized and was used here as a phylogenetic framework (Fig. 1). The crown‐group Crocodylia is encompassed by successively more inclusive clades Eusuchia, Neosuchia, Mesoeucrocodylia and Crocodyliformes.
In spite of the general pattern, there are major points of conflict related to the initial radiation of Mesoeucrocodylia that should be addressed to clarify the phylogenetic framework; in particular, the alternative positions of Thalattosuchia either as a group of basal Mesoeucrocodylia, the sister group of other semi‐aquatic longirostrine groups dyrosaurids and pholidosaurids (Tethysuchia; sensu Andrade et al. 2011) within Neosuchia, or even as a sister‐clade to Crocodyliformes (Benton & Clark, 1988; Clark, 1994; Jouve, 2009; Pol & Gasparini, 2009; Pol & Larsson, 2011; Wilberg, 2015a); the position of Araripesuchus (Price, 1959) as a basal Notosuchia or sister group of Peirosauridae (Turner, 2006; Pol et al. 2009, 2014; Turner & Sertich, 2010; Soto et al. 2011; Montefeltro et al. 2013; Sertich & O'Connor, 2014); and the placement of Sebecidae as the sister group of Baurusuchidae forming Sebecosuchia or closer to Peirosauridae forming Sebecia (Turner & Calvo, 2005; Larsson & Sues, 2007; Sereno & Larsson, 2009; Montefeltro et al. 2011, 2013; Pol & Powell, 2011; Pol et al. 2012, 2014). Here, the basal position of Thalattosuchia as the sister group of all other mesoeucrocodylians (Metasuchia; sensu Benton & Clark, 1988), Araripesuchus as a basal notosuchian, and Sebecidae closer to Peirosauridae forming Sebecia is assumed (Fig. 1). This phylogenetic framework is used as the primary template for the analyses. However, the phylogenetic position of Thalattosuchia is arguably among the most disputed controversy in Crocodyliformes studies. This labile position has great impact on the large‐scale interpretation of crocodyliform palaeodiversity, palaeoecology, biogeography and evolution. Because of that, alternative scenarios for the MC evolution are also addressed in the discussion in which Thalattosuchia is considered as the sister group of Crocodyliformes (Benton & Clark, 1988; Jouve, 2009; Wilberg, 2015a,b) and a member of the Neosuchia sister to Tethysuchia (Pol & Gasparini, 2009; Young et al. 2010, 2011; Pol & Larsson, 2011).
Results
Extant forms
Osteology
The MC in crocodyliforms is mostly contained by bones of the temporal region and skull roof (Fig. 2). Dorsally, the chamber is overhung by lateral shelves of the postorbital and squamosal expanding from the skull roof. A continuous longitudinal sulcus is present at the ventrolateral edge of both bones. The sulcus is developed greatest at the midpoint and posterior portion of its extension, and becomes weaker anteriorly at the level of the postorbital bar (Fig. 2B,C). The dorsoventral expansion of the sulcus is variable among species, tending to be wider in larger specimens of horned crocodylids, such as Crocodylus rhombifer (Cuvier, 1807) and Crocodylus siamensis (Schneider, 1801), and it flares anteriorly in Tomistoma schlegelii (Müller, 1838; Brochu, 2006, 2007; Brochu et al. 2010).
Figure 2.
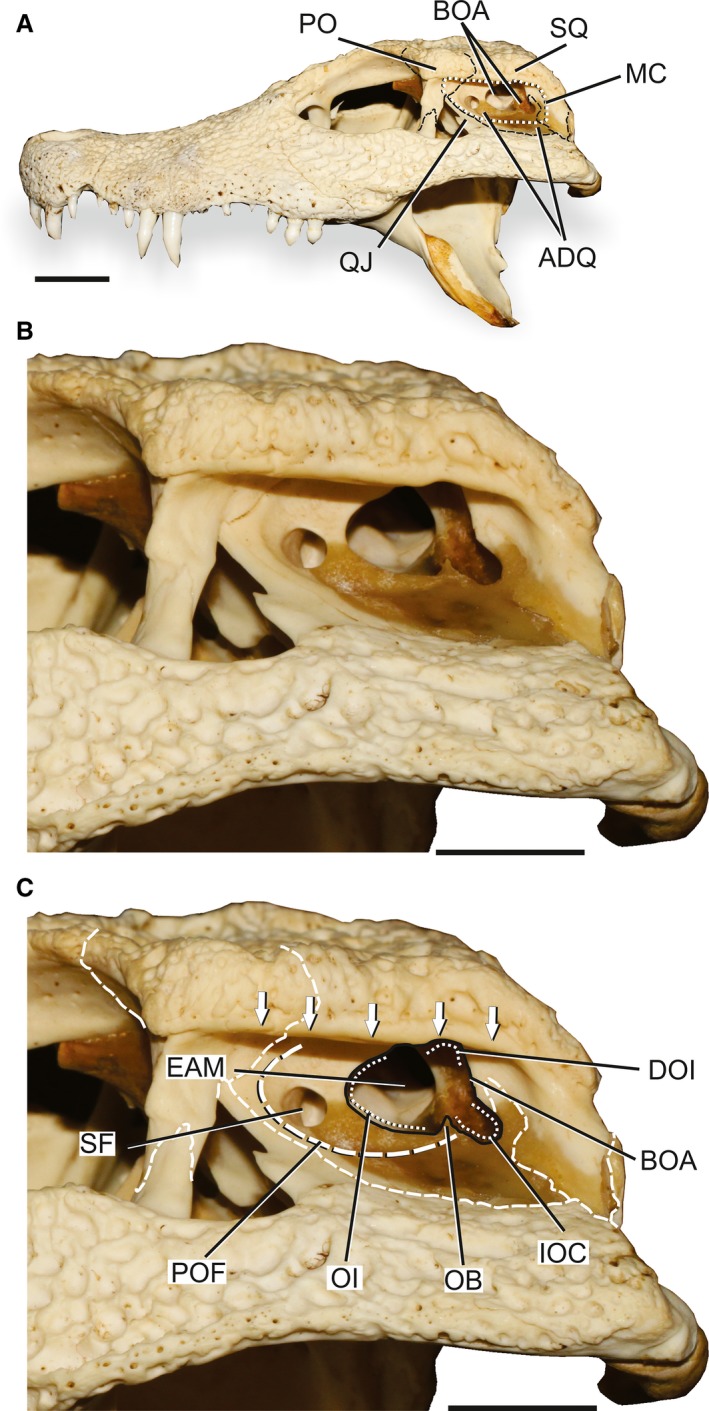
Skull of Osteolaemus tetraspis (ROM R6533) illustrating the osteology of the MC of extant crocodyliforms. (A) Overview of the skull in lateral left view. (B) Detail of the posterior portion of the skull. (C) Detail of the posterior portion of the skull, with selected MC structures highlighted. Arrows indicate the extension of the sulcus for the attachment of the upper earlid. ADQ, anterodorsal process of the quadrate; BOA, bony otic aperture; DOI, dorsal otic incisure; EAM, external auditory meatus; IOC, incisure of the otic aperture of the cranioquadrate passage; MC, meatal chamber; OB, otic buttress; OI, otic incisure; PO, postorbital bone; QJ, quadratojugal; POF; periotic fossa; SF, subtympanic foramen; SQ, squamosal bone. Scale bars: 2 cm.
Anteriorly, the chamber is bounded by the descending process of the postorbital and the anterodorsal process of the quadratojugal, both contributing to the dorsal edge of the infratemporal fenestra. Posteriorly, the MC is limited by the deflected posteroventral prong of the squamosal contacting the posterodorsal surface of the quadrate. This contact encloses the cranioquadrate passage for most of its length, before exiting posteriorly between the quadrate, squamosal and otoccipital (Iordansky, 1973). Anteriorly, the cranioquadrate passage opens medially to the quadrate‐squamosal contact to enter the extreme medial portion of the MC, internal to the TM.
Medially, the MC is limited by the wide lateral surface of the anterodorsal process of the quadrate (ADQ). The ADQ surface has a distinct semicircular depression, the periotic fossa (POF; Fig. 2C; sensu Pol et al. 2014). This fossa occupies most of the anterior portion of the lateral surface of the MC, but fades out posterodorsally and posteroventrally becoming indistinct from the remaining area of the ADQ.
Two apertures are identified in the MC. The anterior‐most apertures have been called the peculiar foramen (sensu Iordansky, 1973) or subtympanic foramen (sensu Dufeau, 2011; Dufeau & Witmer, 2015) and reside just medial to the anteroventral margin of the TM. Because neither term has been well defined and the pneumatic structures associated with this foramen not well described, the term subtympanic foramen is used here because its reference to topology will aid in the description and discussion. The subtympanic foramen is located anteriorly in the MC within the area of the POF and is a superficial extension of the complex quadrate diverticulum. This structure is observed in all extant taxa analysed except Gavialis gangeticus (Gmelin, 1789). In the analysed specimens of the taxon, the aperture is either completely closed over or never developed. In addition, among the dissected Caiman latirostris, there is variation in the number of subtympanic foramina from none to two in each POF. The size of the subtympanic foramina ranges from a reduced pin‐hole aperture, observed in the dissected Caiman latirostris, to more developed ovate apertures, as in Osteolaemus tetraspis (Cope, 1861) and hatchling Alligator mississippiensis.
The bony otic aperture (BOA) is formed largely by the ADQ, but its dorsal and posterodorsal edges are formed by the squamosal. This aperture has a complex outline, and its variation in Crocodyliformes is best described by dividing the aperture into three main portions (Fig. 2C). The greatest portion of the BOA is formed by the external auditory meatus (EAM) that is limited anteriorly by the semilunar otic incisure of the quadrate (OI; Kley et al. 2010). The EAM is limited posteriorly by a dorsomedial projection of the quadrate, herein named the otic buttress (OB), at the level of the posteroventral limit of the POF (Fig. 2C). The dorsal extension of the OB is variable among the studied extant taxa. It ranges from a spur in C. niloticus (Laurenti, 1768); Crocodylus siamensis and Gavialis gangeticus to a more developed projection in Caiman latirostris. The OB limits anterolaterally the incisure of the otic aperture of the cranioquadrate passage (IOC; Iordansky, 1973). The IOC marks the ventrolateral corner of the BOA at the suture between quadrate and squamosal (Fig. 2B,C; Brochu, 1999). The IOC also varies from a slightly marked incisure in Gavialis gangeticus to a deep notch in Osteolaemus tetraspis and Paleosuchus palpebrosus (Cuvier, 1807). The posterior margin of the IOC is formed by the squamosal in Alligatoridae, and by the quadrate and squamosal in Crocodylidae and Gavialis gangeticus (Brochu, 1999). Dorsally, the posterior wall of the IOC is confluent to the posterior wall of the dorsal otic incisure (DOI) bordered by the squamosal (Fig. 2B,C). The DOI represents the dorsal apex of the BOA and is associated with the aperture of tempororbital vessels (Sedlmayr, 2002).
Soft tissue anatomy
Two asymmetrical earlids laterally limit the MC in life and shape the slit‐like fleshy otic aperture of the outer ear in crocodylians (Fig. 3). This aperture is the airborne path for sound to the MC and TM when the crocodylian is not submersed (Higgs et al. 2002; Bierman et al. 2014). The dorsal earlid is attached to the lateral edge of the head and extends ventrally perpendicularly to the dorsal surface of the skull roof (Fig. 3A). In a similar fashion, the smaller ventral earlid extends dorsally on the lateral surface of the head. The dorsal earlid is strengthened internally by a dense connective tissue, the auricular plate (Shute & Bellairs, 1955), which is more developed at the posterior portion of the earlid and is connected to the squamosal and postorbital periosteum by the hinge ligaments (Wever, 1978).
Figure 3.
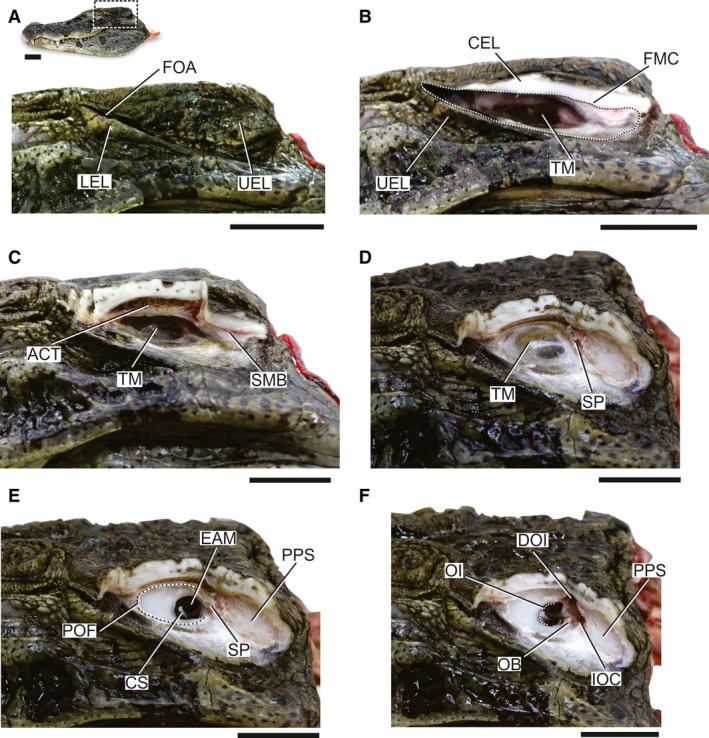
Progressive gross dissection of the MC of a Caiman latirostris illustrating the soft tissue anatomy of this region. (A) Overview of the left lateral view of the head and posterior portion of the head magnified. (B) Left lateral view of the MC after upper earlid removed. (C) Left lateral view of the MC after sections of squamosal and postorbital removed. (D) Left dorsolateral view of the MC after the musculature of the upper earlid removed. (E) Left dorsolateral view of the MC after TM removed. (F) Left dorsolateral view of the MC after suspensory plate removed. ACT, auricular cavernous tissue; CEL, cross‐section of upper earlid; CS, columellar shaft; DOI, dorsal otic incisure; EAM, external auditory meatus; FMC, fleshy meatal chamber; FOA, fleshy otic aperture; IOC, incisure of the otic aperture of the cranioquadrate passage; LEL, lower earlid; OB, otic buttress; OI, otic incisure; POF; periotic fossa; PPS, posteroventral prong of the squamosal; SMB, sectioned muscle bellies of upper earlid; SP, suspensory plate; TM, tympanic membrane; UEL, upper earlid. Scale bars: 2 cm.
The large roughly oval TM is oriented parasagittally and occupies most of the floor of the MC in the three species analysed. The MC extends a short distance anterior to the TM and forms a shallow concavity coincident with the greatest widening of the fleshy otic aperture. Posteriorly, the TM almost reaches the posterior border of the MC at the level of the posteroventral prong of the squamosal (Fig. 3B). The TM ends anteromedially to the bellies of m. depressor auricular superior, m. levator auricular superior and the lining muscle responsible for movements of the upper earlids (Shute & Bellairs, 1955; Wever, 1978; Fig. 3C). The floor of the MC not enclosed by the TM is covered by a light coloured and scale‐less skin. In all Caiman latirostris analysed, the entire dorsolateral surface of the MC under the squamosal and postorbital is covered by a thick auricular cavernous tissue (Sedlmayr, 2002; Fig. 3C). However, invasion of the upper earlid as in Alligator mississippiensis could not be verified (Sedlmayr, 2002).
The transition of the TM and the surrounding tissues is abrupt, and the borders of the membrane are delimited from the remaining tissues (Fig. 3D). The anterior, dorsal and ventral limits of the TM are in association with the ADQ and attached along the edges of the POF. Posteriorly, the TM is firmly attached to a mass of dense connective tissue, the suspensory plate (Wever, 1978; Fig. 3E). The plate is attached ventrally to the OB and dorsomedially to the anterior edge of the DOI. The IOC and DOI are accessible only after the removal of the suspensory plate and the auricular artery (Fig. 3F). In the dissected Caiman latirostris, the TM only covers the EAM whereas the other two incisures are isolated from this aperture by the suspensory plate (Fig. 3E,F).
Osteological correlates for the MC
Osteological correlates for the soft tissue structures in the MC are based on the osteology of extant crocodyliforms and the dissected Caiman latirostris. Table 1 presents a summary of the osteological correlates for each of the soft tissues analysed. In general, the osteological portion of the MC in the crown‐group reliably estimates its soft tissue counterparts. The large upper earlid is associated with the longitudinal sulcus on the ventrolateral edge of the squamosal and postorbital, as previously proposed (Nash, 1975; Hecht & Tarsitano, 1983; Gow, 2000; Clark & Sues, 2002). The greater development of the posterior portion of the sulcus is associated to the attachment of the auricular plate by the hinge ligaments on these bones (Wever, 1978). In addition, the less marked anterior region of the sulcus approximately corresponds to the fleshy otic aperture in Caiman latirostris. The ventral extension of the upper earlid cannot be inferred by osteological correlates, but its vertical extension from the lateral edge of the skull provides an adequate inference of the lateral limits of the MC (Fig. 3A). The lateral limits of the MC are delimited by a plane extending ventrally from the sulcus for the upper earlid reaching the lateral edge of the ADQ and quadratojugal. The dissections did not reveal any osteological correlates for the lower earlid as in Shute & Bellairs (1955) and Wever (1978).
Table 1.
Soft tissues in the MC in extant crocodyliforms and respective osteological correlates
| Soft tissue | Osteological correlate |
|---|---|
| Upper earlid | Longitudinal sulcus at the ventrolateral edge of postorbital and squamosal |
| TM | POF |
| OB | |
| DOI | |
| Suspensory plate | OB |
| DOI |
DOI, dorsal otic incisure; OB, otic buttress; POF, periotic fossa; TM, tympanic membrane.
