Abstract
We examined the effects of exercise on diaphragm degeneration and cardiomyopathy in dystrophin‐deficient mdx mice. Mdx mice (11 months of age) were exercised (swimming) for 2 months to worsen diaphragm degeneration. Control mdx mice were kept sedentary. Morphological evaluation demonstrated increased fibrosis in the diaphragm of exercised mdx mice (33.3 ± 6.0% area of fibrosis) compared with control mdx mice (20.9 ± 1.7% area of fibrosis). Increased (26%) activity of MMP‐2, a marker of fibrosis, was detected in the diaphragms from exercised mdx mice. Morphological evaluation of the heart demonstrated a 45% increase in fibrosis in the right ventricle (8.3 ± 0.6% in sedentary vs. 12.0 ± 0.6% of fibrosis in exercised) and in the left ventricle (35% increase) in the exercised mdx mice. The density of inflammatory cells–degenerating cardiomyocytes increased 95% in the right ventricle (2.3 ± 0.6 in sedentary vs. 4.5 ± 0.8 in exercised) and 71% in the left ventricle (1.4 ± 0.6 sedentary vs. 2.4 ± 0.5 exercised). The levels of both active MMP‐2 and the pro‐fibrotic factor transforming growth factor beta were elevated in the hearts of exercised compared with sedentary mdx mice. The wall thickness to lumen diameter ratio of the pulmonary trunk was significantly increased in the exercised mdx mice (0.11 ± 0.04 in sedentary vs. 0.28 ± 0.12 in exercised), as was the thickness of the right ventricle wall, which suggests the occurrence of pulmonary hypertension in those animals. It is suggested that diaphragm degeneration is a main contributor to right ventricle dystrophic pathology. These findings may be relevant for future interventional studies for Duchenne muscular dystrophy‐associated cardiomyopathy.
Keywords: cardiomyopathy, Duchenne muscular dystrophy, fibrosis, metalloproteinases‐2
Introduction
Duchenne muscular dystrophy (DMD) is lethal and is the most common form of myopathy, affecting approximately 1 in 6000 live male births (Mendell et al. 2012). DMD is caused by the loss of dystrophin, which is a structural protein of the skeletal and cardiac muscle fibers. Loss of dystrophin results in necrosis and progressive weakness in appendicular and diaphragm (DIA) muscles (Engel et al. 1994; De Bruin et al. 1997; Humbertclaude et al. 2012).
In certain diseases, diaphragm weakness causes functional and structural changes in the cardiac muscle (Coats, 1996; Voelkel et al. 2006; Versteegh et al. 2007; Alonso‐Gonzalez et al. 2013). In DMD, diaphragm weakness is usually observed (De Bruin et al. 1997; Humbertclaude et al. 2012). More than 90% of DMD patients display cardiomyopathy, and nearly 10–20% die from heart failure (Finsterer & Stollberger, 2003; Fayssoil et al. 2010; Spurney, 2011; Mosqueira et al. 2013).
Understanding the natural history of progressive cardiomyopathy is relevant because it can influence the therapy to be used. In DMD, it is not clear whether DIA injury can contribute to cardiac damage. The high variability in the degrees of DIA involvement in DMD, in addition to the obvious risks in obtaining heart and DIA biopsies from these patients, has limited our understanding of the interplay between diaphragm and cardiac damage in this disease (Humbertclaude et al. 2012).
In this study, we used histological and histomorphometric methods to examine whether worsening degeneration in the dystrophic diaphragm by using physical exercise could affect cardiac structure in mdx mice, which is the experimental model of DMD (Stedman et al. 1991). The levels of metalloproteinases and transforming growth factor beta, molecular markers of fibrosis and inflammation (Bernasconi et al. 1995; Gosselin et al. 2004; Kumar et al. 2010), were evaluated in dystrophic DIA and heart. Preliminary results were presented at the European Muscle Conference (Barbin et al. 2014).
Materials and methods
Animals
Male mdx (C57BL/10‐Dmd mdx/PasUnib) mice that were 11 months old at the beginning of experiments were used. Some male normal (C57BL/10) mice that were 11 months old were used for focused experiments. All experiments were performed in accordance with the guidelines of the Brazilian College for Animal Experimentation (COBEA; protocol #2377‐1) and the guidelines set forth by our Institution.
Experimental design
Two groups of animals (exercised mdx and exercised normal) were made to swim for 60 min once a day, 6 days a week, for 2 months. Mice start swimming for 10 min (day 1) with a 10‐min increase each day, reaching the 60‐min session by the end of the first week. We used a swimming apparatus (Evangelista et al. 2003) in which the water was maintained at 30–32 °C. Another group of mice was kept sedentary (herein called sedentary mdx and sedentary normal mice). Twenty hours after the last swimming exercise session, the mice were anesthetized with a mixture of ketamine hydrochloride (130 mg kg−1; Francotar, Virbac, São Paulo, Brazil) and xylazine hydrochloride (6.8 mg kg−1, 2% Virbaxyl; Virbac). Hearts and diaphragm (DIA) muscles were dissected out.
