Key Clinical Message
We report the first case of pregnancy in a pediatric patient with catecholiminergic polymorphic ventricular tachycardia (CPVT). Pregnant adolescents with CPVT are at high risk for NSVT and malignant VT during pregnancy, despite antiarrhythmic medication. They may receive multiple implantable cardioverter defibrillator (ICD) therapies. Such patients require close monitoring with special care during the first trimester.
Keywords: Adolescent, catecholimergic polymorphic ventricular tachycardia, discharges, implantable cardioverter defibrillator, pediatric, pregnancy, pregnant, teenage pregnancy
Introduction
There is a paucity of information regarding pregnant women with an implantable cardioverter defibrillator (ICD) who have received documented discharges during pregnancy. Catecholiminergic polymorphic ventricular tachycardia (CPVT) is a calcium channelopathy that causes malignant polymorphic ventricular tachycardia (PVT) with adrenergic stimulation, and can be triggered by emotional or physical stress such as may be experienced in pregnancy 1, 2. We report a case of a pregnant teenager with CPVT who received documented ICD discharges during her pregnancy.
Case Report
In early 2004 a 12‐year‐old female with a history of syncope during exercise was evaluated. Physical examination was normal. Her resting 12‐lead electrocardiogram and echocardiogram were within normal limits. Her cardiac MRI demonstrated normal findings without evidence of arrythmogenic right ventricular dysplasia (ARVD). Her pattern of highly malignant PVT triggered by adrenergic stimulation in the absence of structural heart disease or ECG abnormalities in a patient less than 40 years of age was thought consistent with CPVT 3.
She underwent an electrophysiologic (EP) study, which demonstrated inducible and reproducible non‐sustained polymorphic ventricular tachycardia. An ICD (Medtronic Marquis defibrillator model # 7230CX, Medtronic plc, Dublin, Ireland) was placed in the left infraclavicular area and a ventricular lead (model 6947) was implanted in the right ventricle via the left subclavian vein. Successful conversion of tachycardia with a 10 J shock was recorded. The patient was placed on daily atenolol.
Since implantation, the patient has had multiple episodes of appropriate device therapy for PVT, all of which were preceded by a provoking event. This was frequently followed by a subsequent shock because of catecholamine release from the first shock.
In early 2009 the patient conceived her first child at the age of 17 years. She was seen in the pacemaker clinic at 10 weeks gestation by last menstrual period (LMP). At that time she was on atenolol, 50 mg twice a day. Her device settings that day were pacing mode VVI with a lower rate of 45 bpm, amplitude of 2.5 V, pulse width of 0.6 msec, and a sensitivity of 0.3 mV. Therapy parameters were VT zone 162–207 bpm and VF zone >207 bpm. Pacing history revealed that she was ventricularly sensed 97.5% of the time and ventricularly paced 2.5% of the time since her last visit.
Interrogation revealed three events of sustained PVT requiring device therapy. The events occurred at approximately 2 weeks gestation, 4 weeks gestation and 9 weeks gestation. Each event was reversed with a single 20 J shock. All events occurred while walking and during the day time. The patient said that she forgot to take atenolol on the day of the third event. For each event, the patient stated that she felt very dizzy, and then the defibrillator went off. She continued to remain dizzy with poor exercise tolerance for approximately 30–60 sec following the discharge. Evaluation of her electrocardiograms revealed that she continued to have erratic rhythm with short bursts of ventricular tachycardia during that period before reverting to normal sinus rhythm (Fig. 1).
Figure 1.