The POF was identified as an osteological correlate for the anterior, dorsal and ventral attachments of the TM on the lateral surface of the ADQ. The posterior portion of the TM was not associated with any osteology because of its attachment to the suspensory plate. The OB and the DOI demarcate the attachment of the dorsal and ventral edges of the suspensory plate, respectively. By extension, these bony structures reliably estimate the posterior extension of the TM and are used here as a proxy for the posterior extension of this membrane.
The extension of the TM in the crown‐group is not coincident with the extension of the BOA. The TM extends onto the anterior position of the ADQ beyond the OI also covering the subtympanic foramen, when it is present; whereas the posterior portion of the BOA is not covered by the TM. The two posterior incisures of the BOA (the IOC and DOI) are isolated from the EAM by the suspensory plate and are occupied by structures not directly related to hearing. These include the cranioquadrate passage, transmitting the temporal artery and the seventh cranial nerve.
Anatomy of the extinct forms
Basal Crocodyliformes
Four basal crocodyliforms were examined first‐hand, sampling portions of a grade leading to Mesoeucrocodylia generally called ‘Protosuchia’ (Figs 1 and 4). Protosuchus haughtoni (Busbey & Gow, 1984) and Hemiprotosuchus leali (Bonaparte, 1971) are members of Protosuchidae (sensu Clark, 1986), which is the basal‐most crocodyliform clade (Pol et al. 2012, 2013; Montefeltro et al. 2013). Orthosuchus strombergi (Nash, 1968) has been considered a member of Protosuchidae (Pol et al. 2009; Turner & Sertich, 2010; Montefeltro et al. 2013; Sertich & O'Connor, 2014) or more derived than protosuchids and sister to a group of the derived ‘protosuchians’ and mesoeucrocodylians (Pol et al. 2012, 2013, 2014). Fruitachampsa callisoni (Clark, 2011) is placed in Shartegosuchidae, which is the next most related clade to Mesoeucrocodylia (Benton & Clark, 1988; Pol et al. 2009, 2012, 2014; Clark, 2011; Montefeltro et al. 2013).
Figure 4.
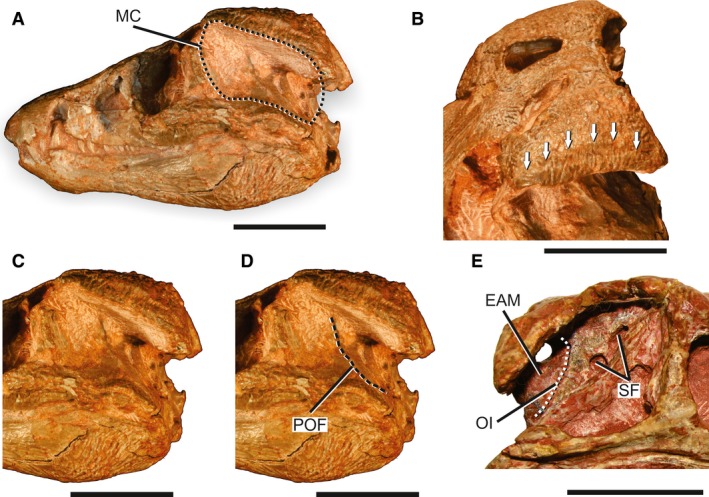
Skull of ‘protosuchians’ to illustrate the MC in basal crocodyliforms. (A) Overview of the skull of Protosuchus haughtoni (BP/1/4770) in left lateral view to illustrate the general morphology of the MC in basal crocodyliforms. (B) Left dorsolateral view of the posterior portion of the skull of Protosuchus haughtoni (BP/1/4770) showing the sulcus on the outer surface of the squamosal and postorbital for the attachment of the upper earlid. (C) Detail of the posterior portion of the skull and MC of Protosuchus haughtoni (BP/1/4770) in left lateral view. (D) Detail of the posterior portion of the skull and MC of Protosuchus haughtoni (BP/1/4770) in left lateral view showing the POF. (E) Detail of the posterior portion of the skull and MC of Hemiprotosuchus leali (PVL 3829) in right lateral view, with selected structures of the MC highlighted. Arrows indicate the extension of the sulcus for the attachment of the upper earlid. EAM, external auditory meatus; MC, meatal chamber; OI, otic incisure; POF; periotic fossa; SF, subtympanic foramina. Scale bars: 2 cm.
All ‘protosuchians’ examined present a well‐defined sulcus at the lateroventral edge of the skull roof for the attachment of the upper earlid (Fig. 4A,B). However, the sulcus extends dorsomedially, forming a broad, smooth, ventrolaterally curved shelf. This shelf is broadest at its midlength and tapers anteriorly and posteriorly (Fig. 4B). The presence of this sulcus indicates that the MC in protosuchians was covered laterally by a broad upper earlid. Considering the plane perpendicular to the skull roof at the lateroventral edge of the squamosal and postorbital, Protosuchus haughtoni, Hemiprotosuchus leali and Fruitachampsa callisoni have a vertically expanded MC. In contrast, Orthosuchus strombergi has a more dorsoventrally compressed skull and a shallower MC (Nash, 1975). The MC in these taxa is formed primarily by the ADQ. The chamber in Protosuchus haughtoni and Hemiprotosuchus leali expands anteriorly into a wide and concave anterodorsal surface of the quadratojugal (Fig. 4A,C–E). This wide surface of the quadratojugal limits greatly the size of the infratemporal fenestrae in these taxa. This configuration of an anteriorly expanded MC is present in many other ‘protosuchians’, such as the gobiosuchids Gobiosuchus kielanae (Osmólska, 1972) and Zaraasuchus shepardi (Pol & Norell, 2004a), and the more derived ‘protosuchians’ Shantungosuchus hangjinensis (Wu et al. 1994) and Hsisosuchus chowi (Peng & Shu, 2005).
Protosuchus haughtoni, Hemiprotosuchus leali and Orthosuchus strombergi lack the squamosal contact with the posterodorsal surface of the quadrate (Busbey & Gow, 1984; Pol et al. 2013). In these taxa the MC is not posteriorly bounded by skeletal elements, which affects the anatomy of the BOA, but was probably delimited by soft tissues. The posterior‐most region of the MC is not preserved in Fruitachampsa callisoni, although the closely related shartegosuchid Zosuchus davidsoni (Pol & Norell, 2004b) and other derived ‘protosuchians’, such as Sichuanosuchus shuhanensis (Wu et al. 1997), present the MC enclosed posteriorly by the squamosal and quadrate as in most Mesoeucrocodylia (Wu et al. 1997; Pol et al. 2013).
The ADQ is notched by the BOA and also pierced by a series of additional apertures in all the four forms analysed first‐hand (Fig. 4). The BOA is formed by an enlarged incisure placed at the rear edge of the ADQ. It has a semilunar anterior margin homologous to the OI of extant crocodylians. Given the absence of the contact of the squamosal and the posterodorsal surface of the quadrate, at least in Protosuchus haughtoni, Hemiprotosuchus leali and Orthosuchus strombergi, the EAM is completely opened posteriorly (Fig. 4C–E). Also, in Protosuchus haughtoni and Orthosuchus strombergi the quadrate, squamosal and otoccipital fail to meet to enclose the occipital aperture of cranioquadrate passage, whereas in Fruitachampsa callisoni they meet and form the occipital aperture of the cranioquadrate passage (Clark, 2011).
The multiple additional apertures in the ADQ, often called the quadrate fenestrae, of these forms open to the periotic sinus (Crompton & Smith, 1980; Hecht & Tarsitano, 1983; Busbey & Gow, 1984). Some of the apertures present internal struts oriented in variable directions that can also form a smaller aperture within a larger aperture (Wu et al. 1994, fig. 3). The number and configuration of the apertures vary intra‐ and interspecific in the analysed taxa as well in other basal crocodyliforms ranging from three in Zosuchus davidsoni to as many as nine in Orthosuchus strombergi (SAM‐PK‐409; Nash, 1975; Pol & Norell, 2004a). In Protosuchus haughtoni, the number of apertures ranges from four to five in the holotype BP/1/4746 to as many as eight in the smaller BP/1/5290. However, in all the studied species, there is larger aperture closer to the posteroventral margin of the OI. The ‘fenestra A’ of Hecht & Tarsitano (1983) was identified first in P. richardsoni (Brown, 1933), but it is also present in Protosuchus haughtoni, Orthosuchus strombergi, Fruitachampsa callisoni, Gobiosuchus kielanae, Shantungosuchus hangjinensis and Hsisosuchus chowi, while it is possibly absent in Hemiprotosuchus leali (PVL 3829). The appearance of this complex of quadrate aperture is abrupt, with no presence of similar pneumatic diverticula in the more basal crocodylomorphs, Sphenosuchia. The position and pneumatic nature of these quadrate apertures suggests they are homologous to the subtympanic foramen of extant crocodylians.
Among the three osteological correlates established for the TM of the crown‐group, only a POF is present in the basal crocodyliforms studied. The OB and DOI are completely absent in Protosuchus haughtoni, Hemiprotosuchus leali, Orthosuchus strombergi and Fruitachampsa callisoni, as well as they could not be identified in published data of any other basal crocodyliform. The POF of Protosuchus haughtoni is identifiable, although not very markedly, at the ADQ in the holotype BP/1/4746, the paratype BP/1/4946, the more complete referred specimens BP/1/4770 and SAM PK 8026, and the specimen previously assigned to Lesotosuchus charigi (NHMUK R8503). In the same way, the ADQ of Hemiprotosuchus leali holotype (PVL 3829) seems to have a slightly marked POF. The putative fossa surrounds, in both forms, the anterior portion of the ADQ, anterior to the subtympanic foramina (Fig. 4D). Additional photographs of the Protosuchus richardsoni (AMNH 3024) also show a POF in this specimen surrounding anteriorly the subtympanic foramina. Peng & Shu (2005, fig. 2) illustrate a semicircular structure in front of the subtympanic foramina of Hsisosuchus chowi; however, it is not clear if this represents the POF.
Based on the POF observed in the analysed taxa, it is suggested that the TM of basal crocodyliforms covered the multiple subtympanic foramina at the ADQ, as suggested by Crompton & Smith (1980) and Hecht & Tarsitano (1983) on Protosuchus richardsoni and Orthosuchus strombergi but contrary to Nash (1975), which limited the TM to the EAM in Orthosuchus strombergi. In addition, the absence of the contact between the squamosal and posterodorsal surface of the quadrate precludes the investigation of the suspensory plate and the estimation of the posterior margin of the TM in these taxa. This study considers the TM of these taxa well developed anteriorly, but refrains to establish a posterior limit for the structure.
Basal Mesoeucrocodylia
Significant variation in the MC among basal mesoeucrocodylians was recognized, and three major patterns established: the ‘thalattosuchian pattern’; the ‘basal notosuchian/sebecian pattern’; and the ‘advanced notosuchian pattern’. The ‘thalattosuchian pattern’ is highly divergent in relation to the other two patterns, which possess anatomies more comparable among them and extant crocodylians.
The ‘thalattosuchian pattern’ is restricted to Thalattosuchia (Fraas, 1901; sensu Young & Andrade, 2009). Thalattosuchians have a modified skull anatomy (Fig. 5; Busbey, 1995; Pierce et al. 2009; Young et al. 2011), which also affects the anatomy of the MC, ADQ and BOA. The skull roof lacks the lateral expansions over the temporal region and the longitudinal sulcus for the attachment of the upper earlid (Fig. 5). However, considering the lateral edge of the skull roof, the MC in thalattosuchians is limited to lateral corners of the posteroventral portion of the skull. Because of the absence of the sulcus for the upper earlid, it was suggested that the MC was not concealed by earlids in these taxa. Therefore, the TM was placed near the lateral edge of the head in life, and the MC, BOA and TM occupied an equivalent position. The size of the MC is particularly small and very often not visible given the dorsoventral compression of their skulls (e.g.: Metriorhynchus superciliosus; Blainville, 1853; NHMUK 1666, NHMUK 2032, Steneosaurus; Geoffroy Saint‐Hilaire, 1825; NHMUK 4771, NHMUK 2074, Suchodus brachyrhynchus; Eudes‐Deslongchamps, 1867; NHMUK R3804, Suchodus durobrivensis; Lydekker, 1890; NHMUK R3321, Gracilineustes leedsi; Andrews, 1913; NHMUK R3015, NHMUK R2042, NHMUK R3899).
Figure 5.
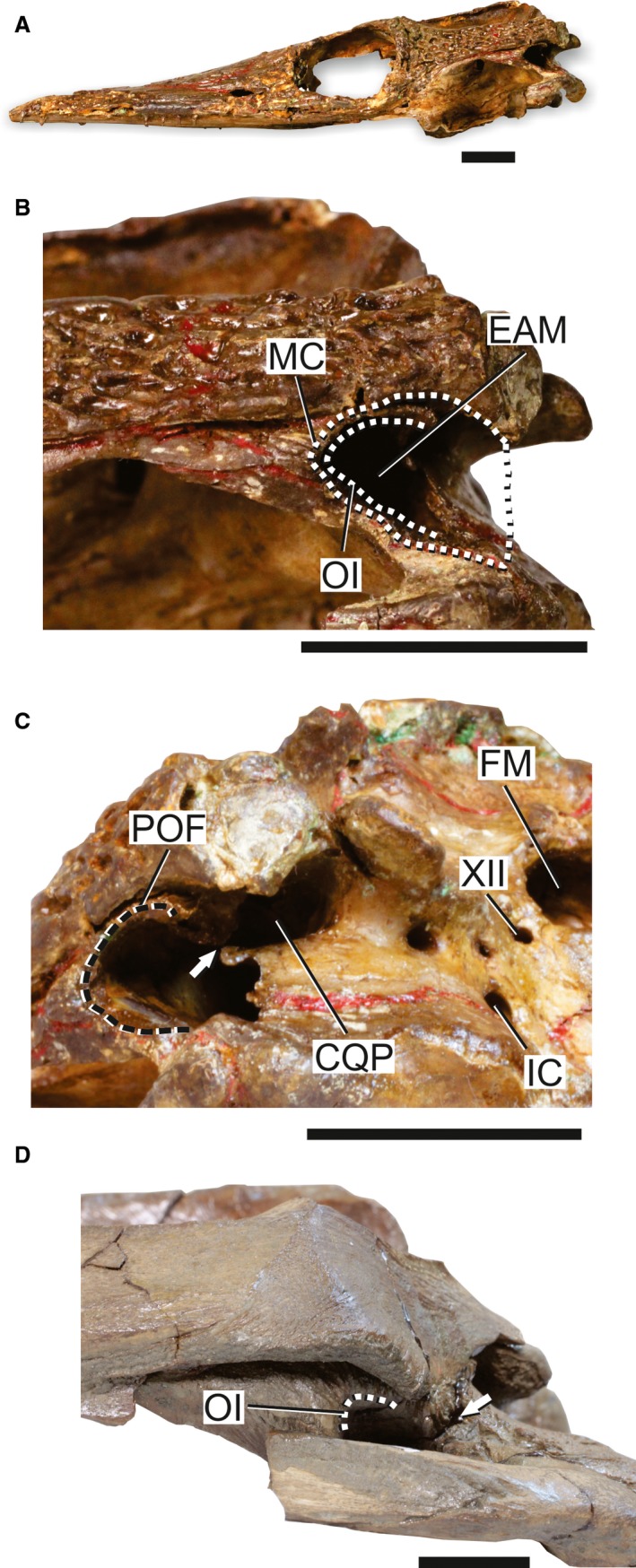
Skull of thallatosuchians to illustrate the ‘thalattosuchian pattern’ of MC. (A) Overview of the skull of Pelagosaurus typus (NHMUK OR 32599) in left lateral view to illustrate the general morphology of the MC in this pattern. (B) detail of the posterior portion of the skull and MC of Pelagosaurus typus (NHMUK OR 32599) in left lateral view. (C) Left lateroposteroventral view of the skull and MC of Pelagosaurus typus (NHMUK OR 32599) to illustrate the possible closure of the occipital portion of the cranioquadrate passage. (D) Detail of the posterior portion of the skull and MC of Suchodus durobrivensis (NHMUK R 2618). The arrow indicates the possible separation of the cranioquadrate passage and EAM. CQP, occipital aperture of cranioquadrate passage;EAM, external auditory meatus; FM, foramen magnum; IC, foramen for the internal carotid artery; MC, meatal chamber; OI, otic incisure; POF; periotic fossa; XII, foramen for cranial nerve XII. Scale bars: 2 cm.
The posterior portion of the squamosal fails to contact the posterodorsal surface of the quadrate (Fig. 5B,C). In addition, there is virtually no posteroventral extension of the postorbital and anterodorsal extension of the quadratojugal (Fig. 5B,D). The ADQ is also anteroposteriorly restricted to a strap of bone oriented anterodorsally to posteroventrally (Fig. 5B). The combination of these features makes the MC and BOA in these taxa very shallow and opened posteriorly (Fig. 5C).
The BOA is the single aperture in the ADQ in the ‘thalattosuchian pattern’. This structure either faces laterally as in Pelagosaurus typus (Bronn, 1841; NHMUK OR 32599), posteriorly as in Dakosaurus andiniensis (Vignaud & Gasparini, 1996; MOZ 6146P; Pol & Gasparini, 2009), or posteroventrally as in Cricosaurus araucanensis (Gasparini & Dellapé, 1976; MLP 72‐IV‐7‐1), Metriorhynchus superciliosus (NHMUK R6859) and Suchodus brachyrhynchus (NHMUK R3700). The BOA is formed only by the EAM, which is limited anteriorly by the semicircular OI notching the posterior edge of ADQ (Fig. 5B–D), and is also opened posteriorly because of the absence of contact between the squamosal and the posterodorsal surface of the quadrate (Fig. 5C). The EAM is bordered by a slightly depressed area along its entire perimeter that is referred to as a restricted POF. In some taxa, within the less inclusive thalattosuchian clade Metriorhynchidae (sensu Young et al. 2010), there is a series of radiating striae in the perimeter of the EAM externally to the POF (Fig. 5D).