Histological and morphometric analysis
We evaluated the following: (1) fibrosis, (2) inflammation‐degeneration in the heart and DIA, (3) the wall/lumen ratio of the pulmonary trunk, and (4) the thickness of the right ventricle wall. Hearts and DIA of exercised (n = 5), sedentary (n = 5) mdx mice, and sedentary (n = 3) and exercised (n = 3) normal mice were flash‐frozen in cooled isopentane before mounting in Tragacanth (Sigma‐Aldrich, St. Louis, MO, USA). Cryostat transverse cross‐sections (6 μm thick) of the whole DIA and heart were stained with Masson's trichrome for the observation of fibrosis. The total cross‐sectional area and fibrosis area from the entire section were measured in dystrophic muscles using a microscope (Nikon Eclipse, Nikon Instruments Inc., Melville, NY, USA) with 10× and 20× magnification (hearts and DIA, respectively) attached to a personal computer. Measurements were taken using imagepro‐express software (Pereira et al. 2012). The mean fibrosis area was expressed as the percentage of the total cross‐sectional area.
The density of patches of inflammatory cell‐degenerating cardiomyocytes from dystrophic heart was evaluated directly using a hand counter on a microscope (Nikon Eclipse) fitted with a graduated eyepiece micrometer at 100× magnification. The results are expressed as patches per mm2.
To estimate the wall/lumen ratio of the main pulmonary trunk, images of the pulmonary trunk were taken using the microscope (Nikon Eclipse) at 10× magnification. Measurements of the wall thickness, major axis (a) and minor axis (b) at right angles to the major axis of the lumen of the trunk were made using imagepro‐express software. The lumen diameter of the sections (Di) was calculated from the equation Di = √ab (Komolafe et al. 2009). The wall to lumen ratio of the pulmonary trunk was calculated as the thickness of the wall/diameter of the lumen (Komolafe et al. 2009).
Zymographic analysis
We examined the following isoforms of metalloproteinases‐2 (MMP‐2): active MMP‐2, pre MMP‐2, and pro MMP‐2. Hearts and DIA from both the exercised (n = 6) and sedentary (n = 6) mdx mice were flash‐frozen in cooled isopentane. Heart and DIA were divided, half used to zimography and the other half used to Western blot. Zymography assays were performed on 7.5% polyacrylamide gels. Gelatinolytic bands were visualized by staining the gels with Coomassie Brilliant Blue dye (Sigma‐Aldrich). Bands were quantified by densitometry (imagej 1.38X software; National Institutes of Health, Bethesda, MD, USA). We performed 2 pools samples, with 3 mice (3 half hearts or 3 half DIA) in each pooled sample. Zymographic analysis was repeated three times, for each pooled sample.
Western blot analysis
The levels of transforming growth factor beta (TGF‐β; Sigma) were quantified using western blotting. As described above, we performed two pool samples, with three mice (three half hearts or three half DIA) in each pooled sample. Western blot was repeated three times for each pooled sample.
As previously described (Ferretti et al. 2011), heart and DIA muscles were lysed in lysis buffer (1% Triton, 10 mm sodium pyrophosphate, 100 mm NaF, 10 g mL−1 aprotinin, 1 mm PMSF and 0.25 mm Na3VO), the samples were centrifuged, and the soluble fraction was resuspended in Laemmli loading buffer [2% sodium dodecyl sulfate (SDS), 20% glycerol, 0.04 mg mL−1 bromophenol blue, 0.12 m Tris·HCl, pH 6.8 and 0.28 m‐mercaptoethanol]. An aliquot (60 μg) of the total protein homogenate from control and exercised mdx DIA and heart was loaded onto 12% SDS polyacrylamide gels and transferred to a nitrocellulose membrane (Bio‐Rad Laboratories submersion electrotransfer apparatus, Hercules, CA, USA). After blocking with 5% skim milk‐Tris·HCl‐buffered saline Tween buffer (TBST; 10 mm Tris·HCl, pH 8, 150 mm NaCl and 0.05% Tween 20), the membranes were incubated with the primary antibody overnight at 4 °C, followed by a peroxidase‐conjugated secondary antibody [peroxidase‐labeled affinity‐purified mouse IgG antibody (H+L, KPL, Gaithersburg, MD, USA], and developed using the SuperSignal West Pico Chemiluminescent Substrate kit (Pierce Biotechnology, Rockford, IL, USA). Ponceau‐stained membranes were used to control for protein loading as described previously (Fogagnolo Mauricio et al. 2013). The luminescent signal from the western blot bands was captured (G: Box iChemi camera; Syngene, Cambridge, UK), and band intensities were quantified using the analysis software provided by the manufacturer (gene tools Version 4.01, Syngene, Cambridge, UK).
Statistical analysis
All data are expressed as the means ± standard deviation (SD). Statistical analysis for direct comparison between the means of two groups was performed using Student's t‐test and anova for comparison between the means of three groups or more, followed by the Bonferroni post hoc test. P < 0.05 was considered statistically significant.
Results
Diaphragm
In normal DIA, exercise did not lead to fibrosis (Fig. 1A). In exercised mdx, fibrosis was increased (Fig. 1C). Quantitative analysis (Fig. 1) showed a significant (P < 0.05) increase (60%) in the fibrotic area in DIA from exercised mdx mice (33.3 ± 6.0) compared with sedentary mdx mice (20.9 ± 6.1; values are expressed as percentage of total sectional area). Zymographic analysis (Fig. 2) revealed that the levels of pro MMP‐2, pre MMP‐2 and active MMP‐2 in the DIA of exercised mdx mice were, respectively, 28, 15 and 26%, each higher than those observed in the sedentary group (P < 0.05).
Figure 1.
