Abstract
Background:
While combined oral contraceptives (COCs) are commonly used to treat polycystic ovary syndrome (PCOS), comparative data regarding metabolic effects of different progestogens on this patient population are missing. This study aimed to compare the different effects of drospirenone (DRP)-containing COCs with cyproterone acetate (CPA)-containing COCs, combined with metformin and lifestyle modifications in women with PCOS and metabolic disorders.
Methods:
Ninety-nine women with PCOS and a metabolic disorder between January 2011 and January 2013 were enrolled into this prospective randomized clinical trial. Participants were randomized into two groups such as DRP-containing COCs, and CPA-containing COCs. Participants took COCs cyclically for 6 months, combined with metformin administration (1.5 g/d) and lifestyle modifications (diet and exercise). Clinical measures and biochemical and hormone profiles were compared. Comparisons for continuous variables were evaluated with paired and unpaired Student's t-tests. The Wilcoxon signed rank test was used when the data were not normally distributed. Analysis of covariance was used to control for age, body mass index (BMI), and baseline data of each analyzed parameter when compared between the two groups.
Results:
A total of 68 patients have completed the study. The combination regimen of COCs, metformin, and lifestyle modifications in these patients resulted in a significant decrease in BMI, acne, and hirsutism scores when compared to baseline levels in both groups (P < 0.05). Blood pressure (BP) was significantly different in the CPA group when compared to baseline (75.14 ± 6.77 mmHg vs. 80.70 ± 5.60 mmHg, P < 0.01), and after 6 months of treatment, only the change in systolic BP was significantly different between the two groups (4.00 [–6.00, 13.00] mmHg vs. –3.50 [–13.00, 9.00] mmHg, P = 0.009). Fasting glucose, fasting insulin, and homeostasis model assessment-insulin resistance decreased significantly in the DRP group (5.40 ± 0.41 mmol/L vs. 5.21 ± 0.32 mmol/L, P = 0.041; 13.90 [10.50, 18.40] μU/ml vs. 10.75 [8.60, 13.50] μU/ml, P = 0.020; 3.74 [2.85, 4.23] vs. 2.55 [1.92, 3.40], P = 0.008) but did not differ between the two groups. While individual lipid profiles increased in both groups, no statistically significant difference was observed.
Conclusions:
DRP-containing COCs combined with metformin and lifestyle modifications could better control BP and correct carbohydrate metabolism in women with PCOS and metabolic disorders compared with CPA-containing COCs.
Trial Registration:
Chinese Clinical Trial Registry, ChiCTR-TRC-11001143; http://www.chictr.org.cn/showproj.aspx?proj=8395.
Keywords: Cyproterone Acetate, Drospirenone, Metabolic Disorder, Oral Contraceptives, Combined, Polycystic Ovary Syndrome
INTRODUCTION
Polycystic ovary syndrome (PCOS) is an anovulatory disease caused by dysfunctional reproductive endocrinology.[1] Insulin resistance (IR) and compensatory hyperinsulinemia are considered as the important pathogenic factors in PCOS. When the compensatory hyperinsulinemia fails to meet the needs of the body, carbohydrate metabolism disorders may develop.[2] Hyperinsulinemia can ultimately result in hyperandrogenism. Obesity is also a characteristic feature in women suffering from PCOS, whereby approximately 40–50% of women with PCOS are overweight or obese.[3,4,5] Obesity, in addition to metabolic syndrome and dyslipidemia, is a risk factor of cardiovascular disease (CVD) in women with PCOS.[6]
Evidence-based guidelines recommend lifestyle modifications as the first line treatment for PCOS,[7] however, the engagement, compliance, and sustainability remain challenging. Metformin, a biguanide used to treat noninsulin-dependent diabetes and the most thoroughly investigated insulin-sensitizing agent, has been used to treat patients with PCOS and IR. Metformin reduces IR and inhibits ovarian androgen production in PCOS patients via effects on steroidogenic acute regulatory protein and 17α-hydroxylase.[8,9,10] It has been suggested that metformin might play a key role in PCOS when combined with lifestyle changes, to assist in weight management and cycle regulation.[11]
Combined oral contraceptives (COCs) have been used for many years in the treatment of PCOS.[12] It is known that COCs may have negative effects on carbohydrate metabolism and the lipid profiles;[13,14] however, it is not well understood. Cyproterone acetate (CPA)-containing COCs are commonly recommended for anti-hyperandrogenism in PCOS, as CPA has high antiandrogenic activity. However, some controversy remains regarding whether CPA has a transiently negative effect on carbohydrate metabolism and lipid profiles.[15,16,17] A number of studies have also investigated the combination of metformin and COCs in women with PCOS and suggested that it may improve the insulin sensitivity.[18,19,20] The addition of metformin to COCs may, therefore, have metabolic benefits in the treatment of women with PCOS.
Drospirenone (DRP), another steroidal progestin, has antiandrogenic and antimineralocorticoid activities which other progestins lack.[21] Combined with 30-μg ethinyl estradiol (EE), it has previously been used as DRP/EE COCs in the treatment of PCOS.[22,23,24,25] In recent years, studies have showed that the DRP-containing COCs had no negative metabolic effects on women with PCOS, and some studies reported favorable metabolic effects.[21,23,24,25,26] However, few studies have investigated the combination of DRP-containing COCs and metformin,[27,28] and the effects of DRP-containing COCs on carbohydrate and lipid metabolism in women with PCOS and dysfunctional metabolism have not, to date, been investigated.
In the current study, we designed a randomized clinical trial to compare the metabolic effect of DRP-containing COCs with the more widely used CPA-containing COCs, combined with metformin and lifestyle modifications, in women with PCOS and dysfunctional metabolism. We also aimed to determine whether DRP-containing COCs have more beneficial effects on carbohydrate metabolism and lipid profiles.
