Abstract
Background:
Chronic obstructive pulmonary disease (COPD) is characterized by progressive loss of lung function and local and systemic inflammation, in which CD8+ T-cells are believed to play a key role. Activated CD8+ T-cells differentiate into distinct subpopulations, including interferon-γ (IFN-γ)-producing Tc1 and interleukin (IL)-17-producing Tc17 cells. Recent evidence indicates that Tc17 cells exhibit considerable plasticity and may convert into IL-17/IFN-γ-double producing (Tc17/IFN-γ) cells when driven by inflammatory conditions. The aim of this study was to investigate the Tc17/IFN-γ subpopulation in peripheral blood of patients with COPD and to evaluate their potential roles in this disease.
Methods:
Peripheral blood samples were collected from 15 never-smokers, 23 smokers with normal lung function, and 25 patients with COPD (Global Initiative for Chronic Obstructive Lung Disease 2–4). Proportions of the IL-17/IFN-γ-double expressing subpopulation were assessed using flow cytometry. Plasma concentrations of cytokines favoring Tc17/IFN-γ differentiation were measured by enzyme-linked immunosorbent assay.
Results:
Patients with COPD had higher proportions of Tc17 cells and Tc17/IFN-γ cells in the peripheral blood than smokers and never-smokers. The plasticity of Tc17 cells was higher than that of Th17 cells. The percentages of Tc17 cells and Tc17/IFN-γ cells showed negative correlations with forced expiratory volume in 1 s % predicted value (r = −0.418, P = 0.03; r = −0.596, P = 0.002, respectively). The plasma concentrations of IL-6, transforming growth factor-β1, and IL-12 were significantly higher in patients with COPD compared with smokers and never-smokers.
Conclusions:
Peripheral Tc17 cells are increased and more likely to convert to Tc17/IFN-γ cells in COPD, suggesting that Tc17 cell plasticity may be involved in persistent inflammation of the disease.
Keywords: CD8+ T-cells, Interferon-γ, Interleukin-17, Plasticity
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is associated with enhanced and chronic inflammatory responses of the lungs to tobacco smoking and other noxious particles or gasses.[1] The inflammation in COPD, both in the lungs and in the systemic circulation, plays critical roles in disease development and progression.[2,3]
CD8+ T-cells are major players in the inflammation and lung destruction of COPD.[4] It is believed that CD8+ T-cells induce lung inflammation and emphysema in COPD by the production of granzyme B, perforin, and many other injurious and pro-inflammatory mediators. Studies in COPD patients have demonstrated that CD8+ T-cells are accumulated in lung parenchyma and airways and correlate with the degree of airway obstruction.[5,6,7,8]
Activated CD8+ T-cells differentiate into distinct subpopulations, including interferon-γ (IFN-γ)-producing Tc1, interleukin (IL)-4 producing Tc2, and IL-17-producing Tc17 cells, defined by selected sets of cytokines and transcription factors production.[9,10,11] Studies have shown that most CD8+ T-cells isolated from the lung parenchyma of COPD patients are IFN-γ-producing Tc1 cells, exhibiting greater cytotoxicity compared with Tc2 cells.[12,13] Compared to Tc1 cells, Tc17 cells exhibit strikingly suppressed cytotoxic activity by secreting low levels of the cytotoxic T lymphocytes markers: T-bet, IFN-γ, perforin, and granzyme B.[14] Tc17 cells are shown to share some phenotypical properties with Th17, including retinoic acid receptor-related orphan receptor γt, CCR6, and IL-23R, and express tumor necrosis factor-α (TNF-α), IL-21, and IL-22.[15,16] Recent studies, however, have revealed that Tc17 cells are increased in several autoimmune diseases, such as rheumatoid arthritis (RA), multiple sclerosis, psoriasis, and are implicated in the pathogenesis of these diseases.[17,18,19]
Tc17 cells possess a high plasticity and can convert to IL-17/IFN-γ-double producing cells (Tc17/IFN-γ cells) permitted by IL-12 signaling, with distinct properties from Tc1 lineage.[20] Tc17/IFN-γ cells are highly cytotoxic and exhibit strong antitumor activity in vitro and in vivo. Interestingly, this unique subpopulation of Tc17 was found implicated in various inflammatory conditions in both humans and mice.[20,21,22,23,24,25,26]
Previous studies reported that IL-17A and IL-17F expressions by CD8+ T-cells were increased in the airways of COPD patients.[27,28] However, little is known about the frequency of circulating Tc17 cells, particularly Tc17/IFN-γ cells and their associations with disease progression in COPD. Given that COPD is a lung disease with significant extrapulmonary effects, exploring the differentiation of peripheral CD8+ T-cells, particularly the newly recognized Tc17 cells, may provide revealing evidence for the understanding of the mechanisms underlying systemic inflammation of the disease. Therefore, we assessed the signature cytokine IL-17 and IFN-γ expressions by CD8+ T lymphocyte in peripheral blood from patients with COPD and analyzed the difference in the plasticity between Tc17 cells and Th17 cells. The cytokines believed to favor Tc17/IFN-γ differentiation were measured in plasma from the study subjects. Our results revealed higher proportions of Tc17 cells and Tc17/IFN-γ cells in peripheral blood from COPD patients, which could be explained by increased concentrations of IL-6, transforming growth factor β1 (TGF-β1) and IL-12. Importantly, the percentages of Tc17 cells and Tc17/IFN-γ cells were correlated negatively with forced expiratory volume in 1 s (FEV1) % predicted. These results indicate that more studies are warranted to reveal the potential involvement of Tc17/IFN-γ cells in the pathogenesis of COPD.
