Abstract
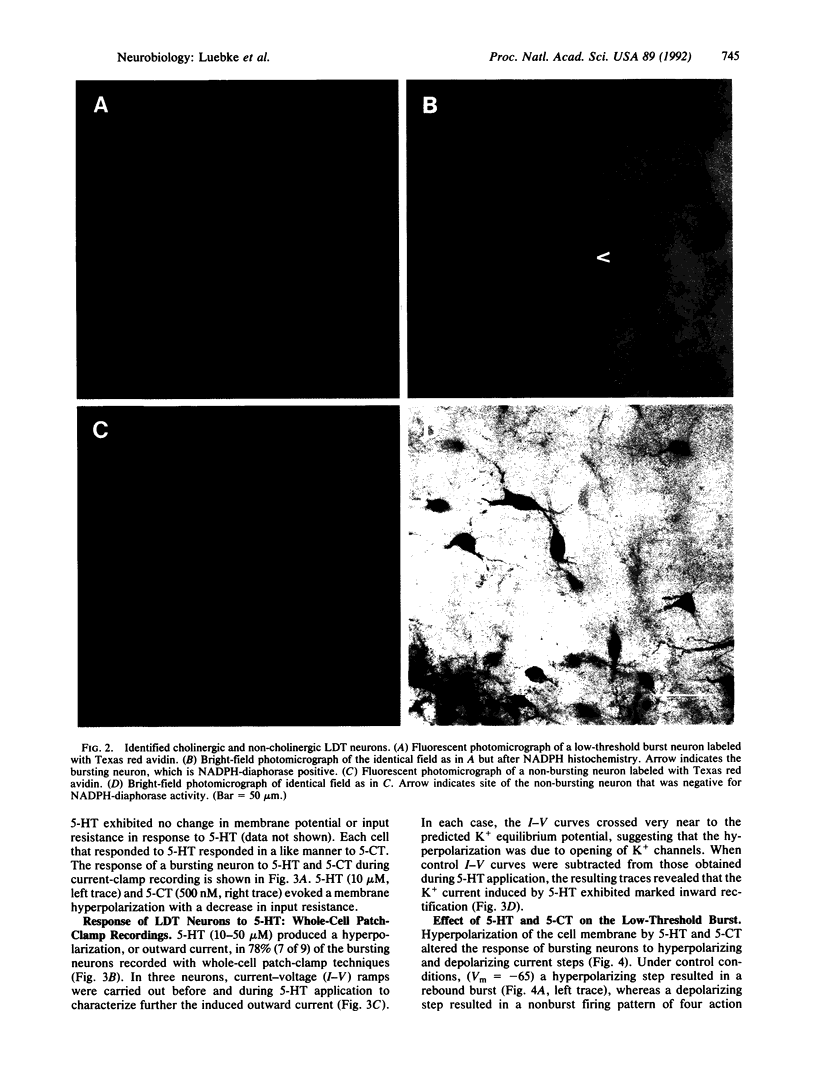
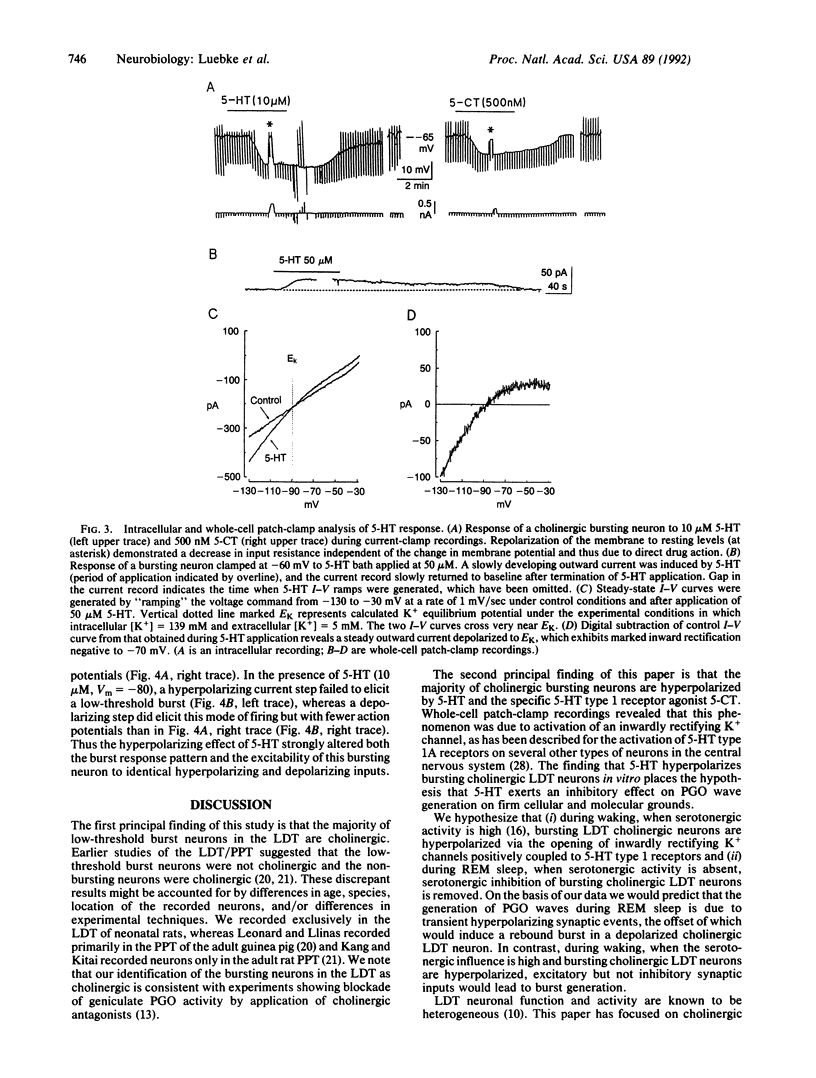
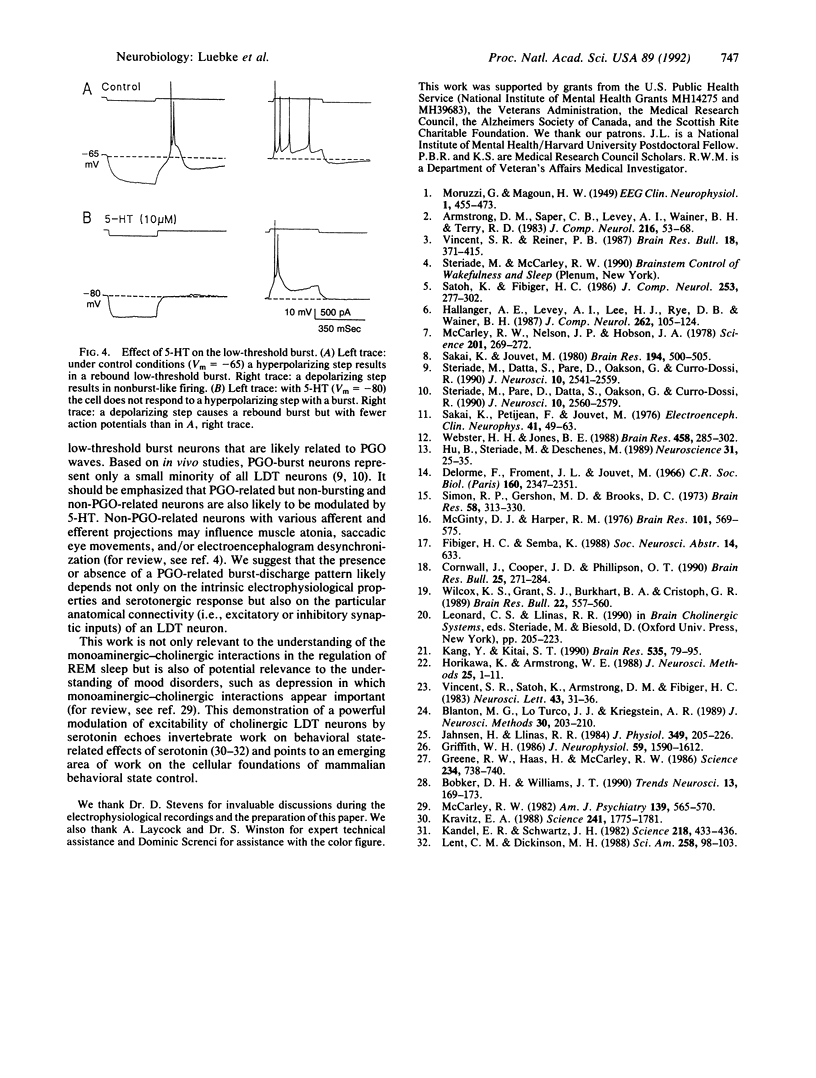
Serotonergic suppression of cholinergic neuronal activity implicated in the regulation of rapid eye movement sleep and its associated phenomenon, pontogeniculooccipital waves, has long been postulated, but no direct proof has been available. In this study, intracellular and whole-cell patch-clamp recording techniques were combined with enzyme histochemistry to examine the intrinsic electrophysiological properties and response to serotonin (5-HT) of identified cholinergic rat laterodorsal tegmental nucleus neurons in vitro. Sixty-five percent of the recorded neurons demonstrated a prominent low-threshold burst, and of these, 83% were cholinergic. In current-clamp recordings 64% of the bursting cholinergic neurons tested responded to the application of 5-HT with a membrane hyperpolarization and decrease in input resistance. This effect was mimicked by application of the selective 5-HT type 1 receptor agonist carboxamidotryptamine maleate. Whole-cell patch-clamp recordings revealed that the hyperpolarizing response was mediated by an inwardly rectifying K+ current. Application of 5-HT decreased excitability and markedly modulated the discharge pattern of cholinergic bursting neurons: during a 5-HT-induced hyperpolarization these neurons exhibited no rebound burst after hyperpolarizing current input and a burst in response to depolarizing current input. In the absence of 5-HT, the relatively depolarized cholinergic bursting neurons responded to an identical hyperpolarizing current input with a burst and did not produce a burst after depolarizing current input. These data provide a cellular and molecular basis for the hypothesis that 5-HT modulates rapid eye movement sleep phenomenology by altering the firing pattern of bursting cholinergic neurons.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. M., Saper C. B., Levey A. I., Wainer B. H., Terry R. D. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol. 1983 May 1;216(1):53–68. doi: 10.1002/cne.902160106. [DOI] [PubMed] [Google Scholar]
