Abstract
Background:
The ultimate goal of hepatitis B treatment is hepatitis B surface antigen (HBsAg) seroclearance. Several factors have been suggested to be associated with the rate of HBsAg reduction in antiviral-naive or lamivudine therapy cohorts. However, there are few studies evaluating the factors during long-term entecavir (ETV) therapy. In the present study, we aimed to evaluate the factors to predict the outcome of ETV therapy for 7 years.
Methods:
A total of 47 chronic hepatitis B (CHB) patients treated with ETV monotherapy were included in this study. Liver biochemistry, hepatitis B virus (HBV) serological markers, serum HBV DNA, and HBsAg titers were tested at baseline, 3 months, 6 months, and yearly from 1 to 7. The associations between factors and HBsAg reduction were assessed using multivariate tests with repeated measure analysis of variance.
Results:
At baseline, serum HBsAg levels showed a positive correlation with baseline HBV DNA levels (r = 0.625, P < 0.001). The mean HBsAg titers after ETV treatment were significantly lower than the baseline titers (P ranges from 0.025 to 0.000,000,6). The HBsAg reduction rate during the 1st year was greater compared to after 1 year of treatment (P < 0.05). Multivariate test showed that hepatitis B e antigen (HBeAg) seroclearance and/or HBsAg reduction ≥0.5 log10 IU/ml at 6 months had a high negative predictive value (96.77%) for HBsAg seroclearance (P = 0.002, P = 0.012, respectively).
Conclusions:
The HBsAg reduction rate during the 1st year was greater than that after 1 year of treatment. Further, HBeAg status and HBsAg levels at month 6 are the optimal factors for the early prediction of HBsAg seroclearance after long-term ETV therapy in CHB patients.
Keywords: Chronic Hepatitis B, Entecavir, Hepatitis B e Antigen, Hepatitis B Surface Antigen
INTRODUCTION
Chronic hepatitis B virus (HBV) infection causes serious damage to human health and must be recognized and treated correctly.[1] Effective antiviral therapy can stop the progression of liver injury and prevent the development of cirrhosis, hepatocellular carcinoma, and complications. In the mid-1970s, interferon-alpha began to be used to treat chronic hepatitis B (CHB), but the response rate to interferon-alpha treatment was low.[2] After 1998, five nucleos(t)ide analogs (NAs)-lamivudine (LAM), adefovir dipivoxil (ADV), telbivudine (LdT), entecavir (ETV), and tenofovir disoproxil fumarate (TDF) were successively introduced, leading to a continuous virologic suppression and control of disease progression in CHB patients.[3] NAs can inhibit viral replication by suppressing the process of reverse transcription, but they have a small effect in reducing intrahepatic covalently closed circular DNA (cccDNA).[4] Therefore, long-term treatment might be beneficial to achieve the ultimate goal of clearance of hepatitis B surface antigen (HBsAg).[5]
cccDNA is the template for viral replication in hepatocytes. There are complex interactions between the HBV DNA level, HBsAg titer, and cccDNA replication markers.[6] A reduction in the cccDNA level after antiviral therapy can reduce the HBsAg level. Clearance of HBsAg is a marker for significant reduction in cccDNA level.[7,8] Quantification of HBsAg has been demonstrated to have a high predictive value in CHB patients receiving pegylated interferon-based therapy.[9] Recent studies also indicate that serum HBsAg levels can predict treatment response to NAs.[10,11] However, information concerning HBsAg dynamics during long-term NAs treatment is limited.[12,13,14]
The NAs vary in resistance barrier and the strength with which they suppress HBV DNA. ETV, a high-potency oral drug, has been shown to efficiently inhibit HBV DNA replication over a prolonged medication time in CHB patients.[15,16] However, the kinetics of HBsAg during long-term (>5 years) ETV treatment remain unknown. Because of this primary requirement, we recruited CHB patients who had received ETV treatment for at least 7 years and who had responded favorably to ETV.