METHODS
Study subjects
Twenty-five male patients with COPD, all current or former smokers, were recruited for the study in the Beijing Tongren Hospital, Capital Medical University, China. Twenty-three smokers and 15 never-smokers with normal lung function were also included. The diagnosis of COPD was made according to clinical symptoms, a history of tobacco smoking, and impaired pulmonary function (postbronchodilator FEV1/forced vital capacity <70%), according to the diagnostic criteria of the Global Initiative for Chronic Obstructive Lung Disease (2013).[1] All subjects with COPD were clinically stable and had not suffered any exacerbations for ≥3 months prior to enrollment. Smokers with normal lung function (FEV1>80% predicted) had a smoking history of ≥20 pack-years. Individuals with asthma, restrictive lung diseases, lung surgery, other chronic systemic inflammatory diseases, such as RA, type 1 diabetes mellitus, and inflammatory bowel disease, were excluded. The demographic and baseline clinical characteristics of the study participants are summarized in Table 1.
Table 1.
The demographic and clinical characteristics of all participants
| Items | Healthy nonsmokers | Smokers | Patients with COPD |
|---|---|---|---|
| Number of subjects | 15 | 23 | 25 |
| Age (years) | 67.3 ± 6.5 | 66.4 ± 8.2 | 67.9 ± 7.7 |
| Male/female (n/n) | 15/0 | 23/0 | 25/0 |
| Current/ex-smokers (n/n) | 0 | 16/7 | 10/15 |
| Pack-years, median (IQR) | 0 | 39 (28–50) | 46 (30–72) |
| FEV1% predicted, mean ± SD | 95.8 ± 6.2 | 91.3 ± 8.7 | 51.7 ± 15.5 |
| FEV1/FVC%, mean ± SD | 83.2 ± 3.4 | 80.8 ± 4.9 | 55.6 ± 11.0 |
| ICS use (n) | 0 | 0 | 19 |
| Bronchodilator use (n) | 0 | 0 | 20 |
| Exacerbations/year, mean ± SD | 0 | 0 | 1.1 ± 0.3 |
Values are presented median (IQR) for smoking history, mean, and standard deviation for all others. COPD: Chronic obstructive pulmonary disease; FEV1: Forced expiratory volume in 1 s; FVC: Forced vital capacity; ICS: Inhaled corticosteroids; IQR: Interquartile range; SD: Standard deviation.
The study was approved by the local research ethics committee (TRECKT 2008-14). Written informed consent was obtained from all subjects.
Cell collection and flow cytometry
Peripheral blood samples were collected into ethylenediaminetetraacetic acid-treated tubes by venipuncture from the subjects after an 8-h fasting and were layered on the Ficoll-Paque Plus solution (Amersham Biosciences, Amersham, Bucks, UK) in a centrifuge tube, centrifuged at 400 × g for 20 min at 21°C, and peripheral blood mononuclear cells (PBMCs) were harvested. Then, divalent cation-free Hanks balanced salt solution was used for washing of cells at 300 × g for 5 min at 4°C. PBMCs were resuspended at 106 cells/ml in RPMI-1640 medium and prepared for the following procedures.
Freshly processed human PBMCs were stimulated with 50 ng/ml of phorbol 12-myristate 13-acetate and 500 ng/ml of ionomycin in the presence of 5 μg/ml brefeldin A for 5 h at 37°C as described by others.[29] The cells were harvested and stained with anti-hCD4-PE (BD Biosciences, San Jose, California, USA) and anti-hCD8-Percp (BD Biosciences) for 30 min at room temperature, followed by staining with anti-hIL-17A-FITC (eBioscience, San Diego, California, USA) and anti-hIFN-γ-APC (eBioscience) after fixation and permeabilization. CD8+ subpopulations were determined using FACS-Calibur (BD Biosciences). A total of 1 × 105 events were collected for each subject and data were analyzed by FlowJo software (Tree Star, Ashland, OR, USA).
Cytokine enzyme-linked immunosorbent assay
The concentrations of IL-6, IL-12, and TGF-β1 in the plasma from the study subjects were measured by enzyme-linked immunosorbent assay (ELISA, eBioscience, San Diego, CA, USA) according to the manufacturer's recommendations with the sensitivity of 2 pg/ml, 2.1 pg/ml, and 8.6 pg/ml, respectively.
Statistical analysis
Group data were depicted as a mean and standard error of the mean or median and interquartile range when appropriate. Comparisons of three groups were performed using one-way analysis of variance (ANOVA) for group data distributed normally, and when the test detected statistical significance, post hoc analysis between two groups was performed by the use of the Tukey test. The correlation was analyzed using Pearson's rank correlation coefficients. A P value < 0.05 was considered statistically significant. All analyses were performed by Prism 5.02 (GraphPad, La Jolla, CA, USA) and SPSS for Windows standard version released 17.0 (SPSS Inc, Chicago, Illinois, USA).