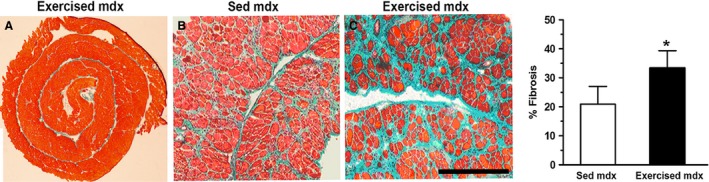
Exercise‐increased fibrosis in the dystrophic diaphragm. Representative transverse histological sections of diaphragm muscles from normal C57BL/10 (A), sedentary (B; Sed) mdx mice and exercised (C) mdx mice. Fibrosis (green, Masson trichrome) fills the spaces between muscle fibers mainly seen in the exercised mdx mice. Histomorphometric analysis (bar graph) revealed that the mean fibrosis area was significantly larger (*P < 0.05) in the diaphragms of exercised mdx mice than in those of sedentary mdx mice. Data are presented as the mean ± SD. Scale bar (shown only in C): 1350 μm (A), 250 μm (B,C).
Figure 2.
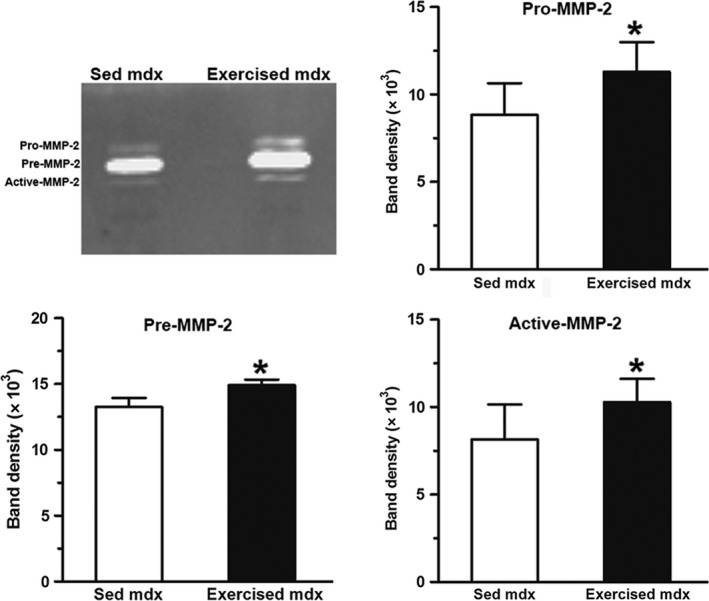
Exercise‐increased metalloproteinases in the diaphragms of mdx mice. Zymographic analysis of metalloproteinases obtained from the diaphragm muscles of control (sedentary, Sed) and exercised mdx mice. The graphs show the activity level of pro‐MMP‐2, pre‐MMP‐2 and active MMP‐2. Data are presented as the mean ± SD. *Significantly different from sedentary mice (P < 0.05).
Heart
In both right and left ventricles (Fig. 3A,B), myocardial fibrosis, as identified by Masson's trichrome stain (Fig. 3C,D), and patches of inflammatory cells–degenerating cardiomyocytes, represented by lymphomononuclear infiltrations and cardiomyocyte lysis (Fig. 3E), were observed in intramyocardial, subendocardial and subepicardial areas in both exercised and sedentary mdx mice.
Figure 3.
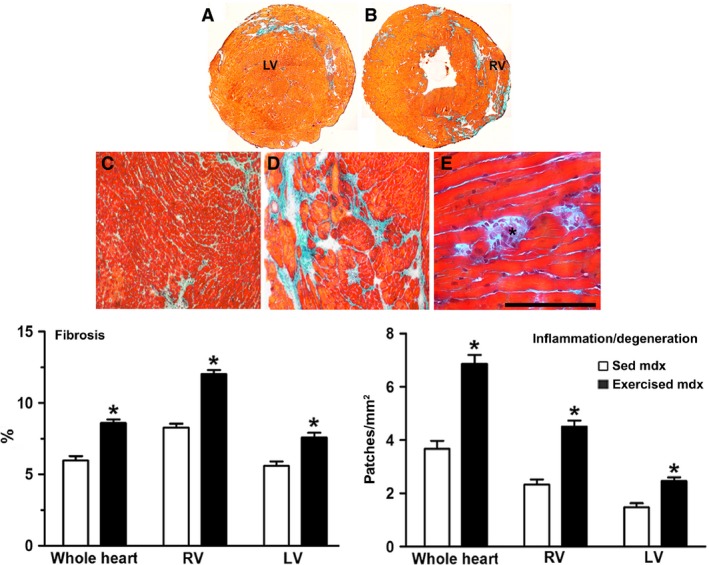
Myocardial fibrosis and inflammation/degeneration were increased by exercise. (A,C) Sedentary mdx. (B,D,E) Exercised mdx. Fibrosis (Masson's trichrome; green) is seen among cardiomyocytes in whole heart sections (A,B) and in histological sections (C,D). Inflammation‐degeneration (asterisk in E). Quantitative analysis (bar graph) revealed that exercise worsened the heart histopathological features, mainly seen in the right ventricle (RV). LV, left ventricle. Data are presented as the mean ± SD. *Significantly different from sedentary mice (P < 0.05). Scale bar (shown only in E): 400 μm (C,D), 100 μm (E).