METHODS
Study subjects
This retrospective study was performed in China. Demographic data were collected from consecutive patients' outpatient service records at the Department of Infectious Diseases of Peking University First Hospital (China). From September 2006 to December 2007, 61 consecutive patients who were chronically monoinfected with HBV were treated with ETV monotherapy. No patient had received interferon-alpha or NAs for at least 6 months prior to ETV treatment. Liver cirrhosis was diagnosed by clinical criteria. The inclusion criteria were as follows: age above 18 years, mono therapy with ETV, and maintained virologic suppression during continuous ETV therapy. The exclusion criteria were as follows: switched to or added another antiviral drug because of poor virological response or virological breakthrough, follow-up time of <7 years, decompensated liver cirrhosis (Child–Pugh class B/C), hepatocellular carcinoma, liver transplantation, co-infection (hepatitis C virus and HIV), causes of liver disease other than HBV, and immunosuppressive treatment.
For the initial treatment, patients received ETV at a dose of 0.5 mg daily. Other patients who had received LAM before the study received ETV at a dose of 1.0 mg daily.
The study was in compliance with the Helsinki Declaration and was approved by the Medical Ethics Committee of Peking University First Hospital. All enrolled patients provided written informed consent.
Laboratory measurements
Liver biochemistry, HBV serological markers, serum HBV DNA, and HBsAg titers were measured before (baseline) and after 3 months, 6 months, 1 year, 2 years, 3 years, 4 years, 5 years, 6 years, and 7 years of ETV therapy.
After overnight (12 h) fasting, venous blood was drawn to determine the serum levels of alanine aminotransferase (ALT) using an automatic biochemical analyzer. Serology markers for HBV, antibody to HBsAg; hepatitis B e antigen (HBeAg); and antibody to HBeAg were measured by enzyme-linked immunosorbent assay (Abbott Laboratories, Chicago, IL, USA).
Serum HBsAg titers were measured using the Elecsys HBsAgII quant assay (Roche Diagnostics, Branchburg, NJ, USA) with a detection limit of 0.05–52,000 IU/ml. Serum HBV DNA levels were determined using the COBAS® TaqMan assay (Roche Diagnostics) with a detection limit of 20 IU/ml.
The HBV genotype was determined by direct sequencing of the preS/S gene. HBV DNA was amplified as previously described.[17] Polymerase chain reaction products were sequenced in both directions using the Big-Dye Terminator version 3.1 Cycle Sequencing kit on the ABI 3730 sequencer (Applied Biosystems, Foster City, CA, USA). HBV genotypes were determined by comparing the generated preS/S gene sequences with prototype sequences from the GenBank using a web-based genotyping tool (National Center for Biotechnology Information).
Definitions
According to the European Association for the Study of the Liver clinical practice guidelines,[18] biochemical response was defined as normalization of serum ALT level (≤40 IU/L). Virological response was defined as undetectable HBV DNA during the treatment period (≤20 IU/ml). Partial virologic response was defined as a decrease in HBV DNA of more than 1 log10 IU/ml, but detectable HBV DNA after at least 6 months of therapy in compliant patients. Virological breakthrough was defined as a confirmed increase in the HBV DNA level of more than 1 log10 IU/ml compared to the nadir (lowest value) HBV DNA level during therapy.
Statistical analysis
Statistical analyses were performed using SPSS version 17.0 (SPSS, Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range). We assumed that the HBsAg titers followed a log-normal distribution. Correlations between different clinical parameters were performed using Spearman rank correlation analysis. The Mann–Whitney U-test was used for continuous variables. Repetitive measure analysis of variance (ANOVA) was used to analyze HBsAg titers at different time points. The interaction between HBsAg and factors was assessed using multivariate testing with repeated measure ANOVA. A value of P < 0.05 was considered statistically significant.