RESULTS
The frequency of Tc1 cells and Tc17 cells is increased in chronic obstructive pulmonary disease patients
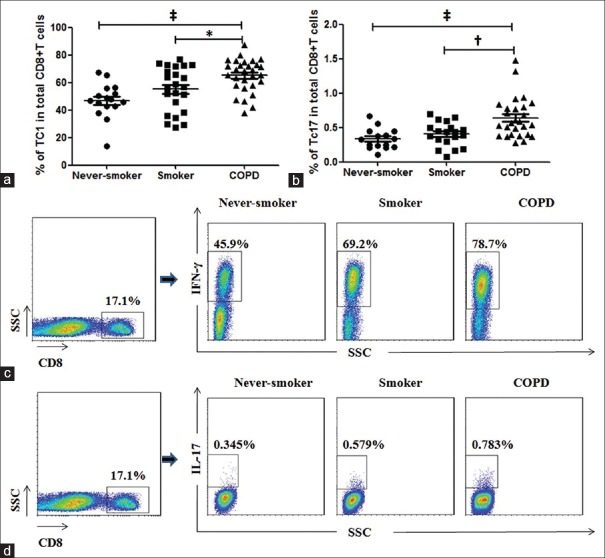
We first examined the frequencies of IFN-γ-producing CD8+ T-cells in peripheral blood from the study subjects using flow cytometry. There was a higher proportion of Tc1 cells in circulating CD8+ T-cells in COPD patients (median, 68.50%) compared with smokers (median, 56.60%, P < 0.05) and never-smokers (median, 47.20%, P < 0.001), and there was a trend for increase in smokers compared with never-smokers [Figure 1a and 1c]. The percentage of Tc17 cells in total circulating CD8+ T lymphocytes was increased in patients with COPD (median, 0.562%) compared with smokers (median, 0.434%, P < 0.01) and never-smokers (median, 0.33%, P < 0.001) [Figure 1b and 1d].
Figure 1.
CD8+ T-cell subpopulations in peripheral blood from patients with the chronic obstructive pulmonary disease, smokers, and never-smokers. CD8+ cells were analyzed for production of interferon-γ or interleukin-17. (a and b) The percentages of Tc1 and Tc17 cells among CD8+ T-cells in peripheral blood from patients with chronic obstructive pulmonary disease, smokers, and never-smokers. (c and d) Representative flow cytometry of Tc1 and Tc17 cells. Horizontal lines indicate median values. SSC: Side scatter. COPD: Chronic obstructive pulmonary disease. *P < 0.05, †P < 0.01, ‡P < 0.001.
The frequency of dual-positive Tc17/interferon-γ cells is increased in chronic obstructive pulmonary disease patients
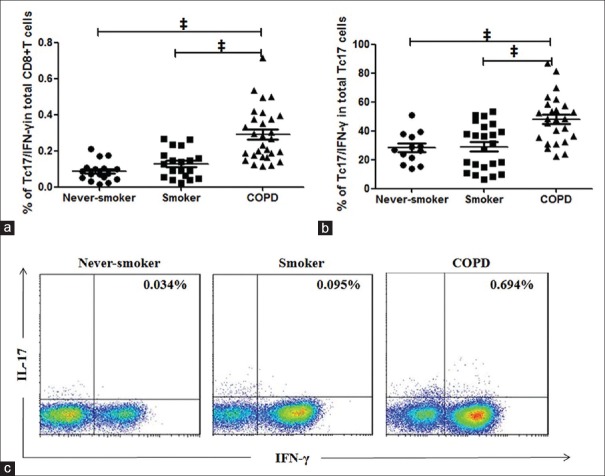
In patients with COPD, a significantly higher percentage of Tc17/IFN-γ cells among CD8+ T-cells (median, 0.268%) in the peripheral blood was found as compared to smokers (median, 0.128%, P < 0.001) and never-smokers (median, 0.074%, P < 0.001) [Figure 2a and 2c]. Furthermore, a significantly higher percentage of Tc17/IFN-γ cells among Tc17 cells was seen in patients with COPD [median, 48.09%; Figure 2b] compared with smokers [median, 31.25%, P < 0.001; Figure 2b] and never-smokers [median, 26.67%, P < 0.001; Figure 2b], which indicated increased differentiation of Tc17 cells to Tc17/IFN-γ cells in COPD.
Figure 2.
Proportions of the Tc17/interferon-γ cell subpopulation in peripheral blood from patients with the chronic obstructive pulmonary disease, smokers, and never-smokers. CD8+ cells were analyzed for production of interleukin-17 and interferon-γ cells after 5 h of stimulation with phorbol 12-myristate 13-acetate/ionomycin and GolgiStop. (a and b) The percentages of Tc17/interferon-γ cells among CD8+ T-cells and Tc17 cells in peripheral blood from patients with the chronic obstructive pulmonary disease, smokers, and never-smokers. (c) Representative flow cytometry of Tc17/interferon-γ cells. COPD: Chronic obstructive pulmonary disease. Horizontal lines indicate median values, ‡P < 0.001.
