INTRODUCTION
Topical corticosteroids (TCs) are among the most commonly used medications in dermatology clinics.[1] They are highly efficacious drugs which are used for the treatment of varied autoimmune and inflammatory dermatological conditions.[1,2] In India, the annual sales figure of TCs was 14 billion rupees in 2013, which accounts for almost 82% of total dermatological product sale in the country.[3] Prescription of TC has become ubiquitous with dermatologists and concerns have also been raised regarding its misuse for non-labeled indications. As per the information available on the Central Drugs Standard Control Organization (CDSCO) website regarding approved dermatological indications of TC (although indications are not mentioned for all the TC molecules), its off-label use seems to be a common clinical practice in India [Table 1].[4,5] However, the more serious concern is its inappropriate use in symptomatic treatment for varied dermatological disorders like acne, primary bacterial and fungal infections, undiagnosed skin rash and as fairness cream by non-registered practitioners or on the advice of pharmacist at chemist shops.[6,7,8] These people are not qualified and competent to treat dermatological disorders and prescribe topical steroids which often provide quick symptomatic relief without treating the underlying pathology of the disease. Ironically, the Indian market is flooded with several fixed-dose combinations (FDCs) of corticosteroids with antibacterial and fungal agents, which in no way can be considered as scientific and rational. Incidentally, two of such irrational FDCs of corticosteroids, antibacterial and antifungal agents, were the top selling formulations of TCs in India in 2013.[3]
Table 1.
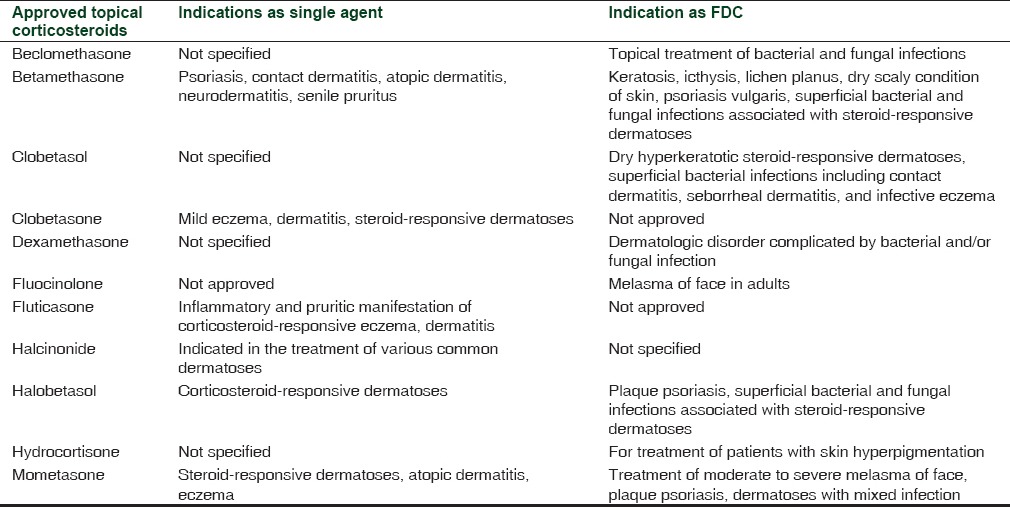
Recently, a study done at rural tertiary care teaching hospital in Maharashtra, India concluded that 28% of 500 prescriptions had TCs, out of which 98% were very potent corticosteroids; and in 85% of cases, the basis of prescribing TCs could not be established.[8] Besides this, a new emerging dangerous pattern regarding use of TCs as fairness cream is being observed in India. A multicenter study conducted in India concluded that 14.8% of patients with facial dermatoses were using TCs, and that fairness or shaving cream (29%) was the most common reason for its use among the study cohort.[6] The topical steroid use on face can lead to many adverse effects like atrophy of the skin, hirsutism, acne, perioral dermatitis, and telangiectasias. Its prolonged and indiscriminate use can lead to development of a condition known as topical steroid-dependent face (TSDF). It is characterized by severe rebound erythema, burning and scaling of the face on attempted stoppage of the TC after prolonged use.[1] It leads to successive use of more and more potent TCs to avoid the rebound effects associated with withdrawal, a condition known as steroid addiction.[9] Moreover, long-term use of potent TCs may also lead to systemic side effects such as adrenal suppression and cushingoid appearance.[10] This is not only a cause of concern for dermatologists, but also poses a challenge to the drug regulators of the country. We have discussed about the implications of this abuse for the drug regulatory body and the possible ways to deal with such challenges.