METHODS
Study population
This randomized controlled trial (Chinese Clinical Trial Registry, ChiCTR-TRC-11001143) was conducted between January 2011 and January 2013, at the West China Second University Hospital of Sichuan University, Chengdu, Sichuan, China. Randomization was performed using a random-number table. The study was not blinded as group allocation was not concealed. The study protocol was approved by the Medical Ethics Committee of the West China Second University Hospital of Sichuan University. Informed written consent was obtained from all participants and/or their parents. A total of 248 women with amenorrhea or oligomenorrhea were screened for PCOS according to the Rotterdam diagnostic criteria,[29] and 27 patients were excluded as not meeting the PCOS diagnostic criteria. The remaining 221 patients were further examined, and women with a body mass index (BMI) ≥25 kg/m2 and/or homeostasis model assessment-insulin resistance (HOMA-IR) of ≥2.77, were included in the study. Patients with contraindications to taking COCs, women aged >40 years, smokers, and women with a history of alcohol abuse were excluded from the study. A total of 99 women were finally included in the study [Figure 1].
Figure 1.
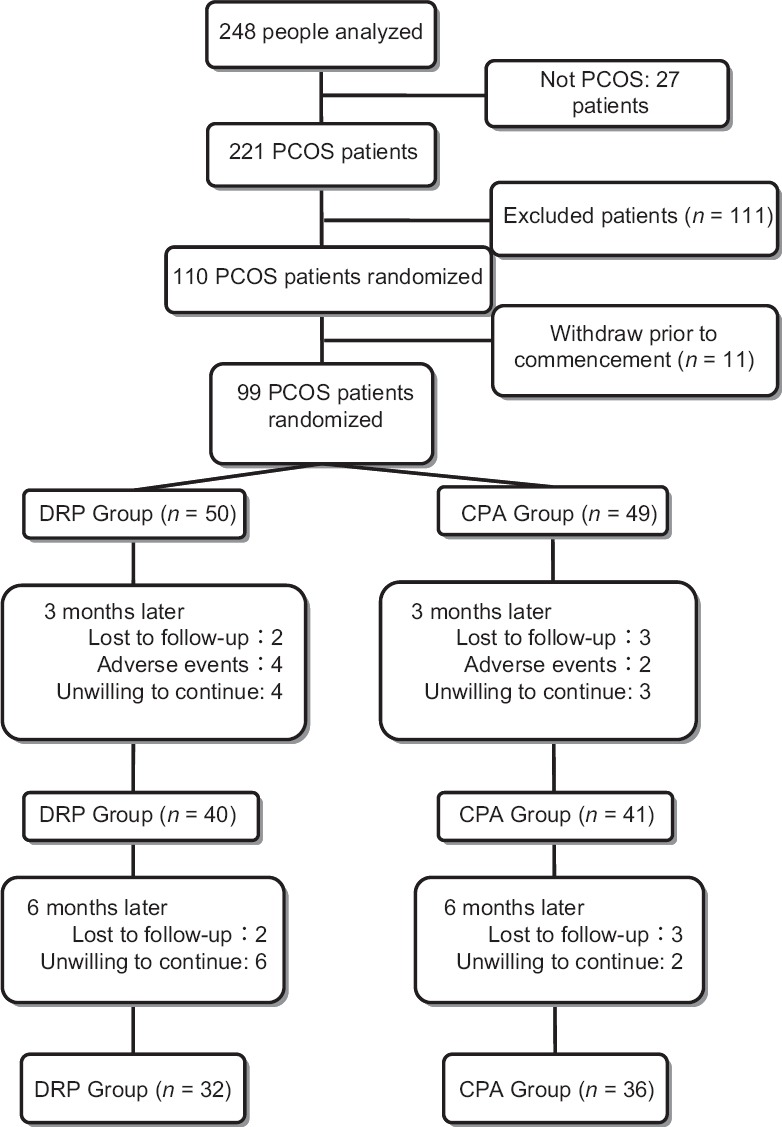
The flowchart of this study. PCOS: Polycystic ovary syndrome; DRP: Drospirenone; CPA: Cyproterone acetate.
Drug administration and implementation of lifestyle modifications
Participants were randomized either to the DRP group (receiving 3 mg DRP plus 30 μg EE/tablet) or the CPA group (receiving 2 mg CPA plus 35 μg EE/tablet). Treatment regimens for both groups were similar: COC administration was commenced on the 1st day of the menstrual cycle or withdrawal of bleeding was continued for 21 consecutive days followed by a 7-day interval and repeated for six cycles. All participants simultaneously took metformin (0.5 g/d), and lifestyle modifications (modification of diet and increased physical activities for control of body weight) were implemented during COC treatment. A low-glycaemic index carbohydrate foods regimen and regular aerobic exercise were recommended to all participants. Meantime, all participants were advised to have a regular aerobic exercise (such as walking and jogging) up to 40 min per session, at least 3 times/week. The starting dose of metformin was 0.5 g/d, and patients gradually adjusted the dose to the full dosage of 1.5 g/d. Any medications known to affect carbohydrate metabolism or sex hormones, including COCs, progestins, and estrogen-progestin combinations, were discontinued for at least 3 months before enrollment in the study. Subjects were not permitted to use any lipid- or blood pressure (BP)-lowering drugs.
Hematological parameters
Blood samples were collected from all subjects during the early follicular phase of their cycles (3–5 days after the onset of spontaneous or progestin-induced menstrual bleeding). Height, weight, and waist and hip circumferences were measured in the morning of fasting-blood collection; BMI and the waist-to-hip ratio (WHR) were calculated. BP was measured in each woman after resting for 30 min. The amount of excess terminal hair growth was assessed using a modified Ferriman-Gallwey (F-G) method.[30] The global acne grading system (GAGS) was used for the severity of acne.[31] All the above measurements and scores were made jointly by two observers throughout the trial.
The measurements of serum estradiol (E2), progesterone (P), testosterone (T), luteinizing hormone, follicle-stimulating hormone, prolactin, and insulin were made by chemiluminescent immunoassay analysis (Advia Centaur, Siemens, Erlangen, Germany). The intra- and inter-assay variability was <6.25%. Plasma glucose was measured by the hexokinase method (ADVI 2400, Siemens, Erlangen, Germany). The intra- and inter-assay variability was <2.5%. An oral glucose tolerance test (OGTT) was also performed. Blood samples were collected before administration of a 75-g oral glucose load and after 30, 60, 120, and 180 min. From the OGTT, area under the curve (AUC) data for insulin and glucose were obtained. The AUCs were calculated using the trapezoidal rule and expressed as μU/ml × 3 h. HOMA-IR was calculated using the following formula: (blood glucose [mmol/L] × insulin [μU/ml])/22.5. The total cholesterol (TC), low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride (TG) concentrations were measured by an enzymatic method (ADVI 2400, Siemens, Germany), for which the intra- and inter-assay variability was <7.5%. The predicted risks for CVD (TC/HDL and LDL/HDL ratios) were calculated from these analyses.