Plasticity of Tc17 cells is higher than that of Th17 cells in chronic obstructive pulmonary disease patients
As demonstrated previously, similar to Th17 cells, the Tc17 phenotype was unstable. Tc17 cell plasticity (converting to Tc17/IFN-γ cells), driven by the inflammatory milieu, especially by IL-12,[22,28] is higher than Th17 plasticity.[10] Therefore, we investigated whether the plasticity of Tc17 cells was also higher in patients with COPD [Figure 3a]. As shown in Figure 3b, the frequency of Tc17/IFN-γ cells (median, 48.09%) in Tc17 cells was significantly higher than the frequency of Th17/Th1 cells (median, 15.44%, P < 0.001) in Th17 cells in COPD patients, indicating that Tc17 plasticity was greater than Th17 plasticity in COPD. Consistent with previous studies[10] similar results were also seen in both smokers and never-smokers (data not shown).
Figure 3.
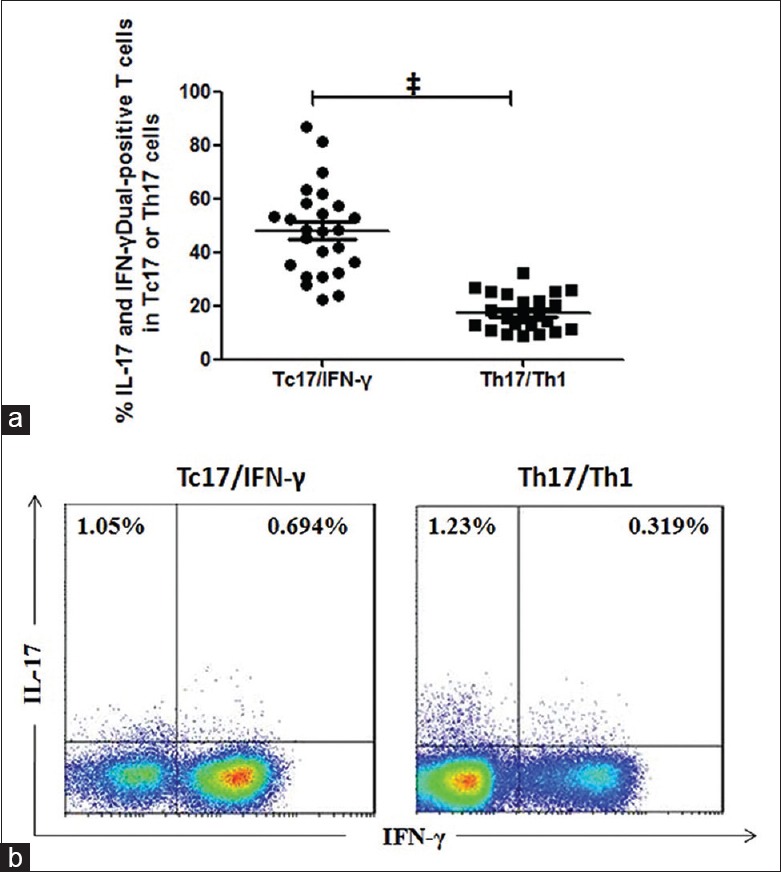
Frequencies of interleukin-17/interferon-γ-double positive subpopulations among Tc17 cells and Th17 cells in peripheral blood from patients with chronic obstructive pulmonary disease. CD4+ cells and CD8+ cells were analyzed for production of interleukin-17 and interferon-γ after 5 h of stimulation with phorbol 12-myristate 13-acetate/ionomycin and GolgiStop. (a) The percentages of Th17/Th1 cells and Tc17/interferon-γ cells among CD4+ T-cells and CD8+ T-cells in peripheral blood from patients with chronic obstructive pulmonary disease, respectively. (b) Representative flow cytometry of Th17/Th1 and Tc17/interferon-γ cells. Horizontal lines indicate median values, ‡P < 0.001.
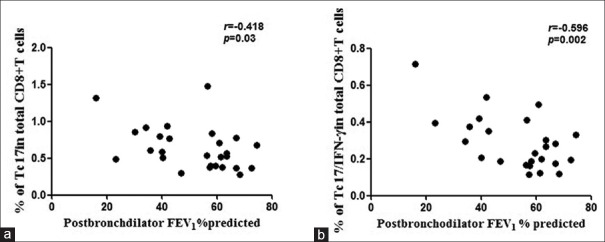
Increased expression of dual-positive Tc17/interferon-γ cells is inversely correlated with forced expiratory volume in 1 s
The increased percentage of Tc17 cells among CD8+ T-cells in peripheral blood from COPD patients was inversely correlated with FEV1% predicted values [r = −0.418, P = 0.03; Figure 4a]. More importantly, the higher frequency of Tc17/IFN-γ cells among CD8+ T-cells in peripheral blood from COPD patients was also inversely correlated with FEV1% predicted values [r = −0.596, P = 0.002; Figure 4b].
Figure 4.