REGULATORY CHALLENGES FOR TOPICAL CORTICOSTEROIDS IN INDIA
In India, hundreds of branded generic versions of FDCs of TCs are available across the pharmacies as over-the-counter (OTC) drugs. The present commentary discusses about the availability and other regulatory issues regarding its use.
Fixed dose combinations of topical corticosteroids
At least 11 TCs of different potencies are available in India [Table 2]. According to best of our knowledge on the basis of Monthly Index of Medical Specialities (MIMS), India and Indian Drug Review (IDR),[11,12] the commonly used drug information sources in India, 119 FDCs of these corticosteroids with other agents (antibacterial, antifungal, keratolytic etc.) are available in the country. Many of the available FDCs, having more than two antibacterial and antifungal drugs along with corticosteroids, are certainly not rational. These kinds of broad-spectrum preparations allow their misuse, as physicians may tend to use them when they are unsure about the diagnosis. Surprisingly, out of the available 119 formulations, only 27 feature among the CDSCO's approved list of FDCs from 1961 to July 2014 in India.[4] Also, none of the three best selling dermatology FDCs in India in 2013 is in the updated list of approved FDCs by the CDSCO, an Indian drug watchdog.[4] This does not necessarily mean that all of them are irrational combinations, as some of them have shown to be quite efficacious in treating dermatological ailments and are approved in other countries. However, their non-inclusion in the list of approved FDCs on Indian regulator's website means that their manufacturing and marketing has not been permitted in the country. Furthermore, the pharmaceutical companies are marketing non-approved FDCs and the regulator needs to take stern steps to regulate dermatological drug market in the country. FDCs having corticosteroids for dermatological use are not common in USA. Also, none of the FDCs available there contains both antibacterial and antifungal agents along with corticosteroids.[13]
Table 2.
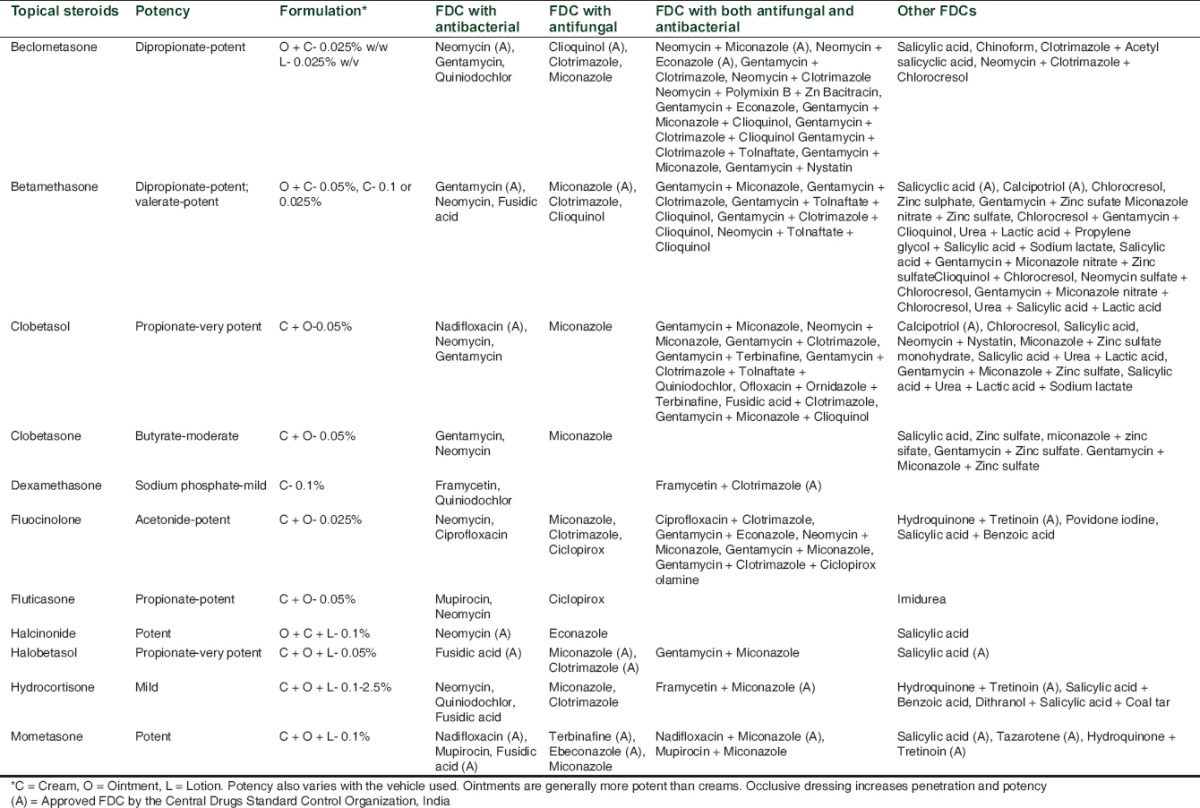
Topical steroid abuse as fairness cream
None of the FDCs in the CDSCO list has been approved for fairness of the skin. However, there are three formulations containing corticosteroids, which are approved for the management of hyper-pigmentation in India.[4] These are:
Hydroquinone 2% + Tretinoin 0.025% + Hydrocortisone 1%: Treatment of skin hyperpigmentation
Hydroquinone 2% + Tretinoin 0.025% + Mometasone 0.1%: Treatment of moderate to severe melasma of face
Hydroquinone 2% + Tretinoin 0.025% + Fluocinolone 0.015%: Treatment of melasma of face in adults.
