Key Clinical Message
The Revivent‐TC™‐system is able to restore LV volumes in patients with severe ischemic cardiomyopathy. We are presenting a case report of successful implantation of the Revivent‐TC™‐system, but postprocedural development of sustained VT. This case report is presenting one way to successfully treat patients with postprocedural frequent VT.
Keywords: Chronic heart failure, Clinical: Cardiac mapping – 3 dimensional systems, Clinical: Catheter ablation – ventricular tachycardia, Clinical: Electrophysiology – ventricular tachycardia, ventricular reconstruction
Introduction
Left ventricle (LV) remodeling and development of LV aneurysms after myocardial infarction (MI) lead to increased LV volumes, reduced LV ejection fraction (LVEF), and subsequent congestive heart failure (CHF) 1, 2. Surgical ventricular reconstruction (SVR) is able to reduce LV volumes and improve LVEF, yet the impact on morbidity and mortality is controversial and a high perioperative risk as well as postoperative malignant ventricular tachycardias (VT) have been reported 3, 4. Recently, interventional strategies have been introduced to treat these high‐risk patients 5. The Less Invasive Ventricular Enhancement (LIVE) technique using the Revivent‐TC™ (transcatheter)‐system (BioVentrix Inc., San Ramon, CA) is able to exclude LV aneurysms and restore LV volumes similar to SVR by performing an off‐pump interventional procedure 6, 7. However, similar to SVR VT may occur after LIVE technique. Catheter ablation (CA) has evolved into an accepted treatment option for patients suffering from scar‐related VT 8, 9. To the best of our knowledge, we report on the first case of CA after LIVE‐based LV restoration and subsequently development of frequent VT.
Case Report
A 77‐year‐old male with a history of CHF (New York Heart Association class III‐IV) and anterior LV aneurysm after MI due to left anterior descending artery occlusion in 10/2000 was subjected for LIVE‐based LV restoration. To exclude progression of coronary artery disease, an angiography was conducted prior LV restoration. The patient underwent a successful MitraClip™‐implantation for severe mitral regurgitation in 06/2014 and a single lead ICD was implanted for primary prevention in 02/2014. No episode of VT or ventricular fibrillation (VF) was detected and no antitachycardial pacing (ATP) or ICD‐shock therapy was documented before the LIVE procedure. The following preinterventional LV parameters were measured by angiography: end‐diastolic volume (EDV): 593 mL, end‐systolic volume (ESV): 545 mL, LVEF: 8%. After informed consent a successful LIVE procedure utilizing the Revivent‐TC™‐system was conducted (Fig. 1). In total four pairs of anchors were placed and LV parameters were improved as the following: EDV: 442 mL, ESV: 377 mL, LVEF: 15%. No periprocedural complications occurred. On the next day the patient developed sustained VT (cycle length: 420 msec, right bundle branch block (RBBB), inferior axis) with subsequently induced cardiogenic shock. He was admitted to the ICU and treated according to current guidelines 2. No ICD therapy was triggered due to relatively slow VT. The patient was initially cardioverted via external defibrillator and intravenous administration of amiodarone 1000 mg/day was started. Although the cycle lengths of VT was prolonged after administration of 9000 mg of amiodarone, the patient still frequently suffered from recurrent sustained and nonsustained VT. Therefore, a CA procedure was planned to abolish clinical VT.
Figure 1.
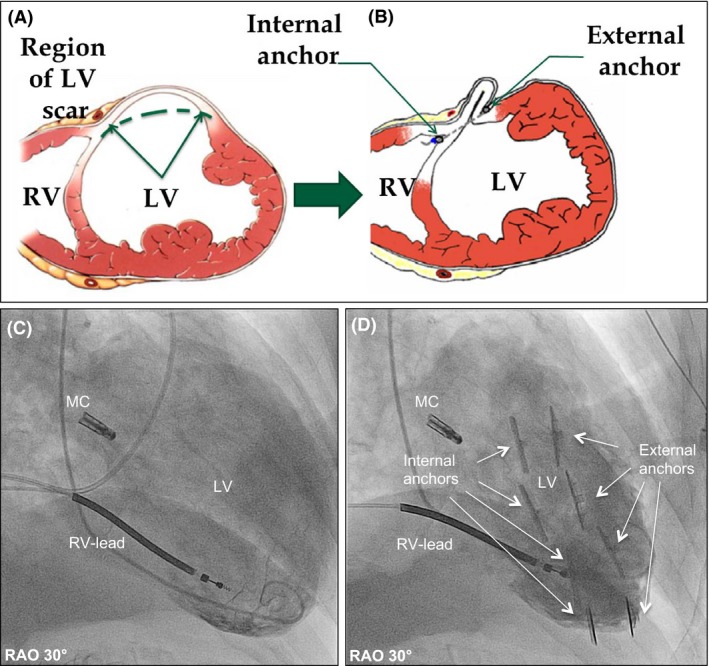
Interventional left ventricular restoration with the Revivent‐TC ™‐system. Principle of the procedure; prior (A) and after (B) LV restoration. Corresponding angiographic images prior (C) and after (D) the procedure. MC, MitraClip™; RV‐Lead, Right ventricular‐lead of ICD; RV, right ventricle; LV, left ventricle.