Correlations of Tc17/interferon-γ cells and lung function (n=25). (a) Frequencies of Tc17 cells in CD8+ T-cells and (b) frequencies of Tc17/interferon-γ cells in CD8+ T-cells correlated with forced expiratory volume in 1 s (FEV1)% predicted values in patients with chronic obstructive pulmonary disease. A P value < 0.05 was considered statistically significant.
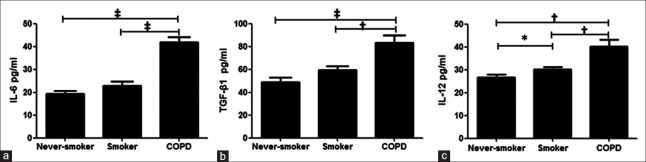
The concentrations of interleukin-6, transforming growth factor-β1, and interleukin-12 are increased in chronic obstructive pulmonary disease
We next examined the concentrations of plasma cytokines believed to drive Tc17/IFN-γ cell differentiation. The concentrations of IL-6, TGF-β1, and IL-12 were significantly higher in plasma from patients with COPD compared with smokers and never-smokers [P < 0.01, Figure 5a–5c]. These suggest that the Tc17 plasticity in COPD may be driven by the inflammatory environment of the disease.
Figure 5.
The concentrations of plasma cytokines. Interleukin-6 (a), transforming growth factor-β1 (b), and interleukin-12 (c) from patients with chronic obstructive pulmonary disease, smokers, and never-smokers. COPD: Chronic obstructive pulmonary disease. Horizontal lines indicate median values, *P < 0.05, †P < 0.01, ‡P < 0.001.
DISCUSSION
CD8+ T-cells have long been recognized as the major pathogenic T-cells in airway inflammation and lung destruction in COPD. In contrast to the plenty of evidence supporting the accumulation and activation of CD8+ T-cells in the lungs of COPD, little is known about the characteristics and functions of circulating CD8+ T-cells in COPD, and the limited findings are often controversial. Domagała-Kulawik et al. reported that frequencies of circulating CD8+ T-cells were increased in COPD while others found no differences in COPD patients compared with healthy subjects.[30,31,32] While these discrepancies might be due to the heterogeneous nature of the disease and different patient populations enrolled, however, functional plasticity and phenotype heterogeneity of CD8+ T-cells are likely to be implicated.
Following the identification of an IL-17-producing subset within CD8+ T-cells, studies have demonstrated that the so-called Tc17 cells are involved in a wide spectrum of immune diseases.[17,18,19,21,23,24,25,26,27,28] More recent evidence confirmed that the Tc17 lineage possessed a late developmental plasticity, that is, converting to Tc17/IFN-γ cells, in response to inflammatory signals.[20,22,26,33] As COPD is a lung disease with significant systemic inflammation, it is likely that circulating CD8+ T-cells and/or their subpopulations are implicated in these pathogenic processes. We therefore hypothesized that Tc17 cells in COPD might show high plasticity and differentiate more to Tc17/IFN-γ cells, which, unlike Tc17 cells in general, are highly toxic, a property similar to Tc1 cells. We demonstrated here that the proportions of Tc17 cells, and more importantly, the multifunctional subpopulation Tc17/IFN-γ cells, were significantly increased in the peripheral blood of patients with COPD, and both were correlated negatively with FEV1, a hallmark of severity of the disease.
Our findings of a higher proportion of Tc1 cells in peripheral blood from COPD patients compared with smokers and never-smokers are consistent with previous reports.[31,34] But our result of increased percentage of Tc17 cells in peripheral blood from patients with COPD was different from the study by Paats et al., who had found that the proportion of IL-17A positive CD8+ T-cells was negligible in the peripheral blood, and no difference existed between COPD patients and healthy controls.[31] This discrepancy may be due to differences in disease severity (our patients had a higher mean FEV1) and gender of the patients (our patients were all males). Moreover, we showed that the elevated frequency of circulating Tc17 cells was correlated significantly with COPD severity, suggesting that these cells may be involved in the pathogenesis of COPD. This is supported by animal studies demonstrating that the number of Tc17 cells was significantly increased in lungs of cigarette smoke-exposed mice, even after smoking cessation, and was correlated with lung emphysematous lesions.[31,35,36]
As mentioned earlier, Tc17 cells are far less cytotoxic as compared to Tc1 cells, and then by what mechanisms they may be pathogenic in COPD? As a novel subset of Tc17 cells, Tc17/IFN-γ cells are capable of acquiring strong cytotoxic function similar to Tc1 cells and expressing pro-inflammatory cytokines similar to their Th17/Th1 counterparts and therefore are believed to augment the pathogenic capability of Tc17 cells and promote exacerbation of a variety of autoimmune diseases.[20,22,24,25,26,37,38,39,40] Saxena et al. revealed that Tc17/IFN-γ cells might be indispensable for the aggravation of diabetes by direct cytotoxicity on the β-islet cells and expressing pro-inflammatory cytokines apart from IFN-γ in an experimental model of autoimmune diabetes.[24] Tajima et al. reported that Tc17/IFN-γ cells were rapidly generated in mesenteric lymph nodes, and IL-17 acted synergistically with IFN-γ to recruit effector CD8+ T-cells and other inflammatory cells to colon tissues in a colitis model.[25] Here, we demonstrated for the first time to our knowledge that the percentages of Tc17/IFN-γ cells among CD8+ T cells and Tc17 cells were significantly higher in peripheral blood from patients with COPD and correlated with FEV1, suggesting that circulating Tc17/IFN-γ cells might be involved in persistent inflammation and loss of lung function in COPD. In addition, we found that Tc17 cells exhibited higher developmental plasticity than Th17 cells, although the implications of Tc17 plasticity in the pathogenesis of COPD remain speculative.