RESULTS
After applying the selection criteria mentioned above, 47 (77.05%) patients with ETV maintained satisfactory virologic suppression during at least 7 years of continuous monotherapy. Among the remaining patients, 4 (6.56%) patients stopped antiviral therapy after achieving a virological response, 4 (6.56%) patients had a virological breakthrough, 1 (1.64%) patient added ADV, 1 (1.64%) patient stopped treatment because of a partial virological response, 2 (3.28%) patients did not take regular medication, 1 (1.64%) patient switched to LdT because of pregnancy, and 1 (1.64%) patient died of pneumonia. Among all patients, only four patients (6.56%) had cirrhosis (Child–Pugh class A). Their baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of patients in this study
| Parameters | All patients (n = 61) | Selected patients (n = 47) |
|---|---|---|
| Male, n (%) | 47 (77.05) | 35 (74.47) |
| Age (years), mean ± SD | 36.13 ± 11.28 | 38.36 ± 11.35 |
| BMI (kg/m2), mean ± SD | 24.42 ± 3.48 | 24.48 ± 3.40 |
| CHB, n (%) | 57 (93.44) | 44 (93.62) |
| ALT >2 × ULN, n (%) | 42 (68.85) | 33 (70.21) |
| HBV DNA level (log10 IU/ml), mean ± SD | 6.92 ± 1.61 | 6.91 ± 1.69 |
| HBsAg (log10 IU/ml), mean ± SD | 3.88 ± 0.74 | 3.85 ± 0.76 |
| HBeAg-positive patients, n (%) | 51 (83.61) | 38 (80.85) |
| HBV genotype, n (%) | ||
| B | 8 (13.11) | 5 (10.64) |
| C | 27 (44.26) | 23 (48.94) |
| Undetermined | 26 (42.62) | 19 (40.42) |
BMI: Body mass index; CHB: Chronic hepatitis B; ALT: Alanine aminotransferase; HBV: Hepatitis B virus; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen; ULN: Upper normal limit; SD: Standard deviation.
Response to treatment
The cumulative ALT normalization rates in patients with an abnormal baseline ALT (n = 41) at 3rd month, 6th month, 1st, 2nd, 3rd, 4th, 5th, 6th, and 7th years of treatment were 51.22%, 65.85%, 87.80%, 85.37%, 87.80%, 90.24%, 95.12%, 90.24%, and 92.68%, respectively. Figure 1a shows changes in the HBV DNA detectable rate during the 7-year period. The cumulative HBeAg seroconversion rates for HBeAg-positive subjects (n = 38) at the above time points were 15.79%, 18.42%, 18.42%, 28.95%, 34.21%, 47.37%, 44.74%, 50.00%, and 50.00%, respectively. Nineteen (50.00%) baseline HBeAg-positive patients achieved HBeAg seroconversion and had undetectable serum HBV DNA after 7 years of treatment. Four (6.55%) patients achieved HBsAg seroclearance.
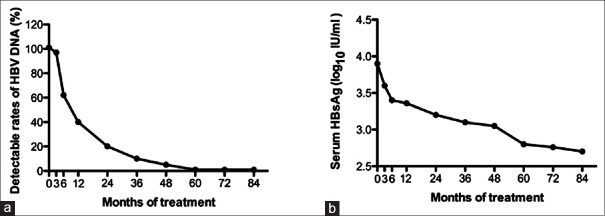
Figure 1.
(a) The detectable rates of HBV DNA and (b) mean HBsAg throughout 7-year treatment period. HBV: Hepatitis B virus; HBsAg: Hepatitis B surface antigen.
Changes in hepatitis B surface antigen titers and factors associated with hepatitis B surface antigen reduction
The average baseline serum HBsAg level of 47 patients was 3.85 ± 0.76 log10 IU/ml. Baseline serum HBsAg levels was positively correlated with baseline HBV DNA level (r = 0.625, P < 0.001). The baseline serum HBsAg level was not correlated with other factors.
Figure 1b shows the changes in serum HBsAg level during the 7-year treatment period. The mean serum HBsAg titer continuously decreased during the treatment. The average HBsAg titer after ETV treatment was significantly reduced when compared to the baseline titer (P ranges from 0.025 to 0.000,000,6). Among the 47 patients, the median rate of HBsAg reduction from baseline to the 7th year was 0.12 log10 IU/ml per year. The HBsAg reduction rates were 0.17, 0.06, 0.03, 0.03, 0.11, 0.02, and 0.14 log10 IU/ml, respectively, after the 1st, 2nd, 3rd, 4th, 5th, 6th, and 7th years of treatment. There was a greater HBsAg reduction rate during the 1st year of ETV therapy compared after 1 year of treatment (P < 0.05), except for the 5th and 7th years (P > 0.05).