Catheter ablation procedure
The CA was performed 10 days after LV restoration and was conducted under endotracheal intubation and intravenous anesthesia without additional LV‐assist device. LV mapping was conducted via a combined antegrade transseptal and retrograde transaortic approach utilizing a three‐dimensional electroanatomical (EA) mapping system (CARTO® 3 System, Biosense Webster Inc., Diamond Bar, CA) and a 8F, 3.5 mm irrigated‐tip catheter (NAVISTAR® Thermocool, Biosense Webster) 10. During mapping two different VT morphologies (VT1 and VT2) were easily induced via catheter manipulation. External cardioversion was performed twice to restore sinus rhythm due to unstable hemodynamic status during VT. In total 213 mapping points were acquired identifying a large anterior scar, which presented as low amplitude (<1.5 mV bipolar voltage) and/or abnormal electrograms (Fig. 2). Through a leakage near the apical septum the ablation catheter was accidentally advanced into a residual part of the previously excluded LV aneurysm, which was not completely excluded 10 days after LV restoration. With pacemapping the entrance site of VT1 (cycle length: 520 msec, RBBB, inferior axis) was found antero‐medial superior, whereas the exit site was identified antero‐lateral inferior at the EA scar border zone. Irrigated radiofrequency (RF) current was delivered in the power‐controlled mode with a maximum power of 40W and a flow rate of 20 mL/min. Via antegrade approach 8 RF applications (total duration of 703 sec) were performed to generate a linear lesion across the slow conduction zone resulted in prolongation and termination of VT1 (Fig. 3). Afterward VT2 (cycle length: 600 msec, RBBB, superior axis) was reproducibly induced. Pacemapping was conducted. The best pacemap (9/12) of VT2 was located at the antero‐medial apex. Utilizing two RF applications via retrograde approach (total duration: 217 sec) VT2 was prolonged and terminated (Fig. 4). Afterward no VT was inducible via electrical programmed stimulation and burst pacing during a waiting period of 60 min. No periprocedural complications occurred. The patient was discharged after 10 days and freedom of any symptoms and no evidence of VT recurrence were observed.
Figure 2.
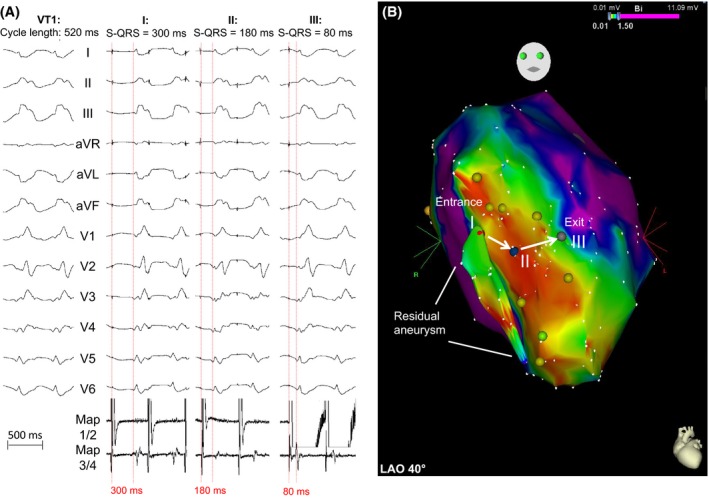
Pacemapping of VT1 (CL = 520 msec, right bundle branch block, inferior axis). (A) Pacemapping with continuous reduction in stimulus‐QRS intervals. Points (I: red, II: blue, III: gray) represent the corresponding locations within the electroanatomical map (B). Entrance and exit site are presented (white arrows). A small residual aneurysm was found during mapping of the left ventricle (white lines).
Figure 3.
