Key Clinical Message
Benign impetigo can progress into a potential fatal staphylococcal scalded skin syndrome (SSSS) if prompt diagnosis and correct therapy is not established rapidly. Local and systematic antibiotics as well as Lactulose are crucial in order to stop SSSS from progressing. Burns units should be involved when skin lesions are extensive.
Keywords: Intensive care, SSSS, staphylococcal scalded skin syndrome
Introduction
Background
Staphylococcal Scalded Skin Syndrome (SSSS) is a vesiculating skin disease often originating from bullous impetigo. SSSS is normally found in children under the age of 5 and is usually caused by the toxins type A or B coming from Staphylococcus aureus 1. The toxin spreads hematogenously and involves the superficial epidermis. It heals within 10–14 days without scarring 1. Mortality in infancy is low (4%) 1. Burn units should be involved when skin lesions are extensive 1.
We present a case of a 5‐year‐old girl with rapidly progressing SSSS covering most of her body's surface area.
Case Presentation
Day 1: A 5‐year‐old healthy girl presented with a sore bullae in the nasolabial line on the right side which measured 1 × 1 cm. The bullae erupted and left a red denuded area. She also had reduced appetite, seemed irritable, and complained of having a sore throat. When drying the nose, the outermost layer of the skin sloughed off (positive Nikolsky's sign. Fig. 1B). Signs of early bilateral conjunctivitis were also present. At this stage, she did not have a fever. The mother consulted the emergency department. With the suspicion of impetigo, the doctor prescribed Fucidin ointment.
Figure 1.
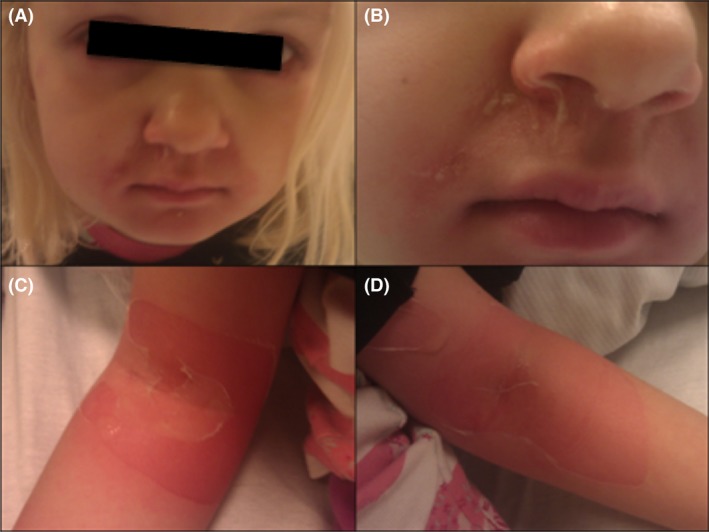
Signs of early conjunctivitis (A) and desquamation of skin (Nikolsky's sign) in the right nasolabial line (B) Desquamation of skin from cubital fossa's on second night.
Day 2: With the symptoms not resolving, the mother took the child to her local GP. On continued suspicion of impetigo, the GP did not change the treatment with Fucidin ointment. Within hours, the bullae spread to the right clavicle and on the backside of the right ear. The patient was still afebrile. With the symptoms advancing, the mother took the child to the emergency department where the child was prescribed a course of Flucloxacillin and sent home. Blood samples clarifying infection counts and fluid electrolytes were not measured.
Same night, the patient awoke with fever, pains, and desquamation of the skin around eyes and mouth, on the back and gluteal area, both axils, cubital fossa (Fig. 1C and D) and popliteal. The rash covered about 10% of the body's surface area. The child was admitted to the local hospital and treated with intravenous Flucloxacillin. Prior to treatment with Flucloxacillin, samples from skin lesions, nasal cavity, perineum, axils, and trachea were obtained and sent to culture growth and microscopy. S. aureus was identified a week later from nasal cavity and pharynx, while samples taken from skin lesions, axils, and perineum were without the presence of S. aureus.
Day 3: After conference with the specialized unit for burn injuries, a treatment of oral Fucidin, intravenous Penicillin, Lactulose, and fluid maintenance was commenced. The patient was then transferred by helicopter to the Clinic of Burn Injury. At arrival, the bullae formation now covered 55% of the body's surface area, including the eye and mouth area, ears, neck, back, gluteal, axilla, cubital fossa's, wrists, hands, genitalia, the groin, popliteal, and ankles (see Fig. 2). The patient seemed fatigued, febrile, and in intense pain. She was placed in a sterile, heated burns injury ward. A central vein catheter was inserted in general anesthesia since a usual peripheral vein catheter could not be inserted. Infection counts and fluid electrolytes were measured in blood samples and results unremarkable. Skin biopsy was performed and SSSS confirmed the following day. Feces and blood were sent to culture growth and microscopy. The presence of S. aureus was confirmed within 2–3 days (gram stain was used after identification of culture growth from blood and prior to microscopy). The patient was given sufficient pain relief. The conjunctivitis was treated with Chloramphenicol eye drops. Fluid maintenance aiming at an hourly diuresis of 1 mL/kg per hour was commenced and the treatment with intravenous Penicillin, oral Fucidin, and Lactulose continued.
Figure 2.