On the third cycle, the participants reported their compliance to drug administration and lifestyle modifications through the telephone or coming back to the hospital. After the sixth cycle, the participants came back to the hospital and all the clinical characteristics and biochemical profiles were tested again. The blood samples for biological parameters were obtained between day 3 and day 5 of the sixth COC withdrawal bleeding.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) or as the median (P25, P75) if the variable was not normally distributed, using the Shapiro–Wilk test. Comparisons for continuous variables were evaluated with the Student's t-test, or the Wilcoxon signed rank test when the data were not normally distributed. Analysis of covariance was used to control for age, BMI, and baseline data of each analyzed parameter when compared between the two groups. P values and confidence intervals were estimated in a two-tailed fashion. A value of P < 0.05 was considered statistically significant. All data were analyzed using Statistical Analysis System version 8.0 software (SAS Institute, Cary, NC, USA).
RESULTS
A total of 99 patients aged 16–33 years were included in the study. Of these women, only 68 participants (DRP: n = 32; CPA: n = 36) completed the 6-month treatment, others withdrew from the study due to side effects, moving away or unwillingness to adhere to therapy guidelines.
All participants had regular withdrawal bleeding during COC treatment. Some participants (four women from the DRP group and five women from the CPA group) experienced spotting during the 1st month of COC use, which subsequently stopped during the second cycle. On the 3rd month's visit, there were 63 patients taking the full dosage (1.5 g/d) of metformin and the left 5 took 1.0 g/d of metformin because of gastrointestinal events. Since the 4th month of treatment, all participants had taken the full dosage of metformin and lasted to the end of the study.
Effects of treatments on clinical and metabolic characteristics before and after treatment
There was no statistically significant difference between baseline clinical, endocrine, and metabolic parameters of the enrolled participants between the DRP and CPA groups [Tables 1 and 2]. The clinical and metabolic parameters before and after treatment in both groups are shown in Table 3. The combination regimen of COC, metformin, and lifestyle modifications in these patients resulted in a significant decrease of BMI when compared to baseline levels in both the DRP and CPA groups (21.76 [20.54, 25.21] kg/m2 vs. 21.42 [19.65, 22.51] kg/m2, P < 0.001;24.01 [21.45, 25.62] kg/m2 vs. 21.62 [20.72, 24.65] kg/m2, P < 0.001, respectively), although the difference in waist circumference and WHR did not reach statistical significance. The GAGS and F-G scores were significantly decreased after treatment in both DRP and CPA groups (2 [0, 4] vs. 0 [0, 0], P < 0.001 and 2.0 [0.5, 5.0] vs. 1.0 [0, 4.0], P = 0.013;3 [0, 8] vs. 0 [0, 0], P < 0.001 and 3.0 [1.0, 4.0] vs. 2.0 [0, 3.0], P = 0.001, respectively). A statistically insignificant trend was observed in falling systolic BP with treatment in the DRP group whereas an upward diastolic BP trend (75.14 ± 6.77 mmHg vs. 80.70 ± 5.60 mmHg, P < 0.001) was observed in the CPA group.
Table 1.
Basal clinical characteristics of patients with polycystic ovary syndrome and metabolic disorders enrolled in the two groups
| Items | DRP group (n = 50) | CPA group (n = 49) | Statistics | P |
|---|---|---|---|---|
| Age (years) | 23.5 ± 4.9 | 24.3 ± 4.0 | 0.863* | 0.391 |
| BMI (kg/m2) | 23.00 (20.77, 26.76) | 24.07 (21.54, 26.71) | −0.846† | 0.398 |
| Hirsutism (F-G) | 2.0 (0, 5.0) | 3.0 (1.0, 4.0) | −0.586† | 0.558 |
| Acne (GAGS) | 2 (0, 5) | 2 (0, 8) | −1.085† | 0.278 |
| WC (cm) | 82.0 (78.0, 92.0) | 87.5 (79.0, 94.0) | −1.440† | 0.150 |
| WHR | 0.89 ± 0.05 | 0.91 ± 0.06 | 1.298* | 0.197 |
| SBP (mmHg) | 117.96 ± 13.29 | 118.06 ± 11.45 | 0.041* | 0.967 |
| DBP (mmHg) | 75.54 ± 8.06 | 76.12 ± 7.73 | 0.362* | 0.718 |
Values were showed as mean ± SD or as the median (P25, P75). *: t values; †: Z values; BMI: Body mass index; WC: Waist circumference; WHR: Waist-to-hip ratio; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; DRP: Drospirenone; CPA: Cyproterone acetate; F-G: Ferriman–Gallwey; GAGS: Global acne grading system; SD: Standard deviation.
Table 2.