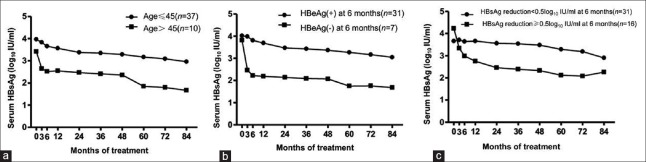
Baseline and during-treatment factors that were associated with HBsAg reduction are shown in Table 2. Multivariate testing with repeated measures ANOVA analysis identified one baseline factor (age, P = 0.003) and two during-treatment factors (HBeAg seroclearance at 6 months, P = 0.002; HBsAg reduction ≥0.5 log10 IU/ml at 6 months, P = 0.012) that were significantly associated with HBsAg reduction. The changes in HBsAg levels in relation to those significant factors are shown in Figure 2a–2c.
Table 2.
Baseline and on-treatment response factors associated with hepatitis B surface antigen reduction
| Factors | F | P |
|---|---|---|
| Baseline factors | ||
| Age (>45 years) | 9.681 | 0.003 |
| Gender | 0.439 | 0.511 |
| HBV genotype | 0.923 | 0.346 |
| HBV DNA (≥7.0 log10 IU/ml) | 3.774 | 0.058 |
| ALT (≥2 × ULN) | 0.730 | 0.397 |
| HBeAg-positive | 0.575 | 0.452 |
| HBsAg (≥3.0 log10 IU/ml) | 2.384 | 0.130 |
| On-treatment response factors | ||
| HBeAg seroconversion at 6 months | 11.018 | 0.002 |
| HBsAg reduction (≥0.5 log10 IU/ml) at 6 months | 6.906 | 0.012 |
HBV: Hepatitis B virus; ALT: Alanine aminotransferase; ULN: Upper normal limit; HBeAg: Hepatitis B e antigen; HBsAg: Hepatitis B surface antigen.
Figure 2.
Mean serum HBsAg reduction in different patients groups stratified by (A) age and (B) HBeAg seroclearance at 6 months (C) HBsAg reduction≥0.5log10 IU/ml at 6 months. HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen.
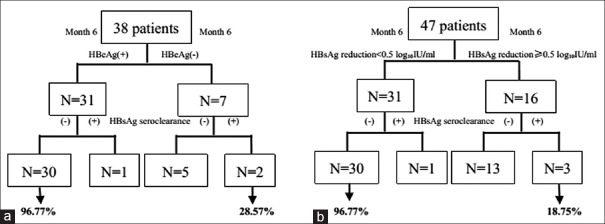
HBeAg seroclearance at 6th month was detected in 7 (18.42%) patients. Thirty-one of 38 (81.58%) patients did not achieve HBeAg seroclearance at 6th month and 30 of those 31 (96.77%) did not achieve HBsAg seroclearance. In contrast, 2 of the 7 (28.57%) patients who achieved HBeAg seroclearance obtained the ultimate goal of HBsAg seroclearance. Failure to achieve HBeAg seroclearance at 6 months has a negative predictive value for HBsAg seroclearance of 96.77% and a positive predictive value of 28.57% [Figure 3a]. We also tested for HBsAg reduction ≥0.5 log10 IU/ml in the 47 patients at 6th month of treatment. Once more, failure to achieve a drop in serum HBsAg had a negative predictive value for HBsAg seroclearance of 96.77% and a positive predictive value of 18.75% [Figure 3b].
Figure 3.
Predictive values of HBeAg seroclearance at month 6 (a) and HBsAg reduction ≥0.5 log10 IU/ml within 6 months (b) of entecavir treatment on HBsAg seroclearance. HBeAg: Hepatitis B e antigen; HBsAg: Hepatitis B surface antigen.