- Blanton M. G., Lo Turco J. J., Kriegstein A. R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989 Dec;30(3):203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Bobker D. H., Williams J. T. Ion conductances affected by 5-HT receptor subtypes in mammalian neurons. Trends Neurosci. 1990 May;13(5):169–173. doi: 10.1016/0166-2236(90)90042-9. [DOI] [PubMed] [Google Scholar]
- Cornwall J., Cooper J. D., Phillipson O. T. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res Bull. 1990 Aug;25(2):271–284. doi: 10.1016/0361-9230(90)90072-8. [DOI] [PubMed] [Google Scholar]
- Delorme F., Froment J. L., Jouvet M. Suppression du sommeil par la p. chlorométhamphétamine et la p. chlorophénylalanine. C R Seances Soc Biol Fil. 1966;160(12):2347–2351. [PubMed] [Google Scholar]
- Greene R. W., Haas H. L., McCarley R. W. A low threshold calcium spike mediates firing pattern alterations in pontine reticular neurons. Science. 1986 Nov 7;234(4777):738–740. doi: 10.1126/science.3775364. [DOI] [PubMed] [Google Scholar]
- Griffith W. H. Membrane properties of cell types within guinea pig basal forebrain nuclei in vitro. J Neurophysiol. 1988 May;59(5):1590–1612. doi: 10.1152/jn.1988.59.5.1590. [DOI] [PubMed] [Google Scholar]
- Hallanger A. E., Levey A. I., Lee H. J., Rye D. B., Wainer B. H. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J Comp Neurol. 1987 Aug 1;262(1):105–124. doi: 10.1002/cne.902620109. [DOI] [PubMed] [Google Scholar]
- Horikawa K., Armstrong W. E. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J Neurosci Methods. 1988 Aug;25(1):1–11. doi: 10.1016/0165-0270(88)90114-8. [DOI] [PubMed] [Google Scholar]