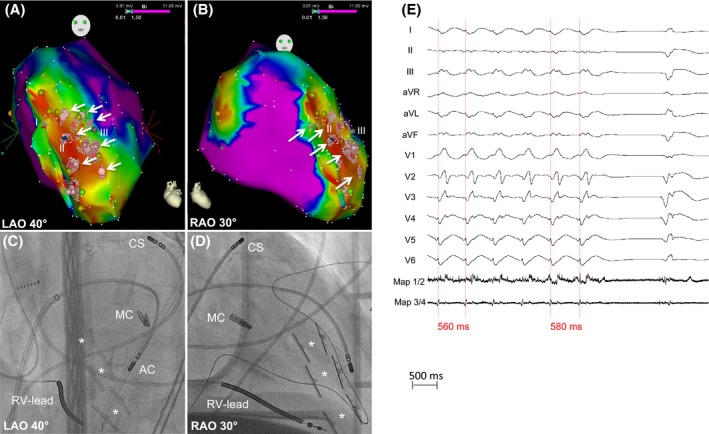
Ablation of VT1. (A, B) Electroanatomical maps. Points (I = red, II = blue, III = gray) represent corresponding pacemapping locations. Light red points and white arrows mark the ablation sights. (C, D) Corresponding angiographic images presenting the sight of ablation (antegrade transseptal approach). AC, Ablation Catheter; CS, Coronary Sinus catheter; MC, MitraClip™; RV‐Lead, Right ventricular‐lead of ICD. The white stars mark the pairs of Revivent‐TC ™ anchors. (E) Prolongation and termination of VT1 during ablation.
Figure 4.
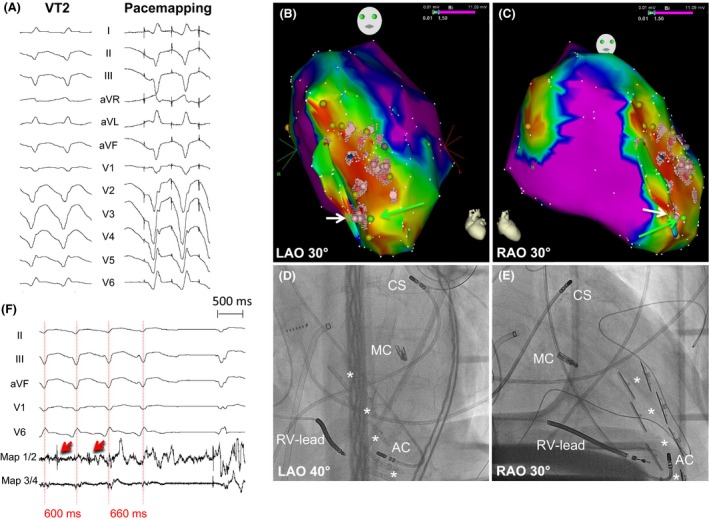
Pacemapping and Ablation of VT2 (CL = 600 msec, right bundle branch block, superior axis). (A) Pacemapping of VT2, (B, C) Electroanatomical maps. Light red points and the white arrow mark the ablation sight. (D, E) Corresponding angiographic images presenting the sight of ablation (retrograde transaortic approach). AC, Ablation Catheter; CS, Coronary Sinus catheter; MC, MitraClip™; RV‐Lead, Right Ventricular‐lead of ICD. The white stars mark the pairs of Revivent‐TC ™ anchors. (F) Prolongation and termination of VT2 during ablation. Red arrows presenting middiastolic potentials.
Discussion
We are presenting a case report of CA after LIVE‐based LV restoration and subsequently development of VT. No VT or VF was observed for >18 month under ICD surveillance until LV restoration while 1 day after the procedure the patient developed VT. We suggest most likely the VT was induced through LV restoration, for example, due to mechanical compression of the scar border zone. Comparing to data after SVR 11; CA after interventional LIVE‐based LV restoration was successfully performed via a combined antegrade and retrograde approach suggesting this treatment strategy to be possibly safe and feasible. However, as recently described for the Parachute™ device the target area of VT ablation may be covered by the device itself and therefore not reachable for endocardial CA 12. Therefore, it should be taken into consideration whether patients who are planned for an interventional or surgical LV restoration should be evaluated for VT ablation prior procedure. In these patients an electrophysiological examination including programmed stimulation could possibly detect whether sustained monomorphic VTs could be induced prior LV restoration. Theoretically LV reduction with this device may potentially produce arrhythmogenic substrate. In clinical practice, programmed stimulation was not performed due to limited survival with the very poor LV function. Furthermore, a preinterventional EA mapping could guide future LV restoration procedures to precisely detect scar area and border zone. In clinical practice, the number of patients with severe CHF due to ischemic cardiomyopathy and the utilization of interventional treatment options are increasing 13. This case report is presenting one way to successfully treat patients with postprocedural frequent VT. However, clinical trials with sufficient patient numbers are needed to draw final conclusions.