Basal hormonal and metabolic levels of patients with polycystic ovary syndrome and metabolic disorders enrolled in the two groups
| Items | DRP group (n = 50) | CPA group (n = 49) | Statistics | P |
|---|---|---|---|---|
| E2 (pg/ml) | 58.68 ± 14.83 | 58.97 ± 17.39 | 1.091* | 0.087 |
| P (ng/ml) | 0.47 (0.34, 0.66) | 0.48 (0.28, 0.72) | −0.078† | 0.938 |
| T (ng/ml) | 0.63 ± 0.18 | 0.63 ± 0.22 | 0.037* | 0.971 |
| LH (mU/ml) | 11.01 ± 6.01 | 10.10 ± 7.06 | −0.641* | 0.523 |
| FSH (mU/ml) | 5.59 ± 1.67 | 5.36 ± 2.26 | −0.575* | 0.567 |
| PRL (ng/ml) | 13.97 ± 8.06 | 15.51 ± 9.06 | 0.883* | 0.379 |
| LH/FSH | 1.76 (1.08, 2.59) | 1.85 (1.20, 2.22) | −0.072† | 0.943 |
| FPG (mmol/L) | 5.44 ± 0.45 | 5.63 ± 0.77 | 1.481* | 0.142 |
| FINS (µU/ml) | 14.90 (12.40, 18.00) | 14.95 (10.38, 20.93) | −0.495† | 0.621 |
| HOMA-IR | 3.73 (2.89, 4.34) | 3.83 (2.90, 5.45) | −0.794† | 0.427 |
| AUCglucose (mmol∙L−1∙min−1) | 421.40 (360.80, 492.60) | 480.60 (372.60, 539.20) | −1.446† | 0.148 |
| AUCinsulin (µU∙ml−1∙ min−1) | 6361.60 (4114.60, 7752.60) | 6694.60 (4100.60, 10931.20) | −1.019† | 0.308 |
| HbA1c (%) | 5.41 ± 0.30 | 5.51 ± 0.44 | 1.261* | 0.210 |
| TC (mmol/L) | 4.23 ± 0.81 | 4.41 ± 0.77 | 1.113* | 0.269 |
| TG (mmol/L) | 1.05 (0.82, 1.38) | 1.42 (0.77, 1.79) | −1.526† | 0.127 |
| HDL-C (mmol/L) | 1.23 (1.08, 1.44) | 1.22 (1.08, 1.40) | −0.152† | 0.880 |
| LDL-C (mmol/L) | 2.51 ± 0.75 | 2.57 ± 0.63 | 0.432* | 0.668 |
| LDL/HDL | 2.05 ± 0.73 | 2.14 ± 0.75 | 0.607* | 0.545 |
| TC/HDL | 3.42 ± 0.83 | 3.62 ± 1.02 | 1.101* | 0.274 |
Values were showed as mean ± SD or as the median (P25, P75). *: t values; †: Z values; E2: Estradiol; P: Progesterone; T: Testosterone; LH: Luteinizing hormone; FSH: Follicle-stimulating hormone; PRL: Prolactin; FPG: Fasting plasma glucose; FINS: Fasting insulin; HOMA-IR: Homeostasis model assessment-insulin resistance; AUCglucose: Area under the curve of glucose; AUCinsulin: Area under the curve of insulin; HbA1c: Glycated hemoglobin; TC: Total cholesterol; TG: Triglycerides; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; DRP: Drospirenone; CPA: Cyproterone acetate; SD: Standard deviation.
Table 3.
Clinical and metabolic characteristics before and after treatment in DRP (n = 32) and CPA groups (n = 36)
| Items | Group | Before treatment | After treatment | Statistics | P |
|---|---|---|---|---|---|
| BMI (kg/m2) | DRP | 21.76 (20.54, 25.21) | 21.42 (19.65, 22.51) | −4.124* | <0.001 |
| CPA | 24.01 (21.45, 25.62) | 21.62 (20.72, 24.65) | −3.857* | <0.001 | |
| Hirsutism (F-G) | DRP | 2.0 (0.5, 5.0) | 1.0 (0, 4.0) | −2.489* | 0.013 |
| CPA | 3.0 (1.0, 4.0) | 2.0 (0, 3.0) | −3.217* | 0.001 | |
| Acne (GAGS) | DRP | 2 (0, 4) | 0 (0, 0) | −3.753* | <0.001 |
| CPA | 3 (0, 8) | 0 (0, 0) | −4.384* | <0.001 | |
| WHR | DRP | 0.89 ± 0.05 | 0.92 ± 0.12 | −1.455† | 0.156 |
| CPA | 0.91 ± 0.07 | 0.91 ± 0.05 | 0.253† | 0.802 | |
| SBP (mmHg) | DRP | 116.10 ± 13.38 | 112.80 ± 10.62 | 1.137† | 0.265 |
| CPA | 118.39 ± 11.06 | 120.30 ± 8.53 | −0.830† | 0.412 | |
| DBP (mmHg) | DRP | 75.09 ± 7.87 | 77.90 ± 9.50 | −1.634† | 0.113 |
| CPA | 75.14 ± 6.77 | 80.70 ± 5.60 | −4.842† | <0.001 | |
| FPG (mmol/L) | DRP | 5.40 ± 0.41 | 5.21 ± 0.32 | 2.141† | 0.041 |
| CPA | 5.52 ± 0.73 | 5.37 ± 0.41 | 1.386† | 0.175 | |
| FINS (µU/ml) | DRP | 13.90 (10.50, 18.40) | 10.75 (8.60, 13.50) | −2.335* | 0.020 |
| CPA | 13.70 (10.30, 22.80) | 17.85 (10.30, 24.40) | −1.462* | 0.144 | |
| HOMA-IR | DRP | 3.74 (2.85, 4.23) | 2.55 (1.92, 3.40) | −2.664* | 0.008 |
| CPA | 3.85 (2.87, 5.10) | 3.90 (2.54, 5.89) | −1.736* | 0.083 | |
| AUCglucose (mmol∙L−1∙ min−1) | DRP | 419.80 (385.80, 486.00) | 467.00 (425.40, 513.40) | −2.822* | 0.005 |
| CPA | 460.60 (394.60, 526.20) | 450.80 (425.00, 524.00) | −0.917* | 0.359 | |
| AUCinsulin (µU∙ml−1∙ min−1) | DRP | 6393.80 (4247.80, 7833.60) | 5094.60 (4292.20, 7240.60) | −1.960* | 0.051 |
| CPA | 6894.60 (4304.60, 10,721.00) | 5264.00 (3060.60, 9504.00) | −2.457* | 0.014 | |
| HbA1c (%) | DRP | 5.37 ± 0.28 | 5.41 ± 0.28 | −0.606† | 0.549 |
| CPA | 5.51 ± 0.52 | 5.51 ± 0.40 | 0.094† | 0.926 | |
| TC (mmol/L) | DRP | 4.18 ± 0.82 | 4.84 ± 0.89 | −5.995† | <0.001 |
| CPA | 4.40 ± 0.80 | 5.20 ± 1.37 | −3.737† | <0.001 | |
| TG (mmol/L) | DRP | 0.95 (0.78, 1.37) | 1.30 (0.87, 1.68) | −2.839* | 0.005 |
| CPA | 1.30 (0.64, 1.73) | 1.32 (0.88, 2.12) | −2.121* | 0.034 | |
| HDL-C (mmol/L) | DRP | 1.24 (1.12, 1.48) | 1.67 (1.45, 1.98) | −4.639* | <0.001 |
| CPA | 1.22 (1.06, 1.48) | 1.59 (1.36, 1.89) | −4.865* | <0.001 | |
| LDL-C (mmol/L) | DRP | 2.43 ± 0.79 | 2.66 ± 0.74 | −2.363† | 0.025 |
| CPA | 2.57 ± 0.66 | 2.72 ± 0.83 | −1.407† | 0.168 | |
| LDL-C/HDL-C | DRP | 1.90 ± 0.72 | 1.64 ± 0.50 | 2.877† | 0.007 |
| CPA | 2.13 ± 0.87 | 1.75 ± 0.70 | 4.026† | <0.001 | |
| TC/HDL-C | DRP | 3.24 ± 0.76 | 2.95 ± 0.54 | 2.711† | 0.011 |
| CPA | 3.61 ± 1.14 | 3.41 ± 1.69 | 0.603† | 0.551 |
Values were showed as mean ± SD or as the median (P25, P75). *: Z values; †: t values. DRP: Drospirenone; CPA: Cyproterone acetate; BMI: Body mass index; WHR: Waist-to-hip ratio; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FPG: Fasting plasma glucose; FINS: Fasting insulin; HOMA-IR: Homeostasis model assessment-insulin resistance; AUCglucose: Area under the curve of glucose; AUCinsulin: Area under the curve of insulin; HbA1c: Glycated hemoglobin; TC: Total cholesterol; TG: Triglycerides; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; F-G: Ferriman–Gallwey; GAGS: Global acne grading system; SD: Standard deviation.