Quantitative analysis (Fig. 3) revealed that the histological parameters differed between hearts from sedentary and exercised mdx mice. The percentage of myocardial fibrosis in total heart (Fig. 3) was 46% higher in the exercised mdx mice (8.6 ± 0. 6% of fibrosis) than in the sedentary mice (5.9 ± 0.7% of fibrosis in sedentary). In the right ventricle (RV), fibrosis increased significantly by 45% (8.3 ± 0.6% of fibrosis in sedentary vs. 12.0 ± 0.6% in exercised mdx mice); in the left ventricle (LV), it was 36% higher (5.6 ± 0.7% in sedentary vs. 7.6 ± 0.8% in exercised mdx mice). The density of patches of inflammatory cells–degenerating cardiomyocytes increased in hearts from exercised mice (Fig. 3). This increase was approximately 95% in the right ventricle (2.3 ± 0.6 patches mm−2 in sedentary vs. 4.5 ± 0.8 patches mm−2 in exercised mdx mice) and 71% in the left ventricle (1.4 ± 0.6 patches mm−2 in sedentary vs. 2.4 ± 0.5 patches mm−2 in exercised mdx mice). The thickness of the right ventricle wall was increased by 40% in the exercised mdx mice (exercised mdx mice: 0.203 ± 0.015* cm; exercised normal mice: 0.129 ± 0.027 cm; sedentary mdx mice: 0.145 ± 0.017; *P < 0.05 compared to exercised normal and sedentary mdx mice; anova).
Figure 4 shows the histological features of the main pulmonary trunk in the mdx mice, focusing on medial wall proliferation. The wall thickness to lumen diameter ratio of the pulmonary trunk (Fig. 4) was significantly increased (P < 0.05) in the exercised mdx mice (0.11 ± 0.04 in sedentary mdx vs. 0.28 ± 0.12 in exercised mdx), which indicated a decrease in the relative lumen diameter in those animals. Exercise did not change the wall thickness to lumen diameter ratio of the pulmonary trunk in normal mice (0.076 ± 0.007 in sedentary normal vs. 0.075 ± 0.014 in exercised normal; P > 0.05).
Figure 4.
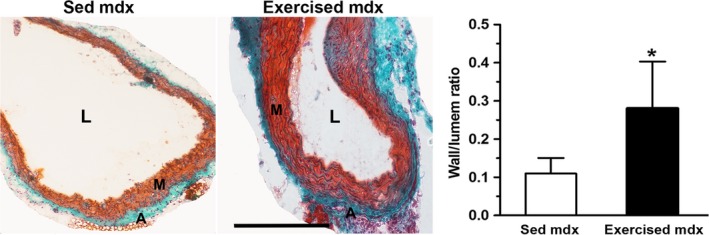
Representative photomicrographs showing histological features of the pulmonary trunk from sedentary (Sed mdx) and exercised mdx mice with Masson staining. Quantitative analysis (bar graphs) of media wall thickness to lumen ratio of pulmonary arteries. Compared with the control (Sed mdx) group, the media thickness of the pulmonary arteries was markedly increased in the exercised mdx group. The development of medial wall thickening suggests the presence of accentuated diaphragm degeneration in the exercised mdx group compared with controls. The results are expressed as the mean ± SD. *P < 0.05. A, adventitial layer; L, lumen; M, media layer. Scale bar: 1.2 μm.
Zymographic analysis (Fig. 5) showed that the expression of pro MMP‐2, pre MMP‐2 and active MMP‐2 were, respectively 28% (7.4 ± 1.7 in sedentary vs. 9.4 ± 1.0 in exercised mdx), 21% (11.3 ± 0.4 in sedentary mdx vs. 13.3 ± 1.3 in exercised mdx) and 32% (6.7 ± 1.3 in sedentary mdx vs. 8.8 ± 1.8 in exercised mdx) higher in hearts from exercised mdx mice than in the sedentary mdx. The levels of TGF‐β (Fig. 6) were significantly elevated (1.8 ± 0.4 in sedentary mdx vs. 3.7 ± 1.3 in exercised mdx; P < 0.05) in the hearts of the mdx mice from the exercised group.
Figure 5.
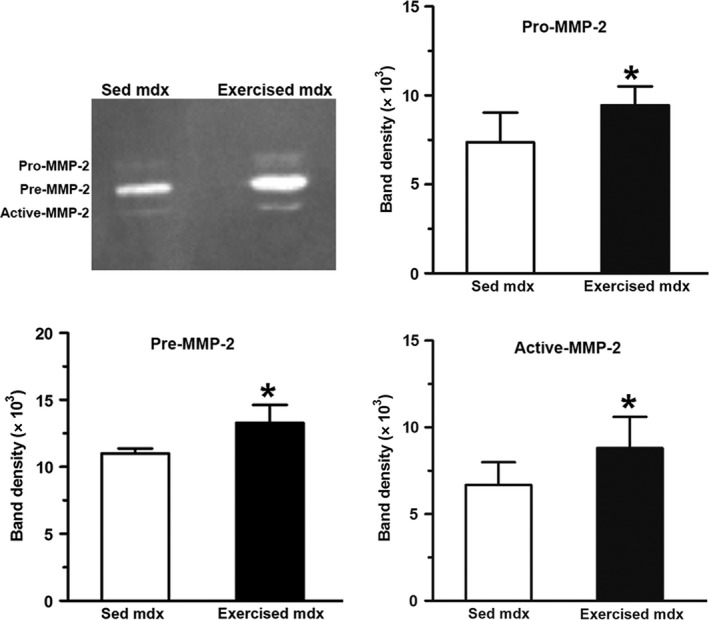
Exercise‐increased metalloproteinases in the hearts of mdx mice. Zymographic analysis of metalloproteinases obtained from the heart of control (sedentary, Sed) and exercised mdx mice. The graphs show the activity level of pro‐MMP‐2, pre‐MMP‐2 and active MMP‐2. The results are presented as the mean ± SD. *Significantly different from sedentary mice (P < 0.05).
