CTLs generated in the presence of IFN-γ mediate intraocular tumor rejection without injuring ocular tissues which cannot regenerate.
Keywords: eye, immune privilege, necrotic inflammation, CTL, IFN-γ
Abstract
The eye is normally an immunosuppressive environment. This condition is better known as immune privilege and protects the eye from immune-mediated inflammation of tissues that cannot regenerate. However, immune privilege creates a dilemma for the eye when intraocular neoplasms arise. In some cases, immune privilege is suspended, resulting in the immune rejection of intraocular tumors. This study employed a mouse model in which interferon-γ–dependent intraocular tumor rejection occurs. We tested the hypothesis that this rejection requires interferon-γ for the generation and functional capacity of cytotoxic T lymphocyte–mediated rejection of intraocular tumors. Tumors grew progressively in the eyes of interferon-γ knockout mice, even though the mice generated tumor-specific cytotoxic T lymphocyte responses in the periphery. However, interferon-γ knockout mice rejected tumors that were introduced into extraocular sites. Subcutaneous tumor immunization before intraocular challenge led to tumor rejection and preservation of the eye in wild-type mice. By contrast, tumors grew progressively in the eyes of interferon-γ knockout mice despite their ability to generate peripheral tumor-specific cytotoxic T lymphocytes as well as the capacity of CD8+ T cells to enter the eye as shown by the presence of CD8 and perforin message and CD3+CD8+ leukocytes within the tumor-bearing eye. We found that cytotoxic T lymphocytes generated in wild-type mice and adoptively transferred into interferon-γ knockout mice mediated the rejection of intraocular tumors in interferon-γ knockout hosts. The results indicate that interferon-γ is critical for the initial priming and differentiation of cytotoxic T lymphocytes residing in the periphery to produce the most effect antitumor function within the eye.
Introduction
Immune responses within the eye differ from responses within other organs or peripheral tissues because of the nonregenerative nature of the ocular tissues. Immune-mediated inflammation is blunted and, in some cases, excluded from the eye—a condition that is classically defined as immune privilege. Multiple processes and anatomic conditions contribute to immune privilege. Antigen that is introduced into the AC of the eye leads to the development of anterior-associated immune deviation, which results in the generation of antigen-specific regulatory T cells that selectively down-regulate immune-mediated inflammation in the eye and thereby lessen the likelihood of irreparable injury to ocular tissues [1, 2]. However, certain conditions, such as severe microbial ocular infections or neoplasms, abolish immune privilege, which allows the host to eliminate life-threatening diseases [3, 4].
Unique anatomic features of the eye are also involved in the maintenance of ocular immune privilege. The nonfenestrated nature of some ocular blood vessels creates a blood–ocular barrier that prevents the extravasation of bloodborne lymphocytes into ocular tissues. However, T cells exposed to IFN-γ gain the ability to breach the blood–ocular barrier and enter the eye [5–7]. Abnormal increases of IFN-γ levels in the eye have been studied in various disorders and are associated with an ocular environment that is more permissive to the influx of inflammatory cells into the eye. Diabetic rats have significant increases of IL-2 and IFN-γ in their retinas, which lead to the retinal damage and inflammation that has been reported in diabetic retinopathy [8]. A single-nucleotide polymorphism in the IFN-γ gene, implicated in higher cytokine production, is significantly associated with disease in patients with diabetic retinopathy [9]. In rats, transgenic expression of the IFN-γ gene in the retina induces an increase in the expression of CAMs, such as LFA-1 on CTLs and ICAM on blood vessels, which culminates in an inflammatory infiltrate and photoreceptor cell death [10]. In light of this, it is not surprising that, intraocular IFN-γ levels are elevated in rodents with experimental autoimmune uveitis and in patients with uveitis [10, 11]. Although some patients with uveitis have increased levels of IFN-γ in the vitreous, patients with primary intraocular lymphoma or oculocerebral lymphoma do not have detectable levels of IFN-γ in the vitreous humor [12], suggesting that not all inflammatory conditions of the eye are marked with increased IFN-γ. Eyes of patients with uveal melanoma contain higher levels of proinflammatory cytokines, including IFN-γ in their aqueous humor, compared with controls [13]. In transgenic rats that constitutively produce IFN-γ in the eye, experimental autoimmune uveitis was more severe and the expression of ICAM-1 increased as disease progressed [11]. Therefore, IFN-γ is important in breaching the blood–ocular barrier and promoting the entry of immune cells into the eye and facilitating intraocular inflammation. However, it is well-documented that IFN-γ is a critical cytokine in suppressing tumor development [14] and, thus, would need to be differentially regulated in the unique immune privileged site of the eye during a neoplasm threat.
We used a well-described intraocular tumor model—Ad5E1 tumor cells injected into syngeneic C57BL/6 mice [15, 16]—to test the hypothesis that IFN-γ is necessary for the function of tumor-specific CTLs in the eye and to determine whether CTLs are essential for the immune rejection of intraocular tumors. The results show that IFN-γ was not only necessary for intraocular tumor rejection but also profoundly affected the nature of tumor rejection in the eye and the fate of the eye itself.
MATERIALS AND METHODS
Mice
C57BL/6 WT mice (H-2b) were obtained from the mouse breeding facility at University of Texas Southwestern Medical Center (Dallas, TX, USA). IFN-γ KO mice (B6.129S7-Ifngtm1Ts /J) breeding pairs and individuals and perforin KO individuals (C57BL/6-Prf1tm1Sdz/J) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). All animals were cared for in compliance with animal protocols approved by University of Texas Southwestern Medical Center’s institutional animal care and use committee standards.
Tumor cell growth and immunization
Ad5E1 clone 2.1 tumor cells undergo phthisical immune rejection within the eye of syngeneic C57BL/6 WT mice and were derived previously in our laboratory [15] from the initial parental Ad5E1 tumor cell line provided by Dr. Rene E.M. Toes [16]. Ad5E1 clone 2.1 tumor cells were chosen as a model of intraocular tumor growth that requires IFN-γ for tumor rejection within the eye [17] and will be referred to as Ad5E1 tumor cells from here on. Tumor cells were cultured in complete DMEM supplemented with 10% heat-inactivated FCS, 1% sodium pyruvate, 1% nonessential amino acids, 1% MEM vitamin mixture, 1% HEPES, 1% antibiotic solution, and 0.1% 2-mercaptoethanol.
Single-cell suspensions were generated and Ad5E1 tumor cells were suspended in complete DMEM for AC and s.c. injections. AC immunizations were conducted on anesthetized mice (0.66 mg/kg ketamine hydrochloride), using a sterile, 30-gauge needle to puncture the AC parallel to the iris. Then, 6 µl of the tumor cell suspension was delivered into the AC using a 0.1-ml, glass Hamilton syringe with a sterile premature infant feeding tube (5 French, Bard, Tyco Healthcare Group, Mansfield, MA, USA) fitted with a glass microneedle inserted into the puncture. Tumor growth was monitored and the percentage of the AC occupied was recorded at least twice per week after immunization. Subcutaneous immunizations were conducted on nonanesthetized mice using a sterile, 30-gauge needle fitted on a 1-ml tuberculin syringe to deliver the tumor cell suspension under the skin of the hind leg flank. Tumor growth was monitored by palpitation and recorded at least twice per week after immunization.
CTL assay
CTL activity of splenocytes in vitro was measured by a standard 4-h [51Cr] release assay, as previously described [18]. Splenocytes from different experimental groups (i.e., naïve vs. 14 d after s.c. immunization) were used as the effector cells, and Ad5E1 tumor cells were the target cells in the CTL assays. Spleens were extracted and transferred to a C tube containing buffer (0.5% BSA, 2mM EDTA in 1× PBS), and single-cell suspensions were created using the gentleMACS Dissociator (Miltenyi Biotec Inc., San Diego, CA, USA). Red blood cells were lysed with ammonium chloride Tris solution, and cells were washed 3 times with HBSS. Ad5E1 tumor cells were collected and treated with 400 µg/ml mitomycin C (Sigma Aldrich, St. Louis, MO, USA) for 45 min in a 37°C CO2 incubator followed by 3 washes with HBSS. Spleen effector cells (3 × 107) and tumor stimulator cells (6 × 106) were mixed together and added to T25 flasks in a final volume of 10 ml complete DMEM. These boosting flasks were incubated upright in a 37°C CO2 incubator for 3 d and observed daily.
After the 3-d incubation, the stimulated effector cells were harvested, washed, and suspended in complete DMEM. Ad5E1 tumor cells (1 × 107) were freshly collected and labeled with 0.5-millicurie [51Cr] (PerkinElmer, Waltham, MA, USA) for 1.5 h in a 37°C CO2 incubator. The excess radionuclide was washed away 3 times with HBSS, and the [51Cr]-labeled target cells were suspended in complete DMEM. Target (T) and effector (E) cells were plated in quadruplet replicate wells in a final volume of 200 µl per well in a 96-well U-bottom plate at 3 E:T ratios (100:1, 50:1, and 25:1). Spontaneous release was detected in wells containing only target cells, and maximal release was detected in wells with target cells treated with Zap-Oglobin lytic reagent (Beckman Coulter, Indianapolis, IN, USA). The plate was briefly centrifuged and incubated in a 37°C CO2 incubator for 4 h. The plate was then centrifuged to pellet the cells, and 100 µl of the supernatant was collected from each well for measuring [51Cr] release on a γ-counter. Cytotoxicity of the effector cells on the tumor target cells was calculated using the following equation and expressed as the percentage of specific lysis: [(Experimental release cpm) − (Spontaneous release cpm)]/[(Maximum release cpm) − (Spontaneous release cpm)] × 100.