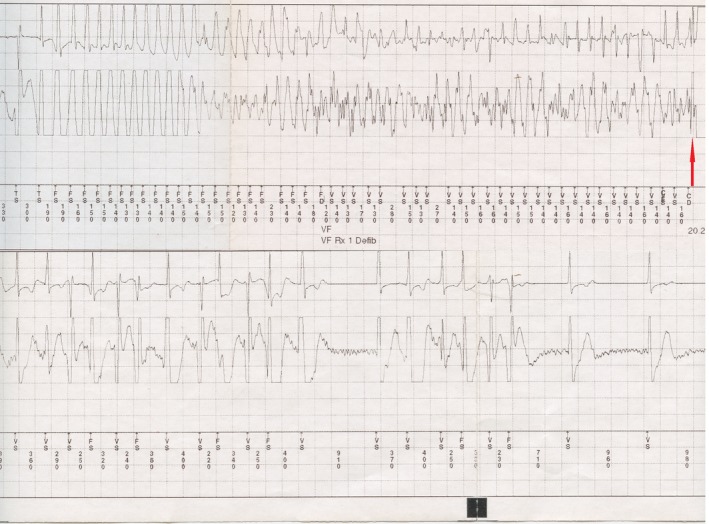
ECG lead showing a ventricular fibrillation event. Failure of antitachycardia pacing was followed by degeneration into ventricular fibrillation and ICD therapy (red arrow). Erratic rhythm with bursts of ventricular tachycardia continued after the ICD therapy before spontaneously reverting to normal sinus rhythm. TS, ventricular sensing of VT zone; FD, ventricular fibrillation detection; FS, ventricular sensing of FVT zone; TF, ventricular sensing of VF zone; TD, ventricular tachycardia detection; Rx, therapy (Defibrillation 20.2J × 1); VF, ventricular fibrillation; VT, ventricular tachycardia; VS, normal R‐wave sensing.
She additionally had thirty events of non‐sustained ventricular tachycardia (NSVT), and two which the device determined to be supraventricular tachycardia (SVT). Eighteen of these events occurred during the third month of pregnancy. At this visit she was switched to metoprolol 50 mg twice a day because of a better safety profile in pregnancy.
She called 4 days later saying metoprolol was making her feel dizzy, and she could feel her pacemaker “pacing her a lot more.” Metoprolol was decreased to 50 mg once daily. She called again a week later, saying she was still feeling unwell and her pacemaker “was pacing all the time.” Both times, she denied any defibrillator therapies. After consultation with the high‐risk obstetric staff, she was resumed on her original dose of atenolol, 50 mg twice daily.
Her 23‐week ultrasound showed a fetal heart rate of 140 bpm and a PR interval of 100 msec. A biophysical profile at 34 weeks gestation revealed a 2416 g fetus which was approximately at the 35th percentile for weight. The amniotic fluid index was estimated to be adequate at 9.5–13 cm. Expected date of delivery by LMP and ultrasound differed by 4 days.
She was seen in the pacemaker clinic a week prior to her planned cesarean section, which was approximately 6 months from her previous visit. Device settings were unchanged. Pacing history revealed that she was ventricularly sensed 99.2% of the time and ventricularly paced 0.8% of the time. She had only three events of NSVT without symptoms, no ventricular fibrillation event and no ICD therapies. She was on atenolol 25 mg in the morning and 50 mg in the evening at this visit.
She underwent planned cesarean section under spinal anesthesia with 20 μg of Fentanyl delivered spinally at 38 weeks gestation. Bupivicaine 1% was used for local anesthesia at site of needle injection. 2 mg of Midazolam and 0.15 mg of Morphine were used preoperatively for mild sedation and pain control. Intravenous bicitra and phenergan were used intraoperatively to relieve patient nausea. The patient delivered a healthy male newborn with a birth weight of 3075 g. Apgar scores were 8 and 9 at 1 and 5 min, respectively. There were no complications during or after delivery, and mother and baby were discharged uneventfully. A 3‐month follow up in the gynecology and cardiac clinics of mother and baby revealed no adverse outcome except a new diagnosis of maternal post‐partum depression.
Discussion
The use of the ICD has allowed an increased number of young women with life‐threatening cardiac arrhythmias to reach their reproductive years. Few studies have examined pregnancy in women with ICD's 4, 5, 6, 7. All have small samples, adult populations and a narrow variety of cardiac disorders. Adolescent pregnancies have a higher propensity for adverse outcome, even in otherwise‐healthy teenagers 8. Such pregnancies may require even closer monitoring in the context of known cardiovascular disease.