Figure 6.
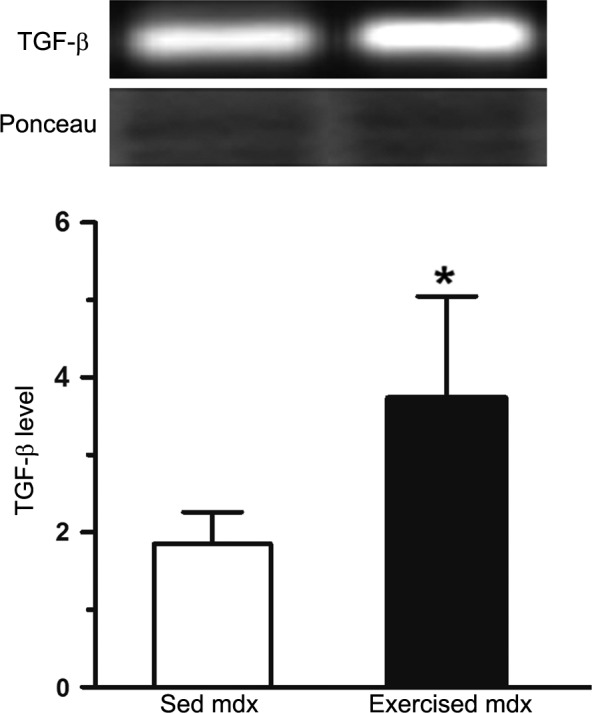
Western blot analysis of transforming growth factor beta (TGF‐β) in crude extracts of hearts from control (sedentary, Sed) and exercised mdx mice. Graphs show the relative value of TGF‐β. The Ponceau‐stained membranes were used to control for protein loading. Data are presented as the mean ± SD. *Significantly different from sedentary mice (P < 0.05).
Discussion
Physical exercise exacerbated diaphragm degeneration
In many studies, physical exercise has been used to accentuate the dystrophic phenotype (Bouchentouf et al. 2006; De Luca et al. 2008; Radley‐Crabb et al. 2012), although it has been reported that physical exercise seems to benefit some skeletal muscles of the mdx mice (Dupont‐Versteegden et al. 1994; Hayes & Williams, 1996; Call et al. 2010; Duong et al. 2011; Baltgalvis et al. 2012). In the present study, we observed a significant increase in the fibrotic area in exercised mdx compared with sedentary mdx mice. Concomitantly, the expression of the isoforms of MMP‐2 was also higher in the DIA of mdx mice submitted to exercise. MMP‐2 is known to degrade basement membrane proteins, collagen, fibronectin and laminin after injury in cardiac and skeletal muscle fibers (Page‐McCaw et al. 2007) and dysregulation of matrix metalloproteinases in mdx mice is associated with a worsened dystrophic phenotype (Li et al. 2009). Therefore, the exercise protocol used here was efficient in exacerbating diaphragm degeneration in mdx mice.
Diaphragm degeneration and cardiac structure in mdx mice
Although improvements in cardiac function have been observed after physical exercise in mdx mice, even in the presence of a failing diaphragm (Selsby et al. 2013), other studies have indicated that cardiac structure is negatively affected by physical exercise (Nakamura et al. 2002; Costas et al. 2010). Cardiac damage in mdx mice is aggravated when voluntary activity is restored, possibly due to cardiac stress (Townsend et al. 2008). In the present study, we observed increased levels of the pro‐fibrotic factor TGF‐β in the cardiac muscle of exercised mdx mice, along with increased fibrosis and inflammatory areas. Therefore, the present exercise protocol also negatively affected the cardiac muscle.
In many conditions, diaphragm weakness produces apneic events and pulmonary vasoconstriction that leads to pulmonary hypertension and right‐sided ventricular damage (Coats, 1996; Voelkel et al. 2006; Versteegh et al. 2007; Alonso‐Gonzalez et al. 2013). Recently, using a biventricular cardiac catheterization technique to measure ventricular function, an early involvement of the right ventricle in the cardiomyopathy of the mdx mice was demonstrated, possibly related to increased constriction of the pulmonary vessels (Meyers & Townsend, 2015). Pulmonary vasoconstriction could be due to abnormal sympathetic activation or to hypoventilation related to respiratory dysfunction (Meyers & Townsend, 2015). In the present study, we demonstrate pulmonary artery remodeling concomitant with an increase in the right ventricle thickness wall, histological evidence of the possible presence of pulmonary hypertension only in the mdx mice. In normal exercised mice, no anatomical changes in DIA (no fibrosis in exercised normal) and heart were detected. Conversely, in mdx mice with worsened diaphragm degeneration (exercised group), histological analysis revealed (1) that RV was more affected than the left ventricle, (2) an increase in the thickness of the right ventricle and (3) an increase in the pulmonary trunk media thickness, as demonstrated by the higher wall thickness to lumen diameter ratio. Increasing media thickness is a histological hallmark predicting the occurrence of pulmonary hypertension (Durmowicz & Stenmark, 1999; Drexler et al. 2008; Sommer et al. 2008). Elevation of the pulmonary pressure induces remodeling of the pulmonary trunk, characterized by an increase in the media thickness (Durmowicz & Stenmark, 1999; Drexler et al. 2008; Sommer et al. 2008). The finding that diaphragm degeneration was concomitant with cardiac morphological changes (fibrosis) argues in favor of the suggestion that respiratory dysfunction and hypoventilation (Meyers & Townsend, 2015) are the main contributors to pulmonary hypertension in mdx mice.