Topical steroid abuse as a fairness cream is widely prevalent in India despite not being approved for this indication.[6] The long-term risk associated with its chronic use certainly outweighs the aesthetic benefit it may offer in some people. Studies suggest that TCs are being used as fairness creams, anti-acne medications and also as general purpose creams.[6,7] The main reason for its abuse as a fairness agent is Indian obsessiveness with fair skin color. This is being exploited by pharmaceutical marketing companies and beauticians, and they are using TCs due to their quick results, without giving any due consideration to their adverse effects. The easy OTC availability of TCs at chemist shops across the country without any valid prescription is further compounding this problem of abuse. According to Drugs and Cosmetics (D and C) Act 1940, the TCs fall under the category of Schedule H drugs, meaning that they should be sold by retail shops only on the valid prescription of a qualified doctor.[14] However, in the absence of proper surveillance mechanisms by the Indian drug watchdog due to various reasons, these rules are routinely flouted by one and all the pharmacies across the country. The drug regulator needs to ensure that the rules as mentioned in D and C Act, 1940 are strictly adhered to by the concerned individuals. An online petition started by Indian Association of Dermatologists, Venereologists and Leprologists (ITATSA) Taskforce Against Topical Steroid Abuse (ITATSA) about OTC availability of TCs is a welcome step in this direction.[15] ITATSA is a special task force which was created by IADVL to look into issues related to TC abuse. It has raised the issue of TC misuse at various fronts including physicians, manufactures, pharmaceutical companies and regulators. One such effort is this online petition, which is being given to the Ministry of Health and Family Welfare, Government of India and CDSCO, stresses upon issues related to the indiscriminate sale of TCs without prescription in India due to unregulated market and also makes an effort to sensitize common people about the risks associated with their use. The drug regulator could also create a new category of OTC TCs, which could include only one or two of the mild potency preparations and the rest all will be sold only on valid prescriptions. Perhaps stringent regulations, similar to the recently issued new Schedule H1 to curb the growing menace of antibiotic resistance in India, are required to regulate TC drug market in India.[16] More importantly, as rules and regulations in the absence of proper surveillance mechanisms are toothless, there is need for well-designed and workable mechanisms to look out for offenders.
Lack of qualified dermatologists, more so in rural areas, is also compounding the problem of irrational use of corticosteroids. There are about 7.5 lakh chemists in India, who, in the absence of qualified medical practitioners, are the point of first contact for majority of Indian population for minor healthcare ailments.[17] They need to be sensitized by the central and state drug regulatory authorities about the potential adverse effects with TC abuse. Also, it has been sometime observed that even trained physicians and dermatologists are prescribing either the wrong strength of TC or for the wrong indication.[8] Regular continuing medical education and workshops to create awareness regarding this important issue will go a long way to ensure rational use of TCs. Along with this, there is need to sensitize patients, the actual user, about the adverse effects of TC abuse on face. Physicians on their part should explain the full details about proper use of corticosteroid. Topical calcineurin inhibitors (like tacrolimus) greatly add to the armamentarium for treatment of inflammatory skin disorders and they should be considered, whenever feasible, as these drugs are free of cutaneous and systemic side effects associated with TCs. Package insert also needs to be made mandatory along with all the TC preparations available in the market. The “fairness of the skin” has been specified under Schedule J of Drugs and Cosmetics Act, 1940, according to which “no drug may purport or claim to prevent or cure or may convey to the intending user thereof any idea that it may prevent or cure one or more of the diseases or ailments specified in this schedule.”[14] Hence, there should be proper check on the advertisement of the drugs which claim to promote fairness of the skin. The media and civil society can also play an active role in spreading this awareness.