Conflict of Interest
CH Heeger received travel grants by St. Jude Medical, Biotronik and Medtronic. KH Kuck received research grants from Biosense Webster, Stereotaxis, Prorhythm, Medtronic, Edwards, Cryocath, and is a consultant to St. Jude Medical, Biosense Webster, Prorhythm, and Stereotaxis. A Metzner received speaker's honoraria from Medtronic. All other authors have no relevant disclosures.
Clinical Case Reports 2016; 4(4): 339–343
References
- 1. Gheorghiade, M. , and Bonow R. O.. 1998. Chronic heart failure in the united states: a manifestation of coronary artery disease. Circulation 97:282–289. [DOI] [PubMed] [Google Scholar]
- 2. McMurray, J. J. , Adamopoulos S., Anker S. D., Auricchio A., Bohm M., Dickstein K., et al. 2012. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur. Heart J. 33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 3. Jones, R. H. , Velazquez E. J., Michler R. E., Sopko G., Oh J. K., O'Connor C. M., et al. 2009. Coronary bypass surgery with or without surgical ventricular reconstruction. N. Engl. J. Med. 360:1705–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castelvecchio, S. , Menicanti L., and Donato M. D.. 2010. Surgical ventricular restoration to reverse left ventricular remodeling. Curr. Cardiol. Rev. 6:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Costa, M. A. , Mazzaferri E. L. Jr, Sievert H., and Abraham W. T.. 2014. Percutaneous ventricular restoration using the parachute device in patients with ischemic heart failure: three‐year outcomes of the parachute first‐in‐human study. Circ. Heart Fail. 7:752–758. [DOI] [PubMed] [Google Scholar]
- 6. Faria, R. , Melica B., Pires‐Morais G., Rodrigues A., Ribeiro J., Guerra M., et al. 2014. New less invasive ventricular reconstruction technique in the treatment of ischemic heart failure. Rev. Port. Cardiol. 33:461–465. [DOI] [PubMed] [Google Scholar]
- 7. Wechsler, A. S. , Sadowski J., Kapelak B., Bartus K., Kalinauskas G., Rucinskas K., et al. 2013. Durability of epicardial ventricular restoration without ventriculotomy. Eur. J. Cardiothorac. Surg. 44:189–192. [DOI] [PubMed] [Google Scholar]
- 8. Priori, S. G. , Blomstrom‐Lundqvist C., Mazzanti A., Blom N., Borggrefe M., Camm J., et al. 2015. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 41:2793–2867. [DOI] [PubMed] [Google Scholar]
- 9. Kuck, K. H. , Schaumann A., Eckardt L., Willems S., Ventura R., Delacretaz E., et al. 2010. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (vtach): a multicentre randomised controlled trial. Lancet 375:31–40. [DOI] [PubMed] [Google Scholar]
- 10. Tilz, R. R. , Makimoto H., Lin T., Rillig A., Metzner A., Mathew S., et al. 2014. In vivo left‐ventricular contact force analysis: comparison of antegrade transseptal with retrograde transaortic mapping strategies and correlation of impedance and electrical amplitude with contact force. Europace 16:1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoshiga, Y. , Mathew S., Wissner E., Tilz R., Fuernkranz A., Metzner A., et al. 2012. Correlation between substrate location and ablation strategy in patients with ventricular tachycardia late after myocardial infarction. Heart Rhythm 9:1192–1199. [DOI] [PubMed] [Google Scholar]
- 12. Wijnmaalen, A. P. , Roberts‐Thomson K. C., Steven D., Klautz R. J., Willems S., Schalij M. J., et al. 2012. Catheter ablation of ventricular tachycardia after left ventricular reconstructive surgery for ischemic cardiomyopathy. Heart Rhythm 9:10–17. [DOI] [PubMed] [Google Scholar]
- 13. Lauschke, J. , Schneider R., and Bansch D.. 2014. Ventricular tachycardia ablation in a patient with a parachute device: a decent word of warning. Europace 16:207. [DOI] [PubMed] [Google Scholar]