Adoptive transfer of CD8+ T cells
Donor mice (WT or perforin KO) were s.c. immunized with 1 × 106 Ad5E1 tumor cells 14 d before harvesting the spleens to allow a CTL response to be generated in vivo. CD8+ T cells were enriched from a single-cell splenocyte suspension according to manufacturer’s recommendations using the negative selection EasySep mouse CD8+ T cell isolation kit (StemCell Technologies, Vancouver, BC, Canada). The CD8+ T cells were stimulated in vitro with anti-CD3/CD28 beads (Dynabeads; Invitrogen, Carlsbad, CA, USA) and 50 ng/ml recombinant mIL-15 (Tonbo, San Diego, CA, USA) in a final volume of 2 ml of complete DMEM per well in a 24-well plate. The plate was incubated in a 37°C CO2 incubator for 3 d and observed daily. This in vitro priming regimen with IL-15 and anti-CD3/CD28 was selected to enhance both in vivo tumor-killing capabilities [19, 20] and peripheral tissue migration [21]. Stimulated CD8+ T cells were removed from the beads using a DynaMag (Invitrogen), washed, and suspended in HBSS. One donor equivalent (2.5 × 106 CD8+ T cells) was injected i.v. into the tail vein of recipient mice. The recipient mice were AC immunized with 1 × 105 (IFN-γ KO) or 3 × 105 (WT) Ad5E1 tumor cells on the same day as the adoptive transfer. The tumor growth in the recipient mice was monitored, and the percentage of the AC occupied was recorded at least twice per week after adoptive transfer.
qPCR
Mice were euthanized, and their eyes were harvested, snap frozen in liquid nitrogen, and stored at −80°C. RNA was isolated using the RNeasy Mini kit according to manufacturer’s recommendations (Qiagen, Valencia, CA, USA). Quality (must have an RNA quality indicator >7.5) and the quantity of the extracted RNA were evaluated using the Experion StdSense RNA chip and regents (BioRad, Hercules, CA, USA). First-strand cDNA was synthesized using the QuantiTect Reverse Transcription kit (Qiagen) and used as template for TaqMan gene expression assays for qPCR, which was run on the QuantStudio 6 Flex system (Applied Biosystems; Life Technologies, Carlsbad, CA, USA). Each gene assayed had 2–3 technical replicates for each of the 5 biologic samples per group, and that process was repeated independently 2–3 times. The following TaqMan gene expression assays (Applied Biosystems; Life Technologies) were used: CD8a (Mm01182107_g1), prf1 (Mm00812512_m1), pdcd1 (Mm01285676_m1), selp (Mm01295931_m1), sele (Mm01310197_m1), CD44 (Mm01277163_m1), selplg (Mm01204601_m1), itgal (Mm00801807_m1), icam1 (Mm00516023_m1), CD4 (Mm00442754_m1), CD19 (Mm00515420_m1), CD3e (Mm00599684_g1), klrb1c (Mm00824341_m1), CD68 (Mm03047340_m1), itgax (Mm00498698_m1), gapdh (Mm99999915_g1). Comparative quantification was done using the log2 transformation of the 2−ΔΔcomparative threshold value by using the lowest-expressing sample (highest comparative threshold value) as the baseline to assess the fold change. GAPDH was used to normalize all genes.
Flow cytometry
Mice were euthanized 14 d after Ad5E1 tumor injection in the AC, and their tumor-bearing eyes and spleens were harvested. Single-cell suspensions of splenocytes were processed in the same manner as performed for the CTL assay above. Single-cell suspensions of tumor-bearing eyes were generated by excising the eye and removing the optic nerve and lens. The eye was digested using collagenase IV (Sigma) in HBSS with gentle agitation for 1 h at 37°C. The cells were filtered through a 70-µm cell strainer and washed. Single-cell suspensions of both eyes and splenocytes were resuspended in flow buffer (1× PBS, 2 mM EDTA, 0.5% BSA, 0.09% sodium azide). Then, 1 × 106 cells of each sample were incubated with anti-CD16/CD32 FcR blocker and then incubated with a master mix containing the following fluorescent antibodies: CD3e PerCP-Cy5.5 (clone 145-2C11), CD8a APC-Cy7 (clone 53-6.7), CD45 rF710 (clone 30-F11), CD62L eF450 (clone MEL-14), and CD44 FITC (clone IM7). Excess Ab was removed from the samples by washing twice with flow buffer before acquisition. Flow cytometry data were acquired using the Attune NxT acoustic focusing cytometer (Applied Biosystems; Life Technologies) and analyzed using FlowJo v10 software (Tree Star, Ashland, OR, USA).
Immunohistochemistry
Mice were euthanized 14 d after Ad5E1 tumor injection in the AC, and their tumor-bearing eyes were harvested and placed in Carson’s formalin for 24 h. The eyes were dehydrated with an alcohol gradient and xylene before being embedding in paraffin. Next, 5-µm sections were cut using a microtome (Shandon Finesse 325 microtome; Thermo Fisher Scientific, Waltham, MA, USA) and adhered to vectabond (Vector Laboratories, Burlingame, CA, USA)–treated glass slides. The paraffin-embedded slides were dewaxed at 65°C for 2 h and rehydrated with an alcohol gradient. Tissues were subjected to antigen retrieval by boiling for 15 min in low pH citric acid–based antigen-unmasking solution (Vector Laboratories). Endogenous peroxidases were blocked with 3% H2O2 for 5 min, washed with PBS, and then, blocked with 3% normal goat serum for 10 min. The eye sections were incubated with a 1:50 dilution of primary rabbit Ab to CD62P (P-selectin) (Abcam, Cambridge, MA, USA) overnight at 4°C. The next day, tissues were washed with PBS and probed with biotinylated goat anti-rabbit secondary Ab (Vectastain Elite ABC Kit; Vector Laboratories) for 1 h at room temperature. Tissues were washed with PBS and incubated with ABC reagent for 30 min at room temperature. The tissues were developed using 3,3′-diaminobenzidine substrate solution (Vector Laboratories) and counterstained with Hematoxylin QS (Vector Laboratories). The slides were dehydrated with an alcohol gradient and cleared with xylenes before coverslip mounting with Permount (Thermo Fisher). Stained eyes were imaged using differential interference contrast microscopy with a ×40 brightfield lens on the Zeiss Observer D1 microscope with AxioVision Imaging System software (Carl Zeiss, Jena, Germany).
Statistical analyses
All statistical analyses were performed using GraphPad Prism software (La Jolla, CA, USA). Student’s t tests assuming equal variance of sd were performed, and P < 0.05 was considered significant.
RESULTS
Cytotoxic T lymphocytes kill tumor cells in vitro, but in the absence of IFN-γ, they are unable to rid the eyes of their tumors
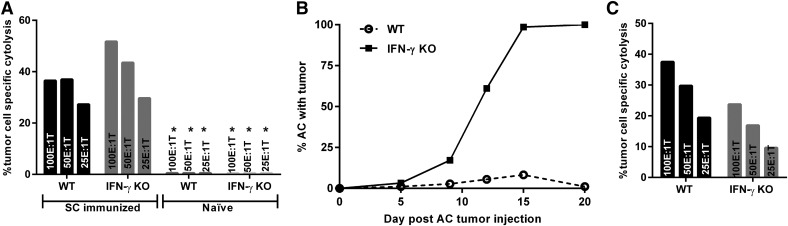
We have previously shown that the Ad5E1 tumor cell line undergoes a necrotizing form of immune rejection in the eyes of WT mice but grows progressively in the eyes of either IFN-γ KO mice or WT mice treated with anti-IFN-γ Ab [22]. By contrast, Ad5E1 tumors transplanted to extraocular sites undergo immune rejection, even in the absence of IFN-γ. Intraocular tumors grew progressively in the AC of all the IFN-γ KO mice, but underwent rejection in WT hosts (data not shown). By contrast, s.c.-injected Ad5E1 tumor cells did not form palpable tumors in either IFN-γ KO or WT mice. Moreover, both groups of mice generated significant CTL responses in response to the s.c. tumor cell injections (Fig. 1A). As expected, naïve IFN-γ KO and WT mice did not produce CTL responses to tumor cells (Fig. 1A). This suggests that the progressive growth of intraocular tumors in IFN-γ–deficient mice is not simply due to the inability of intraocular tumors to induce CTL responses.
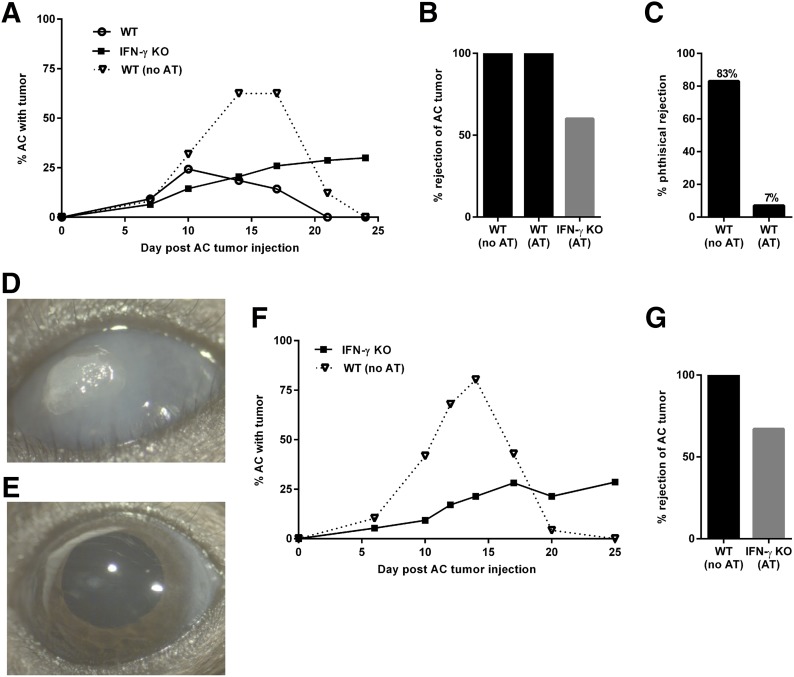
Figure 1. IFN-γ KO mice generate CTL responses following s.c. immunization, yet the CTL responses do not provide protection against subsequent tumor challenge in the eye.