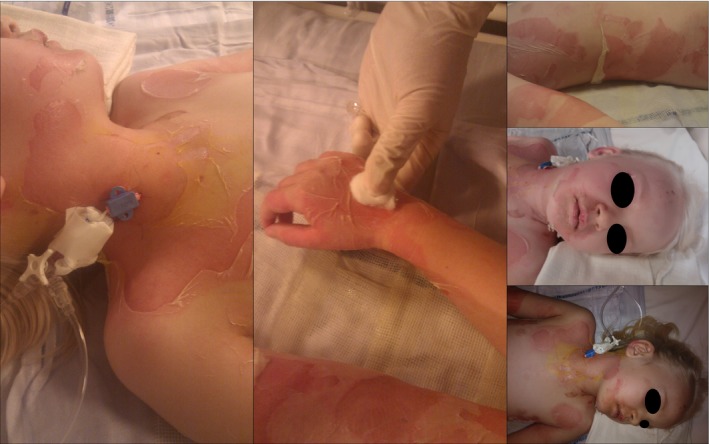
Pictures illustrates the progression of SSSS on the third day. Desquamation of skin covering 55% of the body's surface area.
The patient was in recovery the following day and stayed hospitalized until the wounds heeled with incrustation. On the 10th day she was discharged. At 1‐month follow‐up examination, no scarring was obtained.
Discussion
Cells in epidermis are held together by the protein desmoglein 1 1. In SSSS, the toxins from S. aureus chop the superficial fragments of desmoglein 1, which results in loosening of epidermis and the distinctive bullae formation 1. The toxins produced by S. aureus can be detected by polymerase chain reaction (PCR) within 3 h ensuring the diagnosis 2. Skin biopsy is also diagnostic revealing an intraepidermal cleavage, with splitting of stratum granulosum, which may contain acantholytic cells. The epidermis appears unremarkable and dermis without inflammatory cells 1, 3. S. aureus toxin‐mediated infection are evident in children. As presented in current case report, S. aureus does not occur in the skin lesions, but are confined to the site of infection, primarily the nasopharynx. Unlike present case, blood cultures do not typically help in diagnosis of a child with SSSS, as they are usually negative. Contrary, S. aureus in an adult's blood cultures are commonly positive 1. The patient's condition can be complicated by a secondary infection (pneumonia, sepsis, and cellulitis), dehydration, and consequential electrolyte imbalances 1, 2. With positive Nikolsky's sign and scalded skin appearance, SSSS may resemble other blistering disorders as toxic epidermal necrolysis (TEN) and Steven–Johnson Syndrome (SJS). However, SSSS can be differentiated from TEN by the lack of mucous membrane involvement as well as by more superficial epidermal peeling, which is in contrast to the full thickness denudation found in TEN and SJS. SSSS is most common in children and not a result of medication as in TEN/SJS. Although similar histopathologically to TEN, SJS needs at least two mucous membrane involvements, and unlike TEN, the skin involvement is generally limited to less than 30% body surface area. Skin biopsy can rapidly differentiate between SSSS and TEN/SJS 4.
Early and correct treatment that includes both local and systemic antibiotics as well as Lactulose is crucial in order to stop SSSS from progressing. In children, the toxins from S. aureus cannot be excreted with the urine, as the kidneys are immature. Even though the patient is not constipated and Lactulose is not a part of the standard treatment against SSSS in most hospitals around the world, it is our opinion that Lactulose and the subsequent acceleration of gastrointestinal transit enable the patient to excrete S. aureus via the colorectal tract faster and easier. One study reported increased titers of S. aureus antibody (indicating increased bacterial load) in serum in 57 functional constipated patients, which was effectively treated with laxatives 5. However, it is not without risk to use laxatives in a nonconstipated patient with the need of well hydration. Thus, healthy persons must know the importance of proper timing, hydration and dosing, and how to administer laxative safely. The present case report motivates further research on the hypothesis that laxatives induce gastrointestinal elimination of S. aureus and its toxins.
The learning from this case report is the imperativeness to have an early diagnosis of SSSS, correct treatment with antibiotics, and fluid maintenance to ensure a prompt and complete recovery. Patients suspected of suffering from this condition should see a medical doctor immediately and burns units should be involved when skin lesions are extensive 1.
Consent
Written informed consent was obtained from the parents for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Conflict of Interest
None declared.
Clinical Case Reports 2016; 4(4): 416–419
References
- 1. Handler, M. Z. , and Schwartz R. A.. 2014. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J. Eur. Acad. Dermatol. Venereol. 28:1418–1423. [DOI] [PubMed] [Google Scholar]
- 2. Sakurai, S. , Suzuki H., and Machida K.. 1995. Rapid identification by polymerase chain reaction of staphylococcal exfoliative toxin serotype A and B genes. Microbiol. Immunol. 39:379–386. [DOI] [PubMed] [Google Scholar]
- 3. Schwartz, R. A. , McDonough P. H., and Lee B. W.. 2013. Toxic epidermal necrolysis: Part II. prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J. Am. Acad. Dermatol. 69:187. [DOI] [PubMed] [Google Scholar]
- 4. Harr, T. , and French L. E.. 2010. Toxic epidermal necrolysis and Stevens‐Johnson syndrome. Orphanet J. Rare Dis. 5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khalif, I. L. , Quigley E. M., Konovitch E. A., and Maximova I. D.. 2005. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig. Liver Dis. 37:838–849. [DOI] [PubMed] [Google Scholar]