Since Tc17 plasticity is driven by inflammatory conditions, we supposed that the higher Tc17 plasticity was related to the systemic inflammation in COPD. Previous studies showed that in the presence of IL-6 and TGF-β1, human naïve CD8+ T-cells acquired the Tc17 phenotype, and IL-12 was able to permit Tc17 cells to acquire the potential to produce IFN-γ, thus differentiating to Tc17/IFN-γ cells.[20,29,33] In the current study, we found increased concentrations of IL-6 and TGF-β1 in the plasma from patients of COPD. More importantly, consistent with other studies, the concentration of IL-12 was higher in patients with COPD as compared to the controls.[41,42] These results indicate that further studies are needed to explore the mechanisms by which these cytokines induce Tc17 plasticity in COPD.
Our study has several limitations. First, we did not examine the frequency of Tc17 and Tc17/IFN-γ cells in the lungs, for example, in bronchoalveolar lavage, which may be more relevant to airway diseases of COPD; hence, further investigations to this issue are needed. Second, we have only shown increased plasma levels of IL-6, TGF-β1, and IL-12 in COPD; whether and by what mechanism these cytokines promote Tc17 plasticity is still speculative. Third, some of our patients had used inhaled corticosteroids, and therefore the possibility of an effect of this medication on our results cannot be excluded, although Paats et al. found no effect of inhaled corticosteroids on peripheral CD8+ T-cells in COPD.[31]
In summary, this study provides a comprehensive analysis of circulating CD8+ T-cells and their subpopulations in COPD, with a novel finding that the circulating Tc17/IFN-γ cells, in addition to Tc17 cells, are significantly increased and correlated to the severity of disease, suggesting that these cells may be involved in the pathogenesis of COPD. Further studies are needed to elucidate the underlying mechanisms of CD8+ T-cell heterogeneity and Tc17 cell plasticity in COPD, which may shed new light on the understanding of local and systemic inflammation characteristic of the disease.
Financial support and sponsorship
This study was supported by grants from the National Natural Science Foundation of China (No. 81170039 and No. 81470239).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
REFERENCES
- 1.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65. doi: 10.1164/rccm.201204-0596PP. doi:10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Willemse BW, ten Hacken NH, Rutgers B, Lesman-Leegte IG, Postma DS, Timens W. Effect of 1-year smoking cessation on airway inflammation in COPD and asymptomatic smokers. Eur Respir J. 2005;26:835–45. doi: 10.1183/09031936.05.00108904. doi:10.1183/09031936.05.00108904. [DOI] [PubMed] [Google Scholar]
- 3.Miller M, Cho JY, Pham A, Friedman PJ, Ramsdell J, Broide DH. Persistent airway inflammation and emphysema progression on CT scan in ex-smokers observed for 4 years. Chest. 2011;139:1380–7. doi: 10.1378/chest.10-0705. doi:10.1378/chest.10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosio MG, Majo J, Cosio MG. Inflammation of the airways and lung parenchyma in COPD: Role of T cells. Chest. 2002;121(5 Suppl):160S–5S. doi: 10.1378/chest.121.5_suppl.160s. doi:10.1378/chest.121.5_suppl.160S. [DOI] [PubMed] [Google Scholar]
- 5.Saetta M, Baraldo S, Corbino L, Turato G, Braccioni F, Rea F, et al. CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:711–7. doi: 10.1164/ajrccm.160.2.9812020. doi:10.1164/ajrccm.160.2.9812020. [DOI] [PubMed] [Google Scholar]
- 6.O'Shaughnessy TC, Ansari TW, Barnes NC, Jeffery PK. Inflammation in bronchial biopsies of subjects with chronic bronchitis: Inverse relationship of CD8+T lymphocytes with FEV1. Am J Respir Crit Care Med. 1997;155:852–7. doi: 10.1164/ajrccm.155.3.9117016. doi:10.1164/ajrccm.155.3.9117016. [DOI] [PubMed] [Google Scholar]
- 7.Saetta M, Di Stefano A, Turato G, Facchini FM, Corbino L, Mapp CE, et al. CD8+T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(3 Pt 1):822–6. doi: 10.1164/ajrccm.157.3.9709027. doi:10.1164/ajrccm.157.3.9709027. [DOI] [PubMed] [Google Scholar]
- 8.Forsslund H, Mikko M, Karimi R, Grunewald J, Wheelock ÅM, Wahlström J, et al. Distribution of T-cell subsets in BAL fluid of patients with mild to moderate COPD depends on current smoking status and not airway obstruction. Chest. 2014;145:711–22. doi: 10.1378/chest.13-0873. doi:10.1378/chest.13-0873. [DOI] [PubMed] [Google Scholar]