Hepatitis B surface antigen loss
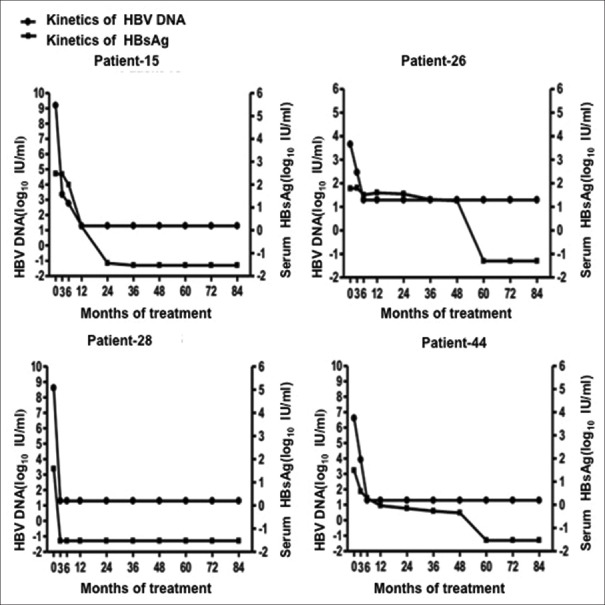
The kinetics of serum HBsAg titers for the four patients (8.51%) who achieved HBsAg seroclearance are shown in Figure 4. Patients 15, 28, and 44 were baseline HBeAg-positive, with HBeAg seroconversion occurring after 2.00, 0.25, and 0.50 years and with HBsAg seroclearance occurring after 3.00, 0.25, and 5.00 years. Patient 26, who was baseline HBeAg-negative, achieved HBsAg seroclearance after 5 years of ETV treatment. Before HBsAg seroclearance, these four patients had an average serum HBsAg reduction rate of 2.01, 18.68, 0.91, and 0.62 log10 IU/ml per year, respectively.
Figure 4.
Kinetics of serum HBsAg titers and HBV DNA in the four patients who achieved HBsAg seroclearance. HBV: hepatitis B virus; HBsAg: Hepatitis B surface antigen.
DISCUSSION
ETV has been applied for the clinical treatment of CHB patients in China since 2006.[19] However, few studies have investigated the kinetics of serum HBsAg titers during long-term ETV treatment of HBV mono-infected patients. Our present study included patients who had received ETV monotherapy for 7 years and who had responded satisfactorily. Those patients who switched to or added other antiviral drugs were excluded. The included patients would be ideal to study the kinetics of serum HBsAg titer during long-term NA therapy and the factors that are associated with the efficacy of NA treatment, for example, HBsAg seroclearance.
In this cohort, 47 patients still had undetectable HBV DNA after 7 years of treatment. This finding of response to ETV treatment is consistent with previous studies,[20] and confirms that ETV is a potent NA for the treatment of Chinese CHB patients.[21] Our results are also consistent with the moderate correlation between baseline HBV DNA levels and HBsAg levels[22] because in our study, baseline serum HBsAg level was positively correlated with baseline HBV DNA levels. A previous study has demonstrated that the HBsAg titer at baseline can help predict the HBsAg seroclearance.[23] However, the baseline HBV DNA level does not influence the declining rate of HBsAg titer. These results are not surprising because serum HBsAg is produced not only from transcriptionally active cccDNA, but also from integrated HBV DNA sequences.[24] Serum HBsAg particles in the peripheral blood are found in two forms: virion-associated HBsAg and empty HBsAg subviral particles, with a much lower proportion of the former than the latter.[25]
During 7 years of ETV treatment, the serum HBsAg titers continuously decreased. The median rate of HBsAg reduction during the 1st year of ETV treatment was significantly higher than in the following years (except the 5th and 7th years), which is similar to the results observed with tenofovir therapy.[26] After the 1st year of ETV therapy, strong suppression of viral replication could lead to a rapid reduction of HBV DNA-containing virions and the synthesis of new cccDNA, resulting in a greater rate of HBsAg reduction. During the following years of treatment, NA therapy inhibits only the HBV reverse transcription step, but has a small effect in reducing intrahepatic cccDNA levels or integrated HBV DNA sequence.[27] In addition, there are more episomal HBV DNA-containing virions entering the hepatocyte than exocytosed into the peripheral circulation, leading to a replenishment of cccDNA.[26] Finally, the production or secretion of empty HBsAg subviral particles is minimally affected by NA therapy.[4] Therefore, the rates of HBsAg reduction in the subsequent years of ETV therapy were slower than in the 1st year.