In several basal thalattosuchians (e.g. Steneosaurus NHMUK R4771, NHMUK R1088), it is not clear if the cranioquadrate passage is isolated from the BOA. However, a short separation between the BOA and the enlarged cranioquadrate passage seems to be present at the occipital portion of the well‐preserved Pelagosaurus typus (NHMUK OR 32599; Fig. 5C), and a separation is also present in Teleosaurus cadomensis (Lamouroux, 1820; Jouve, 2009). In Metriorhynchidae, there is a clear separation between those two apertures in the occipital surface as in Suchodus brachyrhynchus (NHMUK R3700), Dakosaurus andiniensis (MOZ 6146P), Torvoneustes coryphaeus (Young et al. 2013) and Maledictosuchus riclaensis (Parrilla‐Bel et al. 2013). However, there is no IOC in Metriorhynchidae and most probably the rest of Thalattosuchia, even though the occipital aperture of the cranioquadrate passage is closed.
Among the osteological correlates for the TM, only the POF is present in the ‘thalattosuchian pattern’ and limited to the perimeter of the reduced OI. The OB is absent and the cranioquadrate passage is not anteriorly opened within the BOA. As such, it is impossible to infer the posterior extension of the TM based on the osteological correlates found in the crown‐group. However, given that the MC is closer to the posterior margin of the skull, it is expected that the TM did not extend posteriorly to the posterior tip of the squamosal. The presence of radiating striae in the perimeter of the OI in some metriorhynchids is likely also related to the soft tissue of the MC in these taxa.
The ‘basal notosuchian/sebecian pattern’ is recognized in basal notosuchians (e.g. Araripesuchus wegeneri; Buffetaut & Taquet, 1979; Anatosuchus minor; Sereno et al. 2003; and Candidodon itapecuruense; Carvalho & Campos, 1988); peirosaurids (e.g. Hamadasuchus rebouli; Buffetaut, 1994; Lomasuchus palpebrosus; Gasparini et al. 1991; Montealtosuchus arrudacamposi; Carvalho et al. 2007; Pepesuchus deiseae; Campos et al. 2011; and Stolokrosuchus lapparenti; Larsson & Gado, 2000); as well as in the sebecid Sebecus icaeorhinus (Simpson, 1937). The ‘advanced notosuchian pattern’ is present in the advanced notosuchians (sensu Pol et al. 2014) and Baurusuchidae (sensu Montefeltro et al. 2011) that might be sister groups (Montefeltro et al. 2013). The ‘basal notosuchian/sebecian pattern’ (Fig. 6) is simpler and is described first addressing some of the variation within the pattern. The modification present in the ‘advanced notosuchian pattern’ (Fig. 7) is explained after.
Figure 6.
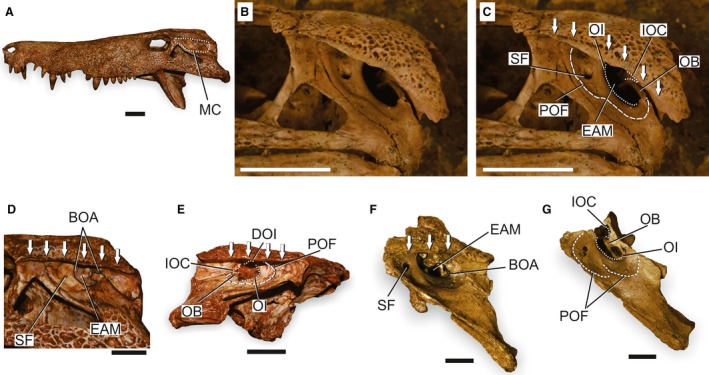
Skull of basal mesoeucrocodylians to illustrate the ‘basal notosuchian/sebecian pattern’ of the MC. (A) Overview of the skull of Hamadasuchus rebouli (ROM 52620) in left lateral view to illustrate the general morphology of the MC in this pattern. (B) Detail of the posterior portion of the skull and MC of Araripesuchus gomesi (AMNH 24450) in left lateral view. (C) Detail of the posterior portion of the skull and MC of Araripesuchus gomesi (AMNH 24450) in left lateral view, with selected structures of the MC highlighted. (D) Detail of the posterior portion of the skull and MC of Hamadasuchus rebouli (ROM 52620) in left lateral view, with selected structures of the MC highlighted. (E) Detail of the posterior portion of the skull and MC of Hamadasuchus rebouli (ROM 54511) in right lateral view, with selected structures of the MC highlighted. (F) Lateral view of the posterior portion of the skull and MC of Sebecus icaeorhinus (AMNH FR 3160), with selected structures of the MC highlighted. (G) Posterolateral view of the posterior portion of the skull and MC of Sebecus icaeorhinus (AMNH FR 3160) after disarticulation of the squamosal, with selected structures of the MC highlighted. Arrows indicate the extension of the sulcus for the attachment of the upper earlid. BOA, bony otic aperture; DOI, dorsal otic incisure; EAM, external auditory meatus; IOC, incisure of the otic aperture of the cranioquadrate passage; MC, meatal chamber; OB, otic buttress; OI, otic incisure; POF; periotic fossa; SF, subtympanic foramen. Scale bars: 2 cm.
Figure 7.
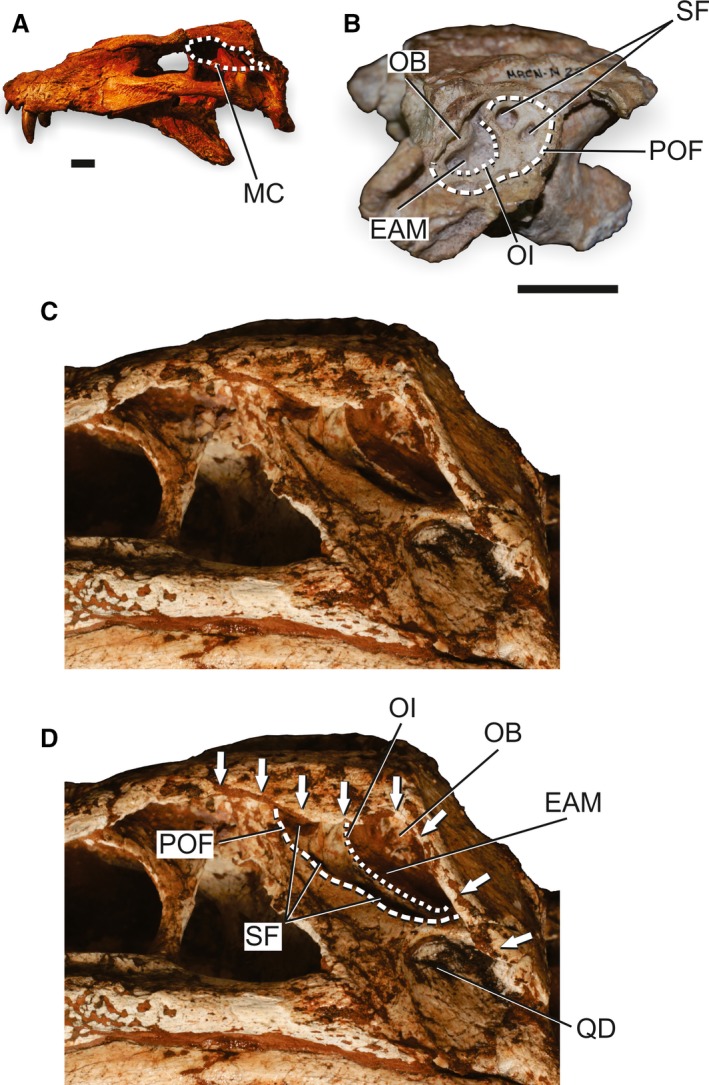
Skull of basal mesoeucrocodylians to illustrate the ‘advanced notosuchian pattern’ of the MC. (A) Overview of the skull Pissarrachampsa sera (LPRP/USP‐0019) in left lateral view to illustrate the general morphology of the MC in this pattern. (B) Detail of the posterior portion of the skull and MC of Notosuchus terrestris (MACN N 22) in left lateral view, with selected structures of the MC highlighted. (C) Detail of the posterior portion of the skull and MC of Pissarrachampsa sera (LPRP/USP‐0049) in left lateral view. (D) Detail of the posterior portion of the skull and MC of Pissarrachampsa sera (LPRP/USP‐0049) in left lateral view, with selected structures of the MC highlighted. Arrows indicate the extension of the sulcus for the attachment of the upper earlid. EAM, external auditory meatus; MC, meatal chamber; OB, otic buttress; OI, otic incisure; POF; periotic fossa; QD, quadrate depression; SF, subtympanic foramina. Scale bars: 2 cm.
All taxa with a ‘basal notosuchian/sebecian pattern’ have the MC overhung by the postorbital and squamosal lateral shelves. These structures expand from the skull roof and possess the longitudinal sulcus for the attachment of the upper earlid at the ventrolateral edge. Therefore, the MC was covered by a muscular upper earlid in these taxa.
Posteriorly, the MC is enclosed by the contact of the squamosal and the posterodorsal surface of the quadrate, which also limits the BOA posteriorly (Pol et al. 2013) and encloses laterally the whole extension of the cranioquadrate passage (Fig. 6). Anteriorly, the MC is limited by the descending process of the postorbital and medially by an expanded lateral surface of the ADQ. In Stolokrosuchus lapparenti (MNN GDF 601) and Pepesuchus deiseae (MN 7005‐V), the squamosal extends anteriorly reaching the MC anterior border (Montefeltro et al. 2013). This type of MC is not greatly expanded dorsoventrally because of the reduced descending process of the postorbital and limited anterodorsal process of the quadratojugal. However, some dorsoventral variation occurs (Fig. 6C,D,F). There are a maximum of two apertures in the MC, a single subtympanic foramen and the BOA. The subtympanic foramen is absent in Stolokrosuchus lapparenti and Pepesuchus deiseae but, in the other taxa, it is moderate in size and completely contained within the ADQ.
The BOA faces laterally and slightly dorsally with an elongated elliptical outline that is obliquely oriented in relation to the skull roof (Fig. 6B,C). This aperture is mostly bounded by the ADQ, but it is bounded posteriorly and posterodorsally by the squamosal. The EAM forms the greatest portion of the BOA and is limited by the OI anteriorly. Posteriorly, the EAM is limited by the OB expanding from the posterior wall of the BOA. In this pattern, the IOC is not expanded and, given the dorsally displaced OB, the aperture is dorsally positioned at the posterior wall of the BOA (Fig. 6C,E,G). This positioning is also consistent with a dorsally positioned quadrate‐squamosal suture in such taxa. The OB in Sebecus icaeorhinus (AMNH FR 3160) is conspicuously dorsally displaced (Fig. 6F,G), and could be a result of the fragmentary nature of the area and the supposed quadrate rotation during the restoration of the specimen (Molnar, 2010). However, a similar dorsally displaced quadrate‐squamosal suture is also present in Sahitisuchus fluminensis (Kellner et al. 2014), suggesting the anatomy of Sebecus icaeorhinus is real.
In peirosaurids (e.g. Hamadasuchus rebouli ROM 52620, Lomasuchus palpebrosus MOZ 4084), the OB expands internally as a robust structure (Fig. 6D,E). Araripesuchus wegneri (MNN GAD19), Hamadasuchus rebouli (ROM 52059 and ROM 54511) and Lomasuchus palpebrosus (MOZ 4084) present an incisure in the dorsal edge of the BOA, interpreted here as homologous to the DOI of extant forms.
A greatly developed POF marks the lateral surface of the ADQ in these taxa. This fossa is expanded in a semicircular margin anteriorly encompassing the subtympanic foramen, when present. This fossa has a sinusoidal ventral margin that is conspicuously marked in Araripesuchus gomesi (Price, 1959; AMNH 24450; Fig. 6B,C). The POF of Sebecus icaeorhinus (AMNH FR 3160) presents a further modification of the ventral margin showing a gap that separates the POF in two different regions (Fig. 6G). In all taxa with the ‘basal notosuchian/sebecian pattern’, the POF curves posterodorsally closer to the squamosal‐quadrate contact at the MC (e.g. Araripesuchus gomesi AMNH 24450) and can invade the anterior portion of the squamosal, as in Sebecus icaeorhinus (AMNH FR 3160). The POF becomes less marked dorsomedially and ends posteriorly at the base of the OB (Fig. 6C,E,F).
The ‘advanced notosuchian pattern’ maintains most of the anatomy present in the ‘basal notosuchian/sebecian pattern’ but with modifications (Fig. 7). The longitudinal sulcus on the ventrolateral edge of the skull roof lateral shelves is modified in baurusuchids, as in Pissarrachampsa sera (Montefeltro et al. 2011; LPRP/USP‐0049). In these taxa, the sulcus is limited to the squamosal bone and extends anteriorly only to the dorsal corner of the infratemporal fenestra (Fig. 7C,D). However, the sulcus extends posteriorly onto the ventral tip of the deflected posteroventral prong of the squamosal that bounds the MC posteriorly (Fig. 7D; Montefeltro et al. 2011; Montefeltro & Larsson, 2011; Godoy et al. 2014). The large posteroventral descending process of the postorbital grants the taxa with this pattern a dorsoventrally expanded MC (Fig. 7A,C; e.g. Mariliasuchus amarali; Carvalho & Bertini, 1999; UFRJ‐DG‐106R; Caipirasuchus montealtensis; Andrade & Bertini, 2008a; MPMA 15‐001/90). This is particularly evident in baurusuchids and sphagesaurids in which the process of the postorbital forms an expanded concave flange (e.g. Baurusuchus salgadoensis; Carvalho et al. 2005; MPMA 62‐0001‐02; Pissarrachampsa sera LPRP/USP‐0019; Fig. 7A,C; Armadillosuchus arrudai; Marinho & Carvalho, 2009; UFRJ DG 303‐R; Montefeltro et al. 2011).
The BOA is enlarged in the ‘advanced notosuchian pattern’ but also presents the basic anatomy of the ‘basal notosuchian/sebecian pattern’, including the DOI in baurusuchids (Baurusuchus salgadoensis MPMA 62‐0001‐02) and the dorsally displaced IOC and quadrate‐squamosal suture (Fig. 7D). The ADQ in the ‘advanced notosuchian pattern’ is pierced by additional apertures at the perimeter of the BOA that are entirely placed within the area of the POF (Fig. 7B,D). These apertures are often called quadrate fenestrae (Andrade & Bertini, 2008b; Montefeltro et al. 2011; Pol et al. 2014), and considering its position and pneumatic nature are also homologized to the subtympanic foramen (see Discussion). The subtympanic foramina are variable in number, particularly in Notosuchus terrestris (Woodward, 1896) and Mariliasuchus amarali (Andrade & Bertini, 2008b). A more consistent pattern is present in baurusuchids, Adamantinasuchus navae (UFRJ‐DG 107‐R) and Caipirasuchus montealtensis (MPMA 15001/90) that have a larger ventral‐most subtympanic foramen. These multiple subtympanic foramina differ from those of more basal crocodyliforms in that the apertures in ‘advanced notosuchian pattern’ lack internal struts. One partial exception is a specimen of Notosuchus terrestris (MUCPv‐198; Fiorelli & Calvo, 2008) that does have some internal struts visible within its anterior‐most foramen. The multiple subtympanic foramina of advanced notosuchians are generally laterally oriented (Fig. 7B,D). However, only the dorsal foramen in Baurusuchinae (sensu Montefeltro et al. 2011; Godoy et al. 2014) is laterally oriented, whereas the other more ventral foramina face perpendicularly to the BOA plane and opening dorsolaterally.
Taxa with the ‘advanced notosuchian pattern’ also have a well‐developed POF and, in some cases, also have a well‐developed ventral sinusoidal margin, as in Armadillosuchus arrudai (UFRJ DG 303‐R), Mariliasuchus amarali (UFRJ‐DG‐106‐R) and Yacarerani boliviensis (Novas et al. 2009; Pol et al. 2014). The POF in Baurusuchidae is particularly recessed from the remaining area of the ADQ with a marked step at its boundary (Fig. 7C,D). Only the anterodorsal portion of the POF is similar to other advanced notosuchians, being slightly recessed from the remaining area of the ADQ (Fig. 7D). The ventral limit of the POF is coincident with the dorsal extension of the quadrate depression in baurusuchids (Montefeltro et al. 2011).
All three osteological correlates for the TM are recognized in taxa with the ‘basal notosuchian/sebecian pattern’ and ‘advanced notosuchian pattern’. The enlarged POF suggests a well‐developed TM in these taxa, which encompassed the single subtympanic foramen in the ‘basal notosuchian/sebecian pattern’ and also the more elaborated foramina in the ‘advanced notosuchian pattern’ (Figs 6C,E,G and 7B,D).
Neosuchia
Neosuchians encompass a relatively large diversity of forms, with variable skull shapes adapted for terrestrial, amphibious and aquatic lifestyles. This variation affects the anatomy of the MC, BOA and EAM. Nevertheless, the structures of the MC of all neosuchians are similar in many aspects to the anatomy of the crown‐group and to the ‘basal notosuchian/sebecian pattern’. All neosuchians have well‐developed squamosal and postorbital shelves overhanging the lateral MC with a well‐defined longitudinal sulcus at the ventrolateral edges. The ADQ and posteroventral extension of the postorbital are reduced, and the infratemporal fenestrae is well developed in most neosuchians; therefore, they have a relatively restricted MC (Fig. 8). Yet, some taxa, such as Shamosuchus djadochtaensis (Mook, 1924; IGM 100/1195; Fig. 8B,C) and Allodaposuchus (Nopcsa, 1928) species (Delfino et al. 2008; Puértolas‐Pascual et al. 2014), have a modified anatomy with a broadly developed ADQ and expanded MC. Additionally, some fossil members within Crocodylia possess a more expanded MC, such as Hassiacosuchus haupti (Weitzel, 1935; HLMD Me4415), planocraniids and meckosuchines (Langston, 1975; Salisbury & Willis, 1996; Buchanan, 2009; Brochu, 2012). The MC is particularly dorsoventrally reduced in the basal eusuchians Hylaeochampsa vectiana (Owen, 1874; NHMUK R177; Fig. 8E) and Aegisuchus witmeri (Holliday & Gardner, 2012; ROM 54530; Fig. 8F). In contrast, some forms with elongated rostra have a low but anterorposteriorly elongated MC, such as the dyrosaurids (Guarinisuchus munizi; Barbosa et al. 2008; DG‐CTG‐UFPE‐5723; Dyrosaurus phosphaticus; Pomel, 1849; Jouve, 2005; Dyrosaurus maghribensis; Jouve et al. 2006; Hyposaurus rogersii; Denton et al. 1997; cf. Rhabdognathus sp.; Swinton, 1930; Brochu et al. 2002; Cerrejoniosuchus improcerus; Hastings et al. 2010), pholidosaurids (Sarcosuchus imperator; Buffetaut & Taquet, 1966; Sereno et al. 2001; Pholidosaurus sp.; Meyer, 1841; NHMUK 36721) and Khoratosuchus jintasakuli (Lauprasert et al. 2009).