Only one other case of pregnancy in a woman with CPVT and an ICD was found in the literature as part of a recent review of 12 adult patients 4. This patient did not receive any ICD discharges, and did not experience any episodes of ventricular arrhythmias or ventricular pacing during her pregnancy (Boule et al., personal communication). Other reports on arrythmogenic conditions during pregnancy such as long‐QT syndrome and ARVD have suggested ventricular events requiring ICD therapy in the second trimester, approximately around 20 weeks 9, 10. In contrast, our patient experienced three appropriate therapies and several events of antitachycardia pacing during the first trimester. Hormonal, cardiovascular and autonomic changes might play a role in exacerbation of arrhythmias during pregnancy 10, 11. In particular, circulating estrogen has been proposed to increase myocardial sensitivity to catecholamines 12. This may make patients with CPVT more sensitive to ventricular electrical events than other arrhythmogenic conditions, including other primary electrical diseases. However, the current scarcity of literature precludes any evidence‐based presumptions regarding the pattern of arrhythmias during pregnancy in CPVT on the basis of other arrhythmogenic conditions 9, 13. Important differences in physiology and endocrinology in adolescent pregnancy from adults are well‐documented 8, 14. These may all be factors in the poor control of ventricular arrhythmias in our patient. We additionally propose that the development of tolerance to the rapid cardiovascular, hormonal or autonomic changes induced by pregnancy may be different in adolescents compared to adults. Adolescent physiology may, in fact, be slower to adapt to these multiple changes thereby increasing their risk of electrical events early on. This premise is supported by our observation that the arrhythmias decreased as the pregnancy progressed despite no significant changes in medical management, possibly due to a better cardiovascular tolerance. A better and quicker adaptation may also be the reason for Boule et al.'s adult patient having a significantly different course of pregnancy.
Our case indicates that the highest risk for ventricular events may be during the first trimester. Also, teenage pregnancies may behave completely differently from adults, as is suggested by the key differences between our patient and the one reported by Boule et al. Based on these observations, we recommend closer monitoring of non‐adult patients with CPVT early in pregnancy. As such, any “high‐risk pregnancy” team for such patients should include a cardiologist on all first trimester visits. These patients may be appropriate candidates for aggressive remote monitoring, suggested by recent reports to offer opportunity for faster action in case of adverse events, and also prevent inappropriate therapies 15.
Our experience suggests metoprolol may not be as effective as atenolol during pregnancy in CPVT, by causing more side effects and patient discomfort. Boule et al.'s patient was maintained successfully on high dose nadolol (personal communication). Our use of atenolol did not result in adverse fetal outcome, suggesting it may be a safe alternative. However, dose optimization of beta blockers during pregnancy may be required to achieve better antiarrhythmic control, and prevent unnecessary ICD therapies. Neither our patient nor that of Boule et al. required antibradycardia pacing, despite close to maximal doses of beta blockers. The importance of adhering to medication should be emphasized at every cardiology and obstetric visit. In our case, missing a single dose may have resulted in symptomatic malignant VT requiring ICD therapy within a few hours. Sustained VT requiring device intervention within a short timeframe of discontinuing beta blockers has also been reported elsewhere 16, 17. Furthermore, it would also be reasonable to assume that nadolol may have been an important factor in preventing malignant VT in Boule et al.'s patient.
Pacemaker and ICD parameters should be individualized for pregnant patients based on the characteristics of their prior events. However, where such information may not be available, our settings may be effective to maintain a patient with CPVT during pregnancy. Boule et al. maintained their patient at somewhat comparable settings of VVI 40 bpm, VT zone 200–250 bpm and VF zone >250 bpm. However, their higher VF and VT thresholds are untested for appropriate response to malignant VT in such a clinical situation.
ICD therapies and frequent ventricular pacing did not result in adverse fetal outcome in our patient. This may be a combined effect of the low amount of current delivered by ICD's and a high fetal fibrillatory threshold 18.
Conclusions
Our case report is the first report of pregnancy in a pediatric patient with CPVT. It is also the first identifiable report of ICD therapies during pregnancy in a CPVT patient of any age. This case report provides information which may be critical in managing such patients. We conclude that adolescents with CPVT are at high risk for both NSVT and malignant VT during pregnancy, despite antiarrhythmic medication. We recommend close monitoring of such patients throughout pregnancy with special care during the first trimester.
Conflict of Interest
None declared.