CTL was isolated from the spleens 14 d following s.c. immunization of WT and IFN-γ KO mice with Ad5E1 tumor cells. (A) Both WT and IFN-γ KO mice developed CTL responses following s.c. immunization. (B) Subcutaneous immunization before AC challenge does not protect against AC tumor growth in IFN-γ KO mice but is protective in the WT mice. (C) Subcutaneous immunization before AC challenge induces peripheral CTL responses in both WT and IFN-γ KO mice, and cytolytic function is retained. Three different effector to target ratios (E:T) were tested. Results are expressed as mean percent cytolysis from 4 replicates. *<1% cytolysis. Data are representative of 2 independent experiments with 5 mice per group.
Because both IFN-γ KO and WT mice rejected their s.c.-injected tumor cells and developed tumor-specific CTL responses, we tested whether s.c. immunization with tumor cells would provide protection against subsequent AC challenge. WT and IFN-γ KO mice were s.c. immunized with tumor cells 14 d before AC challenge. Although none of the s.c.-immunized IFN-γ KO mice developed s.c. tumors, all mice developed progressively growing intraocular tumors (N = 18 of 18) (Fig. 1B). By contrast, 79% (N = 15 of 19) of the s.c.-immunized WT mice did not develop either s.c. or AC tumors and remained tumor free beyond d 20 after AC challenge (Fig. 1B). The resistance of tumor development in the AC of the WT mice was associated with a robust CTL response that was still present 20 d after the AC tumor injection (Fig. 1C). However, CTL responses similar to those found in WT mice were also detected in the s.c.-immunized IFN-γ KO mice, even though the latter mice developed progressively growing AC tumors (Fig. 1C). Thus, even in the face of tumor-specific CTLs generated and retained after s.c. immunization, IFN-γ KO mice were unable to restrain intraocular tumor growth after AC challenge.
IFN-γ is not required for endogenously generated CD8+ CTLs to enter the tumor-bearing eye
Because CTLs were generated in the absence of IFN-γ, we suspected that the progressive growth of intraocular tumors in IFN-γ KO mice was due to the inability of CTLs to enter the eye. We interrogated the tumor-bearing eyes of WT mice and IFN-γ KO mice for the expression of CD8, perforin, and PD-1 mRNA. Because IFN-γ can also affect the expression of key CAMs that facilitate the extravasation of lymphocytes, we also examined these eyes for their expression of P-selectin, E-selectin, ICAM-1, LFA-1, and PSGL-1 mRNA.
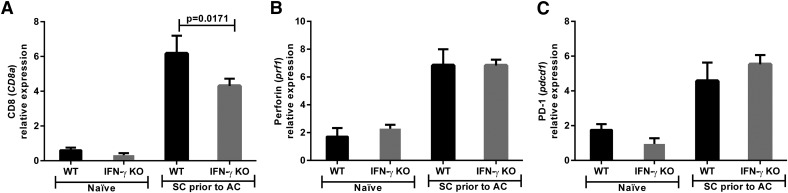
IFN-γ KO and WT mice were immunized s.c. with tumor cells, and 14 d later were challenged with AC tumor injections. Eyes were removed 20 d after AC tumor challenge and were examined for the expression of the previously mentioned transcripts. As expected, WT mice did not develop large AC tumors, whereas the IFN-γ KO mice developed progressively growing AC tumors (Fig. 1B). Both WT and IFN-γ KO mice expressed CD8 transcripts within the tumor-bearing eyes (Fig. 2A). Although the CD8 transcripts were significantly increased in WT mice compared with IFN-γ KO mice (6.2 vs. 4.3 relative expression; P = 0.0171), there was no difference in perforin expression, a functional cytolytic marker [23], at this time point (Fig. 2A and B). It is possible that the CD8+ T cells that entered the IFN-γ–deficient eye failed to eliminate the tumor because they were part of an “exhausted” immune response from the persistence of tumor cells and the absence of cytokine help. Although expression of PD-1 has been defined as a signature of immune cell exhaustion and reduced effector function [24–33], both the WT and IFN-γ KO mice had similar increases in PD-1 expression at d 20 after AC challenge when compared with naïve mice (Fig. 2C).
Figure 2. Endogenously generated CD8+ T cells induced by s.c. immunization enter tumor-containing eyes in both WT and IFN-γ KO mice.
WT and IFN-γ KO mice were immunized s.c. with Ad5E1 tumor cells and challenged in the AC with Ad5E1 tumor cells 14 d later. Tumor-containing eyes were removed 20 d after AC tumor challenge and examined by qPCR. The following relative gene expressions were determined by qPCR: (A) CD8 (CD8a), (B) perforin (prf1), and (C) PD1 (pdcd1). Log2-transformed means of 5 biologic replicates in each of the 4 mouse groups are shown with sds. The least-expressing sample was used as the baseline for each gene assayed. Data are representative of 3 independent qPCR experiments with 5 mice per group. Groups were compared using the Student’s t test, and P < 0.05 is shown.
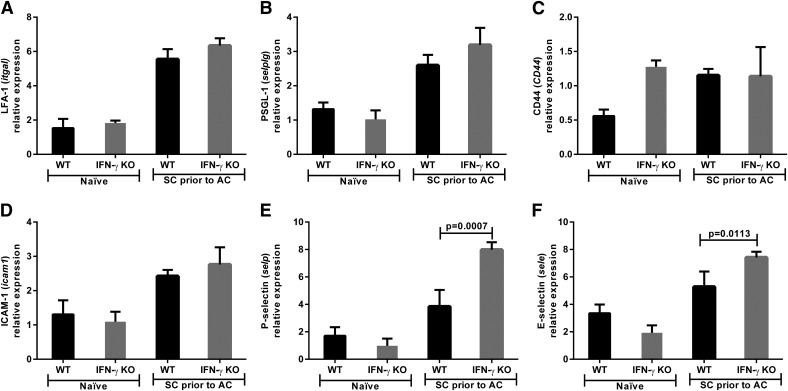
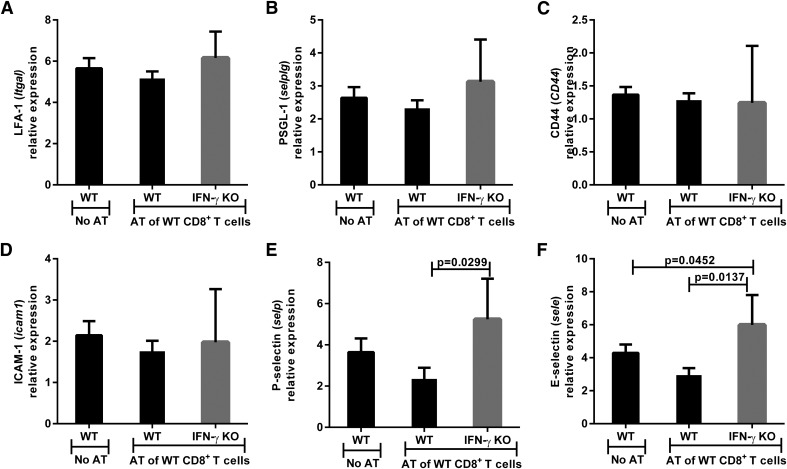
The same eyes were also assessed for CAMs to determine whether lymphocyte migration into the eye was affected by the absence of IFN-γ. However, there was no difference in LFA-1, PSGL-1, CD44, or ICAM-1 expression in IFN-γ KO and WT mice (Fig. 3A–D). Contrary to our prediction, both P-selectin (3.8 vs. 7.9 relative expression; P = 0.0007) and E-selectin (5.3 vs. 7.4 relative expression; P = 0.0113) were significantly increased in IFN-γ KO mice compared with WT mice (Fig. 3E and F). Immunohistochemistry of tumor-bearing eyes isolated 14 d after AC tumor injection demonstrated P-selectin expression within the intraocular tumor (Fig. 4A–C) and ciliary body (Fig. 4D–F) but no staining within the retina (Fig. 4G–I) of both WT and IFN-γ KO mice. Thus, loss of IFN-γ does not negatively affect the expression of key CAMs involved in leukocyte trafficking into tumor-challenged eyes.
Figure 3. IFN-γ KO mice express similar levels of CAM in the eye to that of WT mice.
WT and IFN-γ KO mice were immunized s.c. with Ad5E1 tumor cells and challenged in the AC with Ad5E1 tumor cells 14 d later. Tumor-containing eyes were removed 20 d after AC tumor challenge and examined by qPCR. The relative expression of the following CAM genes was determined by qPCR: (A) LFA-1 (itga1), (B) PSGL-1 (selplg), (C) CD44 (CD44), (D) ICAM-1 (icam1), (E) P-selectin (selp), and (F) E-selectin (sele). Log2-transformed means of 5 biologic replicates in each of the 4 mouse groups are shown with SDs. The least-expressing sample was used as the baseline for each gene assayed. Results are representative of 3 independent qPCR experiments. There were 5 mice per group. Groups were compared using the Student’s t test, and P < 0.05 is shown.
Figure 4. P-selectin is present in the intraocular tumor and along the ciliary body in IFN-γ KO and WT tumor-bearing eyes.