- Hu B., Steriade M., Deschênes M. The cellular mechanism of thalamic ponto-geniculo-occipital waves. Neuroscience. 1989;31(1):25–35. doi: 10.1016/0306-4522(89)90028-6. [DOI] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol. 1984 Apr;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Kang Y., Kitai S. T. Electrophysiological properties of pedunculopontine neurons and their postsynaptic responses following stimulation of substantia nigra reticulata. Brain Res. 1990 Dec 3;535(1):79–95. doi: 10.1016/0006-8993(90)91826-3. [DOI] [PubMed] [Google Scholar]
- Kravitz E. A. Hormonal control of behavior: amines and the biasing of behavioral output in lobsters. Science. 1988 Sep 30;241(4874):1775–1781. doi: 10.1126/science.2902685. [DOI] [PubMed] [Google Scholar]
- Lent C. M., Dickinson M. H. The neurobiology of feeding in leeches. Sci Am. 1988 Jun;258(6):98–103. doi: 10.1038/scientificamerican0688-98. [DOI] [PubMed] [Google Scholar]
- McCarley R. W., Nelson J. P., Hobson J. A. Ponto-geniculo-occipital (PGO) burst neurons: correlative evidence for neuronal generators of PGO waves. Science. 1978 Jul 21;201(4352):269–272. doi: 10.1126/science.663656. [DOI] [PubMed] [Google Scholar]
- McCarley R. W. REM sleep and depression: common neurobiological control mechanisms. Am J Psychiatry. 1982 May;139(5):565–570. doi: 10.1176/ajp.139.5.565. [DOI] [PubMed] [Google Scholar]
- McGinty D. J., Harper R. M. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976 Jan 23;101(3):569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- Sakai K., Jouvet M. Brain stem PGO-on cells projecting directly to the cat dorsal lateral geniculate nucleus. Brain Res. 1980 Aug 4;194(2):500–505. doi: 10.1016/0006-8993(80)91231-7. [DOI] [PubMed] [Google Scholar]
- Sakai K., Petitjean F., Jouvet M. Effects of ponto-mesencephalic lesions and electrical stimulation upon PGO waves and EMPs in unanesthetized cats. Electroencephalogr Clin Neurophysiol. 1976 Jul;41(1):49–63. doi: 10.1016/0013-4694(76)90214-5. [DOI] [PubMed] [Google Scholar]