Figure 8.
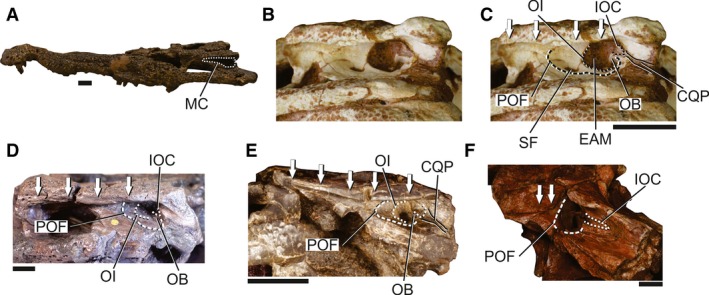
Skull of neosuchians to illustrate the MC in these forms. (A) Overview of the skull of Amphicotylus lucasii (AMNH FARB 5782) in left lateral view to illustrate the general morphology of the MC in neosuchians. (B) Detail of the posterior portion of the skull and MC of Shamosuchus djadochtaensis (IGM 100/1195). (C) Detail of the posterior portion of the skull and MC of Shamosuchus djadochtaensis (IGM 100/1195), with selected structures of the MC highlighted. (D) Detail of the posterior portion of the skull and MC of Anterophthalmosuchus hooleyi (NHMUK R 3876), with selected structures of the MC highlighted. (E) Detail of the posterior portion of the skull and MC of Hylaeochampsa vectiana (NHMUK R 177), with selected structures of the MC highlighted. (F) Detail of the posterior portion of the skull and MC of Aegisuchus witmeri (ROM 54530), with selected structures of the MC highlighted. Arrows indicate the extension of the sulcus for the attachment of the upper earlid. CQP, cranioquadrate passage; EAM, external auditory meatus; IOC, incisure of the otic aperture of the cranioquadrate passage; MC, meatal chamber; OB, otic buttress; OI, otic incisure; POF; periotic fossa; SF, subtympanic foramen. Scale bars: 2 cm.
The squamosal bears a ventrally directed lamina that in most taxa contacts the posterodorsal surface of the quadrate posteriorly. In this configuration, both the MC and BOA are posteriorly enclosed by skeletal structures as in the crown‐group (Fig. 8). The BOA and subtympanic foramen are present in the ADQ, but some taxa lack the latter aperture (e.g. Guarinisuchus munizi DG‐CTG‐UFPE‐5723, Aegisuchus witmeri ROM 54530).
As in the crown‐group, the BOA is formed mostly by the ADQ except on its dorsal and posterodorsal edges, which are formed by the squamosal. The complex outline of the BOA and division of the aperture in the EAM, IOC, and DOI is present in many extinct neosuchians. The EAM forms the greatest portion of the BOA, and is limited by the OI anteriorly and by the OB posteroventrally. However, the anatomy and the position of the OB, as well as the position of the quadrate‐squamosal suture, are variable among neosuchians. The OB forms a stout structure positioned at the posteroventral corner of the BOA in Anterophthalmosuchus hooleyi (Salisbury & Naish, 2011; NHMUK R3876; Fig. 8D) and cf. Rhabdognathus sp. (Brochu et al. 2002), whereas in Shamosuchus djadochtaensis (IGM 100/1195; Fig. 8C,D) it is a more developed and dorsally displaced structure. The basal eusuchians also have a variable anatomy of the OB from virtually no anterodorsal extension in Aegisuchus witmeri (ROM 54530) to a well‐developed anterodorsally directed structure in Hylaeochampsa vectiana (NHMUK R177; Fig. 8E).
The anatomy of the IOC is the most variable structure in the MC of neosuchians. The arrangement of this structure is similar to that of extant forms in dyrosaurids (Guarinisuchus munizi DG‐CTG‐UFPE‐5723, cf. Rhabdognathus sp.; Brochu et al. 2002), pholidosaurids (Sarcosuchus imperator; Sereno et al. 2001; NHMUK 36721), Paluxysuchus newmani (Adams, 2013) and some taxa closer to Crocodylia, such as Anterophthalmosuchus hooleyi (NHMUK R3876; Salisbury & Naish, 2011), Wannchampsus kirpachi (Adams, 2014) and Isisfordia duncani (Salisbury et al. 2006; but see Turner & Pritchard, 2015). In these forms, the IOC is limited to a slightly marked sulcus at the posteroventral edge of the BOA and the squamosal contacts the quadrate along its entire lateroventral edge. In this arrangement, the quadrate and squamosal bound the cranioquadrate passage along its entire extension (Fig. 8D). By contrast, in some derived neosuchians, the IOC extends posteroventrally in a laterally opened cranioquadrate passage at the quadrate‐squamosal suture, which exposes the medial wall of the passage. Most of the taxa with an opened cranioquadrate passage are included in Goniopholididae, such as Amphicotylus lucasii (Cope, 1878; AMNH FARB 5782), Eutretauranosuchus delfsi (Mook, 1967; Pritchard et al. 2013), Hulkepholis willetti (Salisbury & Naish, 2011) and Goniopholis baryglyphaeus (Schwarz, 2002). Taxa from different groups also exhibit a similar anatomy, such as Shamosuchus djadochtaensis (IGM 100/1195; Turner, 2015; Fig. 8D), the eusuchians Hylaeochampsa vectiana (NHMUK R177; Fig. 8E), and the species in Allodaposuchus (Delfino et al. 2008; Buscalioni et al. 2011). Aegisuchus witmeri (ROM 54530) has a unique anatomy of the cranioquadrate passage that is opened anteriorly but closed posteriorly near to the occipital surface of the skull (Fig. 8F).
The posterior wall of the IOC within the BOA is confluent to the posterior wall of the DOI in all neosuchians with a laterally closed cranioquadrate passage. In most taxa, the DOI represents the dorsal apex of the BOA. However, most of the specimens have this portion damaged or not fully prepared (e.g. Anterophthalmosuchus hooleyi NHMUK R3876).
A POF marks the lateral surface of the ADQ in neosuchians. This fossa is expanded in a semicircular margin anteriorly encompassing the subtympanic foramen, when present, and merging with the remaining ADQ surface at the base of the OB (Fig. 8C). In Aegisuchus witmeri (ROM 54530), the POF is limited to an area slightly larger than the BOA (Fig. 8F), whereas in taxa such as Shamosuchus djadochtaensis (IGM 100/1195; Fig. 8B,C), Anterophthalmosuchus hooleyi (NHMUK R3876; Fig. 8D) and Hylaeochampsa vectiana (NHMUK R177; Fig. 8E), the POF extends anteriorly to the BOA and occupies most of the floor of the MC, as in the crown‐group.
All the osteological correlates found in the extant forms are also present in neosuchians. The longitudinal sulcus on the ventrolateral edge of the skull roof shelves and the POF are invariably present in these taxa. This suggests the presence of a MC concealed by an upper earlid, and implies variable sizes of the MC and TM. Additionally, the TM encompassed the subtympanic foramen when present. Considering the posteroventral extension of the POF at the base of the OB and the anterior edge of the DOI, the TM would not have encompassed the IOC and its posteroventral extension in forms with an opened cranioquadrate passage.
Discussion
Evolution of the MC in Crocodyliformes
The origins of Crocodyliformes and Mesoeucrocodylia are associated with an extensive reorganization of the temporal region of the skull (Whetstone & Whybrow, 1983; Clark, 1994; Clark et al. 2000, 2004; Holliday & Witmer, 2009; Pol et al. 2013). The hearing apparatus of extant crocodyliforms is unique among amniotes and includes a well‐developed external ear, something only present elsewhere in mammals. However, comparatively little effort has been made to investigate the anatomy of these structures and their evolutionary origins (Shute & Bellairs, 1955; Wever, 1978; Saunders et al. 2000; Vergne et al. 2009). The review of crocodyliform posterior skulls and osteological proxies of associated soft tissue structures of the ear map a complex pattern of evolution (Fig. 9).
Figure 9.
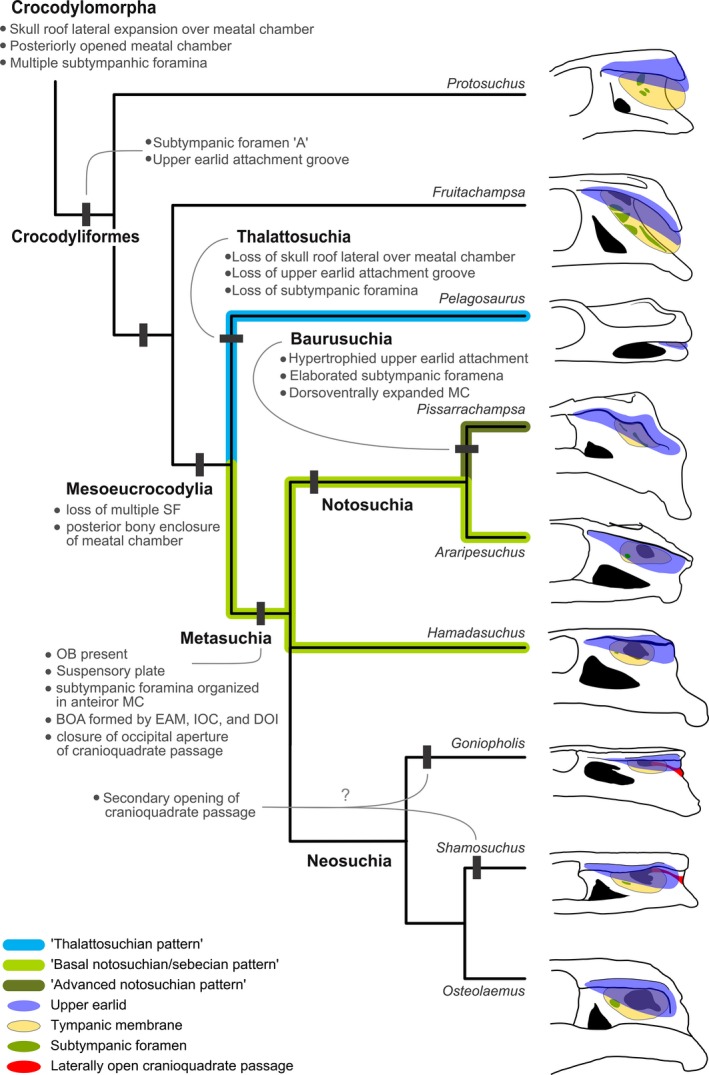
Simplified phylogenetic framework with major stages in the evolution of the MC of Crocodyliformes discussed in the text. BOA, bony otic aperture; DOI, dorsal otic incisure; EAM, external auditory meatus; IOC, incisure of the otic aperture of the cranioquadrate passage; MC, meatal chamber; SF, subtympanic foramen.
The early forms, Protosuchus haughtoni, Hemiprotosuchus leali, Orthosuchus strombergi and Fruitachampsa callisoni, present the ancestral state of the MC in crocodyliforms. The broad, lateral expansions of the skull roof overhanging the temporal region in most crocodyliforms represents a plesiomorphy, present in a much broader phylogenetic assemblage, possibly at the base of Crocodylomorpha (Pol et al. 2013). A similar anatomy is also present in the paraphyletic array of closely related forms, such as Litargosuchus leptorhynchus (Clark & Sues, 2002; BP/1/5237), Pseudhesperosuchus jachaleri (Bonaparte, 1969), Hesperosuchus agilis (Colbert, 1952), Saltoposuchus connectens (von Huene, 1921), Terrestrisuchus gracilis (Crush, 1984), Dibothrosuchus elaphros (Simmons, 1965), Sphenosuchus acutus (Haughton, 1915), Kayentasuchus walkeri (Clark & Sues, 2002), Junggarsuchus sloani (Clark et al. 2004) and Almadasuchus figarii (Clark et al. 2000, 2004; Clark & Sues, 2002; Pol et al. 2013).
In addition, the sulcus on the outer surface of the squamosal and postorbital for the attachment of the upper earlid is also present in Kayentasuchus walkeri (Clark & Sues, 2002; Clark et al. 2004; Pol et al. 2013). Kayentasuchus walkeri has a variable position among Crocodylomorpha, recovered in a basal position in most topologies (Clark & Sues, 2002; Sues et al. 2003; Clark et al. 2004; Pol et al. 2013; Wilberg, 2015b), but also as a sister group of Crocodyliformes (Nesbitt, 2011) or as a basal member of that clade (Jouve, 2009). If future work confirms the basal position of Kayentasuchus walkeri outside Crocodyliformes, it implies that the muscular upper earlid was homoplastically acquired twice during the Crocodylomorpha evolution, once by Kayentasuchus walkeri and once at the Crocodyliformes node. Also, the recently described Almadasuchus figarii might have the sulcus for an upper earlid taphonomically obscured (Pol et al. 2013). If confirmed, the upper earlid represents a slightly more inclusive synapomorphy. The simultaneous occurrence of an inset BOA and the upper earlid bounding the MC laterally were already present at the origins of Crocodyliformes, as previous work has suggested (Nash, 1975; Hecht & Tarsitano, 1983; Busbey & Gow, 1984; Busbey, 1995; Gow, 2000; Clark & Sues, 2002; Clark et al. 2004; Pol et al. 2013).
The posteriorly opened MC, BOA and cranioquadrate passage present in the basal crocodylomorphs and other pseudosuchians, such as Terrestrisuchus gracilis, Dibothrosuchus elaphros and Sphenosuchus acutus (SAM PK K3014) presents the plesiomorphic state for Crocodyliformes (Walker, 1970; Clark et al. 2000, 2004; Benton & Walker, 2002; Nesbitt, 2011; Nesbitt et al. 2013; Pol et al. 2013; Butler et al. 2014a). The cranioquadrate passage is restricted between the quadrate and otoccipital early on, in non‐crocodyliform taxa such as Junggarsuchus and Almadasuchus, and persists throughout Crocodyliformes (Pol et al. 2013). The contact between the squamosal and the quadrate behind the MC occurred along the ‘protosuchian’ grade (Fig. 9). Some taxa do not have this region completely preserved, such as Fruitachampsa callisoni (LACM 120455a), Zosuchus davidsoni and Shantungosuchus hangjinensis, which hampers the complete understanding of the phylogenetic origin of this closure. Almadasuchus figarii may shed some support for a single, early origin of this closure, given the support for this taxon as a sister‐taxon to Crocodyliformes and the presence of a partially closed posterior MC (Pol et al. 2013), but see below alternative scenarios regarding Thalattosuchia. The closure is not as complete as that of Mesoeucrocodylia, and the posteroventrally downturned squamosal of this species differs greatly from that of mesoeucrocodylians. However, this anatomy suggests that at least a functional closure was present before Crocodyliformes and may have persisted throughout all protosuchians. The ‘protosuchians’ Protosuchus haughtoni, Hemiprotosuchus leali and Orthosuchus strombergi have more posteriorly open MCs than Almadasuchus. The squamosal in some of these taxa does bend posteroventrally, perhaps homologous to that of Almadasuchus, but the MC remains open posteriorly. Taxa closer to Mesoeucrocodylia, such as Hsisosuchus dashanpuensis (Gao, 2001) and Hsisosuchus chungkingensis (Young & Chow, 1953; Li et al. 1994; Gao, 2001), have fully closed posterior MCs as in extant crocodylians.
The BOA formed only by the EAM with the OI and lacking the OB, IOC and DOI exhibits very little change from its ancestral anatomy present in pseudosuchians (Benton & Clark, 1988; Benton & Walker, 2002; Nesbitt et al. 2009, 2013; Nesbitt, 2011; Butler et al. 2014a,b). On the other hand, the extra apertures, named quadrate fenestrae or quadrate foramina at the anterior perimeter of the OI, are uncommon and need a closer inspection for assessing the homology of its component across crocodyliforms. Another foramen, called the quadrate foramen, is commonly bounded between the quadrate and quadratojugal in archosauromorphs (Ewer, 1965; Sereno & Novas, 1992; Dilkes, 1998; Nesbitt et al. 2009; Nesbitt, 2011; Butler et al. 2014a,b). This foramen is closed at the origin of Crocodylomorpha (Benton & Clark, 1988; Nesbitt, 2011), but the same nomenclature has been applied to other quadrate structures within crocodylomorph ADQs (Walker, 1970; Busbey & Gow, 1984; Pol et al. 2013). Discussing the homology of the quadrate foramen across Archosauromorpha is beyond the scope of this paper. However, to avoid any implicit homology statement confusions, it is suggested to discard the term quadrate foramen for crocodylomorphs. The openings in the crocodylomorph ADQ are associated with the pneumatic diverticulae of the periotic sinus (Iordansky, 1973, Wever, 1978; Witmer, 1997; Hetherington, 2008; Montefeltro et al. 2011; Tahara & Larsson, 2011; Pol et al. 2014). In extant crocodylians, this diverticulum only exits the quadrate via the subtympanic foramen on the ADQ to return into the middle ear near the inner contact between the tympanum and quadrate. These openings are not covered in extant taxa and were probably uncovered in extinct taxa as well, and therefore should not be called fenestrae. In keeping with the preferred terminology, the multiple foramina in the ‘protosuchian’ ADQ are called here the subtympanic foramina. The presence of multiple subtympanic foramina is the plesiomorphic state for crocodyliforms and also presents two successive outgroups of the clade, Junggarsuchus sloani and Almadasuchus figarii (Clark et al. 2004; Pol et al. 2013). The number and configuration of these foramina is highly variable in crocodyliforms, and establishing the homology of each foramen across taxa is currently uncertain. However, a large subtympanic foramen, called fenestra ‘A’ by Hecht & Tarsitano (1983), seems to be a reliable landmark and is considered homologous in Protosuchus richardsoni, Protosuchus haughtoni, Orthosuchus strombergi, Fruitachampsa callisoni, Gobiosuchus kielanae, Shantungosuchus hangjinensis, Hsisosuchus dashanpuensis, Hsisosuchus chungkingensis and Hsisosuchus chowi. None of the basal crocodylomorphs has a putative subtympanic foramen ‘A’, and this structure on the ADQ might be an autapomorphy of crocodyliforms. The developed POF at the ADQ shows that the TM would have occupied a greater portion of the head in basal crocodyliforms even though the posterior limits of the membrane cannot be inferred from osteological correlates.