Mice were injected with Ad5E1 tumor cells into the AC, and the eyes were removed for histology 14 d later. P-selectin–positive staining is brown (3,3′-diaminobenzidine), and the cellular counterstain is blue (Hematoxylin QS). Intraocular P-selectin staining is not seen in the secondary control alone (A) but is seen in the AC tumor of WT (B) and IFN-γ KO (C) mice. The ciliary body of the secondary section alone shows background melanin staining (D). Positive P-selectin staining (red arrows) borders the ciliary body in WT (E) and IFN-γ KO (F) mice. There is no appreciable staining in the retina above the background expressed in specimens treated with the secondary Ab alone (G) or both WT (H) and IFN-γ KO (I) mice. Images are at ×40 magnification and are representative of 3 eye sections each from 3 to 6 mice per group.
IFN-γ is not required for CD3+CD8+ CTLs to enter the tumor-bearing eye after intraocular tumor injection
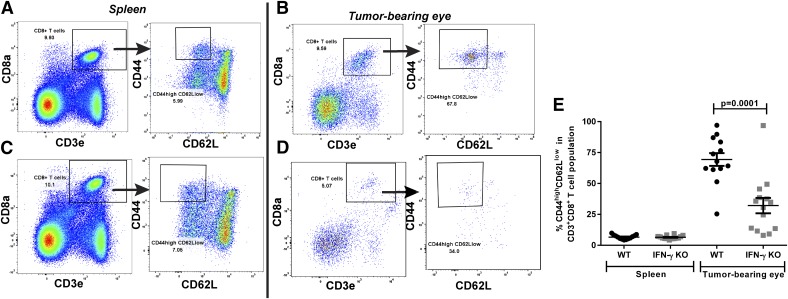
CD8 message was present in the tumor-bearing eyes of both WT and IFN-γ KO mice that were s.c. immunized before AC challenge (Fig. 2A), even though CD8+ T cells were only effective at eliminating the intraocular tumor in the WT mice. Furthermore, WT mice rejected their intraocular tumors pristinely and preserved the architecture of the ocular tissues. We next used flow cytometry to determine the phenotype of the infiltrating CD8+ T cells present in the spleen and tumor-bearing eye 14 d after AC tumor injection in both WT and IFN-γ KO mice (Fig. 5). CD45+ leukocytes compromised 6.5% and 2.0% of the cells present in the tumor-bearing eyes of WT and IFN-γ KO mice, respectively, in contrast to naïve eyes, which expressed <1% CD45+ leukocytes. In the tumor-bearing eyes, CD3+CD8+ T cells represented a similar amount of the leukocyte population in both WT mice and IFN-γ KO mice (8.6% vs. 6.0%; P > 0.05). However, there was a significant decrease in CD44highCD62Llow CD3+CD8+ effector T cells in the tumor-bearing eyes of the IFN-γ KO mice compared with the WT mice (32.1% vs. 69.4%; P = 0.0001) (Fig. 5E). Furthermore, this >50% reduction was only seen in the tumor-bearing eyes because the spleens from the same AC tumor–injected mice had similar levels of CD44highCD62Llow effector CTLs (6.4% vs. 6.6%) (Fig. 5E). Thus, CD3+CD8+ T cells enter the tumor-bearing eyes of both WT and IFN-γ KO mice during an intraocular tumor challenge, but the absence of IFN-γ is associated with a significant decrease in the proportion that are CD44highCD62Llow effector CTLs.
Figure 5. Tumor-bearing eyes in IFN-γ KO mice display a decreased percentage of effector CTL when compared with WT mice.
Mice were injected with Ad5E1 tumor cells into the AC, and the spleens and tumor-bearing eyes were removed for flow cytometric immunophenotyping of the infiltrating CD8+ T cells 14 d later. Single cells were gated on CD45+ leukocytes followed by a CD3+CD8+ gate for CTLs. These were further phenotyped by CD44 and CD62L expression to gate on CD44high CD62Llow effector CTLs. Representative gating is shown for WT splenocytes (A) and tumor-bearing eyes (B) and for IFN-γ KO splenocytes (C) and tumor-bearing eyes (D). (E) The percentage of CD3+CD8+ T cells expressing CD44high CD62Llow in spleens and tumor-bearing eyes of WT (N = 13) and IFN-γ KO (N = 14) mice. The means and sem of the groups are shown. The experiment was performed 3 times with similar results.
CD8+ T cells generated in the presence of IFN-γ protect against tumor growth in IFN-γ–deficient eyes
Despite both WT and IFN-γ KO mice developing CTLs that are capable of killing tumor cells in vitro, a protective response after an AC challenge in vivo was only observed in the WT mice. IFN-γ may be required in situ for tumor-specific CTLs to function in the eye. Conversely, IFN-γ might endow CTLs with a functional capacity that is not present in CTLs generated in the absence of IFN-γ. To address this possibility, we adoptively transferred 1 donor equivalent of CD8+ T cells from s.c.-immunized WT donor mice into WT and IFN-γ KO recipient mice, which subsequently received an AC tumor cell injection the same day. WT mice that received CD8+ T cells from WT s.c.-immunized donors had smaller tumors than did WT mice that did not receive cells (average maximal growth: 23% vs. 60% of the AC, respectively) (Fig. 6A). Intraocular tumors underwent rejection in 100% of WT mice that received adoptively transferred CTLs and in 60% of the IFN-γ KO recipients (Fig. 6B). More important, intraocular tumor rejection in the WT recipients of CD8+ CTLs was qualitatively different from the intraocular rejection that occurred in WT mice that did not receive adoptive cell transfers. That is, spontaneous intraocular tumor rejection in WT mice culminated in widespread necrosis and complete destruction of the eye (phthisis). By contrast, intraocular tumors in WT mice that received adoptively transferred CTLs from s.c. immunized WT donors underwent a nonnecrotizing form of immune rejection that left the eye intact and produced no necrosis or injury to intraocular tissues that could be detected by histologic examination (data not shown and Fig. 6C–E). Thus, CTLs generated by s.c. immunization in WT mice promoted protective antitumor responses that were able to rid the eyes of their intraocular tumor challenge while preserving the anatomic integrity of the eye.
Figure 6. CD8+ T cells generated in the presence of IFN-γ eradicate intraocular tumors in IFN-γ KO hosts.
(A) CD8+ T cells isolated from s.c.-immunized WT mice were adoptively transferred (AT) (2.5 × 106 CD8+ T cells/mouse) to WT (N = 15) and IFN-γ KO (N = 15) mice. Mice were challenged with AC tumor injections on the same day that they received 1 donor equivalent of stimulated CD8+ T cells. WT mice that did not receive CD8+ T cells from s.c. immunized WT mice served as controls (no AT; N = 12). Results are representative of 2 independent experiments. All mice in each group initially developed intraocular tumors. (B) Percentage of mice from those in (A), which rejected their AC tumors. (C) Percentage of WT mice that had pristine AC tumor rejection in the AT (N = 15) and the no AT (N = 12) groups. (D) Representative images of a phthisical rejected AC tumor in the WT that did not receive AT CD8+ T cells (E) and a pristine rejected AC tumor in the WT mice that received adoptively transferred CD8+ T cells. (F) An aggressive adoptive cell transfer protocol was implemented in which the number of CD8+ T cells from s.c.-immunized WT mice was doubled (5 × 106 CD8+ T cells/mouse) compared to (A) above, and 2 rounds of adoptive cell transfers were performed (on the day of intraocular tumor injection and 12 d later). (G) Percentage of mice from (F) that rejected their AC tumor. All mice developed intraocular tumors after AC challenge.
Tumors were not rejected in 40% (N = 6 of 15) of the IFN-γ KO mice that received WT s.c.-immunized CD8+ T cells, whereas the remaining 60% (N = 9 of 15) rejected their intraocular tumor (Fig. 6B). This increase in tumor protection in the IFN-γ KO mice, compared with the 100% progressive intraocular growth rate without adoptive cell transfer, suggests that in more than one-half of the mice, CTLs generated in the presence of IFN-γ are able to reject the tumor, even in the absence of IFN-γ in the IFN-γ KO mouse eye.
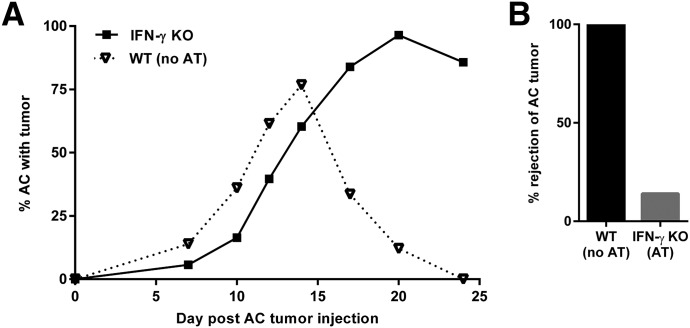
We suspected that the failure of adoptively transferred CD8+ CTLs to produce tumor rejection in 100% of the IFN-γ KO recipients was due to insufficient numbers of CTLs being transferred in the IFN-γ KO host. Accordingly, CD8+ CTLs were isolated as described earlier; however, the number of cells transferred was doubled (5 × 106 cells/recipient), and 2 adoptive cell transfers (on d 0 and 12 after AC tumor injection), rather than a single cell transfer, were performed. The results indicated that even doubling the number of CD8+ CTLs transferred and doubling the number of transfers did not significantly increase the incidence of rejection. Tumors were completely eradicated in 67% (N = 10 of 15) (compared with 60%) in the IFN-γ KO recipients of adoptively transferred CD8+ CTLs from WT donors (Fig. 6F and G). Thus, the intraocular milieu in the IFN-γ–deficient eye failed to support the maximal expression of CTL activity that occurs in the eyes of mice with intact IFN-γ expression.