CONCLUSION
Topical steroids are very important and efficacious drugs for management of various dermatological disorders. However, strict implementation of the existing regulations is the need of the hour to prevent their widespread abuse. Indian drug regulatory agency has to take proactive steps to ensure the availability of only approved FDCs in the country. Any new FDC approval has to be based on the quality evidence generated by the clinical trials. Along with this, a robust surveillance mechanism, to look out for any irregularities in implantation of approved guidelines, will go a long way to ensure safety of the patients. There is an urgent need to curb easy OTC availability of TCs. Awareness needs to be created among public regarding the harmful effects of prolonged and improper use of corticosteroids. A coordinated approach among all the stakeholders, viz. drug regulator, physicians, pharmacists and patients is vital to prevent misuse of TCs.
REFERENCES
- 1.Rathi SK, D'Souza P. Rational and ethical use of topical corticosteroids based on safety and efficacy. Indian J Dermatol. 2012;57:251–9. doi: 10.4103/0019-5154.97655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ference JD, Last AR. Choosing topical corticosteroids. Am Fam Physician. 2009;79:135–40. [PubMed] [Google Scholar]
- 3.Verma SB. Sales, status, prescriptions and regulatory problems with topical steroids in India. Indian J Dermatol Venereol Leprol. 2014;80:201–3. doi: 10.4103/0378-6323.132246. [DOI] [PubMed] [Google Scholar]
- 4.Fixed Dose Combinations approved By DCG (I) since 1961 till July, 2014. Central Drugs Standard Control Organization, India. [Last accessed on 2015 Jan 20]. Available from: http://cdsco.nic.in/writereaddata/approved%20FDC%20list%20by%20DCG(I)%20Till%20July%202014.pdf .
- 5.Approval Status of New Drug during 1971-1998. Central Drugs Standard Control Organization, India. [Last accessed on 2015 May 28]. Available from: http://cdsco.nic.in/writereaddata/1971-98.doc .
- 6.Saraswat A, Lahiri K, Chatterjee M, Barua S, Coondoo A, Mittal A, et al. Topical corticosteroid abuse on the face: A prospective, multicenter study of dermatology outpatients. Indian J Dermatol Venereol Leprol. 2011;77:160–6. doi: 10.4103/0378-6323.77455. [DOI] [PubMed] [Google Scholar]
- 7.Ambika H, Vinod CS, Yadalla H, Nithya R, Babu AR. Topical corticosteroids abuse on face: A prospective, study on outpatients of dermatology. Our Dermatol Online. 2014;5:5–8. [Google Scholar]
- 8.Rathod SS, Motghare VM, Deshmukh VS, Deshpande RP, Bhamare CG, Patil JR. Prescribing practices of topical corticosteroids in the outpatient dermatology department of a rural tertiary care teaching hospital. Indian J Dermatol. 2013;58:342–5. doi: 10.4103/0019-5154.117293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh A, Sengupta S, Coondoo A, Jana AK. Topical corticosteroid addiction and phobia. Indian J Dermatol. 2014;59:465–8. doi: 10.4103/0019-5154.139876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Böckle BC, Jara D, Nindl W, Aberer W, Sepp NT. Adrenal insufficiency as a result of long-term misuse of topical corticosteroids. Dermatology. 2014;228:289–93. doi: 10.1159/000358427. [DOI] [PubMed] [Google Scholar]
- 11.Monthly Index of Medical Specialities – Online version, India. [Last accessed on 2015 Feb 2]. Available from: https://www.mims.com/India/home/Index .
- 12.Indian Drug Review 2013. Vol. 19. UBM Medica India Private Limited, Mumbai. 2013:249–52. [Google Scholar]
- 13.Monthly Index of Medical Specialities – Online version, USA. [Last accessed on 2015 Feb 2]. Available from: https://www.mims.com/USA/home/Index .
- 14.The Drugs and Cosmetics Rules, 1945. Ministry of Health and Family Welfare, Government of India. [Last accessed on 2015 Feb 2]. Available from: http://cdsco.nic.in/html/copy%20of%201.%20d and cact121.pdf .
- 15.Petitioning the Drug Controller of India-Stop indiscriminate OTC sale of topical steroid without prescription, most are Schedule H drugs. [Last accessed on 2015 Feb 2]. Available from: http://www.change.org/p/the-drug-controller-of-india-stop-indiscriminate-otc-sale-of-topicalsteroid-without-prescription-most-are-schedule-h-drugs .
- 16.Ministry of Health and Family Welfare. GSR 588(E). Central Drugs Standard Control Organization, India. [Last accessed on 2015 Feb 2]. Available from: http://cdsco.nic.in/writereaddata/588E30thAug2013.pdf .
- 17.All India Organization of Chemists and Druggists. [Last accessed on 2015 Feb 2]. Available from: http://www.aiocd.net/index.html .