The phylogenetic context of the three MC patterns in basal mesoeucrocodylian indicates great modifications in this section of the crocodyliform evolutionary history (Fig. 9). The interpretation of the ancestral anatomy at this node is greatly biased by the discrepant ‘thalattosuchian pattern’ positioned in the primary phylogenetic framework as a basal Mesoeucrocodylia. In this scenario, a series of traits established in ‘protosuchians’ and present in other basal mesoeucrocodylian are absent or modified in this group. However, its alternative positions (Wilberg, 2015a,b) also imply a great amount of reversions and homoplasies to accommodate the interpretation of the ‘thalattosuchian pattern’ (see below).
The absence of the lateral shelves of skull roof over the MC in thallatosuchians and the absence of the longitudinal sulcus for the attachment of the upper earlid are unique traits among crocodyliforms. This anatomy represents a reversion from what is usually observed in basal Crocodylomorpha. It implies that in life, the TM in thalattosuchians was exposed and attached to the lateral edge of the head as in lizards and turtles today (Wever, 1978; Dooling et al. 2000; Werner et al. 2005; Christensen‐Dalsgaard & Carr, 2008; Christensen‐Dalsgaard et al. 2012; Willis et al. 2013).
The closure of the posterior portion of the MC and the otic aperture of the cranioquadrate canal occurred within the ‘protosuchian’ grade, and it is present in the ‘basal notosuchian/sebecian pattern’, the ‘advanced notosuchian pattern’ and plesiomorphically in neosuchians. In spite of the early advances in basal crocodyliforms to enclose the posterior MC, Thalattosuchia have a posteriorly opened MC, and a large but enclosed occipital aperture of the cranioquadrate passage. Accordingly, these modifications may have occurred as reversions to the plesiomorphic state, perhaps in association with reduced hearing function and a fully marine lifestyle. It is not clear if all basal thalattosuchians have the occipital aperture of the cranioquadrate passage enclosed by the quadrate, the squamosal and the otoccipital. However, the large clade of metriorhynchids certainly do (Fig. 5D). In addition, a closed occipital aperture of the cranioquadrate passage does not require the simultaneous occurrence of related structures as IOC and OB. In thalattosuchians, the posterior attachment of the TM did not occur in the suspensory plate or exhibit a different configuration not requiring an OB. Differently from other mesoeucrocodylians, the BOA represents the only aperture in the thalattosuchian ADQ, and implies an apomorphic state of the group, in which all the subtympanic foramina are closed.
The distribution of the ‘basal notosuchian/sebecian pattern’ along the phylogenetic framework shows this type of MC as the most widespread anatomy for the basal metasuchians (Fig. 9). During this section of the crocodyliform evolution, general traits were for the first time established and kept with minor subsequent changes along the later evolution of the group.
The inset BOA and longitudinal sulcus at the lateral edge of squamosal and postorbital (implying the presence of earlids) is ubiquitously present in metasuchians. This condition is shared with taxa with the ‘advanced notosuchian pattern’ and all neosuchians, including the crown‐group. In a similar way, the MC and BOA are closed posteriorly by the contact of the squamosal and the quadrate and the occipital aperture of the cranioquadrate passage closed by the quadrate, squamosal and the otoccipital.
The complex anatomy of the BOA, including the IOC, DOI and OB, is present for the first time in metasuchian, representing apomorphies for this group, and maintained in more derived forms. However, a closer inspection of the BOA anatomy of some ‘protosuchians’ (e.g. Hsisosuchus dashanpuensis and Hsisosuchus chungkingensis) can potentially change the interpretation for the origins of these structures. The presence of the IOC suggests that at the metasuchian node, the cranioquadrate passage becomes closer to the BOA, extensively enclosed in the canal formed laterally by the quadrate and squamosal, while the courses of the tempororbital vessels and seventh cranial nerve are closer to the condition of extant crocodyliforms (Benton & Clark, 1988; Sedlmayr, 2002). The dorsally displaced OB extends from the posterior wall of the BOA and restricts the IOC to a dorsal position (Fig. 6). It supports that the BOA was mostly covered by the TM in the ‘basal notosuchian/sebecian pattern’, which is different from the extant forms that have a greater portion of the BOA positioned posteriorly to the TM (Figs 2 and 3).
The subtympanic foramen is the only extra aperture in the ADQ of basal metasuchians with the ‘basal notosuchian/sebecian pattern’. The homology of this structure with one of the multiple apertures present in the ADQ of basal crocodyliforms and the ‘advanced notosuchian pattern’ was proposed. This proposition fulfils the homology criteria of similarity, conjunction and congruence (Patterson, 1982; Pinna, 1991), even though the variable configuration of the foramina discourages the assignment of the exact aperture that the subtympanic foramen is homologous to. However, the subtympanic ‘A’ (fenestra ‘A’) is not the best candidate given its posteroventral position at the margin of the OI, whereas the single subtympanic foramen of the ‘basal notosuchian/sebecian pattern’ is located anterodorsally to its equivalent position at the ADQ. The ventral margin of the POF suggests a complex morphology of the TM. This morphology might have been related to a unique shape for the TM different from the oval structure of the extant forms.
The greater modifications in ‘advanced notosuchian pattern’ are related to the extension of the sulcus at the lateral shelves of the skull for the upper earlid in baurusuchids, the dimensions of the MC, and the pattern of the multiple subtympanic foramina on the quadrate. The sulcus for the upper earlid in bauruschids suggests an orientation of the upper earlid that would have also expanded from the back of the MC, differently from the strictly vertical orientation from the skull roof in extant crocodyliforms and other fossil groups. The MC is dorsoventrally increased in ‘advanced notosuchian pattern’ due to a well‐developed postorbital descending flange, as in Pissarrachampsa sera (LPRP/USP‐0049), Notosuchus terrestris (MUCPv‐147), Mariliasuchus amarali (UFRJ DG 106‐R) and Caipirasuchus montealtensis (MPMA 15‐001/90). This is rather different from ‘protosuchians’ in which the expansion of the MC is achieved by a well‐developed anterodorsal process of the quadratojugal (e.g. Protosuchus haughtoni BP/1/4770).
The quadrate pneumatization in the ‘advanced notosuchian pattern’ is similar to the configuration of some of the basal crocodyliforms previously discussed, including the presence of a larger ventral‐most aperture in some taxa. The same discussion regarding the homology among the multiple extra apertures and the subtympanic foramen is valid here. Therefore, it is proposed that the structures called quadrate fenestrae in advanced notosuchians and baurusuchids are homologous to the single subtympanic foramina of the ‘basal notosuchian/sebecian pattern’, neosuchians, and ultimately to the multiple apertures in basal crocodyliforms. The phylogenetic distribution of the ‘advanced notosuchian pattern’ suggests an independent acquisition of the multiple pneumatic apertures from ‘basal notosuchian/sebecian pattern’. Thus, the direct homology among each of the extra subtympanic foramen in basal crocodyliforms and advanced notosuchians is not feasible. The unique orientation of the subtympanic foramina present in Baurusuchinae, in which most of the foramen are not facing laterally but perpendicularly to the BOA, creates a shelf at the anterior perimeter of the OI and a further inset BOA that ultimately works as a secondary BOA.
The taxonomical sampling was focused on basal Crocodyliformes, basal Mesoeucrocodylia and basal Metasuchia, in which the greater modifications in MC are concentrated. Accordingly, the current analysis did not embody the great diversity of fossil neosuchians, eusuchians and crocodylians. Further analyses with broader samples of neosuchians are desirable for a better understanding of the modifications in the MC of specific groups, such as dyrosaurids, goniopholidids and paralligatorids, as well as basal eusuchians and crocodylians.
The current analysis indicates that many characters of the MC in crown‐group Crocodylia were already established at the origins of Neosuchia. The neosuchian ADQ is ancestrally pierced by two apertures, the BOA and a single subtympanic foramen. The latter is absent in some forms and represents multiple evolutionary losses of this structure. The complex BOA including EAM, IOC and DOI is also the plesiomorphic state for the clade but the IOC assumes two anatomies among the neosuchians. The widespread condition is the same as described for ‘basal notosuchian/sebecian pattern’ and extant forms. It is present in an array of basal neosuchians and represents the plesiomorphic state for the clade.
The other condition is found in some derived groups of neosuchians in which the IOC assumes a unique morphology extending posteroventrally at the posteroventral corner of the BOA and forming a laterally and posteriorly opened cranioquadrate passage (Delfino et al. 2008). However, the configuration of the MC and BOA is achieved by secondary modifications of a typical neosuchian BOA. In these neosuchians, the squamosal is not limited to the skull roof and has a ventrally directed lamina bounding the MC and BOA posteriorly (Fig. 8C,E). The opened canal is formed dorsally by this squamosal lamina, ventrally by the quadrate, and posteromedially by the otoccipital. Anteriorly, the OB limits the canal as in neosuchians with closed cranioquadrate passage. Posteriorly, the otoccipital squamosal and quadrate fail to meet and enclose the occipital aperture of the passage.
The phylogeny of advanced neosuchians is in a state of flux. The hypotheses diverge in the position of neosuchians with secondarily opened cranioquadrate passage and there is no complete taxonomical overlapping among the hypotheses (Andrade et al. 2011; Buscalioni et al. 2011; Figueiredo et al. 2011; Adams, 2013, 2014; Montefeltro et al. 2013; Pritchard et al. 2013; Puértolas‐Pascual et al. 2014; Turner, 2015). In addition, key taxa, both in basal and derived positions in Neosuchia, lack well‐preserved MCs (e.g. Bernissartia fagesii; Dollo, 1883; IRScNBr 46; Theriosuchus pusillus; Owen, 1879; NHMUK 48330; Pholidosaurus purberckensis; NHMUK 36721). These circumstances preclude a detailed analysis of the changes in the morphology of the cranioquadrate passage along neosuchian evolutionary history. However, all hypotheses imply a closed cranioquadrate passage as the ancestral state for Neosuchia and Crocodylia, but not necessarily for Eusuchia, and also imply multiple shifts in this character along the evolutionary history of neosuchians (Delfino et al. 2008; Martin, 2010; Andrade et al. 2011; Brochu, 2011, 2012; Buscalioni et al. 2011; Figueiredo et al. 2011; Puértolas et al. 2011; Conrad et al. 2013; Puértolas‐Pascual et al. 2014; Turner, 2015).
Addressing alternative positions of Thalattosuchia
To understand the influences of alternative positions of Thalattosuchia, the two most common alternative positions for the group (within Neosuchia and the sister‐clade of Crocodyliformes) were emulated in the phylogenetic framework and the evolution of this anatomical system was evaluated. Both alternative scenarios still imply that Thalattosuchia lost the lateral expansions of the skull roof overhanging the temporal region and the inset BOA. Both traits were established at different levels of the basal crocodylomorphs and are optimized as the plesiomorphic state for Crocodylomorpha (Clark & Sues, 2002; Pol et al. 2013). In addition, both alternative positions require the closure of all subtympanic foramina in Thalattosuchia. The crocodyliform sister‐clade scenario requires the full closure of the multiple subtympanic foramina present in Junggarsuchus sloani, Almadasuchus figarii and basal crocodyliforms. On the other side, a Neosuchia affinity requires the closure of the single subtympanic foramen present plesiomorphicaly in Neosuchia and Tethysuchia.
The other traits of this anatomical system imply different interpretations depending on the alternative phylogenetic position for Thalattosuchia taken into consideration. In view of the neosuchian position for Thalattosuchia (Turner & Sertich, 2010; Pol et al. 2012, 2013, 2014), optimization of the posteriorly opened MC and BOA is better interpreted as a reversion to the state present in basal crocodyliforms and crocodylomorphs (Clark & Sues, 2002; Pol et al. 2013). This condition departs from the widespread anatomy in Neosuchia and Tethysuchia in which the outer ear is bounded posteriorly by the quadrate and squamosal (Pol et al. 2013). Such rearrangement of the MC could also have drastically affected the course of the cranioquadrate passage and eliminated the IOC. However, even in eusuchians with a laterally opened cranioquadrate passage (e.g. Hylaeochampsa vectiana NHMUK R177), the IOC and OB are present. The absence of the sulcus on the outer surface of the squamosal and postorbital for the attachment of the upper earlid has a similar evolutionary interpretation. This structure is ubiquitously present in Neosuchia and the possible association of Thalattosuchia to this clade points to the loss of this structure.
Assuming a sister‐clade relationship of Thalattosuchia and Crocodyliformes (Benton & Clark, 1988; Jouve, 2009; Pol & Gasparini, 2009; Wilberg, 2015b), the posteriorly opened MC and BOA presented by this clade, basal crocodyliforms and basal crocodylomorphs match the plesiomorphic condition for Crocodylomorpha. Thus, this scenario does not require extra evolutionary steps in the interpretation of the evolution of these structures. However, Almadasuchus figarii has a partially closed MC and BOA (Pol et al. 2013). Therefore, the putative Thalattosuchia + Crocodyliformes node partially lost the somewhat more advanced anatomy present in Almadasuchus figarii, or there was not a single, early origin of this closure as suggested by the primary phylogenetic framework. The optimization of the absence of the sulcus for the upper earlid in Thalattosuchia depends on Kayentasuchus walkeri and Almadasuchus figarii. If Kayentasuchus walkeri is considered closely related to crocodyliforms and subsequent analyses of Almadasuchus figarii confirm the presence of this sulcus, then the absence in Thalattosuchia is better interpreted as a reversion to the condition present in more basal crocodylomorphs.
The greatest impacts of a putative sister‐clade relationship of Thalattosuchia and Crocodyliformes in the evolution of the MC are related to the cranioquadrate passage and the IOC. In this alternative scenario, the likely occipital closure of the cranioquadrate passage in all thalattosuchians (Jouve, 2009; Parrilla‐Bel et al. 2013) is better interpreted as homoplastically acquired with a similar condition appearing in derived ‘protosuchians’ and maintained in Mesoeucrocodylia. In such a scenario, the absence of IOC in thalatosuchians would support a different arrangement of the cranioquadrate passage in this clade that does not require an otic aperture of the passage.
Considering the two alternatives scenarios, the unique anatomy of the ‘thalattosuchian pattern’ is greatly divergent from the MC of all putative closely related clades. These differences force Thalattosuchia to significantly interfere on character polarization of this anatomical system in each alternative phylogenetic position. The unique morphology of Thalattosuchia is equally incongruent with the generally accepted phylogenetic framework used here, so does not provide any further insight into the phylogenetic position of this enigmantic clade. The profound rearrangements in the MC at the origins of this clade, which were associated with reduced tympanic hearing, are probably associated with their fully marine lifestyle and have no comparisons throughout Crocodylomorpha. A greater effort is necessary to investigate other traits in the MC in detail before claiming for a better fit to any phylogenetic position for Thalattosuchia supported by this anatomical system. However, it is noted that other derived marine taxa, such as Dyrosauridae, do not modify their external ears to the same degree, suggesting that Thalattosuchia evolved either independently from Dyrosauridae or that auditory functions were quite divergent in these taxa.
Palaeobiological implications
Functional anatomy of the hearing apparatus is strongly linked to habitat occupation in tetrapods (Thewissen & Nummela, 2008; Christensen‐Dalsgaard et al. 2011, 2012; Willis et al. 2013). Evolutionary transitions between terrestrial, semi‐aquatic and aquatic habitats impose large constraints to hearing due to the different sound transmission properties of these different media (Clack, 2002a,b; Christensen‐Dalsgaard & Carr, 2008; Christensen‐Dalsgaard et al. 2011). Crocodyliformes explored all these habitats multiple times and each transition is associated with modifications in their outer ear anatomy. Overall, the MC in crocodyliforms indicates that the outer ear is strongly linked to clade‐specific habitat occupation and emphasizes that auditory abilities were a major factor in evolution within this group. The pattern of anatomical evolution in the MC was not a progressive accumulation toward that in extant forms. Instead, important changes are concentrated at particular nodes with some exceptional modifications in divergent lineages.