Fasting glucose, AUC of glucose, and fasting insulin levels changed in both groups, but only reached statistical significance in the DRP group (5.40 ± 0.41 mmol/L vs. 5.21 ± 0.32 mmol/L, P = 0.041; 419.80 [385.80, 486.00] mmol·L−1·min−1 vs. 467.00 [425.40, 513.40] mmol·L−1·min−1, P = 0.005; 13.90 [10.50, 18.40] µU/ml vs. 10.75 [8.60, 13.50] µU/ml, P = 0.020, respectively). AUC of insulin significantly decreased after treatment in the CPA group (6894.60 [4304.60, 10,721.00] µU·ml−1·min−1 vs. 5264.00 [3060.60, 9504.00] µU·ml−1·min−1, P = 0.014) but did not reach statistical significance in the DRP group. HOMA-IR significantly decreased in the DRP group (3.74 [2.85, 4.23] vs. 2.55 [1.92, 3.40], P = 0.008), but not in the CPA group.
In the DRP group, lipid profiles, TC, TG, HDL, and LDL were significantly increased after treatment (4.18 ± 0.82 mmol/L vs. 4.84 ± 0.89 mmol/L, P < 0.001; 0.95 [0.78, 1.37] mmol/L vs. 1.30 [0.87, 1.68] mmol/L, P = 0.005; 1.24 [1.12, 1.48] mmol/L vs. 1.67 [1.45, 1.98] mmol/L, P < 0.001; 2.43 ± 0.79 mmol/L vs. 2.66 ± 0.74 mmol/L, P = 0.025, respectively) while LDL/HDL and TC/HDL ratios decreased significantly (1.90 ± 0.72 vs. 1.64 ± 0.50, P = 0.007; 3.24 ± 0.76 vs. 2.95 ± 0.54, P = 0.011, respectively). In the CPA group, there was an increase in TC, TG, and HDL (4.40 ± 0.80 mmol/L vs. 5.20 ± 1.37 mmol/L, P < 0.001; 1.30 [0.64, 1.73] mmol/L vs. 1.32 [0.88, 2.12] mmol/L, P < 0.034; 1.22 [1.06, 1.48] mmol/L vs. 1.59 [1.36, 1.89] mmol/L, P < 0.001, respectively) and a decrease of LDL/HDL ratio (2.13 ± 0.87 mmol/L vs. 1.75 ± 0.70 mmol/L, P < 0.001).
Effects of treatments on clinical and metabolic profiles between the two groups
The relative changes in all studied parameters after 6 months of treatment, compared with the baseline levels in both study groups, are shown in Table 4. A statistically significant difference was observed in the systolic BP (4.00 [–6.00, 13.00] mmHg vs. –3.50 [–13.00, 9.00] mmHg, P = 0.009) after treatment between the two groups, but in none of the remaining clinical and metabolic parameters in either study group.
Table 4.
Clinical and metabolic changes from baseline after 6 months of treatment in DRP and CPA groups
| Items | DRP group (n = 32) | CPA group (n = 36) | F | P |
|---|---|---|---|---|
| BMI (kg/m2) | 1.06 (0.42, 2.55) | 1.56 (0.12, 2.59) | 0.179 | 0.674 |
| Hirsutism (F-G) | 0 (0, 1) | 0 (0, 1) | 1.496 | 0.226 |
| Acne (GAGS) | 2 (0, 4) | 3 (0, 8) | 1.268 | 0.265 |
| WHR | −0.01 (−0.03, 0.03) | 0 (−0.02, 0.03) | 1.081 | 0.303 |
| SBP (mmHg) | 4.00 (−6.00, 13.00) | −3.50 (−13.00, 9.00) | 7.348 | 0.009 |
| DBP (mmHg) | −4.00 (−9.00, 1.00) | −4.50 (−9.75, −0.25) | 1.706 | 0.196 |
| FPG (mmol/L) | 0.07 (−0.07, 0.31) | 0.01 (−0.11, 0.33) | 0.933 | 0.338 |
| FINS (μU/ml) | 2.20 (−0.20, 5.90) | 2.00 (−1.20, 7.60) | 0.001 | 0.977 |
| HOMA-IR | 0.56 (0.04, 1.56) | 0.56 (−0.43, 1.64) | 0.000 | 0.983 |
| AUCglucose (mmol∙L−1∙ min−1) | −49.66 (−85.90, 3.26) | −11.44 (−84.54, 48.34) | 0.016 | 0.899 |
| AUCinsulin (μU∙ml−1∙ min−1) | 938.00 (−858.00, 191.00) | 1063.50 (−639.50, 2255.00) | 0.006 | 0.938 |
| HbA1c (%) | 0 (−0.23, 0.10) | 0 (−0.20, 0.20) | 0.398 | 0.531 |
| TC (mmol/L) | −0.57 (−1.14, −0.31) | −0.62 (−0.91, −0.32) | 1.077 | 0.304 |
| TG (mmol/L) | −0.34 (−0.62, 0.00) | −0.18 (−0.58, 0.22) | 0.054 | 0.817 |
| HDL-C (mmol/L) | −0.33 (−0.55, −0.09) | −0.34 (−0.55, −0.1) | 0.999 | 0.322 |
| LDL-C (mmol/L) | −0.15 (−0.51, 0.11) | −0.24 (−0.44, 0.29) | 0.017 | 0.897 |
| LDL-C/HDL-C | 0.27 (−0.06, 0.54) | 0.34 (0.09, 0.76) | 0.084 | 0.773 |
| TC/HDL-C | 0.23 (−0.13, 0.65) | 0.18 (−0.09, 1.03) | 1.169 | 0.283 |
Values were showed as mean ± SD, or as the median (P25, P75). DRP: Drospirenone; CPA: Cyproterone acetate; BMI: Body mass index; F-G: Ferriman-Gallwey; GAGS: Global acne grading system; WHR: Waist-to-hip ratio; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FPG: Fasting plasma glucose; FINS: Fasting insulin; HOMA-IR: Homeostasis model assessment-insulin resistance; AUCglucose: Area under the curve of glucose; AUCinsulin: Area under the curve of insulin; HbA1c: Glycated hemoglobin; TC: Total cholesterol; TG: Triglycerides; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; SD: Standard deviation.