We next aimed to confirm that CD8+ T cell–derived perforin was necessary for the increased incidence of AC tumor rejection in IFN-γ KO mice that received an adoptive transfer of WT primed CTLs. CD8+ CTLs were isolated from s.c.-immunized perforin-KO mice donors, and a double dose of cells was transferred to IFN-γ KO recipients (5 × 106 cells/recipient) on d 0 and 12 after AC tumor injection. Intraocular tumors grew progressively in 86% (N = 6 of 7) of the IFN-γ KO recipient mice that received the perforin-deficient CTLs (Fig. 7). Therefore, CD8+ T cell–derived perforin is important for the adoptively transferred WT CTLs to function within the IFN-γ KO mouse eye to eliminate the tumor.
Figure 7. Perforin KO CD8+ T cells do not provide intraocular tumor protection in IFN-γ KO hosts.
(A) CD8+ T cells isolated from s.c.-immunized perforin KO mice were adoptively transferred (AT) (5 × 106 CD8+ T cells/mouse) to IFN-γ KO (N = 8) mice. Mice were challenged with AC tumor injections on the same day that they received 2 donor equivalent of stimulated CD8+ T cells, and 2 rounds of adoptive cell transfers were performed (on the day of intraocular tumor injection and 12 d later). WT mice that did not receive CD8+ T cells from s.c.-immunized perforin KO mice served as controls (no AT; N = 8). (B) Percentage of mice from (A) that rejected their AC tumor. All mice developed intraocular tumors after AC challenge.
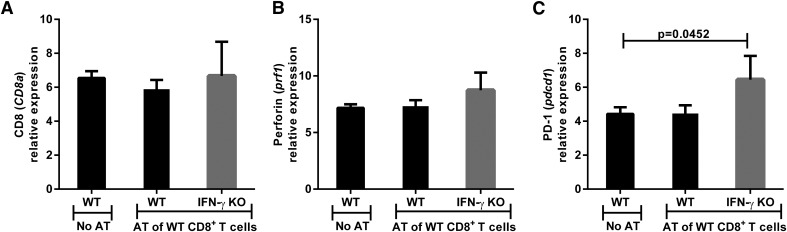
IFN-γ is not required in the eye for adoptively transferred CD8+ T cells to enter the tumor-bearing eye but is needed for the promotion of CTL activity
The adoptive transfer results suggested that IFN-γ may be required in the periphery to generate protective CTLs that function in vivo at the intraocular tumor site. Although IFN-γ was not necessary for the generation of CTLs that produce cytolytic activity against tumor cells in vitro, WT CTLs had a limited capacity to restrain tumor growth in the eyes of IFN-γ KO mice because 40% of the IFN-γ KO mice developed progressively growing intraocular tumors following the adoptive transfer of WT tumor-specific CTLs (Fig. 6A). Tumor-bearing eyes from WT and IFN-γ KO mice were assessed by qPCR for CTL and CAM markers following the adoptive transfer of WT CD8+ CTLs and AC tumor challenge. The results indicated that the absence of IFN-γ did not inhibit the entry of CD8+ CTLs into the tumor-bearing eyes because there were no differences in the levels of CD8 message among the adoptive transfer recipients (Fig. 8A), indicating that the adoptively transferred CD8+ T cells had an equal ability to migrate into and survive within the eyes of the groups of recipient mice. There was also no difference in the expression of perforin within the tumor-bearing eyes between the WT and IFN-γ KO groups (Fig. 8B). However, expression of PD-1, a marker associated with immune cell exhaustion, was increased in the eyes of IFN-γ KO mice compared with WT mice following the adoptive transfer of CD8+ T cells (6.4 vs. 4.4 relative expression; P = 0.0452) (Fig. 8C). However, the conclusion that the failure of adoptively transferred CD8+ T cells to mediate rejection in 40% of the intraocular tumors in IFN-γ KO mice was a result of “exhaustion” should be viewed with caution because the results shown in Fig. 2 indicate that PD-1 levels were the same in the eyes of IFN-γ KO mice and WT mice that were immunized s.c. before intraocular tumor challenge. Thus, it remains to be seen whether T cell exhaustion is the basis for the reduced capacity of adoptively transferred CD8+ T cells to mediate tumor rejection in the eyes of IFN-γ KO mice (Fig. 6).
Figure 8. Adoptively transferred WT CD8+ T cells enter the tumor-challenged eye in IFN-γ KO mice, but there is an increase in expression of the PD-1 immune cell exhaustion markers in the eye.
Eyes were removed from WT and IFN-γ KO mice 24 d after the mice received adoptively transferred CD8+ T cells from s.c.-immunized WT donors and were challenged in the AC with Ad5E1 tumor cells. Eyes were examined by qPCR for expression of the following genes: (A) CD8 (CD8a), (B) perforin (prf1), and (C) PD1 (pdcd1). Log2 transformed means of 4–5 biologic replicates in each of the 4 mouse groups are shown with sds. The least-expressing sample (including naïve animals) was used as the baseline for each gene assayed. Results are representative of 2 independent qPCR experiments. There were 4–5 mice per group. Groups were compared using the Student’s t test, and P < 0.05 is shown.
The same eyes were examined for CAMs to assess the potential for lymphocyte migration into the eye. However, there was no difference in LFA-1, PSGL-1, CD44, or ICAM-1 expression among the 3 groups of mice (Fig. 9). Unexpectedly, both P-selectin (2.4- vs. 5.2-fold change; P = 0.0299) and E-selectin (2.9- vs. 6.0-fold change; P = 0.0137) were significantly increased, rather than decreased, in IFN-γ KO mice compared with WT recipients (Fig. 9E and F). Thus, the absence of IFN-γ did not adversely affect the expression of CAMs that are involved in lymphocyte trafficking into tumor sites.
Figure 9. The absence of IFN-γ does not cause a decrease in the levels of cell adhesion molecules (CAM) in the eyes of adoptive transfer recipients.
Eyes were removed from WT and IFN-γ KO mice 24 d after the mice received adoptively transferred CD8+ T cells from s.c.-immunized WT donors and were challenged in the AC with Ad5E1 tumor cells. Eyes were examined by qPCR for expression of the following CAM genes: (A) LFA-1 (itga1), (B) PSGL-1 (selplg), (C) CD44 (CD44), (D) ICAM-1 (icam1), (E) P-selectin (selp), and (F) E-selectin (sele). Log2-transformed means of 4–5 biologic replicates in each of the 4 mouse groups are shown with sds. The least-expressing sample (including naïve animals) was used as the baseline for each gene assayed. Data are representative of 2 independent qPCR experiments. There were 4–5 mice per group. Groups were compared using the Student’s t test, and P < 0.05 is shown.
IFN-γ KO recipient mice of WT CD8+ T cells that reject their intraocular tumor have increased CD4 message
The earlier results indicated that either intraocular or s.c. tumor cell injection induced CTL responses, yet intraocular tumors were not rejected in either circumstance. Moreover, adoptively transferred CD8+ T cells mediated the rejection of 100% of the intraocular tumors in WT mice but failed to promote rejection in 40% of IFN-γ KO recipients (Fig. 6F and G). In addition, intraocular tumor rejection in WT recipients of adoptively transferred CD8+ CTL did not culminate in phthisis of the affected eye. Although 60% of the IFN-γ KO recipients mimicked the WT recipients of CD8+ CTL and rejected their intraocular tumors in a pristine manner, the remaining 40% of the IFN-γ KO recipients of the same CD8+ CTL failed to reject their intraocular tumors. We suspected that this inability of the same CD8+ CTL from WT donors to mediate nonphthisical tumor rejection in IFN-γ KO recipients may be due to the inability of the IFN-γ–deficient eye to support sustained CTL function in situ. We have previously shown that rejection of this tumor cell line requires the presence of an intact CD4+ T cell repertoire [17]. Moreover, tumor-specific CD4+ T cell help has been found to reactivate memory CTLs to kill tumor cells [34] and enhance the efficacy of adoptively transferred antitumor CTLs [35]. With this in mind, we interrogated the eyes of WT and IFN-γ KO mice following adoptive transfer of CD8+ T cells from WT donors for the presence of Th cell marker CD4. The rationale for this approach is based on the observation that CD4+ T cells can promote dendritic cells to prime CTL responses and to enhance their cytokine production [36].
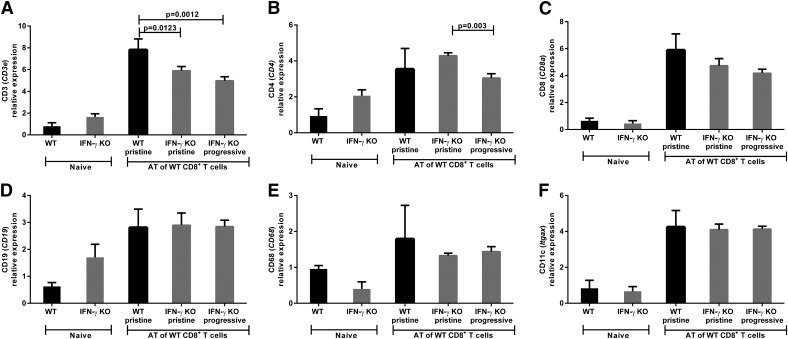
We hypothesized that there would be a distinction in the infiltrating immune cell population present in the IFN-γ KO recipient mice that rejected their intraocular tumors compared with those mice that continued to develop progressive tumors. Tumor-challenged eyes were assessed by qPCR for immune cell population markers following adoptive transfer of WT CD8+ CTLs and AC tumor challenge. Three groups of recipient mice were analyzed based on their intraocular rejection phenotype: WT recipients with pristine rejection, IFN-γ KO recipients with pristine rejection, and IFN-γ KO recipients with progressive tumor growth. There was an increase in the T cell marker CD3e mRNA expression in WT recipients compared with IFN-γ KO recipients (WT pristine 7.8 vs. IFN-γ KO pristine 5.9; P = 0.0123; vs. IFN-γ KO progressive 5.0; P = 0.0012) (Fig. 10 A). The Th cell marker CD4 was significantly increased in the IFN-γ KO recipients displaying pristine rejection compared with the IFN-γ KO recipients with progressive tumors (4.3- vs. 3.0-fold change; P = 0.003) (Fig. 10B). Furthermore, recipients with pristine rejection from either the WT or IFN-γ KO group expressed similar levels of CD4 mRNA (Fig. 10B). All 3 groups of recipient mice had similar levels of the remaining immune cell population markers: CD8 CTL marker, CD19 B cell marker, CD68 macrophage marker, and CD11c dendritic cell marker (Fig. 10C–F). Therefore, the distinguishing immune cell difference within the tumor-challenged eyes between the IFN-γ KO recipients that pristinely rejected their intraocular tumors compared with those with progressive growth was the significant increase in the CD4+ Th cell–associated mRNA marker expression.