The first Crocodyliformes begin to show a marked departure from the plesiomorphic archosauromorph state. The plesiomorphic morphology was a moderately sized TM probably supported behind the posteriorly concave quadrate with little to no accessory musculoskeletal modifications to the outer ear, similar to extant squamates. ‘Protosuchians’ altered this morphology by evolving a complex dorsal ear flap musculature that presumably functioned to protect their greatly expanded TM (Busbey & Gow, 1984; Gow, 2000). This membrane was stretched anteriorly toward the postorbital and bounded dorsally by the lateral shelf of the squamosal. Posteriorly, the large tympanum was also partially isolated from the depressor mandibulae musculature by a laterally expanded otoccipital and posteroventrally curved squamosal. The quadrate is also extensively pneumatized by quadrate diverticula from the middle ear. Internal to the tympanum, these diverticula expand to re‐enter the middle ear via a complex set of subtympanic foramina. Internally, the periotic sinuses expand throughout the braincase to connect both middle ears via diverticula travelling dorsally through the mastoid antrum of the supraoccipital (Crompton & Smith, 1980; Hecht & Tarsitano, 1983; Busbey & Gow, 1984; Montefeltro & Larsson, 2011). This complex pneumatic morphology has been associated with the advanced auditory sensitivity and directionality of extant crocodylians (Bierman et al. 2014).
The origin of Crocodyliformes was thus accompanied with a sophisticated hearing system that may have facilitated the rapid radiation of the clade. The extension of the TM is a key factor discriminating properties of the hearing apparatus, and an enlarged TM is evidence for efficient impedance matching and an increased hearing ability (Wever, 1978; Müller & Tsuji, 2007; Nummela & Thewissen, 2008). The extraordinarily large TM of basal crocodyliforms was partially isolated from the depressor mandibulae musculature and roofed by novel dorsal ear musculature for protection and/or sound focusing and, internal to the tympanum, evolved a complex set of pneumatic chambers that acoustically couple both middle ears. This coupling probably aided to augment certain frequencies via a pressure difference receiver mechanism described for extant crocodylians (Bierman et al. 2014).
Regardless of the phylogenetic position of Thalattosuchia, it implies that their evolution toward a marine lifestyle is accompanied by a nearly complete loss of any modifications of outer ear anatomy. The dorsal earlid is absent, the posterior enclosure of the tympanum by the squamosal and quadrate is absent, the TM is greatly reduced and displaced toward the surface of the head, and the periotic diverticula are reduced to eliminate any subtympanic foramen and mastoid antrum, thus obviating any interaural communication. This departure from the crocodyliform anatomy was certainly attributed to the trade‐offs of hearing and living in water (Pol & Gasparini, 2009; Young et al. 2010). Impedance‐matching middle ears are less effective in water where sounds in the dense water media translate easily through dense tissues (Nummela & Thewissen, 2008). However, at this point it is not possible to address any potentail variation in hearing capabilities within the clade.
The presence of well‐developed upper earlids in the terrestrial sebecids, notosuchians and bauruschians suggests a further elaboration on the ‘protosuchian’ state. These former taxa are all terrestrial, evolved a posteriorly closed MC to completely encircle the tympanum by bone and retained a mastoid antrum (Colbert, 1946; Sereno & Larsson, 2009; Montefeltro et al. 2011; Pol & Powell, 2011; Godoy et al. 2014; Kellner et al. 2014; Pol et al. 2014). Baurusuchians evolved perhaps the most sophisticated hearing anatomy. This clade is characterized by greatly elaborated upper ear musculature, a laterally extended posterior squamosal‐quadrate closure of the MC, extensive periotic pneumatization including a complex of subtympanic foramina, and an expanded TM that would have exceeded the area of each orbit.
This study raises a possible relation of the MC and upper earlid to a device for the maximization of sound wave reception in basal crocodyliforms and baurusuchids, and to more basal notosuchians to some extent. In these groups, the MC is expanded and occupies a great portion at the back of the head. The combination of the concave and expanded MC to the arrangement of the upper earlid at the borders of the MC may have functioned as a resonance device analogous to the mammalian pinna. A further modification in the attachment of the upper earlid of Baurusuchia supports the presence of a resonance device. The upper earlid would have expanded from the back of the MC and along the attachment sulcus restricted on the squamosal. The large size of the MC and TM implies that the fleshy otic aperture was much larger in these taxa and possibly lacked the mechanism to seal off the MC with its upper earlids. The multiple subtympanic foramina present in baurusuchians are also in alignment with a putative increased hearing capability of these taxa. Recent work by Dufeau & Witmer (2015) has shown that in extant forms the single subtympanic foramen and its associated infundibular diverticulum may have resonant functions. In this sense, the multiple subtympanic foramina may have functioned as multiple resonance structures in the ear of these terrestrial taxa.
The hypothesis of a resonance device in these crocodyliforms has yet to be tested. However, if present, it may have its origins in basal crocodyliforms that may have also been experimenting with acoustic resonance devices. This scenario would imply that the closing outer ear of extant crocodylians was exapted from a terrestrial resonance function for an amphibious function to close and protect their TM while submerged. The adaptation to a semi‐aquatic lifestyle may also have driven the reduced resonance properties of the MC in extant forms. This change most likely occurred within Neosuchia when the amphibious bodyplan originated. Such an exaptation for using ear musculature to protect the TM underwater may have evolved at least twice, if the semiaquatic Stolokrosuchus is a derived peirosaurid (Larsson & Gado, 2000; Montefeltro et al. 2013; Sertich & O'Connor, 2014), or once if it is a basal neosuchian (Turner & Sertich, 2010; Andrade et al. 2011). Analysis of the intrinsic musculature of the upper earlid is beyond the scope of this study, but future investigation of its osteological correlates could offer important clues on their function. In addition, osteological correlates for the lower earlid have not been proposed. The lower earlid is responsible for most of the earlid movements in extant crocodylians (Shute & Bellairs, 1955), and its analysis in fossil forms might also help to understand the function of the structures at the crocodyliform origins.
Concluding remarks
Osteological and the soft tissue components of the MC, including the complex anatomy of the BOA formed by three incisures (OI, IOC and the DOI) and the limited covering of the TM on the posterior portion of the BOA are described in extant crocodyliforms in an attempt to further clarify this understudied anatomical region.
Osteological correlates for the soft anatomy of the MC in extant crocodyliforms were established. Among others, the anterior and posterior attachment limits of the TM at the POF and OB, respectively, were established.
The osteological correlates were mapped through the primary phylogenetic framework to trace the pattern of character acquisition in the MC of Crocodyliformes. Some traits hitherto believed to be unique for crocodyliforms were found to be already present as ancestral characters at the base of the clade, for instance the subtympanic foramina were already present in some Crocodylomorpha during the Triassic. At the origin of Neosuchia sometime during the Jurassic, all characters present in the MC of extant crocodyliforms were already established. The phylogenetic congruence of many osteological correlates was used to propose homologies among structures of the MC of extant forms to those of fossil groups, such as the homology of the multiple subtympanic foramina present in basal crocodyliforms, advanced notosuchians and baurusuchids.
Also, the evolution of this complex anatomical system in the alternative positions for Thalattosuchia (within Neosuchia and sister to Crocodyliformes) was evaluated. Both scenarios also lead to the proposition of great rearrangements of the MC early on the evolution of Thalattosuchia. Such drastic rearrangements can also be associated to the adaptation of the sensory functions to a fully aquatic lifestyle. However, at this point there is no clear phylogenetic scenario to fit the evolutionary history of the MC in Thalattosuchia, and outer ear morphology does not suggest any more parsimonious phylogenetic position.
Palaeobiological implications for the modified anatomy of the MC in fossil groups and its components describes a more complex scenario of the evolution of the crocodyliform external ear and auditory function. For instance, it is suggested that the origins of the earlids were not linked to an amphibious lifestyle or protection of the TM, but were rather evolved as a feature associated with the improvement of sound wave collection and a possible analogous to the mammalian pinna in baurusuchians. Similarly, the presence of the subtympanic foramina is interpreted as a mechanism of increased sound localization capacity. Together, these suggested palaeobiological implications suggest that airborne hearing played an important role in the origin of Crocodyliformes and facilitated a broad range of evolutionary endpoints within the clade. These evolutionary trajectories are now available to be tested in future works.
Author contributions
FCM, HCEL and DVA conceived and designed the experiments. FCM performed the dissections. FCM, HCEL and DVA analysed the data. FCM and HCEL wrote the paper.
Supporting information
Data S1. Complete list of taxa analysed first‐hand in this study with their taxonomical assignment and collection numbers.
Acknowledgements
The authors are greatly indebted to Ari Palomo Del’ Alamo and Luis Antonio B. Basseti (Criatório Caiman Ltda) for the donation of the Caiman latirostris heads used in this study. The authors thank Stéphane Jouve and the anonymous reviewer for their comments, and Julia Clarke for handling the manuscript. The authors thank Martin Ezcurra, Caio Geroto and Paulo Nascimento for additional images of taxa mentioned here. FCM thanks São Paulo Research Foundation (grant 2013/11358‐3) for the postdoctoral fellowship, and Brazil Visiting Fellows Scheme of the University of Birmingham for additional funding for collection visits. DVA was supported by the National Council for Scientific and Technological Development (CNPq, proc. 302045/2012‐0) and by the São Paulo Research Foundation (FAPESP, multiple research grants). The authors thank Max Langer (Universidade de São Paulo) and Richard Butler (University of Birmingham) for advice and discussions along the development of the research. The authors thank the following curators, collections managers and researchers who provided access to specimens in their care: Mark Norell and Carl Mehling (AMNH), Bernhard Zipfel and Bruce Rubdge (BPI), Juliana Sayão and Edison Vicente Oliveira (DG‐CTG‐UFPE), Norbert Micklich (HLMD), Annelise Folie (IRScNBr), Luis Chiappe and Maureen Walsh (LACM), Max Langer (LPRP/USP), Lucas Pomi and Marcelo Reguero (MLP), Alexander Kellner, Deise Henriques and Rodrigo Giesta Figueiredo (MN), Alberto Garrido (MOZ), Antonio Celso Arruda‐Campos, Fabiano Iori and Sandra Tavares (MPMA), Domenica Santos, Flávia Coelho, Jorge Calvo and Juan Porfiri (MUCP), Lorna Steel and Paul Barret (NHMUK), Jaime Powell (PVL), David Evans (ROM), Sheena Kaal (SAM), and Paul Sereno (UCRC), Felipe Vasconcellos, Ismar Carvalho and Thiago S. Marinho (UFRJ).
References
- Adams TL (2013) A new neosuchian crocodyliform from the Lower Cretaceous (late Aptian) Twin Mountains formation of North‐Central Texas. J Vert Paleontol 33, 85–101. [Google Scholar]
- Adams TL (2014) Small crocodyliform from the Lower Cretaceous (late Aptian) of central Texas and its systematic relationship to the evolution of Eusuchia. J Paleontol 88, 1031–1049. [Google Scholar]
- Andrade MB, Bertini RJ (2008a) A new Sphagesaurus (Mesoeucrocodylia: Notosuchia) from the Upper Cretaceous of Monte Alto City (Bauru Group, Brazil), and a revision of the Sphagesauridae. Historical Biol 20, 101–136. [Google Scholar]
- Andrade MB, Bertini RJ (2008b) Morphological and anatomical observations about Mariliasuchus amarali and Notosuchus terrestris (Mesoeucrocodylia) and their relationships with other South American notosuchians. Arch Mus Nac 66, 5–62. [Google Scholar]
- Andrade MB, Edmonds R, Benton M, et al. (2011) A new Berriasian species of Goniopholis (Mesoeucrocodylia, Neosuchia) from England, and a review of the genus. Zool J Lin Soc 163, S66–S108. [Google Scholar]
- Andrews CW (1913) A Descriptive Catalogue of the Marine Reptiles of the Oxford Clay, Part Two London: British Museum (Natural History). [Google Scholar]
- Barbosa JB, Kellner AWA, Vianna MSS (2008) New dyrosaurid crocodylomorph and evidences for faunal turnover at the K‐P transition in Brazil. Proc R Soc Lond B Biol Sci 275, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumel JJ, Witmer LM (1993) Osteology In: Handbook of Avian Anatomy: Nomina Anatomica Avium (ed. Baumel JJ.), pp. 45–132. Cambridge: Publication of the Nuttal Ornithological Club. [Google Scholar]
- Benton M, Clark JM (1988) Archosaur phylogeny and the relationships of the Crocodylia In: The Phylogeny and Classification of the Tetrapods (ed. Benton M.), pp. 295–338. Oxford: Clarendon Press. [Google Scholar]
- Benton MJ, Walker AD (2002) Erpetosuchus, a crocodile‐like basal archosaur from the Late Triassic of Elgin, Scotland. Zool J Lin Soc 136, 25–47. [Google Scholar]
- Bierman HS, Thornton JL, Jones HG, et al. (2014) Biophysics of directional hearing in the American alligator (Alligator mississippiensis). J Exp Biol 2017, 1094–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blainville HD (1853) Lettres sur les crocodiles vivants et fossiles In: Bulletin de la Société Linnéenne de Normandie (ed. Deslongchamps E.), pp. 109–120. Caen, France: Société Linnéenne de Normandie. [Google Scholar]
- Bolt JR, Lombard RE (1985) Evolution of the amphibian tympanic ear and the origin of frogs. Biol J Linn Soc 24, 83–99. [Google Scholar]
- Bonaparte J (1969) Dos nuevos “faunas” de reptiles triásicos de Argentina In: Gondwana Stratigraphy. I.U.G.S. Symposium, pp. 283–306. Paris: UNESCO. [Google Scholar]
- Bonaparte JF (1971) Los vertebrados fósiles de la Formación Rio Colorado, de la Ciudad de Neuquén y cercanías, Cretácico Superior, Argentina. Rev Mus Argent Cienc Nat 4, 17–123. [Google Scholar]
- Brochu CA (1999) Phylogenetics, taxonomy, and historical biogeography of Alligatoroidea. J Vert Paleontol Memoir 6, 9–100. [Google Scholar]
- Brochu CA (2006) A new miniature horned crocodile from the Quaternary of Aldabra Atoll, Western Indian Ocean. Copeia 2006, 149–158. [Google Scholar]
- Brochu CA (2007) Systematics and taxonomy of Eocene tomistomine crocodylians from Britain and Northern Europe. Palaeontology 50, 917–928. [Google Scholar]
- Brochu CA (2011) Phylogenetic relationships of Necrosuchus ionensis Simpson, 1937 and the early history of caimanines. Zool J Lin Soc 163, S228–S256. [Google Scholar]
- Brochu CA (2012) Phylogenetic relationships of Palaeogene ziphodont eusuchians and the status of Pristichampsus Gervais, 1853. Earth Environ Sci Trans R Soc Edinb 103, 521–550. [Google Scholar]
- Brochu CA, Bouaré ML, Sissoko F, et al. (2002) A dyrosaurid crocodyliform braincase from Mali. J Paleontol 76, 1060–1071. [Google Scholar]
- Brochu CA, Wagner JR, Jouve S, et al. (2009) A correction corrected: consensus over the meaning of Crocodylia and why it matters. Syst Biol 58, 1–7. [DOI] [PubMed] [Google Scholar]
- Brochu CA, Njau J, Blumenschine RJ, et al. (2010) A new horned crocodile from the Plio‐Pleistocene hominid sites at Olduvai Gorge, Tanzania. PLoS ONE 5, e9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronn HG (1841) Über die fossilen Gaviale der Lias‐Formation und der Oolithe. Arch Naturgesch 8, 77–82. [Google Scholar]
- Bronzati MF, Montefeltro FC, Langer MC (2012) A species‐level supertree of Crocodyliformes. Hist Biol 24, 598–606. [Google Scholar]
- Bronzati MF, Montefeltro FC, Langer MC (2015) Diversification events and the effects of mass extinction on Crocodyliformes evolutionary history. R Soc Open Sci 2, 140385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B (1933) An ancestral crocodile. Am Mus Novit 638, 1–4. [Google Scholar]
- Buchanan LA (2009) Kambara taraina sp.nov. (Crocodylia, Crocodyloidea), a new Eocene mekosuchine from Queensland, Australia, and a revision of the genus. J Vert Paleontol 29, 473–486. [Google Scholar]
- Buffetaut E (1994) A new crocodilian from the Cretaceous of southern Morocco. Comptes Rendu Acad Sci 319, 1563–1568. [Google Scholar]
- Buffetaut E, Taquet P (1979) Early Cretaceous terrestrial crocodilian and the opening of the South‐Atlantic. Nature 280, 486–487. [Google Scholar]
- Busbey AB (1995) The structural consequences of skull flattening in crocodilians In: Functional Morphology in Vertebrate Paleontology (ed. Thomason J.), pp. 173–192. New York, NY: Cambridge University Press. [Google Scholar]
- Busbey AB III, Gow CE (1984) A new protosuchian crocodile from the Upper Elliot formation of South Africa. Palaeontol Afr 25, 127–149. [Google Scholar]
- Buscalioni AD, Piras P, Vullo R, et al. (2011) Early eusuchia crocodylomorpha from the vertebrate‐rich Plattenkalk of Pietraroia (Lower Albian, southern Apennines, Italy). Zool J Lin Soc 163, S199–S227. [Google Scholar]
- Butler RJ, Sullivan C, Ezcurra MD, et al. (2014a) New clade of enigmatic early archosaurs yields insights into early pseudosuchian phylogeny and the biogeography of the archosaur radiation. BMC Evol Biol 14, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler RJ, Rauhut OWM, Stocker MR, et al. (2014b) Redescription of the phytosaurs Paleorhinus (‘Francosuchus’) angustifrons and Ebrachosuchus neukami from Germany, with implications for Late Triassic biochronology. Zool J Lin Soc 170, 155–208. [Google Scholar]
- Campos DA, Oliveira GR, Figueiredo RG, et al. (2011) On a new peirosaurid crocodyliform from the Upper Cretaceous, Bauru Group, southeastern Brazil. An Acad Bras Ciênc 83, 317–327. [DOI] [PubMed] [Google Scholar]
- Carvalho IS, Bertini RJ (1999) Mariliasuchus, um novo Crocodylomorpha (Notosuchia) do Cretáceo da Bacia Bauru, Brasil. Geol Colomb 24, 83–105. [Google Scholar]
- Carvalho IS, Campos DA (1988) Um mamífero triconodonte do Cretáceo Inferior do Maranhão, Brasil. An Acad Bras Ciênc 60, 437–446. [Google Scholar]
- Carvalho IS, Campos ACA, Nobre PH (2005) Baurusuchus salgadoensis, a new Crocodylomorpha (Cretaceous), Brazil. Gondwana Res 8, 11–30. [Google Scholar]
- Carvalho IS, Vasconcellos FM, Tavares SAS (2007) Montealtosuchus arrudacamposi, a new peirosaurid crocodile (Mesoeucrocodylia) from the Late Cretaceous Adamantina Formation of Brazil. Zootaxa 1607, 35–46. [Google Scholar]
- Christensen‐Dalsgaard J (2005) Directional hearing in non mammalian tetrapods In: Sound Source Localization (eds Popper AN, Fay RR.), pp. 67–123. New York, NY: Springer. [Google Scholar]
- Christensen‐Dalsgaard J, Carr CE (2008) Evolution of a sensory novelty: tympanic ears and the associated neural processing. Brain Res Bull 75, 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen‐Dalsgaard J, Brandt C, Wilson M, et al. (2011) Hearing in the African lungfish (Protopterus annectens): pre‐adaptation to pressure hearing in tetrapods? Biol Lett 7, 139–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen‐Dalsgaard J, Brandt C, Willis KL, et al. (2012) Specialization for underwater hearing by the tympanic middle ear of the turtle, Trachemys scripta elegans . Proc R Soc Lond B Biol Sci 279, 2816–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack JA (1997) The evolution of tetrapod ears and the fossil record. Brain Behav Evol 50, 198–2012. [DOI] [PubMed] [Google Scholar]
- Clack JA (2002a) Patterns and processes in the early evolution of the tetrapod ear. J Neurobiol 53, 251–264. [DOI] [PubMed] [Google Scholar]
- Clack JA (2002b) Gaining Ground: The Origin and Evolution of Tetrapods. Bloomington, IN: University Press. [Google Scholar]
- Clark JM (1986) Phylogenetic Relationships of the Crocodylomorph Archosaurs, pp. 556 Chicago, IL: University of Chicago. [Google Scholar]
- Clark JM (1994) Patterns of evolution in Mesozoic Crocodyliformes In: In the Shadow of Dinosaurs (eds Fraser NC, Sues H‐D.), pp. 84–97. Cambridge: Cambridge University. [Google Scholar]
- Clark JM (2011) A new shartegosuchid crocodyliform from the Upper Jurassic Morrison Formation of western Colorado. Zool J Lin Soc 163, S152–S172. [Google Scholar]
- Clark JM, Sues H‐D (2002) Two new basal crocodylomorph archosaurs from the Lower Jurassic and the monophyly of the Sphenosuchia. Zool J Lin Soc 136, 77–95. [Google Scholar]
- Clark JM, Sues H‐D, Berman D (2000) A new specimen of Hesperosuchus agilis from the Upper Triassic of New Mexico and the interrelationships of basal crocodylomorph archosaurs. J Vert Paleontol 20, 683–740. [Google Scholar]
- Clark JM, Xu X, Forster CA, et al. (2004) A Middle Jurassic ‘sphenosuchian’ from China and the origin of the crocodylian skull. Nature 430, 1021–1024. [DOI] [PubMed] [Google Scholar]
- Colbert EH (1946) Sebecus, representative of a peculiar suborder of fossil crocodilia from Patagonia. Bull Am Mus Nat Hist 87, 217–270. [Google Scholar]
- Colbert EH (1952) A pseudosuchian reptile from Arizona. Bull Am Mus Nat Hist 99, 561–592. [Google Scholar]
- Conrad JL, Jenkins K, Lehmann T, et al. (2013) New specimens of ‘Crocodylus’ pigotti (Crocodylidae) from Rusinga Island, Kenya, and generic reallocation of the species. J Vert Paleontol 33, 629–646. [Google Scholar]
- Cope ED (1861) Recent species of emydosaurian reptiles represented in the Museum of the Academy. Proc Acad Nat Sci Phila 1860, 549–551. [Google Scholar]
- Cope ED (1878) Descriptions of new extinct Vertebrata from the Upper Tertiary and Dakota Formations. Bull U S Geol Geogr Surv Terr 4, 379–396. [Google Scholar]
- Crompton A, Smith KK (1980) A new genus and species of crocodilian from the Kayenta Formation (Late Triassic) of Northern Arizona In Aspects of Vertebrate (ed. Jacobs LL.), pp. 193–217. Flagstaff: Museum of Northern Arizona Press. [Google Scholar]
- Crush P (1984) A Late Upper Triassic sphenosuchid crocodilian from Wales. Palaeontology 27, 131–157. [Google Scholar]
- Cuvier G (1807) Sur les différentes especes de Crocodiles vivans et sur leurs caracteres distinctifs. Ann Mus Hist Nat Paris 10, 8–86. [Google Scholar]
- Daudin FM (1802) Histoire naturelle, générale et particulière, des reptiles: ouvrage faisant suite à l'Histoire naturelle générale et particulière, composée par Leclerc de Buffon, et rédigée par C.S. Sonnini. De l'Imprimerie de F. Dufart,an X‐XI.