Side effects
The main adverse events resulting from metformin included gastrointestinal reactions such as nausea, vomiting, diarrhea, or poor appetite, and 6/99 (6.1%) of patients canceled their treatment due to these adverse events during the first 3 months of treatment. As such, a step by step incremental regimen is recommended for the introduction of metformin therapy in future studies.
DISCUSSION
Here, we investigated the combined effects of metformin and lifestyle modifications in addition to DRP- or CPA-containing COCs on clinical and metabolic parameters in women with PCOS and metabolic disorders. As predicted, there was significant decrease in BMI in both study groups following treatment, and studies have associated reduced BMI with improved carbohydrate metabolism and decreased related CVD risk, in obese women with PCOS. In the current study, both types of COCs were able to relieve hyperandrogenic symptoms such as hirsutism and acne, in line with previous reports.[32,33,34,35,36,37] Furthermore, the decline in F-G score observed in the present study, which has not been previously reported,[38,39] may be due to the possibility that metformin and lifestyle modifications combined with COCs can further promote the antiandrogenic effects of COCs. As previously demonstrated, BP (the most important predictor of CVD) showed a declining trend in DRP group, as DRP has antimineralocorticoid activities.[21,26]
To date, a limited number of short-term studies have assessed the effects of different COCs on carbohydrate metabolism in women with PCOS, and there is still debate on the metabolic effects of COCs. Some studies have suggested that COCs may aggravate IR.[34,35] However, a study by Cagnacci et al.[16] reported ameliorated insulin sensitivity with CPA-containing COC treatment. Moreover, while some studies have reported no correlation between COCs and carbohydrate metabolism,[36,37] others have demonstrated favorable effects on carbohydrate metabolism when combined COCs with metformin.[18,19,20] In the current study, fasting glucose, insulin, and HOMA-IR were significantly improved in the DRP/EE regimen and had no negative effects on the carbohydrate metabolism in women with PCOS and metabolic disorders. Furthermore, the lipid profiles increased significantly in both groups in the present study, increased levels of TC, TG, and LDL cholesterol may increase the risk of CVD. This finding of our study is similar to several previous reports.[6,32,33] The deterioration in lipid profiles typically relates to the dose of EE and the androgenicity of the progestin used. However, in women with normal baseline lipid levels, the profiles remained within the normal range in the present study although the cardiovascular impact of this remains unknown.[40] In addition, the level of HDL cholesterol in our study was also significantly increased and may, to some extent, counterbalance the negative effects of the other lipid profiles. Furthermore, decreased levels of atherosclerosis markers and TC/HDL and LDL/HDL ratios were also observed in the current study, which can further reduce CVD risk in women with PCOS and metabolic disorders.
In the current study, while there were more superficial benefits of DRP-containing COCs compared with CPA-containing COCs, when each regimen was combined with metformin and lifestyle modifications, the effects on metabolic parameters were almost identical, with only the change in systolic BP identified as statistically significant between the two regimens. Further studies involving larger sample sizes and longer follow-up periods are necessary to fully elucidate the effects of these treatment regiments on carbohydrate and lipid profiles in women with PCOS and metabolic disorders.