- 9.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 2005;136:2348–57. doi 0022-1767/86/1367-2348802.00/0. [PubMed] [Google Scholar]
- 10.Liang Y, Pan HF, Ye DQ. IL-17A-producing CD8(+) T cells as therapeutic targets in autoimmunity. Expert Opin Ther Targets. 2015;19:651–61. doi: 10.1517/14728222.2014.997710. doi:10.1517/14728222.20 14.997710. [DOI] [PubMed] [Google Scholar]
- 11.Mosmann TR, Li L, Sad S. Functions of CD8 T-cell subsets secreting different cytokine patterns. Semin Immunol. 1997;9:87–87. doi: 10.1006/smim.1997.0065. doi:10.1006/smim.1997.0065. [DOI] [PubMed] [Google Scholar]
- 12.Gill D, Tan PH. Induction of pathogenic cytotoxic T lymphocyte tolerance by dendritic cells: A novel therapeutic target. Expert Opin Ther Targets. 2010;14:797–824. doi: 10.1517/14728222.2010.499360. doi:10.1517/14728222.2010.499360. [DOI] [PubMed] [Google Scholar]
- 13.Chrysofakis G, Tzanakis N, Kyriakoy D, Tsoumakidou M, Tsiligianni I, Klimathianaki M, et al. Perforin expression and cytotoxic activity of sputum CD8+lymphocytes in patients with COPD. Chest. 2004;125:71–6. doi: 10.1378/chest.125.1.71. doi:10.1378/chest.125.1.71. [DOI] [PubMed] [Google Scholar]
- 14.Huber M, Heink S, Grothe H, Guralnik A, Reinhard K, Elflein K, et al. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur J Immunol. 2009;39:1716–25. doi: 10.1002/eji.200939412. doi:10.1002/eji.200939412. [DOI] [PubMed] [Google Scholar]
- 15.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. doi:10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–46. doi: 10.1038/ni1467. doi:10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 17.Henriques A, Gomes V, Duarte C, Pedreiro S, Carvalheiro T, Areias M, et al. Distribution and functional plasticity of peripheral blood Th(c) 17 and Th(c) 1 in rheumatoid arthritis. Rheumatol Int. 2013;33:2093–9. doi: 10.1007/s00296-013-2703-6. doi:10.1007/s00296-013-2703-6. [DOI] [PubMed] [Google Scholar]
- 18.Peelen E, Thewissen M, Knippenberg S, Smolders J, Muris AH, Menheere P, et al. Fraction of IL-10+and IL-17+CD8 T cells is increased in MS patients in remission and during a relapse, but is not influenced by immune modulators. J Neuroimmunol. 2013;258:77–84. doi: 10.1016/j.jneuroim.2013.02.014. doi:10.1016/j.jneuroim.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Cheuk S, Wikén M, Blomqvist L, Nylén S, Talme T, Ståhle M, et al. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J Immunol. 2014;192:3111–20. doi: 10.4049/jimmunol.1302313. doi:10.4049/jimmunol.1302313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tajima M, Wakita D, Satoh T, Kitamura H, Nishimura T. IL-17/IFN-g double producing CD8+T (Tc17/IFN-γ) cells: A novel cytotoxic T-cell subset converted from Tc17 cells by IL-12. Int Immunol. 2011;23:751–9. doi: 10.1093/intimm/dxr086. doi:10.1093/intimm/dxr086. [DOI] [PubMed] [Google Scholar]
- 21.Ortega C, Fernández AS, Carrillo JM, Romero P, Molina IJ, Moreno JC, et al. IL-17-producing CD8+T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J Leukoc Biol. 2009;86:435–43. doi: 10.1189/JLB.0109046. doi:10.1189/JLB.0109046. [DOI] [PubMed] [Google Scholar]
- 22.Hamada H, Garcia-Hernandez Mde L, Reome JB, Misra SK, Strutt TM, McKinstry KK, et al. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009;182:3469–81. doi: 10.4049/jimmunol.0801814. doi:10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henriques A, Inês L, Couto M, Pedreiro S, Santos C, Magalhães M, et al. Frequency and functional activity of Th17, Tc17 and other T-cell subsets in systemic lupus erythematosus. Cell Immunol. 2010;264:97–103. doi: 10.1016/j.cellimm.2010.05.004. doi:10.1016/j.cellimm.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Saxena A, Desbois S, Carrié N, Lawand M, Mars LT, Liblau RS. Tc17 CD8+T cells potentiate Th1-mediated autoimmune diabetes in a mouse model. J Immunol. 2012;189:3140–9. doi: 10.4049/jimmunol.1103111. doi:10.4049/jimmunol.1103111. [DOI] [PubMed] [Google Scholar]
- 25.Tajima M, Wakita D, Noguchi D, Chamoto K, Yue Z, Fugo K, et al. IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8+T cells. J Exp Med. 2008;205:1019–27. doi: 10.1084/jem.20071133. doi:10.1084/jem.20071133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satoh T, Tajima M, Wakita D, Kitamura H, Nishimura T. The development of IL-17/IFN-g-double producing CTLs from Tc17 cells is driven by epigenetic suppression of Socs3 gene promoter. Eur J Immunol. 2012;42:2329–42. doi: 10.1002/eji.201142240. doi:10.1002/eji.201142240. [DOI] [PubMed] [Google Scholar]