- Delfino M, Codrea V, Folie A, et al. (2008) A complete skull of Allodaposuchus precedens Nopsca, 1928 (Eusuchia) and a reassessment of the morphology of the taxon based on the Romanian remains. J Vert Paleontol 28, 111–122. [Google Scholar]
- Denton RK, Dobie JL, Parris DC (1997) The marine crocodilian Hyposaurus in North America In: Ancient Marine Reptiles (eds Callaway JM, Nicholls EL.). San Diego, CA: Academic Press. [Google Scholar]
- Dilkes DW (1998) The Early Triassic rhynchosaur Mesosuchus browni and the interrelationships of basal archosauromorph reptiles. Philos Trans R Soc Lond B Biol Sci 353, 501–541. [Google Scholar]
- Dollo L (1883) Première note sur les crocodiliens de Bernissart. Bull Mus R Hist Nat Belg 1883, 309–340. [Google Scholar]
- Dooling RJ, Lohr B, Dent ML (2000) Hearing in Birds and Reptiles In: Comparative Hearing: Birds and Reptiles (eds Dooling RJ, Fay RR, Popper AN.), pp. 308–359. New York, NY: Springer. [Google Scholar]
- Dufeau DL (2011) The evolution of cranial pneumaticity in Archosauria: Patterns of paratympanic sinus development [dissertation] Athens (OH): Ohio University. 174 pp. [Google Scholar]
- Dufeau DL, Witmer LM (2015) Ontogeny of the middle‐ear air‐sinus system in Alligator mississippiensis (Archosauria: Crocodylia). PLoS ONE 10, e0137060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eudes‐Deslongchamps E (1867) Notes sur les Téléosauriens. Bull Soc Linn Normandie 3, 124–221. [Google Scholar]
- Ewer RF (1965) The anatomy of the thecodont reptile Euparkeria capensis Broom. Philos Trans R Soc Lond B Biol Sci 248, 379–435. [Google Scholar]
- Figueiredo RG, Moreira JKR, Saraiva AAF, et al. (2011) Description of a new specimen of Susisuchus anatoceps (Crocodylomorpha: Mesoeucrocodylia) from the Crato Formation (Santana Group) with comments on Neosuchia. Zool J Lin Soc 163, S273–S288. [Google Scholar]
- Fiorelli LE, Calvo JO (2008) New remains of Notosuchus terrestris Woodward 1896 (Crocodyliformes: Mesoeucrocodylia) from Late Cretaceous Neuquèn, Patagonia, Argentina. Arch Mus Nac 66, 83–124. [Google Scholar]
- Fraas E (1901) Die Meerkrokodile (Thalattosuchia n. g.) eine neue sauriergruppe der Juraformation. Jahresh Ve vaterl Naturkd Wb 57, 409–418. [Google Scholar]
- Gao Y‐H (2001) A new species of Hsisosuchus from Dashanpu, Zigong, Sichuan. Vert Palasiat 7, 177–184. [Google Scholar]
- Gasparini Z, Dellapé D (1976) Un nuevo cocodrilo marino (Thalattosuchia, Metriorhynchidae) de la Formación Vaca Muerta (Jurásico, Tithoniano) de la provincia de Neuquén In Actas I Congresso Geológico Chileno, pp. C1–C21. Santiago, Chile. [Google Scholar]
- Gasparini Z, Chiappe LM, Fernandez M (1991) A new Senonian peirosaurid (Crocodylomorpha) from Argentina and a synopsis of the South American Cretaceous crocodilian. J Vert Paleontol 11, 316–333. [Google Scholar]
- Gasparini Z, Pol D, Spalletti LA (2006) An unusual marine crocodyliform from the Jurassic‐Cretaceous boundary of Patagonia. Science 311, 70–73. [DOI] [PubMed] [Google Scholar]
- Geoffroy Saint‐Hilaire E (1825) Recherches sur ‘organisation des Gavialis, sur leurs affinités naturelles desquelles résulte la nécessité d'une autre distribution générique: Gavialis, Teleosaurus, Steneosaurus . Mem Mus Hist Nat 12, 97–155. [Google Scholar]
- Gmelin J (1789) Linnei Systema Naturae. Beer, G.E: Leipzig. [Google Scholar]
- Godoy PL, Montefeltro FC, Norell MA, et al. (2014) An additional baurusuchid from the Cretaceous of Brazil with evidence of interspecific predation among Crocodyliformes. PLoS ONE 9, e97138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M, Brusatte S, Norell M (2013) The cranial pneumatic sinuses of the tyrannosauraurid Alioramus (Dinosauria: Theropoda) and the evolution of cranial pneumaticity in theropod dinosaurs. Am Mus Novit 3790, 1–46. [Google Scholar]
- Gow CE (2000) The skull of Protosuchus haughtoni, an Early Jurassic crocodyliform from Southern Africa. J Vert Paleontol 20, 49–56. [Google Scholar]
- Hastings AK, Bloch JI, Cadena EA, et al. (2010) A new small short‐snouted dyrosaurid (crocodylomorpha, Mesoeucrocodylia) from the Paleocene of northeastern Colombia. J Vert Paleontol 30, 139–162. [Google Scholar]
- Haughton SH (1915) A new thecodont from Stormberg Beds. Ann S Afr Mus 12, 98–105. [Google Scholar]
- Hecht BM, Tarsitano SF (1983) On the cranial morphology of the Protosuchia, Notosuchia and Eusuchia. Neues Jahrb Geol Palaontol Abh 11, 657–668. [Google Scholar]
- Hetherington T (2008) Comparative anatomy and function of hearing in aquatic amphibians, reptiles and birds In: Sensory Evolution on the Threshold (eds Thewissen JGM, Nummela S.), pp. 183–209. London: University of California Press. [Google Scholar]
- Higgs DM, Brittan‐Powell EF, Soares D, et al. (2002) Amphibious auditory responses of the American alligator (Alligator mississippiensis). J Comp Physiol A 188, 217–223. [DOI] [PubMed] [Google Scholar]
- Hogg D (1990) The development of pneumatization in the skull of the domestic‐fowl (Gallus‐Gallus domesticus). J Anat 169, 139–151. [PMC free article] [PubMed] [Google Scholar]
- Holliday CM, Gardner NM (2012) A new Eusuchian crocodyliform with novel cranial integument and its significance for the origin and evolution of Crocodylia. PLoS ONE 7, e30471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday CM, Witmer LM (2009) The epipterygoid of crocodyliforms and its significance for the evolution of the orbitotemporal region of eusuchians. J Vert Paleontol 29, 715–733. [Google Scholar]
- von Huene F (1921) Neue pseudosuchier und coelurosaurier aus dem württembergischen Keuper. Acta Zool 2, 329–403. [Google Scholar]
- Iordansky NN (1973) The skull of the Crocodylia In: Biology of Reptilia (ed. Gans C.), pp. 201–262. New York, NY: Academic Press. [Google Scholar]
- Jouve S (2005) A new description of the skull of Dyrosaurus phosphaticus (Thomas, 1893) (Mesoeucrocodylia: Dyrosauridae) from the Lower Eocene of North Africa. Can J Earth Sci 42, 323–337. [Google Scholar]
- Jouve S (2009) The skull of Teleosaurus camodensis (Crocodylomorpha; Thalattosuchia), and phylogenetic analysis of Thalattosuchia. J Vert Paleontol 29, 88–102. [Google Scholar]
- Jouve S, Iarochène M, Bouya B, et al. (2006) A new species of Dyrosaurus (Crocodylomorpha, Dyrosauridae) from the early Eocene of Morocco: phylogenetic implications. Zool J Lin Soc 148, 603–656. [Google Scholar]
- Kellner AWA, Pinheiro AEPC, Campos DA (2014) A new sebecid from the Paleogene of Brazil and the crocodyliform radiation after the K‐Pg boundary. PLoS ONE 9, e81386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kley NJ, Sertich JJW, Turner A, et al. (2010) Craniofacial morphology of Simosuchus clarki (Crocodyliformes: Notosuchia) from the Late Cretaceous of Madagascar. J Vert Paleontol 30, 13–98. [Google Scholar]
- Kundrat M, Janacek J (2007) Cranial pneumatization and auditory perceptions of the oviraptorid dinosaur Conchoraptor gracilis (Theropoda, Maniraptora) from the Late Cretaceous of Mongolia. Naturwissenschaften 94, 769–778. [DOI] [PubMed] [Google Scholar]
- Lamouroux M (1820) Sur le crocodile fossile trouvé dans les carriè res du bourg d'Allemagne, à un quart de lieue de Cean. Ann Gen Sci Phys 3, 160–164. [Google Scholar]
- Langston JW (1975) Ziphodont crocodiles: Pristichampsus vorax (Troxell), new combination, from the Eocene of North America. Fieldiana Geol 33, 291–314. [Google Scholar]
- Larsson HCE, Gado B (2000) A new Early Cretaceous crocodyliform from Niger. Neues Jahrb Geol Palaontol Abh 217, 131–141. [Google Scholar]
- Larsson HCE, Sues H‐D (2007) Cranial osteology and phylogenetic relationships of Hamadasuchus rebouli (Crocodyliformes: Mesoeucrocodylia) from the Cretaceous of Morocco. Zool J Lin Soc 149, 533–567. [Google Scholar]
- Lauprasert K, Cuny G, Thirakhupt K, et al. (2009) Khoratosuchus jintasakuli gen. et sp. nov., an advanced neosuchian crocodyliform from the Early Cretaceous (Aptian–Albian) of NE Thailand. Geol Soc Spec Pub 315, 175–187. [Google Scholar]
- Laurenti JN (1768) Specimen medicum, exhibens synopsin reptilium emendatam cum experimentis circa venena et antidota reptilium austracorum, quod authoritate et consensu. Vindobonae, Vienna: Joan Thomae. [Google Scholar]
- Li J, Wu X‐C, Li X (1994) New material of Hsisosuchus chungkingensis from Sichuan, China. Vert Palasiat 4, 107–126. [Google Scholar]
- Lombard RE, Bolt JR (1979) Evolution of the tetrapod ear – analysis and reinterpretation. Biol J Linn Soc 11, 19–76. [Google Scholar]
- Lydekker R (1890) On a crocodilian jaw from the Oxford Clay of Peterborough. Q J Geol Soc London 46, 284–288. [Google Scholar]
- Marinho TS, Carvalho IS (2009) An armadillo‐like sphagesaurid crocodyliform from the Late Cretaceous of Brazil. J South Am Earth Sci 27, 36–41. [Google Scholar]
- Martin JE (2010) Allodaposuchus Nopsca, 1928 (Crocodylia, Eusuchia), from the Late Cretaceous of southern France and its relationships to Alligatoroidea. J Vert Paleontol 30, 756–767. [Google Scholar]
- Mason M, Farr M (2013) Flexibility within the middle ears of vertebrates. J Laryngol Otol 127, 2–14. [DOI] [PubMed] [Google Scholar]
- Meyer H (1841) Pholidosaurus schaumbergensis n. gen. n. sp. Neues Miner 1841, 343–345. [Google Scholar]
- Molnar R (2010) A new reconstruction of the skull of Sebecus icaeorhinus (Crocodyliformes: Sebecosuchia) from the Eocene of Argentina. Braz Geogr J Geosci Hum Res Medium 2, 314–330. [Google Scholar]
- Montefeltro FC, Larsson HCE (2011) Evolution of the otic region of fossil Crocodyliformes. J Vert Paleontol 2–5, 161. [Google Scholar]
- Montefeltro FC, Larsson HCE, Langer MC (2011) A new Baurusuchid (Crocodyliformes, Mesoeucrocodylia) from the Late Cretaceous of Brazil and the phylogeny of Baurusuchidae. PLoS ONE 6, e21916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefeltro FC, Larsson HCE, França MAG, et al. (2013) A new neosuchian with Asian affinities from the Jurassic of northeastern Brazil. Naturwissenschaften 100, 835–841. [DOI] [PubMed] [Google Scholar]
- Mook CC (1924) A new crocodilian from Mongolia. Am Mus Novit 117, 1–5. [Google Scholar]
- Mook C (1967) Preliminary description of a new goniopholid crocodilian. Kirtlandia 2, 1–10. [Google Scholar]
- Müller L (1838) Waarnemingen over de Indische krokodillen en Beschrijving van eene nieuwe sort. Tijdschr Nat Geschied 5, 61–87. [Google Scholar]
- Müller J, Tsuji L (2007) Impedance‐matching hearing in Paleozoic reptiles: evidence of advanced sensory perception at an early stage of amniote evolution. PLoS ONE 2, e889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash D (1968) A crocodile from the Upper Triassic of Lesotho. Proc Zool Soc Lond 156, 163–179. [Google Scholar]
- Nash D (1975) The morphology and relationships of a crocodilian, Orthosuchus stormbergi, from the Upper Triassic of Lesotho. Ann S Afr Mus 67, 227–329. [Google Scholar]
- Nesbitt S (2011) The early evolution of archosaurs: relationships and the origin of major clades. Bull Am Mus Nat Hist 352, 1–352. [Google Scholar]
- Nesbitt S, Stocker MR, Small BJ, et al. (2009) The osteology and relationships of Vancleavea campi (Reptilia: Archosauriformes). Zool J Lin Soc 157, 814–864. [Google Scholar]
- Nesbitt S, Turner A, Weinbaum JC (2013) A survey of skeletal elements in the orbit of Pseudosuchia and the origin of the crocodylian palpebral. Earth Env Sci T R S 103, 365–381. [Google Scholar]
- Nopcsa FB (1928) Palaeontological notes on Reptilia In: 7. Classification of the Crocodilia, pp. 75–84. Acta Geol Hungarica, Series Palaeontologica Volume 1. [Google Scholar]
- Novas F, Pais DF, Pol D, et al. (2009) Bizarre notosuchian crocodyliform with associated eggs from the Upper Cretaceous of Bolivia. J Vert Paleontol 29, 1316–1320. [Google Scholar]
- Nummela S, Thewissen JGM (2008) The physics of sound in air and water In: Sensory Evolution on the Threshold (eds Thewissen JGM, Nummela S.), pp. 175–181. London: University of California Press. [Google Scholar]
- O'Connor PM, Sertich JJW, Stevens NJ, et al. (2010) The evolution of mammal‐like crocodyliforms in the Cretaceous period of Gondwana. Nature 466, 748–751. [DOI] [PubMed] [Google Scholar]
- Osmólska H (1972) Preliminary note on a crocodilian from the Upper Cretaceous of Mongolia. Palaeontol Pol 27, 43–47. [Google Scholar]
- Owen R (1850) On the communications between the cavity of the tympanum and the palate in the crocodilia (Gavialis, Alligators and Crocodiles). Philos Trans R Soc Lond B Biol Sci 140, 521–527. [Google Scholar]
- Owen R (1874) Monograph on the fossil Reptilia of the Wealden and Purbeck formations. Suppl. no. 6 (Hylaeochampsa.). Palaeontogr Soc Monogr 27, 1–7. [Google Scholar]
- Owen R (1879) Monograph on the fossil Reptilia of the Wealden and Purbeck formations. Supplement No. IX (Goniopholis, Brachydectes, Nannosuchus, Theriosuchus and Nuthetes). Palaeontogr Soc Monogr 33, 1–15. [Google Scholar]
- Parrilla‐Bel J, Young MT, Moreno‐Azanza M, et al. (2013) The first metriorhynchid crocodylomorph from the Middle Jurassic of Spain, with implications for evolution of the subclade Rhacheosaurini. PLoS ONE 8, e54275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson C (1982) Morphological characters and homology In Problems of Phylogenetic Reconstruction, Systematics Association Special Volume (eds Joysey KA, Friday AE.), pp. 21–74. London: Academic Press. [Google Scholar]