Clinical significance
Although caution is required when the results obtained with the mdx mice are transposing to human dystrophy, the present findings may be of clinical importance. Regarding the time‐course of the progressive cardiac damage, it has been mostly portrayed that the left ventricle is the primary site of injury in DMD, with the right ventricle being spared (Finsterer & Stollberger, 2003). However, a predominance of right ventricular failure with the left ventricle being only slightly affected has also been observed in DMD, even in terminal stages of the disease (Sanyal et al. 1978; Yotsukura et al. 1988; Nishimura et al. 2001). Furthermore, although some DMD studies have indicated that the risk of cardiac failure is inversely related to diaphragm damage, other studies noted that failure of the left heart correlates with diaphragm degeneration (Yotsukura et al. 1988; Backman & Nylander, 1992; Finder et al. 2004). Interestingly, diaphragm weakness and restrictive respiratory insufficiency are highly heterogeneous in DMD (De Bruin et al. 1997; Humbertclaude et al. 2012). The present findings motivated us to hypothesize that the degree of diaphragm degeneration may play a role in the natural history of the cardiomyopathy associated with DMD. Possibly, in patients with a diaphragm more severely affected, the right ventricle may be compromised earlier and more intensely compared with the left ventricle.
The present findings may also be of interest for the treatment of DMD‐associated cardiomyopathy. Adrenergic beta‐antagonists and angiotensin‐converting enzyme inhibitors have been the most commonly recommended as first‐line treatments for all DMD patients, independent of their clinical signs (Fayssoil et al. 2010). However, in patients with a more accentuated diaphragm dysfunction, targeting pulmonary hypertension should result in a better outcome for cardiac function. In a recent study, treatment with sildenafil citrate, which was successfully used as a pulmonary anti‐hypertensive drug (Galie et al. 2005), failed to improve cardiac function in DMD patients. However, in that study there was a lack of information regarding diaphragm status, and in right ventricular function in particular (Leung et al. 2014). Therefore, the present results suggest that the evaluation of diaphragm degeneration and respiratory function is of great relevance when designing a better therapy for cardiomyopathy in DMD, which is in line with the concept that treatment of DMD‐associated cardiomyopathy requires diaphragm preservation, as shown in the mdx mice (Townsend et al. 2008; Crisp et al. 2011; Wasala et al. 2013; Betts et al. 2015).
Concluding remarks
In this morphometric study, we demonstrated that swimming exercise induces structural changes in the heart of the mdx mouse. The cardiac changes could be due to a direct effect of the exercise on the heart of the mdx mouse, since it is well described that swimming exercise induces physiological or pathological remodeling of the heart, mainly in the left ventricle, depending on the intensity of the exercise (Fernandes et al. 2015; Tosic et al. 2015). Alternatively, cardiac changes could be related to diaphragm degeneration, which was exacerbated in the exercised mdx mice. Supporting this alternative, we found that the right ventricle was preferentially affected, in agreement with a recent functional study (Meyers & Townsend, 2015). Furthermore, exercise did not affect the right ventricle of the normal mice, with no diaphragm degeneration. Although there is no direct evidence of changes in pulmonary pressure in mdx mouse, the significant increase in the thickness of the media wall of the pulmonary trunk and of the right ventricle wall, possibly related to hypoventilation due to diaphragm degeneration, may be consistent with increased pulmonary pressure in the mdx. These findings may be relevant for future interventional studies for DMD‐associated cardiomyopathy.
Conflict of interest
None.
Author contributions
Concept/design (H.S.N., I.C.C.B.), acquisition of data (I.C.C.B., J.A.P., M.R., D.O.M.), data analysis/interpretation (H.S.N., M.J.M., I.C.C.B., J.A.P., D.O.M.), drafting of the manuscript (H.S.N., M.J.M.), critical revision of the manuscript (H.S.N., M.J.M., J.A.P.) and approval of the article (all authors).
Acknowledgements
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grants 04/15526‐9, 08/58491‐1, 2021/03498‐7 and 14/04782‐6). H.S.N. and M.J.M. are recipients of fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grants 302831/2013‐4, 303320/2013‐3). J.A.P. was the recipient of a Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes)‐Proex fellowship.