We found that old age is associated with HBsAg reduction. It is well known that the HBsAg seroclearance rate increased with increasing age in untreated CHB patients,[28,29] indicating that old age is independent of ETV treatment. The specificity of the host immune response in enhanced with age increasing, accompanied by destruction of HBV infectious liver cells, which causes a decline in functional liver cells combined with an increase in liver fibrosis. Finally, liver intracellular HBV particles gradually reduce, causing a decrease in the level of HBsAg.[30]
Our results also showed that two during-treatment factors influenced the rate of HBsAg reduction: HBeAg seroclearance and HBsAg reduction ≥0.5 log10 IU/ml at 6 months. HBeAg seroclearance at the 6th month of ETV therapy was found in 18.42% of patients and an HBsAg reduction ≥0.5 log10 IU/ml at 6 months was found in 34.04% of patients. Both factors had a high negative predictive value for HBsAg seroclearance. In addition, among 4 patients who achieved HBsAg seroclearance, patients 28 and 44 achieved HBeAg seroclearance at 6 months and patients 15, 28, and 44 had a serum HBsAg drop ≥0.5 log10 IU/ml at 6 months. The HBsAg reduction rate in the early stage was also correlated with the subsequent serum HBsAg reduction rates, which supports a similar previous finding.[14,31] The HBeAg seroclearance and/or the serum HBsAg reduction ≥0.5 log10 IU/ml at 6 months indicate a rapid decrease in cccDNA levels and a strong host immune response. Therefore, measurement of HBeAg status and HBsAg levels at 6 months of therapy is the optimal timing for the early prediction of HBsAg seroclearance after 7 years of ETV therapy in CHB patients.
The major limitation of our study is the small number of patients. This might have influenced our estimates of factors associated with the HBsAg reduction rate, and might also have influenced the predictive validity of HBeAg seroclearance or HBsAg reduction ≥0.5 log10 IU/ml at 6 months. Therefore, these observations in a small sample size should be further assessed in larger sample prospective studies.
While our study included a few patients who underwent previous treatment with LAM, none of those patients had taken any antiviral drugs for at least 6 months prior to ETV treatment. Furthermore, those patients who had received LAM before the study received ETV at a dose of 1.0 mg daily. According to previous studies, treatment history and the dose of ETV (1.0 mg) might not significantly affect the results.[32,33] Moreover, our study included only those patients who had responded favorably during ETV therapy.
In conclusion, serum HBsAg levels decreased gradually during 7 years of ETV treatment. The median rate of HBsAg reduction during the 1st year of treatment was higher than that in the following years. HBeAg seroclearance and/or an HBsAg reduction ≥0.5 log10 IU/ml at 6 months had a high negative predictive value for HBsAg seroclearance. If prospectively validated in a larger number patient cohort, HBeAg status and HBsAg level measurements at an early stage would represent a useful additional tool to optimize antiviral therapy, thereby achieving superior clinical results and preserving medical resources.
Financial support and sponsorship
This study was supported by the grants from the 12th Five-Year Plan (No. 2012ZX10002003-004-003 and No. 2013ZX10002004-001-003), the National Natural Science Foundation of China (No. 81373056), and the Beijing Municipal Science and Technology Commission of Major Projects (No. D121100003912003).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The content of this publication does not necessarily reflect the views or policies of the Department of the Health and Human Service, nor does the mention of trade names or commercial products. We would also like to sincerely acknowledge all individuals who contributed to this study.