- Peng G‐Z, Shu C‐K (2005) A new species of Hsisosuchus from the Late Jurassic of Zigong, Sichuan, China. Vert Palasiat 10, 312–324. [Google Scholar]
- Pierce SE, Angielczyk KD, Rayfield EJ (2009) Morphospace occupation in thalattosuchian crocodylomorphs: skull shape variation, species delineation and temporal patterns. Palaeontology 52, 1057–1097. [Google Scholar]
- Pinna MGG (1991) Concepts and tests of homology in the cladistic paradigm. Cladistics 7, 367–394. [Google Scholar]
- Pol D, Gasparini Z (2009) Skull anatomy of Dakosaurus andiniensis (Thalattosuchia: Crocodylomorpha) and the phylogenetic position of Thalattosuchia. J Syst Palaeontol 7, 163–197. [Google Scholar]
- Pol D, Larsson HCE (2011) 1st Symposium on the evolution of crocodyliforms. Zool J Lin Soc 163, S1–S6. [Google Scholar]
- Pol D, Norell MA (2004a) A new gobiosuchid crocodyliform taxon from the Cretaceous of Mongolia. Am Mus Novit 3458, 1–31. [Google Scholar]
- Pol D, Norell MA (2004b) A new crocodyliform from Zos Canyon, Mongolia. Am Mus Novit 3445, 1–36. [Google Scholar]
- Pol D, Powell J (2011) A new sebecid mesoeucrocodylian from the Rio Loro formation (Palaeocene) of north‐western Argentina. Zool J Lin Soc 163, S7–S36. [Google Scholar]
- Pol D, Turner A, Norell MA (2009) Morphology of the Late Cretaceous crocodylomorph Shamosuchus djadochtaensis and a discussion of neosuchian phylogeny as related to the origin of Eusuchia. Bull Am Mus Nat Hist 324, 1–103. [Google Scholar]
- Pol D, Leardi JM, Lecuona A, et al. (2012) Postcranial anatomy of Sebecus icaeorhinus (Crocodyliformes, Sebecidae) from the Eocene of Patagonia. J Vert Paleontol 32, 328–354. [Google Scholar]
- Pol D, Rauhut OWM, Lecuona A, et al. (2013) A new fossil from the Jurassic of Patagonia reveals the early basicranial evolution and the origins of Crocodyliformes. Biol Rev 88, 862–872. [DOI] [PubMed] [Google Scholar]
- Pol D, Nascimento ER, Carvalho AB, et al. (2014) A new notosuchian from the Late Cretaceous of Brazil and the phylogeny of advanced notosuchians. PLoS ONE 9, e93105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomel A (1849) Découverte de champsosauriens dans les gisements de phosphorite du suessonien de l'Algérie. Comptes Rendu Acad Sci Paris Ser II 118, 1309–1310. [Google Scholar]
- Price LI (1959) Sobre um crocodilídeo notossúquio do Cretácico brasileiro. Bol Div Geol Min Dep Nac Prod Min 188, 1–55. [Google Scholar]
- Pritchard AC, Turner A, Allen ER, et al. (2013) Osteology of a North American goniopholidid (Eutretauranosuchus delfsi) and palate evolution in Neosuchia. Am Mus Novit 3783, 1–56. [Google Scholar]
- Puértolas E, Canudo JI, Cruzado‐Caballero P (2011) A new Crocodylian from the Late Maastrichtian of Spain: implications for the initial radiation of crocodyloids. PLoS ONE 6, e20011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puértolas‐Pascual E, Canudo JI, Moreno‐Azanza M (2014) The eusuchian crocodylomorph Allodaposuchus subjuniperus sp. nov., a new species from the latest Cretaceous (upper Maastrichtian) of Spain. Hist Biol 26, 91–109. [Google Scholar]
- Salisbury SW, Naish D (2011) Crocodilians In: English Wealden Fossils (ed. Batten DJ.), pp. 305–369. London: The Palaeontological Association. [Google Scholar]
- Salisbury SW, Willis PMA (1996) A new crocodylian from the Early Eocene of southeastern Queensland and a preliminary investigation of the phylogenetic relationship of crocodyloids. Alcheringa 20, 179–227. [Google Scholar]
- Salisbury SW, Molnar R, Frey E, et al. (2006) The origin of modern crocodyliforms: new evidence from the Cretaceous of Australia. Proc R Soc Lond B Biol Sci 273, 2439–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson S, Witmer L (2007) Craniofacial anatomy of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the Late Cretaceous of Madagascar. J Vert Paleontol 27, 32–102. [Google Scholar]
- Saunders JC, Duncan RK, Doan DE, et al. (2000) The middle ear of reptiles and birds In: Comparative Hearing: Birds and Reptiles (eds Dooling RJ, Fay RR.), pp. 13–69. New York, NY: Springer. [Google Scholar]
- Schneider JG (1801) Historiae Amphibiorum naturalis et literariae. Fasciculus Secundus Continens Crocodilos, Scincos, Chamaesauras, Boas. Pseudoboas, Elapes, Angues, Amphisbaenas et Caecilias. Jena: Frommanni. [Google Scholar]
- Schwarz D (2002) A new species of Goniopholis from the Upper Jurassic of Portugal. Palaeontology 45, 185–208. [Google Scholar]
- Sedlmayr JC (2002) Anatomy, evolution, and functional significance of cephalic vasculature in Archosauria PhD. Thesis: College of Arts and Sciences, Athens OH: University of Ohio, 398 pp. [Google Scholar]
- Sereno PC, Larsson HCE (2009) Cretaceous crocodyliforms from the Sahara. ZooKeys 28, 1–143. [Google Scholar]
- Sereno PC, Novas F (1992) The complete skull and skeleton of an early dinosaur. Science 258, 1137–1140. [DOI] [PubMed] [Google Scholar]
- Sereno PC, Larsson HCE, Sidor CA, et al. (2001) The giant Crocodyliform Sarcosuchus from the Cretaceous of Africa. Science 294, 1516–1519. [DOI] [PubMed] [Google Scholar]
- Sereno PC, Sidor CA, Larsson HCE, et al. (2003) A new notosuchian from the Early Cretaceous of Niger. J Vert Paleontol 23, 477–482. [Google Scholar]
- Sertich JJW, O'Connor PM (2014) A new crocodyliform from the Middle Cretaceous Galula Formation, southwestern Tanzania. J Vert Paleontol 34, 576–596. [Google Scholar]
- Shute CCD, Bellairs AA (1955) The external ear in Crocodylia. Proc Zool Soc Lond 124, 741–748. [Google Scholar]
- Simmons DJ (1965) The non‐therapsid reptiles of the Lufeng Basin, Yunnan, China. Fieldiana Geol 15, 1–93. [Google Scholar]
- Simpson GG (1937) New reptiles from the Eocene of South America. Am Mus Novit 927, 1–3. [Google Scholar]
- Soto M, Pol D, Perea D (2011) A new specimen of Uruguaysuchus aznarezi (Crocodyliformes: Notosuchia) from the middle Cretaceous of Uruguay and its phylogenetic relationships. Zool J Lin Soc 163, S173–S198. [Google Scholar]
- Stubbs TL, Pierce SE, Rayfield EJ, et al. (2013) Morphological and biomechanical disparity of crocodile‐line archosaurs following the end‐Triassic extinction. Proc R Soc Lond B Biol Sci 283, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sues H‐D, Olsen PE, Carter JG, et al. (2003) A new crocodylomorph archosaur from the Upper Triassic of North Carolina. J Vert Paleontol 23, 329–343. [Google Scholar]
- Swinton WE (1930) On fossil Reptilia from Sokoto Province. Bull Geol Surv Nigeria 13, 9–34. [Google Scholar]
- Tahara R, Larsson HCE (2011) Cranial pneumatic anatomy of Ornithomimus edmontonicus (Ornithomimidae: Theropoda). J Vert Paleontol 31, 127–143. [Google Scholar]
- Thewissen JGM, Nummela S (2008) Sensory Evolution on the Threshold. London: University of California Press. [Google Scholar]
- Tolijagic' O, Butler RJ (2013) Triassic–Jurassic mass extinction as trigger for the Mesozoic radiation of crocodylomorphs. Biol Lett 9, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A (2006) Osteology and phylogeny of a new species of Araripesuchus (Crocodyliformes: Mesoeucrocodylia) from the Late Cretaceous of Madagascar. Hist Biol 18, 255–369. [Google Scholar]
- Turner A (2015) A review of Shamosuchus and Paralligator (Crocodyliformes, Neosuchia) from the Cretaceous of Asia. PLoS ONE 10, e0118116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A, Calvo JO (2005) A new sebecosuchian crocodyliform from the late Cretaceous of Patagonia. J Vert Paleontol 25, 87–98. [Google Scholar]
- Turner A, Pritchard A (2015) The monophyly of Susisuchidae (Crocodyliformes) and its phylogenetic placement in Neosuchia. PeerJ 3, e759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A, Sertich JJW (2010) Phylogenetic history of Simosuchus clarki (Crocodyliformes: Notosuchia) from the Late Cretaceous of Madagascar. J Vert Paleontol Memoir 30, 177–236. [Google Scholar]
- Vergne AL, Pritz MB, Mathevon N (2009) Acoustic communication in crocodilians: from behaviour to brain. Biol Rev 84, 391–411. [DOI] [PubMed] [Google Scholar]
- Vignaud P, Gasparini Z (1996) New Dakosaurus (Crocodylomorpha, Thalattosuchia) in the upper Jurassic of Argentina. Comptes Rendu Acad Sci Paris Ser II 322, 245–250. [Google Scholar]
- Walker AD (1970) A revision of the Jurassic reptile Hallopus victor (Marsh), with remarks on the classification of crocodiles. Philos Trans R Soc Lond B Biol Sci 257, 323–372. [Google Scholar]
- Walsh SA, Barrett P, Milner AC, et al. (2009) Inner ear anatomy is a proxy for deducing auditory capability and behaviour in reptiles and birds. Proc R Soc Lond B Biol Sci 276, 1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzel K (1935) Hassiacosuchus haupti n. g n. s. ein durophages Krokodilaus dem Mitteleozän von Messel Landesanst. Notizbl Ver geol Darmstadt 16, 40–49. [Google Scholar]
- Werner Y, Safford SD, Seifan M, et al. (2005) Effects of age and size in the ears of gekkonomorph lizards: middle‐ear morphology with evolutionary implications. Anat Rec A Discov Mol Cell Evol Biol 283A, 212–223. [DOI] [PubMed] [Google Scholar]
- Wever EG (1978) The Reptile Ear: its Structure and Function. Princeton, NJ: Princeton University Press. [Google Scholar]
- Whetstone KN, Whybrow P (1983) A cursorial crocodilian from the Triassic of Lesotho (Basutiland, South Africa). Occas Pap Mus Nat Hist 106, 1–37. [Google Scholar]
- Wilberg E (2015a) A new metriorhynchoid (Crocodylomorpha, Thalattosuchia) from the Middle Jurassic of Oregon and the evolutionary timing of marine adaptations in thalattosuchian crocodylomorphs. J Vert Paleontol 35, e902846. [Google Scholar]
- Wilberg E (2015b) What's in an outgroup? The impact of outgroup choice on the phylogenetic position of Thalattosuchia (Crocodylomorpha) and the origin of Crocodyliformes. Syst Biol 64, 621–637. [DOI] [PubMed] [Google Scholar]
- Willis KL, Christensen‐Dalsgaard J, Ketten D, et al. (2013) Middle ear cavity morphology is consistent with an aquatic origin for Testudines. PLoS ONE 8, e54086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer LM (1990) The craniofacial air sac system of mesozoic birds (Aves). Zool J Lin Soc 100, 327–378. [Google Scholar]
- Witmer LM (1997) The evolution of the antorbital cavity of archosaurus: a study in soft‐tissue reconstruction in the foissl record with analysis of the function of pneumaticity. J Vert Paleontol Memoir 3, 1–75. [Google Scholar]
- Witmer L (1999) The narial anatomy of extant amniotes and its significance for the interpretation of narial novelty in dinosaurs. Am Zool 39, 98A–99A. [Google Scholar]
- Witmer LM, Ridgely R (2008) The paranasal air sinuses of predatory and armored dinosaurs (Archosauria: Theropoda and Ankylosauria) and their contribution to cephalic structure. Anat Rec 291, 1362–1388. [DOI] [PubMed] [Google Scholar]
- Witmer L, Ridgely R (2009) New insights into the brain, braincase, and ear region of tyrannosaurs (Dinosauria, Theropoda), with implications for sensory organization and behavior. Anat Rec 292, 1266–1296. [DOI] [PubMed] [Google Scholar]
- Witmer LM, Ridgely R, Dufeau DL, et al. (2008) Using CT to peer into the past: 3D visualization of the brain and ear regions of birds, crocodiles, and nonavian dinosaurs In: Anatomical Imaging: Towards a New Morphology (eds Endo H, Frey R.), pp. 67–88. Tokyo: Springer. [Google Scholar]
- Woodward AS (1896) On two Mesozoic crocodilians, Notosuchus (genus novum) and Cynodontosuchus (genus novum) from the red sandstones of the territory of Neuquén (Argentina). An Mus La Plata 4, 1–20. [Google Scholar]
- Wu X‐C, Brinkman DB, Lu J‐C (1994) A new species of Shantungosuchus from the Lower Cretaceous of Inner Mongolia (China), with comments on S. chuhsienensis Young, 1961 and the phylogenetic position of the genus. J Vert Paleontol 14, 210–229. [Google Scholar]
- Wu X‐C, Sues H‐D, Dong Z‐M (1997) Sichuanosuchus shuhanensis, a new? Early Cretaceous protosuchian (Archosauria: Crocodyliformes) from Sichuan (China), and the monophyly of Protosuchia. J Vert Paleontol 17, 89–103. [Google Scholar]
- Young MT, Andrade MB (2009) What is Geosaurus? Redescription of Geosaurus giganteus (Thalattosuchia: Metriorhynchidae) from the Upper Jurassic of Bayern, Germany. Zool J Lin Soc 157, 551–585. [Google Scholar]
- Young CC, Chow MC (1953) New fossil reptiles from Szechuan China. Acta Paleontol Sin 1, 1–87. [Google Scholar]
- Young MT, Brussatte SL, Ruta M, et al. (2010) The evolution of Metriorhynchoidea (Mesoeucrocodylia, Thalattosuchia): an integrated approach using geometric morphometrics, analysis of disparity, and biomechanics. Zool J Lin Soc 158, 801–859. [Google Scholar]
- Young MT, Bell MA, Brussatte SL (2011) Craniofacial form and function in Metriorhynchidae (Crocodylomorpha: Thalattosuchia): modelling phenotypic evolution with maximum likelihood methods. Biol Lett 7, 913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MT, Andrade MB, Etches S, et al. (2013) A new metriorhynchid crocodylomorph from the Lower Kimmeridge Clay Formation (Late Jurassic) of England, with implications for the evolution of dermatocranium ornamentation in Geosaurini. Zool J Lin Soc 169, 820–848. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Complete list of taxa analysed first‐hand in this study with their taxonomical assignment and collection numbers.