- 27.Chang Y, Nadigel J, Boulais N, Bourbeau J, Maltais F, Eidelman DH, et al. CD8 positive T cells express IL-17 in patients with chronic obstructive pulmonary disease. Respir Res. 2011;12:43. doi: 10.1186/1465-9921-12-43. doi:10.1186/1465-9921-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eustace A, Smyth LJ, Mitchell L, Williamson K, Plumb J, Singh D. Identification of cells expressing IL-17A and IL-17F in the lungs of patients with COPD. Chest. 2011;139:1089–100. doi: 10.1378/chest.10-0779. doi:10.1378/chest.10-0779. [DOI] [PubMed] [Google Scholar]
- 29.Nistala K, Adams S, Cambrook H, Ursu S, Olivito B, de Jager W, et al. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc Natl Acad Sci U S A. 2010;107:14751–6. doi: 10.1073/pnas.1003852107. doi:10.1073/pnas.1003852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domagala-Kulawik J, Hoser G, Dabrowska M, Chazan R. Increased proportion of Fas positive CD8+cells in peripheral blood of patients with COPD. Respir Med. 2007;101:1338–43. doi: 10.1016/j.rmed.2006.10.004. doi:10.1016/j.rmed.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Paats MS, Bergen IM, Hoogsteden HC, van der Eerden MM, Hendriks RW. Systemic CD4+and CD8+T-cell cytokine profiles correlate with GOLD stage in stable COPD. Eur Respir J. 2012;40:330–7. doi: 10.1183/09031936.00079611. doi:10.1183/09031936.00079611. [DOI] [PubMed] [Google Scholar]
- 32.BarcelóB Pons J, Fuster A, Sauleda J, Noguera A, Ferrer JM, et al. Intracellular cytokine profile of T lymphocytes in patients with chronic obstructive pulmonary disease. Clin Exp Immunol. 2006;145:474–9. doi: 10.1111/j.1365-2249.2006.03167.x. doi:10.1111/j.1365-2249.2006.03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yen HR, Harris TJ, Wada S, Grosso JF, Getnet D, Goldberg MV, et al. Tc17 CD8 T cells: Functional plasticity and subset diversity. J Immunol. 2009;183:7161–8. doi: 10.4049/jimmunol.0900368. doi:10.4049/jimmunol.0900368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirai T, Suda T, Inui N, Chida K. Correlation between peripheral blood T-cell profiles and clinical and inflammatory parameters in stable COPD. Allergol Int. 2010;59:75–82. doi: 10.2332/allergolint.09-OA-0126. doi:10.2332/allergo lint.09-OA-0126. [DOI] [PubMed] [Google Scholar]
- 35.Duan MC, Tang HJ, Zhong XN, Huang Y. Persistence of Th17/Tc17 cell expression upon smoking cessation in mice with cigarette smoke-induced emphysema. Clin Dev Immunol 2013. 2013:162–71. doi: 10.1155/2013/350727. doi:10.1155/2013/350727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou H, Hua W, Jin Y, Zhang C, Che L, Xia L, et al. Tc17 cells are associated with cigarette smoke-induced lung inflammation and emphysema. Respirology. 2015;20:426–33. doi: 10.1111/resp.12486. doi:10.1111/resp. 12486. [DOI] [PubMed] [Google Scholar]
- 37.Yeh N, Glosson NL, Wang N, Guindon L, McKinley C, Hamada H, et al. Tc17 cells are capable of mediating immunity to vaccinia virus by acquisition of a cytotoxic phenotype. J Immunol. 2010;185:2089–98. doi: 10.4049/jimmunol.1000818. doi:10.4049/jimmunol.1000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–9. doi: 10.1038/ni.2416. doi:10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boniface K, Blumenschein WM, Brovont-Porth K, McGeachy MJ, Basham B, Desai B, et al. Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol. 2010;185:679–87. doi: 10.4049/jimmunol.1000366. doi:10.4049/jimmunol.1000366. [DOI] [PubMed] [Google Scholar]
- 40.Kondo T, Takata H, Matsuki F, Takiguchi M. Cutting edge: Phenotypic characterization and differentiation of human CD8+T cells producing IL-17. J Immunol. 2009;182:1794–8. doi: 10.4049/jimmunol.0801347. doi:10.4049/jimmunol.0801347. [DOI] [PubMed] [Google Scholar]
- 41.Bade G, Khan MA, Srivastava AK, Khare P, Solaiappan KK, Guleria R, et al. Serum cytokine profiling and enrichment analysis reveal the involvement of immunological and inflammatory pathways in stable patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:759–73. doi: 10.2147/COPD.S61347. doi:10.2147/COPD.S61347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinto-Plata V, Toso J, Lee K, Park D, Bilello J, Mullerova H, et al. Profiling serum biomarkers in patients with COPD: Associations with clinical parameters. Thorax. 2007;62:595–601. doi: 10.1136/thx.2006.064428. doi:10.1136/thx.2006.064428. [DOI] [PMC free article] [PubMed] [Google Scholar]