Footnotes
Edited by: Li-Min Chen
REFERENCES
- 1.Ganem D, Prince AM. Hepatitis B virus infection –Natural history and clinical consequences. N Engl J Med. 2004;350:1118–29. doi: 10.1056/NEJMra031087. doi:10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 2.Jiang DK, Wu X, Qian J, Ma XP, Yang J, Li Z, et al. Genetic variation in STAT4 predicts response to interferon-alpha therapy for hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2015 doi: 10.1002/hep.28423. [Epub ahead of print]. doi:10.1002/hep.28423. [DOI] [PubMed] [Google Scholar]
- 3.Wang YJ, Yang L, Zuo JP. Recent developments in antivirals against hepatitis B virus. Virus Res. 2015;213:205–13. doi: 10.1016/j.virusres.2015.12.014. doi:10.1016/j.virusres.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Wong DK, Seto WK, Fung J, Ip P, Huang FY, Lai CL, et al. Reduction of hepatitis B surface antigen and covalently closed circular DNA by nucleos(t)ide analogues of different potency. Clin Gastroenterol Hepatol. 2013;11:1004–10.e1. doi: 10.1016/j.cgh.2013.01.026. doi:10.1016/j.cgh.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Li GJ, Yu YQ, Chen SL, Fan P, Shao LY, Chen JZ, et al. Sequential combination therapy with pegylated interferon leads to loss of hepatitis B surface antigen and hepatitis B e antigen (HBeAg) seroconversion in HBeAg-positive chronic hepatitis B patients receiving long-term entecavir treatment. Antimicrob Agents chemother. 2015;59:4121–8. doi: 10.1128/AAC.00249-15. doi:10.1128/AAC.00249-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guner R, Karahocagil M, Buyukberber M, Kandemir O, Ural O, Usluer G. Correlation between intrahepatic hepatitis B virus cccDNA levels and other activity markers in patients with HBeAg-negative chronic hepatitis B infection. Eur J Gastroenterol Hepatol. 2011;23:1185–91. doi: 10.1097/MEG.0b013e32834ba13a. doi:10.1097/MEG.0b013e32834ba13a. [DOI] [PubMed] [Google Scholar]
- 7.Chan HL, Wong VW, Tse AM, Tse CH, Chim AM, Chan HY, et al. Serum hepatitis B surface antigen quantitation can reflect hepatitis B virus in the liver and predict treatment response. Clin Gastroenterol Hepatol. 2007;5:1462–8. doi: 10.1016/j.cgh.2007.09.005. doi:10.1016/j.cgh.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Sonneveld MJ, Zoutendijk R, Janssen HL. Hepatitis B surface antigen monitoring and management of chronic hepatitis B. J Viral Hepat. 2011;18:449–57. doi: 10.1111/j.1365-2893.2011.01465.x. doi:10.1111/j.1365-2893.2011.01465.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen GY, Zhu MF, Zheng DL, Bao YT, Wang J, Zhou X, et al. Baseline HBsAg predicts response to pegylated interferon-a2b in HBeAg-positive chronic hepatitis B patients. World J Gastroenterol. 2014;20:8195–200. doi: 10.3748/wjg.v20.i25.8195. doi:10.3748/wjg.v20.i25.8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CH, Chiu YC, Lu SN, Lee CM, Wang JH, Hu TH, et al. Serum hepatitis B surface antigen levels predict treatment response to nucleos(t)ide analogues. World J Gastroenterol. 2014;20:7686–95. doi: 10.3748/wjg.v20.i24.7686. doi:10.3748/wjg.v20.i24.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Xi H, Wang Q, Hou F, Huo N, Zhang X, et al. Kinetics of serum HBsAg in Chinese patients with chronic HBV infection with long-term adefovir dipivoxil treatment. Chin Med J. 2014;127:2101–4. doi:10.3760/cma.j.issn.0366-6999.20132826. [PubMed] [Google Scholar]
- 12.Shin JW, Jung SW, Park BR, Kim CJ, Eum JB, Kim BG, et al. Prediction of response to entecavir therapy in patients with HBeAg-positive chronic hepatitis B based on on-treatment HBsAg, HBeAg and HBV DNA levels. J Viral Hepat. 2012;19:724–31. doi: 10.1111/j.1365-2893.2012.01599.x. doi:10.1111/j.1365-2893.2012.01599.x. [DOI] [PubMed] [Google Scholar]