In conclusion, the current study demonstrated that a combination regimen with DRP-containing COCs was beneficial for improving glucose metabolic profiles which, in turn, resulted in reduced BMI and may, therefore lessen CVD risk in women with PCOS and metabolic disorders.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Azziz R, Dumesic DA, Goodarzi MO. Polycystic ovary syndrome: An ancient disorder? Fertil Steril. 2011;95:1544–8. doi: 10.1016/j.fertnstert.2010.09.032. doi:10.1016/j.fertnstert.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2010;16:347–63. doi: 10.1093/humupd/dmq001. doi:10.1093/humupd/dmq001. [DOI] [PubMed] [Google Scholar]
- 3.Chun-Sen H, Chien-Hua W, Wan-Chun C, Ching-Tzu L, Chun-Jen C, Ming IH. Obesity and insulin resistance in women with polycystic ovary syndrome. Gynecol Endocrinol. 2011;27:300–6. doi: 10.3109/09513590.2010.488776. doi:10.3109/09513590.2010.488776. [DOI] [PubMed] [Google Scholar]
- 4.Liou TH, Yang JH, Hsieh CH, Lee CY, Hsu CS, Hsu MI. Clinical and biochemical presentations of polycystic ovary syndrome among obese and nonobese women. Fertil Steril. 2009;92:1960–5. doi: 10.1016/j.fertnstert.2008.09.003. doi:10.1016/j.fertnstert.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Pasquali R. Obesity and androgens: Facts and perspectives. Fertil Steril. 2006;85:1319–40. doi: 10.1016/j.fertnstert.2005.10.054. doi:10.1016/j.fertnstert.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 6.El-Mazny A, Abou-Salem N, El-Sherbiny W, El-Mazny A. Insulin resistance, dyslipidemia, and metabolic syndrome in women with polycystic ovary syndrome. Int J Gynaecol Obstet. 2010;109:239–41. doi: 10.1016/j.ijgo.2010.01.014. doi:10.1016/j.ijgo.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Teede HJ, Misso ML, Deeks AA, Moran LJ, Stuckey BG, Wong JL, et al. Assessment and management of polycystic ovary syndrome: Summary of an evidence-based guideline. Med J Aust. 2011;195:S65–112. doi: 10.5694/mja11.10915. doi:10.5694/mja11.10915. [DOI] [PubMed] [Google Scholar]
- 8.Diamanti-Kandarakis E, Christakou CD, Kandaraki E, Economou FN. Metformin: An old medication of new fashion: Evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eur J Endocrinol. 2010;162:193–212. doi: 10.1530/EJE-09-0733. doi:10.1530/EJE-09-0733. [DOI] [PubMed] [Google Scholar]
- 9.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–92. doi: 10.1210/jc.2013-2350. doi:10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao L, Tian YJ, Zhao JJ, Xin Y, Xing HY, Dong JJ. Metformin versus metformin plus rosiglitazone in women with polycystic ovary syndrome. Chin Med J. 2011;124:714–8. doi:10.3760/cma.j.issn.0366-6999.2011.05.015. [PubMed] [Google Scholar]
- 11.Naderpoor N, Shorakae S, de Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in polycystic ovary syndrome: Systematic review and meta-analysis. Hum Reprod Update. 2015;21:560–74. doi: 10.1093/humupd/dmv025. doi:10.1093/humupd/dmv025. [DOI] [PubMed] [Google Scholar]
- 12.Vrbíková J, Cibula D. Combined oral contraceptives in the treatment of polycystic ovary syndrome. Hum Reprod Update. 2005;11:277–91. doi: 10.1093/humupd/dmi005. doi:10.1093/humupd/dmi005. [DOI] [PubMed] [Google Scholar]
- 13.Morin-Papunen LC, Vauhkonen I, Koivunen RM, Ruokonen A, Martikainen HK, Tapanainen JS. Endocrine and metabolic effects of metformin versus ethinyl estradiol-cyproterone acetate in obese women with polycystic ovary syndrome: A randomized study. J Clin Endocrinol Metab. 2000;85:3161–8. doi: 10.1210/jcem.85.9.6792. doi:10.1210/jc.85.9.3161. [DOI] [PubMed] [Google Scholar]
- 14.Nader S, Diamanti-Kandarakis E. Polycystic ovary syndrome, oral contraceptives and metabolic issues: New perspectives and a unifying hypothesis. Hum Reprod. 2007;22:317–22. doi: 10.1093/humrep/del407. doi:10.1093/humrep/del407. [DOI] [PubMed] [Google Scholar]
- 15.Morin-Papunen L, Vauhkonen I, Koivunen R, Ruokonen A, Martikainen H, Tapanainen JS. Metformin versus ethinyl estradiol-cyproterone acetate in the treatment of nonobese women with polycystic ovary syndrome: A randomized study. J Clin Endocrinol Metab. 2003;88:148–56. doi: 10.1210/jc.2002-020997. doi:10.1097/01.OGX.0000070134.96377.E6. [DOI] [PubMed] [Google Scholar]
- 16.Cagnacci A, Paoletti AM, Renzi A, Orrù M, Pilloni M, Melis GB, et al. Glucose metabolism and insulin resistance in women with polycystic ovary syndrome during therapy with oral contraceptives containing cyproterone acetate or desogestrel. J Clin Endocrinol Metab. 2003;88:3621–5. doi: 10.1210/jc.2003-030328. doi:10.1210/jc.2003-030328. [DOI] [PubMed] [Google Scholar]
- 17.Luque-Ramírez M, Alvarez-Blasco F, Botella-Carretero JI, Martínez-Bermejo E, Lasunción MA, Escobar-Morreale HF. Comparison of ethinyl-estradiol plus cyproterone acetate versus metformin effects on classic metabolic cardiovascular risk factors in women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:2453–61. doi: 10.1210/jc.2007-0282. doi:http://dx.doi.org/10.1210/jc.2007-0282. [DOI] [PubMed] [Google Scholar]
- 18.Wu J, Zhu Y, Jiang Y, Cao Y. Effects of metformin and ethinyl estradiol-cyproterone acetate on clinical, endocrine and metabolic factors in women with polycystic ovary syndrome. Gynecol Endocrinol. 2008;24:392–8. doi: 10.1080/09513590802217027. doi:10.1080/09513590802217027. [DOI] [PubMed] [Google Scholar]
- 19.Elter K, Imir G, Durmusoglu F. Clinical, endocrine and metabolic effects of metformin added to ethinyl estradiol-cyproterone acetate in non-obese women with polycystic ovarian syndrome: A randomized controlled study. Hum Reprod. 2002;17:1729–37. doi: 10.1093/humrep/17.7.1729. doi:10.1093/humrep/17.7.1729. [DOI] [PubMed] [Google Scholar]
- 20.Glintborg D, Altinok ML, Mumm H, Hermann AP, Ravn P, Andersen M. Body composition is improved during 12 months’ treatment with metformin alone or combined with oral contraceptives compared with treatment with oral contraceptives in polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99:2584–91. doi: 10.1210/jc.2014-1135. doi:10.1210/jc.2014-1135. [DOI] [PubMed] [Google Scholar]