References
- Alonso‐Gonzalez R, Borgia F, Diller GP, et al. (2013) Abnormal lung function in adults with congenital heart disease: prevalence, relation to cardiac anatomy, and association with survival. Circulation 127, 882–890. [DOI] [PubMed] [Google Scholar]
- Backman E, Nylander E (1992) The heart in Duchenne muscular dystrophy: a non‐invasive longitudinal study. Eur Heart J 13, 1239–1244. [DOI] [PubMed] [Google Scholar]
- Baltgalvis KA, Call JA, Cochrane GD, et al. (2012) Exercise training improves plantar flexor muscle function in mdx mice. Med Sci Sports Exerc 44, 1671–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbin ICC, Pereira JA, Taniguti APT, et al. (2014) Diaphragm degeneration aggravates cardiac muscle injury in dystrophin‐deficient mouse. J Muscle Res Cell Motil 35, 114. [Google Scholar]
- Bernasconi P, Torchiana E, Confalonieri P, et al. (1995) Expression of transforming growth factor‐beta 1 in dystrophic patient muscles correlates with fibrosis. J Clin Invest 96, 1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts CA, Saleh AF, Carr CA, et al. (2015) Implications for cardiac function following rescue of the dystrophic diaphragm in a mouse model of Duchenne muscular dystrophy. Sci Rep 5, 11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchentouf M, Benabdallah BF, Mills P, et al. (2006) Exercise improves the success of myoblast transplantation in mdx mice. Neuromuscul Disord 16, 518–529. [DOI] [PubMed] [Google Scholar]
- Call JA, McKeehen JN, Novotny SA, et al. (2010) Progressive resistance voluntary wheel running in the mdx mouse. Muscle Nerve 42, 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats AJ (1996) The ‘muscle hypothesis’ of chronic heart failure. J Mol Cell Cardiol 28, 2255–2262. [DOI] [PubMed] [Google Scholar]
- Costas JM, Nye DJ, Henley JB, et al. (2010) Voluntary exercise induces structural remodeling in the hearts of dystrophin‐deficient mice. Muscle Nerve 42, 881–885. [DOI] [PubMed] [Google Scholar]
- Crisp A, Yin H, Goyenvalle A, et al. (2011) Diaphragm rescue alone prevents heart dysfunction in dystrophic mice. Hum Mol Genet 20, 413–421. [DOI] [PubMed] [Google Scholar]
- De Bruin PF, Ueki J, Bush A, et al. (1997) Diaphragm thickness and inspiratory strength in patients with Duchenne muscular dystrophy. Thorax 52, 472–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Nico B, Rolland JF, et al. (2008) Gentamicin treatment in exercised mdx mice: identification of dystrophin‐sensitive pathways and evaluation of efficacy in work‐loaded dystrophic muscle. Neurobiol Dis 32, 243–253. [DOI] [PubMed] [Google Scholar]
- Drexler ES, Bischoff JE, Slifka AJ, et al. (2008) Stiffening of the extrapulmonary arteries from rats in chronic hypoxic pulmonary hypertension. J Res Natl Inst Stand Technol 113, 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong T, Iantorno M, Sali A, et al. (2011) Submaximal exercise effects on mdx mouse model. Neuromuscul Disord 21, 658. [Google Scholar]
- Dupont‐Versteegden EE, McCarter RJ, Katz MS (1994) Voluntary exercise decreases progression of muscular dystrophy in diaphragm of mdx mice. J Appl Physiol (1985) 77, 1736–1741. [DOI] [PubMed] [Google Scholar]
- Durmowicz AG, Stenmark KR (1999) Mechanisms of structural remodeling in chronic pulmonary hypertension. Pediatr Rev 20, e91–e102. [PubMed] [Google Scholar]
- Engel AG, Yamamoto M, Fischbeck KH (1994) Muscular dystrophies In: Myology (eds Engel AG, Franzini‐Armstrong C.), pp. 1133–1187. New York: McGraw‐Hill. [Google Scholar]
- Evangelista FS, Brum PC, Krieger JE (2003) Duration‐controlled swimming exercise training induces cardiac hypertrophy in mice. Braz J Med Biol Res 36, 1751–1759. [DOI] [PubMed] [Google Scholar]
- Fayssoil A, Nardi O, Orlikowski D, et al. (2010) Cardiomyopathy in Duchenne muscular dystrophy: pathogenesis and therapeutics. Heart Fail Rev 15, 103–107. [DOI] [PubMed] [Google Scholar]
- Fernandes T, Barauna VG, Negrao CE, et al. (2015) Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs. Am J Physiol Heart Circ Physiol 309, H543–H552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti R, Neto HS, Marques MJ (2011) Expression of utrophin at dystrophin‐deficient neuromuscular synapses of mdx mice: a study of protected and affected muscles. Anat Rec (Hoboken) 294, 283–286. [DOI] [PubMed] [Google Scholar]
- Finder JD, Birnkrant D, Carl J, et al. (2004) Respiratory care of the patient with Duchenne muscular dystrophy – ATS Consensus Statement. Am J Respir Crit Care Med 170, 456–465. [DOI] [PubMed] [Google Scholar]
- Finsterer J, Stollberger C (2003) The heart in human dystrophinopathies. Cardiology 99, 1–19. [DOI] [PubMed] [Google Scholar]
- Fogagnolo Mauricio A, Minatel E, Santo Neto H, et al. (2013) Effects of fish oil containing eicosapentaenoic acid and docosahexaenoic acid on dystrophic mdx mice. Clin Nutr 32, 636–642. [DOI] [PubMed] [Google Scholar]
- Galie N, Ghofrani HA, Torbicki A, et al. (2005) Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 353, 2148–2157. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Williams JE, Deering M, et al. (2004) Localization and early time course of TGF‐β1 mRNA expression in dystrophic muscle. Muscle Nerve 30, 645–653. [DOI] [PubMed] [Google Scholar]