Figure 10. Increased Th cell–associated CD4 message distinguishes adoptive transfer (AT) recipient IFN-γ KO mice that pristinely rejected their intraocular tumor when compared with those that developed progressive intraocular tumors.
Eyes were removed from WT and IFN-γ KO mice 24 d after the mice received AT CD8+ T cells from s.c.-immunized WT donors and were challenged in the AC with Ad5E1 tumor cells. Eyes were examined by qPCR for expression of the following immune cell phenotype genes: (A) CD3 (CD3e), (B) CD4 (CD4), (C) CD8 (CD8a), (D) CD19 (CD19),(E) CD68 (CD68), and (F) CD11c (itgax). Log2-transformed means of 5 biologic replicates in each of the 5 groups are shown with sds. The least-expressing sample (including naïve animals) was used as the baseline for each gene assayed. Results are representative of 2 independent qPCR experiments. There were 5 mice per group. Groups were compared using the Student’s t test, and P < 0.05 is shown.
DISCUSSION
We employed a well-characterized model of intraocular tumor rejection to address 6 fundamental questions about the role of CD8+ T cells in the rejection of tumors in an immune privilege site: 1) is IFN-γ necessary for the generation of CD8+ CTL by intraocular tumors, 2) can CTL, induced by intraocular tumors, mediate tumor rejection outside of the eye but not inside of the eye, 3) is IFN-γ needed for CTL to enter the tumor-bearing eye, 4) is IFN-γ necessary for CTL to mediate tumor rejection in the eye, 5) does CD8+ CTL-mediated tumor rejection in the eye require perforin 6) Does IFN-γ affect the immunopathologic sequelae of tumor rejection differently in the immune privileged eye compared with a nonimmune privileged site?
First, and most noteworthy, is the finding that, even though IFN-γ was required for intraocular tumor rejection, induction of tumor-specific CD8+ CTL was independent of IFN-γ. Both WT mice and IFN-γ KO mice developed similar levels of CTL and were equally capable of rejecting tumors in an s.c. nonprivileged site. Furthermore, both qPCR analysis of CD8 and perforin message and flow cytometric analysis of protein expression of CD3 and CD8 on the surface of infiltrating CD45+ leukocytes in the tumor-bearing eyes indicated that the absence of IFN-γ still permitted the infiltration of CTLs. The data also strongly support the conclusion that CD8+ T cells mediate tumor rejection in the eye by a classic perforin-mediated process because almost 90% of the mice receiving CD8+ T cells from perforin KO mice failed to reject their intraocular tumors. Phenotypic analysis of these CD3+CD8+ T cells revealed a significant decrease in the proportion that were CD44highCD62Llow effector CTL populations within the IFN-γ KO tumor-bearing eyes compared with the WT mice. Furthermore, adoptive transfer studies revealed that only CTLs generated in WT donors with an intact IFN-γ gene could eradicate the intraocular tumors in IFN-γ–deficient mice.
The present results also revealed a curious dichotomy in the pathophysiology of tumor rejection in the absence and presence of peripherally immunized, tumor-specific CTLs. In other studies, CTLs generated after AC immunization with soluble antigen were not deleted, but instead, exhibited reduced lytic activity [37]. In the present study, intraocular Ad5E1 tumors underwent immune rejection in WT mice. However, the immune response that was elicited by intraocular tumors in WT mice inflicted extensive necrosis that culminates in phthisis of the eye. By contrast, a remarkably different outcome occurred when the primary tumor response was elicited by peripheral s.c.-injected tumor cells in WT mice or when in the form of adoptively transferred CD8+ CTLs from s.c.-immunized WT donors. In these cases, tumor rejection did not culminate in necrosis; instead, the tumor was eliminated with no discernible injury to the eye. The most plausible explanation to account for both of these conditions is based on the observation that, in both cases, CTL responses were generated for 14 d before either AC tumor challenge (s.c. immunization group) or adoptive transfer of primed CTL before AC tumor challenge. In both of these groups, the intraocular tumors grew to a smaller peak tumor size and the histopathologic features of intraocular tumor rejection were characterized by piecemeal necrosis and no apparent ischemia (Figs. 1 and 5; data not shown). In the case of AC tumor injection into naïve WT mice, the intraocular tumors underwent significant growth and expansion in volume in the eye while the primary immune response was being generated, and histopathologic inspection of these eyes revealed significant ischemic necrosis and injury to innocent bystander cells [22].
The lack of correlation between in vitro CTL activity and protection against in vivo intraocular tumors in IFN-γ KO mice might relate to the role of CAMs and the trafficking of CTLs into the tumor-containing eye. CAMs are important for the homing and extravasation of lymphocytes to sites of infections or neoplasms. IFN-γ–producing Th1 cells have been found to penetrate the blood–retina barrier in experimental autoimmune uveitis because of an up-regulation of P-selectin and E-selectin on the retinal vascular endothelial cells [38]. Interestingly, we detected higher amounts of both P-selectin and E-selectin in tumor-containing eyes of IFN-γ KO mice compared with WT mice, which rules out this explanation for the inability of CTLs from IFN-γ KO mice to rid the eyes of tumors in IFN-γ KO hosts. It is possible that these adhesion molecules were elevated in the IFN-γ KO eye because of other cytokines produced in the inflammatory response in the highly vascularized tumor. In fact, increased CAM expression, including P-selectin and E-selectin, has been detected on the vascular endothelium of breast and gastric cancers [39, 40]. Immunohistochemistry of the AC tumor, ciliary body, and retina 14 d after tumor injection revealed P-selectin staining within the AC tumor and bordering the ciliary body, but not in the retina. P-selectin could be weakly expressed within these retinas, but it was undetectable by immunohistochemistry. Therefore, the increased mRNA expression of the 2 selectins detected in the IFN-γ KO eyes may be due to the larger intraocular tumor present in the eye. However, the present results clearly indicate that the increased expression of P-selectin and E-selectin in the eyes of IFN-γ KO mice did not endow the CTLs with the ability to reject the intraocular tumors or to migrate in larger numbers compared with the WT mice.
Studies have demonstrated that IFN-γ is critical for trafficking of bloodborne immune cells into the eye. This could explain why some ocular diseases have milder presentations in IFN-γ KO mice. Goblet cell loss during experimental dry eye in mice is associated with increased T cell infiltrate and expression of IFN-γ within the conjunctival epithelium, which is absent in IFN-γ KO mice [41]. During allergic conjunctivitis, IFN-γ KO mice have much-reduced clinical signs because of an absence of VCAM-1 expression on the conjunctiva of IFN-γ KO mice compared with WT mice, which prevents the infiltration of inflammatory cells into the conjunctiva [42]. Conversely, in the present study, we found that, in the absence of IFN-γ, CD3+CD8+ T cells were still able penetrate the blood–ocular barrier and enter the intraocular tumor environment. A potential explanation for this difference is that the immune cells enter the tumor-containing eye through a disorganized, leaky vasculature that frequently occurs in growing neoplasms [43]. Although the CTLs generated in s.c.-immunized IFN-γ KO were able to breach the blood–ocular barrier, they were incapable of ridding the eye of the tumor, which reinforces the notion that the inability CTLs to fully eliminate intraocular tumors in the eyes of IFN-γ KO mice is not simply due to the CTLs being unable to enter the intraocular tumor-containing eye.
Our study has shown that CTLs primed in the presence of IFN-γ in WT mice are more potent in their abilities to reject tumors within the eye compared with CTLs generated in IFN-γ KO mice. Both WT and IFN-γ KO mice generated endogenous CD8+ CTLs that were able to migrate into tumor-bearing eyes. IFN-γ has a role in regulating survivin expression, which is known to promote both CTL survival and greater tumor-rejection efficacy [43, 44]. Our present study revealed that the infiltrating CD8+ T cells in the intraocular tumors in IFN-γ KO mice, compared with WT mice, expressed a lower number of CD44highCD62Llow effector CD8+ T cells, suggesting that the CD8+ T cells in WT mice were better poised to mediate a productive antitumor response in eyes replete with IFN-γ.