- 21.Oelkers W. Drospirenone, a progestogen with antimineralocorticoid properties: A short review. Mol Cell Endocrinol. 2004;217:255–61. doi: 10.1016/j.mce.2003.10.030. doi:10.1016/j.mce.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Pehlivanov B, Mitkov M. Efficacy of an oral contraceptive containing drospirenone in the treatment of women with polycystic ovary syndrome. Eur J Contracept Reprod Health Care. 2007;12:30–5. doi: 10.1080/13625180600983082. doi:10.1080/13625180600983082. [DOI] [PubMed] [Google Scholar]
- 23.Guido M, Romualdi D, Giuliani M, Suriano R, Selvaggi L, Apa R, et al. Drospirenone for the treatment of hirsute women with polycystic ovary syndrome: A clinical, endocrinological, metabolic pilot study. J Clin Endocrinol Metab. 2004;89:2817–23. doi: 10.1210/jc.2003-031158. doi:10.1210/jc.2003-031158. [DOI] [PubMed] [Google Scholar]
- 24.Kriplani A, Periyasamy AJ, Agarwal N, Kulshrestha V, Kumar A, Ammini AC. Effect of oral contraceptive containing ethinyl estradiol combined with drospirenone vs. desogestrel on clinical and biochemical parameters in patients with polycystic ovary syndrome. Contraception. 2010;82:139–46. doi: 10.1016/j.contraception.2010.02.009. doi:10.1016/j.contraception.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Fruzzetti F, Perini D, Lazzarini V, Parrini D, Gambacciani M, Genazzani AR. Comparison of effects of 3 mg drospirenone plus 20 mg ethinyl estradiol alone or combined with metformin or cyproterone acetate on classic metabolic cardiovascular risk factors in nonobese women with polycystic ovary syndrome. Fertil Steril. 2010;94:1793–8. doi: 10.1016/j.fertnstert.2009.10.016. doi:10.1016/j.fertnstert.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Oelkers W, Foidart JM, Dombrovicz N, Welter A, Heithecker R. Effects of a new oral contraceptive containing an antimineralocorticoid progestogen, drospirenone, on the renin-aldosterone system, body weight, blood pressure, glucose tolerance, and lipid metabolism. J Clin Endocrinol Metab. 1995;80:1816–21. doi: 10.1210/jcem.80.6.7775629. doi:10.1210/jc.80.6.1816. [DOI] [PubMed] [Google Scholar]
- 27.Ilie IR, Marian I, Mocan T, Ilie R, Mocan L, Duncea I, et al. Ethinylestradiol30mg-drospirenone and metformin: Could this combination improve endothelial dysfunction in polycystic ovary syndrome? BMC Endocr Disord. 2012;12:9. doi: 10.1186/1472-6823-12-9. doi:10.1186/1472-6823-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun X, Wu X, Zhou Y, Yu X, Zhang W. Evaluation of apelin and insulin resistance in patients with PCOS and therapeutic effect of drospirenone-ethinylestradiol plus metformin. Med Sci Monit. 2015;21:2547–52. doi: 10.12659/MSM.894926. doi:10.12659/MSM.894926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotterdam ESHRE/ASRM-Sponsored PCOS, Consensus Workshop, Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. doi:10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 30.Yildiz BO, Bolour S, Woods K, Moore A, Azziz R. Visually scoring hirsutism. Hum Reprod Update. 2010;16:51–64. doi: 10.1093/humupd/dmp024. doi:10.1093/humupd/dmp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36:416–8. doi: 10.1046/j.1365-4362.1997.00099.x. doi:10.1046/j.1365-4362.1997.00099.x. [DOI] [PubMed] [Google Scholar]
- 32.Villaseca P, Hormaza P, Cárdenas I, Oestreicher E, Arteaga E. Ethinylestradiol/cyproterone acetate in polycystic ovary syndrome: Lipid and carbohydrate changes. Eur J Contracept Reprod Health Care. 2004;9:155–65. doi: 10.1080/13625180400007751. doi:10.1080/13625180400007751. [DOI] [PubMed] [Google Scholar]
- 33.Prelevic GM, Würzburger MI, Trpkovic D, Balint-Peric L. Effects of a low-dose estrogen-antiandrogen combination (Diane-35) on lipid and carbohydrate metabolism in patients with polycystic ovary syndrome. Gynecol Endocrinol. 1990;4:157–68. doi: 10.3109/09513599009009803. doi:10.3109/09513599009009803. [DOI] [PubMed] [Google Scholar]
- 34.Batukan C, Muderris II, Ozcelik B, Ozturk A. Comparison of two oral contraceptives containing either drospirenone or cyproterone acetate in the treatment of hirsutism. Gynecol Endocrinol. 2007;23:38–38. doi: 10.1080/09637480601137066. doi:10.1080/09637480601137066. [DOI] [PubMed] [Google Scholar]
- 35.Mastorakos G, Koliopoulos C, Deligeoroglou E, Diamanti-Kandarakis E, Creatsas G. Effects of two forms of combined oral contraceptives on carbohydrate metabolism in adolescents with polycystic ovary syndrome. Fertil Steril. 2006;85:420–7. doi: 10.1016/j.fertnstert.2005.07.1306. doi:10.1016/j.fertnstert.2005.07.1306. [DOI] [PubMed] [Google Scholar]
- 36.Halperin IJ, Kumar SS, Stroup DF, Laredo SE. The association between the combined oral contraceptive pill and insulin resistance, dysglycemia and dyslipidemia in women with polycystic ovary syndrome: A systematic review and meta-analysis of observational studies. Hum Reprod. 2011;26:191–201. doi: 10.1093/humrep/deq301. doi:10.1093/humrep/deq301. [DOI] [PubMed] [Google Scholar]
- 37.Kahraman K, Sükür YE, Atabekoglu CS, Ates C, Taskin S, Cetinkaya SE, et al. Comparison of two oral contraceptive forms containing cyproterone acetate and drospirenone in the treatment of patients with polycystic ovary syndrome: A randomized clinical trial. Arch Gynecol Obstet. 2014;290:321–8. doi: 10.1007/s00404-014-3217-5. doi:10.1007/s00404-014-3217-5. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharya SM, Ghosh M, Basu R. Effects of ethinyl estradiol and desogestrel on clinical and metabolic parameters in Indian patients with polycystic ovary syndrome. J Obstet Gynaecol Res. 2012;38:285–90. doi: 10.1111/j.1447-0756.2011.01682.x. doi:10.1111/j.1447-0756.2011.01682.x. [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharya SM, Jha A. Comparative study of the therapeutic effects of oral contraceptive pills containing desogestrel, cyproterone acetate, and drospirenone in patients with polycystic ovary syndrome. Fertil Steril. 2012;98:1053–9. doi: 10.1016/j.fertnstert.2012.06.035. doi:10.1016/j.fertnstert.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 40.Meyer C, McGrath BP, Teede HJ. Effects of medical therapy on insulin resistance and the cardiovascular system in polycystic ovary syndrome. Diabetes Care. 2007;30:471–8. doi: 10.2337/dc06-0618. doi:10.2337/dc06-0618. [DOI] [PubMed] [Google Scholar]