- Hayes A, Williams DA (1996) Beneficial effects of voluntary wheel running on the properties of dystrophic mouse muscle. J Appl Physiol (1985) 80, 670–679. [DOI] [PubMed] [Google Scholar]
- Humbertclaude V, Hamroun D, Bezzou K, et al. (2012) Motor and respiratory heterogeneity in Duchenne patients: implication for clinical trials. Eur J Paediatr Neurol 16, 149–160. [DOI] [PubMed] [Google Scholar]
- Komolafe OA, Adeyemi DO, Adewole OS, et al. (2009) Morphological and morphometric studies of the aorta, pulmonary trunk, and heart of streptozotocin‐induced diabetic Wistar rats. Folia Morphol (Warsz) 68, 207–214. [PubMed] [Google Scholar]
- Kumar A, Bhatnagar S, Kumar A (2010) Matrix metalloproteinase inhibitor batimastat alleviates pathology and improves skeletal muscle function in dystrophin‐deficient mdx mice. Am J Pathol 177, 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DG, Herzka DA, Thompson WR, et al. (2014) Sildenafil does not improve cardiomyopathy in Duchenne/Becker muscular dystrophy. Ann Neurol 76, 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Mittal A, Makonchuk DY, et al. (2009) Matrix metalloproteinase‐9 inhibition ameliorates pathogenesis and improves skeletal muscle regeneration in muscular dystrophy. Hum Mol Genet 18, 2584–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell JR, Shilling C, Leslie ND, et al. (2012) Evidence‐based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol 71, 304–313. [DOI] [PubMed] [Google Scholar]
- Meyers TA, Townsend DW (2015) Early right ventricular fibrosis and reduction in biventricular cardiac reserve in the dystrophin‐deficient mdx heart. Am J Physiol Heart Circ Physiol 308, H303–H315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosqueira M, Zeiger U, Forderer M, et al. (2013) Cardiac and respiratory dysfunction in Duchenne muscular dystrophy and the role of second messengers. Med Res Rev 33, 1174–1213. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Yoshida K, Takeda S, et al. (2002) Progression of dystrophic features and activation of mitogen‐activated protein kinases and calcineurin by physical exercise, in hearts of mdx mice. FEBS Lett 520, 18–24. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Yanagisawa A, Sakata H, et al. (2001) Thallium‐201 single photon emission computed tomography (SPECT) in patients with Duchenne's progressive muscular dystrophy: a histopathologic correlation study. Jpn Circ J 65, 99–105. [DOI] [PubMed] [Google Scholar]
- Page‐McCaw A, Ewald AJ, Werb Z (2007) Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8, 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JA, Taniguti AP, Matsumura C, et al. (2012) Doxycycline ameliorates the dystrophic phenotype of skeletal and cardiac muscles in mdx mice. Muscle Nerve 46, 400–406. [DOI] [PubMed] [Google Scholar]
- Radley‐Crabb H, Terrill J, Shavlakadze T, et al. (2012) A single 30 min treadmill exercise session is suitable for ‘proof‐of concept studies’ in adult mdx mice: a comparison of the early consequences of two different treadmill protocols. Neuromuscul Disord 22, 170–182. [DOI] [PubMed] [Google Scholar]
- Sanyal SK, Johnson WW, Thapar MK, et al. (1978) An ultrastructural basis for electrocardiographic alterations associated with Duchenne's progressive muscular dystrophy. Circulation 57, 1122–1129. [DOI] [PubMed] [Google Scholar]
- Selsby JT, Acosta P, Sleeper MM, et al. (2013) Long‐term wheel running compromises diaphragm function but improves cardiac and plantarflexor function in the mdx mouse. J Appl Physiol (1985) 115, 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer N, Dietrich A, Schermuly RT, et al. (2008) Regulation of hypoxic pulmonary vasoconstriction: basic mechanisms. Eur Respir J 32, 1639–1651. [DOI] [PubMed] [Google Scholar]
- Spurney CF (2011) Cardiomyopathy of Duchenne muscular dystrophy: current understanding and future directions. Muscle Nerve 44, 8–19. [DOI] [PubMed] [Google Scholar]
- Stedman HH, Sweeney HL, Shrager JB, et al. (1991) The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature 352, 536–539. [DOI] [PubMed] [Google Scholar]
- Tosic JTS, Jakovljevic VL, Zivkovic VV, et al. (2015) Biphasic response of cardiodynamic adaptations to swimming exercise in rats. Gen Physiol Biophys 34, 301–310. [DOI] [PubMed] [Google Scholar]
- Townsend D, Yasuda S, Li S, et al. (2008) Emergent dilated cardiomyopathy caused by targeted repair of dystrophic skeletal muscle. Mol Ther 16, 832–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteegh MI, Braun J, Voigt PG, et al. (2007) Diaphragm plication in adult patients with diaphragm paralysis leads to long‐term improvement of pulmonary function and level of dyspnea. Eur J Cardiothorac Surg 32, 449–456. [DOI] [PubMed] [Google Scholar]
- Voelkel NF, Quaife RA, Leinwand LA, et al. (2006) Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 114, 1883–1891. [DOI] [PubMed] [Google Scholar]
- Wasala NB, Bostick B, Yue Y, et al. (2013) Exclusive skeletal muscle correction does not modulate dystrophic heart disease in the aged mdx model of Duchenne cardiomyopathy. Hum Mol Genet 22, 2634–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsukura M, Miyagawa M, Tsuya T, et al. (1988) Pulmonary hypertension in progressive muscular dystrophy of the Duchenne type. Jpn Circ J 52, 321–326. [DOI] [PubMed] [Google Scholar]