CD8+ T cells are a heterogeneous assemblage of T cells that have different effector functions depending on the environment and cytokine milieu in which they are primed [21]. Conventional CD8+ CTLs produce perforin- and granzyme-mediated cytotoxicity, which is associated with IFN-γ production. Our laboratory has previously shown that, after s.c. immunization with the Ad5E1 tumor in IFN-γ KO mice, peripheral CD4+ T cells differentiate into Th17 cells in the presence of IL-6 [17]. This environment could also promote the development of IL-17–associated CTLs that have reduced cytolytic functions, compared with conventional CTLs [39]. However, in a lung-tumor model, adoptively transferred IL-17-associated CTLs were still able to exert an antitumor response independent of IFN-γ, but overall, they were inferior to the conventional IFN-γ–associated CTLs [40]. Therefore, in the ocular environment it may be necessary for CTLs to be augmented by IFN-γ for effective tumor clearance, whereas IL-17-associated CTLs may be unable to reject intraocular tumors. This is supported by the finding that endogenously generated CTLs, induced by s.c. immunization of IFN-γ KO, enter the eyes but are unable to reject the intraocular tumors, which results in 100% of mice developing progressive intraocular tumors, compared with the 40% incidence of progressive intraocular tumors in IFN-γ KO mice that receive an adoptive transfer of CTLs generated in WT mice. Moreover, the protective antitumor function of these adoptively transferred CTLs requires perforin, as evidenced by the 87% progressive intraocular tumor rate in IFN-γ KO mice that received perforin-KO CTLs. Interestingly, IFN-γ is not required in situ for WT CTLs to function in the IFN-γ KO mice.
Even in an IFN-γ–replete environment, the originally transferred CD8+ T cells from WT mice may not provide long-term protection against the intraocular tumor without additional help from endogenous CD4+ T cells [36]. The IFN-γ KO mice that received adoptively transferred CTLs that fully rejected their intraocular tumor had higher levels of the Th cell–associated CD4 message in the tumor-bearing eye than did their IFN-γ KO recipient counterparts which continued to develop progressive intraocular tumors. Tumor-specific CD4+ T cell help has been found to reactivate memory CTLs to kill tumor cells [34] and to enhance the efficacy of adoptively transferred antitumor CTLs [35]. Interestingly, in a syngeneic tumor model of pancreatic cancer, CD4+ T cell help was most important in the tumor milieu for the expression of a robust antitumor CTL response, rather than during the initial priming phase [45].
A portion of memory CD8+ T cells are able to produce IFN-γ during the early phase of infection through bystander activation dependence on multiple factors produced by macrophages and dendritic cells [46]. It is possible that the adoptively transferred CD8+ T cells that enter the IFN-γ–deficient eye do not receive all the necessary factors to produce sufficient amounts of IFN-γ during the early immune response. Additionally, we found that the IFN-γ KO mice that received adoptively transferred CTLs had increased levels of PD-1 message in the tumor-bearing eye. Although we do not know which immune cells are expressing increased PD-1 in the tumor-bearing eye, PD-1 is a well-known immune exhaustion marker, characterized by reduced cellular functionality in a wide range of immune cells other than CTLs, including NKT cells [26, 27], CD4+ T cells [28, 29], B cells [30, 31], NK cells [32], and macrophages [33]. The increased PD-1 message present in the IFN-γ KO adoptive transfer recipients could be a marker of immune cell exhaustion involving many cells, in addition to the adoptively transferred CTLs, culminating in an insufficient antitumor response. PD-1 is an established CTL exhaustion marker defined by reduced cytokine production, proliferation, and cytotoxicity [24, 25], which is most suppressive within the tumor environment [47]. The increase of a global immune cell exhaustion marker may be indicative of failure to support a robust antitumor response in the eye. Furthermore, in a chronic viral infection model, CD8+ T cells that did not receive CD4+ T cell help expressed significantly higher levels of PD-1 on memory CD8+ T cells with lowered recall responses, which could be reversed by simulating CD4+ T cell help by administering IL-2 complexes [48]. This may account for the inability of the WT s.c.-immunized, primed CTLs to fully protect the eye against intraocular tumor growth in the absence of additional IFN-γ and reduced levels of CD4+ T cell help in situ in the IFN-γ KO mice. Recently, costimulation provided by CD4+ T cells was reported to prevent CD8+ memory T cells from becoming exhausted [49]. This is consistent with our findings of reduced CD4 message in the eyes of IFN-γ KO mice that failed to reject their tumors even after receiving adoptively transferred CD8+ T cells. However, confirmation of reduced CD4 message because of a commensurate reduction in CD4+ T cells is beyond the scope of the present study and requires more-extensive investigation to be confirmed.
The environment of the eye is unique when compared with the periphery and requires endogenous IFN-γ for CTLs primed in the periphery to be effective antitumor agents within the intraocular milieu. Although the exact mechanisms are unknown for the lack of CTL functionality in the eyes of IFN-γ KO mice, we know that this dysfunction is specific to the eye because peripheral tumors can be rejected whereas intraocular tumors cannot. Even though equal proportions of infiltrating leukocytes are CD8+CD3+ CTLs in both WT and IFN-γ KO tumor-bearing eyes, there is a significant decrease in the CD44highCD62Llow CD8+ effector CTL populations in the IFN-γ KO eyes. More than one-half of IFN-γ KO mice that received an adoptive transfer of WT, primed CTLs able to produce perforin were able to reject their intraocular tumors. Even with an aggressive adoptive transfer protocol, 100% rejection of intraocular tumors was not reached in the IFN-γ KO recipient mice. Therefore, unlike extraocular body sites, the IFN-γ-deficient intraocular milieu is an inefficient environment for promoting sustained and effective CD8+ T cell–mediated rejection of intraocular tumors.
AUTHORSHIP
A.J.L. performed in vitro and in vivo experiments and contributed to the design of experiments, analysis of the results, and writing of the manuscript. J.R.B. assisted with the in vivo adoptive transfer experiments and in the statistical analysis of the experiments. J.Y.N. contributed to the designing of experiments, analysis of the results, and writing of the manuscript.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health Grants EY005631 and EY020799 and by Research to Prevent Blindness. We appreciate the technical assistance and training of Jessamee Mellon, Tracy Gray, and Neema Lakshman.
Glossary
- AC
anterior chamber
- AT
adoptive transfer
- CAM
cell adhesion molecule
- KO
knockout
- qPCR
quantitative PCR
- WT
wild-type C57BL/6 mice
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Niederkorn J. Y. (2006) See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat. Immunol. 7, 354–359. [DOI] [PubMed] [Google Scholar]
- 2.Niederkorn J. Y. (2002) Immune privilege in the anterior chamber of the eye. Crit. Rev. Immunol. 22, 13–46. [PubMed] [Google Scholar]
- 3.Niederkorn J. Y. (1991) The immunopathology of intraocular tumour rejection. Eye (Lond.) 5, 186–192. [DOI] [PubMed] [Google Scholar]
- 4.McKenna K. C., Kapp J. A. (2004) Ocular immune privilege and CTL tolerance. Immunol. Res. 29, 103–112. [DOI] [PubMed] [Google Scholar]
- 5.Devine L., Lightman S., Greenwood J. (1996) Lymphocyte migration across the anterior and posterior blood–retinal barrier in vitro. Cell. Immunol. 168, 267–275. [DOI] [PubMed] [Google Scholar]
- 6.Devine L., Lightman S. L., Greenwood J. (1996) Role of LFA-1, ICAM-1, VLA-4 and VCAM-1 in lymphocyte migration across retinal pigment epithelial monolayers in vitro. Immunology 88, 456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yannariello-Brown J., Hallberg C. K., Häberle H., Brysk M. M., Jiang Z., Patel J. A., Ernst P. B., Trocme S. D. (1998) Cytokine modulation of human corneal epithelial cell ICAM-1 (CD54) expression. Exp. Eye Res. 67, 383–393. [DOI] [PubMed] [Google Scholar]
- 8.Johnsen-Soriano S., Sancho-Tello M., Arnal E., Navea A., Cervera E., Bosch-Morell F., Miranda M., Javier Romero F. (2010) IL-2 and IFN-gamma in the retina of diabetic rats. Graefes Arch. Clin. Exp. Ophthalmol. 248, 985–990. [DOI] [PubMed] [Google Scholar]
- 9.Paine S. K., Basu A., Mondal L. K., Sen A., Choudhuri S., Chowdhury I. H., Saha A., Bhadhuri G., Mukherjee A., Bhattacharya B. (2012) Association of vascular endothelial growth factor, transforming growth factor beta, and interferon gamma gene polymorphisms with proliferative diabetic retinopathy in patients with type 2 diabetes. Mol. Vis. 18, 2749–2757. [PMC free article] [PubMed] [Google Scholar]
- 10.Geiger K., Howes E., Gallina M., Huang X. J., Travis G. H., Sarvetnick N. (1994) Transgenic mice expressing IFN-gamma in the retina develop inflammation of the eye and photoreceptor loss. Invest. Ophthalmol. Vis. Sci. 35, 2667–2681. [PubMed] [Google Scholar]
- 11.Egwuagu C. E., Sztein J., Mahdi R. M., Li W., Chao-Chan C., Smith J. A., Charukamnoetkanok P., Chepelinsky A. B. (1999) IFN-gamma increases the severity and accelerates the onset of experimental autoimmune uveitis in transgenic rats. J. Immunol. 162, 510–517. [PubMed] [Google Scholar]