- Satoh K., Fibiger H. C. Cholinergic neurons of the laterodorsal tegmental nucleus: efferent and afferent connections. J Comp Neurol. 1986 Nov 15;253(3):277–302. doi: 10.1002/cne.902530302. [DOI] [PubMed] [Google Scholar]
- Simon R. P., Gershon M. D., Brooks D. C. The role of the raphe nuclei in the regulation of ponto-geniculo-occipital wave activity. Brain Res. 1973 Aug 30;58(2):313–330. doi: 10.1016/0006-8993(73)90004-8. [DOI] [PubMed] [Google Scholar]
- Steriade M., Datta S., Paré D., Oakson G., Curró Dossi R. C. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990 Aug;10(8):2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., Paré D., Datta S., Oakson G., Curró Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci. 1990 Aug;10(8):2560–2579. doi: 10.1523/JNEUROSCI.10-08-02560.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S. R., Reiner P. B. The immunohistochemical localization of choline acetyltransferase in the cat brain. Brain Res Bull. 1987 Mar;18(3):371–415. doi: 10.1016/0361-9230(87)90015-3. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Satoh K., Armstrong D. M., Fibiger H. C. NADPH-diaphorase: a selective histochemical marker for the cholinergic neurons of the pontine reticular formation. Neurosci Lett. 1983 Dec 23;43(1):31–36. doi: 10.1016/0304-3940(83)90124-6. [DOI] [PubMed] [Google Scholar]
- Webster H. H., Jones B. E. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. II. Effects upon sleep-waking states. Brain Res. 1988 Aug 23;458(2):285–302. doi: 10.1016/0006-8993(88)90471-4. [DOI] [PubMed] [Google Scholar]
- Wilcox K. S., Grant S. J., Burkhart B. A., Christoph G. R. In vitro electrophysiology of neurons in the lateral dorsal tegmental nucleus. Brain Res Bull. 1989 Mar;22(3):557–560. doi: 10.1016/0361-9230(89)90111-1. [DOI] [PubMed] [Google Scholar]