Clinical Case Reports 2016; 4(4): 361–365
References
- 1. Kim, J. B. 2014. Channelopathies . Korean J. Pediatr. 57:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miyake, C. Y. , Webster G., Czosek R. J., Kantoch M. J., Dubin A. M., Avasarala K., et al. 2013. Efficacy of implantable cardioverter defibrillators in young patients with catecholaminergic polymorphic ventricular tachycardia: success depends on substrate. Circ. Arrhythm. Electrophysiol. 6:579–587. [DOI] [PubMed] [Google Scholar]
- 3. Priori, S. G. , Wilde A. A., Horie M., Cho Y., Behr E. R., Berul C., et al. 2013. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 10:1932–1963. [DOI] [PubMed] [Google Scholar]
- 4. Boule, S. , Ovart L., Marquie C., Botcherby E., Klug D., Kouakam C., et al. 2014. Pregnancy in women with an implantable cardioverter‐defibrillator: is it safe? Europace 16:1587–1594. [DOI] [PubMed] [Google Scholar]
- 5. Natale, A. , Davidson T., Geiger M. J., and Newby K.. 1997. Implantable cardioverter‐defibrillators and pregnancy: a safe combination? Circulation 96:2808–2812. [DOI] [PubMed] [Google Scholar]
- 6. Schuler, P. K. , Herrey A., Wade A., Brooks R., Peebles D., Lambiase P., et al. 2012. Pregnancy outcome and management of women with an implantable cardioverter defibrillator: a single centre experience. Europace 14:1740–1745. [DOI] [PubMed] [Google Scholar]
- 7. Miyoshi, T. , Kamiya C. A., Katsuragi S., Ueda H., Kobayashi Y., Horiuchi C., et al. 2013. Safety and efficacy of implantable cardioverter‐defibrillator during pregnancy and after delivery. Circ. J. 77:1166–1170. [DOI] [PubMed] [Google Scholar]
- 8. Torvie, A. J. , Callegari L. S., Schiff M. A., and Debiec K. E.. 2015. Labor and delivery outcomes among young adolescents. Am. J. Obstet. Gynecol. 213:95 e1–8. [DOI] [PubMed] [Google Scholar]
- 9. Agir, A. , Bozyel S., Celikyurt U., Argan O., Yilmaz I., Karauzum K., et al. 2014. Arrhythmogenic right ventricular cardiomyopathy in pregnancy. Int. Heart J. 55:372–376. [DOI] [PubMed] [Google Scholar]
- 10. Silversides, C. K. , Harris L., Haberer K., Sermer M., Colman J. M., and Siu S. C.. 2006. Recurrence rates of arrhythmias during pregnancy in women with previous tachyarrhythmia and impact on fetal and neonatal outcomes. Am. J. Cardiol. 97:1206–1212. [DOI] [PubMed] [Google Scholar]
- 11. Ekholm, E. M. , Piha S. J., Erkkola R. U., and Antila K. J.. 1994. Autonomic cardiovascular reflexes in pregnancy. A longitudinal study. Clin. Auton. Res. 4:161–165. [DOI] [PubMed] [Google Scholar]
- 12. Page, R. L. 1995. Treatment of arrhythmias during pregnancy. Am. Heart J. 130:871–876. [DOI] [PubMed] [Google Scholar]
- 13. Piacenza, J. M. , Kirkorian G., Audra P. H., and Mellier G.. 1998. Hypertrophic cardiomyopathy and pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 80:17–23. [DOI] [PubMed] [Google Scholar]
- 14. Aguilar‐Moreno, M. , Galicia‐Castillo O. R., Aguilera‐Reyes U., Varea‐Gonzalez C., Bernis‐Carro C., and Garcia‐Lopez G. I. 2015. Hormonal state comparison (progesterone, estradiol, and leptin) of body fat and body mass indices in mexican women as a risk factor for neonatal physiologic condition. J. Pediatr. Adolesc. Gynecol. 28:149–156. [DOI] [PubMed] [Google Scholar]
- 15. Guedon‐Moreau, L. , Kouakam C., Klug D., Marquie C., Brigadeau F., Boule S., et al. 2014. Decreased delivery of inappropriate shocks achieved by remote monitoring of ICD: a substudy of the ECOST trial. J. Cardiovasc. Electrophysiol. 25:763–770. [DOI] [PubMed] [Google Scholar]
- 16. Lee, L. C. , Bathgate S. L., and Macri C. J.. 2006. Arrhythmogenic right ventricular dysplasia in pregnancy: a case report. J. Reprod. Med. 51:725–728. [PubMed] [Google Scholar]
- 17. Bonini, W. , Botto G. L., Broffoni T., and Dondina C.. 2000. Pregnancy with an ICD and a documented ICD discharge. Europace 2:87–90. [DOI] [PubMed] [Google Scholar]
- 18. Page, R. L. , Hamdan M. H., and Joglar J. A.. 2002. Arrhythmias occurring during pregnancy. Card. Electrophysiol. Rev. 6:136–139. [DOI] [PubMed] [Google Scholar]