- 12.Fisson S., Ouakrim H., Touitou V., Baudet S., Ben Abdelwahed R., Donnou S., Miloudi A., Galand C., Bodaghi B., Lehoang P., Brissard M., Le Garff-Tavernier M., Fridman W. H., Sautès-Fridman C., Cassoux N., Merle-Béral H. (2013) Cytokine profile in human eyes: contribution of a new cytokine combination for differential diagnosis between intraocular lymphoma or uveitis. PLoS One 8, e52385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee C. S., Jun I. H., Kim T. I., Byeon S. H., Koh H. J., Lee S. C. (2012) Expression of 12 cytokines in aqueous humour of uveal melanoma before and after combined ruthenium-106 brachytherapy and transpupillary thermotherapy. Acta Ophthalmol. 90, e314–e320. [DOI] [PubMed] [Google Scholar]
- 14.Dunn G. P., Koebel C. M., Schreiber R. D. (2006) Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 6, 836–848. [DOI] [PubMed] [Google Scholar]
- 15.Coursey T. G., Chen P. W., Niederkorn J. Y. (2011) Abrogating TNF-α expression prevents bystander destruction of normal tissues during iNOS-mediated elimination of intraocular tumors. Cancer Res. 71, 2445–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toes R. E., Offringa R., Blom R. J., Brandt R. M., van der Eb A. J., Melief C. J., Kast W. M. (1995) An adenovirus type 5 early region 1B-encoded CTL epitope-mediating tumor eradication by CTL clones is down-modulated by an activated ras oncogene. J. Immunol. 154, 3396–3405. [PubMed] [Google Scholar]
- 17.Coursey T. G., Chen P. W., Niederkorn J. Y. (2011) IL-17-dependent, IFN-gamma-independent tumor rejection is mediated by cytotoxic T lymphocytes and occurs at extraocular sites, but is excluded from the eye. J. Immunol. 187, 4219–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hegde S., Niederkorn J. Y. (2000) The role of cytotoxic T lymphocytes in corneal allograft rejection. Invest. Ophthalmol. Vis. Sci. 41, 3341–3347. [PubMed] [Google Scholar]
- 19.Mueller K., Schweier O., Pircher H. (2008) Efficacy of IL-2- versus IL-15-stimulated CD8 T cells in adoptive immunotherapy. Eur. J. Immunol. 38, 2874–2885. [DOI] [PubMed] [Google Scholar]
- 20.Klebanoff C. A., Gattinoni L., Torabi-Parizi P., Kerstann K., Cardones A. R., Finkelstein S. E., Palmer D. C., Antony P. A., Hwang S. T., Rosenberg S. A., Waldmann T. A., Restifo N. P. (2005) Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl. Acad. Sci. U. S. A. 102, 9571–9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mittrücker H. W., Visekruna A., Huber M. (2014) Heterogeneity in the differentiation and function of CD8+ T cells. Arch. Immunol. Ther. Exp. (Warsz.) 62, 449–458. [DOI] [PubMed] [Google Scholar]
- 22.Coursey T. G., Chen P. W., Niederkorn J. Y. (2012) IFN-γ-independent intraocular tumor rejection is mediated by a macrophage-dependent process that leaves the eye intact. J. Leukoc. Biol. 92, 939–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trapani J. A., Smyth M. J. (2002) Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2, 735–747. [DOI] [PubMed] [Google Scholar]
- 24.Keir M. E., Butte M. J., Freeman G. J., Sharpe A. H. (2008) PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wherry E. J., Ha S. J., Kaech S. M., Haining W. N., Sarkar S., Kalia V., Subramaniam S., Blattman J. N., Barber D. L., Ahmed R. (2007) Molecular signature of CD8++ T cell exhaustion during chronic viral infection. Immunity 27, 670–684. [DOI] [PubMed] [Google Scholar]
- 26.Chang W. S., Kim J. Y., Kim Y. J., Kim Y. S., Lee J. M., Azuma M., Yagita H., Kang C. Y. (2008) Cutting edge: Programmed death-1/programmed death ligand 1 interaction regulates the induction and maintenance of invariant NKT cell anergy. J. Immunol. 181, 6707–6710. [DOI] [PubMed] [Google Scholar]
- 27.Kee S. J., Kwon Y. S., Park Y. W., Cho Y. N., Lee S. J., Kim T. J., Lee S. S., Jang H. C., Shin M. G., Shin J. H., Suh S. P., Ryang D. W. (2012) Dysfunction of natural killer T cells in patients with active Mycobacterium tuberculosis infection. Infect. Immun. 80, 2100–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antoine P., Olislagers V., Huygens A., Lecomte S., Liesnard C., Donner C., Marchant A. (2012) Functional exhaustion of CD4+ T lymphocytes during primary cytomegalovirus infection. J. Immunol. 189, 2665–2672. [DOI] [PubMed] [Google Scholar]
- 29.Sachdeva M., Fischl M. A., Pahwa R., Sachdeva N., Pahwa S. (2010) Immune exhaustion occurs concomitantly with immune activation and decrease in regulatory T cells in viremic chronically HIV-1-infected patients. J. Acquir. Immune Defic. Syndr. 54, 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thibult M. L., Mamessier E., Gertner-Dardenne J., Pastor S., Just-Landi S., Xerri L., Chetaille B., Olive D. (2013) PD-1 is a novel regulator of human B-cell activation. Int. Immunol. 25, 129–137. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura H., Minato N., Nakano T., Honjo T. (1998) Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int. Immunol. 10, 1563–1572. [DOI] [PubMed] [Google Scholar]
- 32.Wiesmayr S., Webber S. A., Macedo C., Popescu I., Smith L., Luce J., Metes D. (2012) Decreased NKp46 and NKG2D and elevated PD-1 are associated with altered NK-cell function in pediatric transplant patients with PTLD. Eur. J. Immunol. 42, 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang X., Venet F., Wang Y. L., Lepape A., Yuan Z., Chen Y., Swan R., Kherouf H., Monneret G., Chung C. S., Ayala A. (2009) PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc. Natl. Acad. Sci. U. S. A. 106, 6303–6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao F. G., Khammanivong V., Liu W. J., Leggatt G. R., Frazer I. H., Fernando G. J. (2002) Antigen-specific CD4+ T-cell help is required to activate a memory CD8+ T cell to a fully functional tumor killer cell. Cancer Res. 62, 6438–6441. [PubMed] [Google Scholar]
- 35.Antony P. A., Piccirillo C. A., Akpinarli A., Finkelstein S. E., Speiss P. J., Surman D. R., Palmer D. C., Chan C. C., Klebanoff C. A., Overwijk W. W., Rosenberg S. A., Restifo N. P. (2005) CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol. 174, 2591–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams M. A., Bevan M. J. (2007) Effector and memory CTL differentiation. Annu. Rev. Immunol. 25, 171–192. [DOI] [PubMed] [Google Scholar]
- 37.McKenna K. C., Xu Y., Kapp J. A. (2002) Injection of soluble antigen into the anterior chamber of the eye induces expansion and functional unresponsiveness of antigen-specific CD8+ T cells. J. Immunol. 169, 5630–5637. [DOI] [PubMed] [Google Scholar]
- 38.Xu H., Manivannan A., Jiang H. R., Liversidge J., Sharp P. F., Forrester J. V., Crane I. J. (2004) Recruitment of IFN-gamma-producing (Th1-like) cells into the inflamed retina in vivo is preferentially regulated by P-selectin glycoprotein ligand 1:P/E-selectin interactions. J. Immunol. 172, 3215–3224. [DOI] [PubMed] [Google Scholar]
- 39.Huber M., Heink S., Grothe H., Guralnik A., Reinhard K., Elflein K., Hünig T., Mittrücker H. W., Brüstle A., Kamradt T., Lohoff M. (2009) A Th17-like developmental process leads to CD8+ Tc17 cells with reduced cytotoxic activity. Eur. J. Immunol. 39, 1716–1725. [DOI] [PubMed] [Google Scholar]
- 40.Yu Y., Cho H. I., Wang D., Kaosaard K., Anasetti C., Celis E., Yu X. Z. (2013) Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms. J. Immunol. 190, 1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Paiva C. S., Villarreal A. L., Corrales R. M., Rahman H. T., Chang V. Y., Farley W. J., Stern M. E., Niederkorn J. Y., Li D. Q., Pflugfelder S. C. (2007) Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest. Ophthalmol. Vis. Sci. 48, 2553–2560. [DOI] [PubMed] [Google Scholar]
- 42.Stern M. E., Siemasko K., Gao J., Duong A., Beauregard C., Calder V., Niederkorn J. Y. (2005) Role of interferon-gamma in a mouse model of allergic conjunctivitis. Invest. Ophthalmol. Vis. Sci. 46, 3239–3246. [DOI] [PubMed] [Google Scholar]
- 43.Zimmerman M., Yang D., Hu X., Liu F., Singh N., Browning D., Ganapathy V., Chandler P., Choubey D., Abrams S. I., Liu K. (2010) IFN-γ upregulates survivin and Ifi202 expression to induce survival and proliferation of tumor-specific T cells. PLoS One 5, e14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao B., Song A., Haque R., Lei F., Weiler L., Xiong X., Wu Y., Croft M., Song J. (2009) Cooperation between molecular targets of costimulation in promoting T cell persistence and tumor regression. J. Immunol. 182, 6744–6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bos R., Sherman L. A. (2010) CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 70, 8368–8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kambayashi T., Assarsson E., Lukacher A. E., Ljunggren H. G., Jensen P. E. (2003) Memory CD8+ T cells provide an early source of IFN-gamma. J. Immunol. 170, 2399–2408. [DOI] [PubMed] [Google Scholar]
- 47.Wu X., Zhang H., Xing Q., Cui J., Li J., Li Y., Tan Y., Wang S. (2014) PD-1+ CD8+ T cells are exhausted in tumours and functional in draining lymph nodes of colorectal cancer patients. Br. J. Cancer 111, 1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fuse S., Tsai C. Y., Molloy M. J., Allie S. R., Zhang W., Yagita H., Usherwood E. J. (2009) Recall responses by helpless memory CD8+ T cells are restricted by the up-regulation of PD-1. J. Immunol. 182, 4244–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKinney E. F., Lee J. C., Jayne D. R., Lyons P. A., Smith K. G. (2015) T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 523, 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]