- 13.Heathcote EJ, Marcellin P, Buti M, Gane E, De Man RA, Krastev Z, et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140:132–43. doi: 10.1053/j.gastro.2010.10.011. doi:10.1053/j.gastro.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Wursthorn K, Jung M, Riva A, Goodman ZD, Lopez P, Bao W, et al. Kinetics of hepatitis B surface antigen decline during 3 years of telbivudine treatment in hepatitis B e antigen-positive patients. Hepatology. 2010;52:1611–20. doi: 10.1002/hep.23905. doi:10.1002/hep. 23905. [DOI] [PubMed] [Google Scholar]
- 15.Pol S, Lampertico P. First-line treatment of chronic hepatitis B with entecavir or tenofovir in 'real-life'settings: From clinical trials to clinical practice. J Viral Hepat. 2012;19:377–86. doi: 10.1111/j.1365-2893.2012.01602.x. doi:10.1111/j.1365-2893.2012.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koffi J, Egounlety R, Pradat P, Lebosse F, Si-Ahmed SN, Lussier V, et al. Impact of lamivudine-resistance mutations on entecavir treatment outcome in hepatitis B. Eur J Gastroenterol Hepatol. 2014;26:146–54. doi: 10.1097/MEG.0b013e328365c3e5. doi:10.1097/MEG.0b013e328365c3e5. [DOI] [PubMed] [Google Scholar]
- 17.Lada O, Benhamou Y, Poynard T, Thibault V. Coexistence of hepatitis B surface antigen (HBs Ag) and anti-HBs antibodies in chronic hepatitis B virus carriers: Influence of “a” determinant variants. J Virol. 2006;80:2968–75. doi: 10.1128/JVI.80.6.2968-2975.2006. doi:10.1128/JVI.80.6.2968-2975.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85. doi: 10.1016/j.jhep.2012.02.010. doi:10.1016/j.jhep2012.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Yao GB, Zhu M, Wang YM, Xu DZ, Tan DM, Chen CW, et al. A double-blind, double-dummy, randomized, controlled study of entecavir versus lamivudine for treatment of chronic hepatitis B (in Chinese) Chin J Intern Med. 2006;45:891–5. [PubMed] [Google Scholar]
- 20.Chen EQ, Wang TT, Bai L, Tao CM, Liang T, Liu C, et al. Quantitative hepatitis B surface antigen titres in Chinese chronic hepatitis B patients over 4 years of entecavir treatment. Antivir Ther. 2013;18:955–65. doi: 10.3851/IMP2579. doi:10.3851/IMP2579. [DOI] [PubMed] [Google Scholar]
- 21.Yu HM, Kwon SY, Kim J, Chung HA, Kwon SW, Jeong TG, et al. Virologic response and safety of tenofovir versus entecavir in treatment-naïve chronic hepatitis B patients. Saudi J Gastroenterol. 2015;21:146–51. doi: 10.4103/1319-3767.157558. doi:10.4103/1319-3767.157558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson AJ, Nguyen T, Iser D, Ayres A, Jackson K, Littlejohn M, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: Disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51:1933–44. doi: 10.1002/hep.23571. doi:10.1002/hep. 23571. [DOI] [PubMed] [Google Scholar]
- 23.Arai M, Togo S, Kanda T, Fujiwara K, Imazeki F, Yokosuka O. Quantification of hepatitis B surface antigen can help predict spontaneous hepatitis B surface antigen seroclearance. Eur J Gastroenterol Hepatol. 2012;24:414–8. doi: 10.1097/MEG.0b013e328350594d. doi:10.1097/MEG.0b013e328350594d. [DOI] [PubMed] [Google Scholar]
- 24.Bill CA, Summers J. Genomic DNA double-strand breaks are targets for hepadnaviral DNA integration. Proc Natl Acad Sci U S A. 2004;101:11135–40. doi: 10.1073/pnas.0403925101. doi:10.1073/pnas.0403925101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia T, Li J, Sureau C, Ito K, Qin Y, Wands J, et al. Drastic reduction in the production of subviral particles does not impair hepatitis B virus virion secretion. J Virol. 2009;83:11152–65. doi: 10.1128/JVI.00905-09. doi:10.1128/JVI.00905-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seto WK, Liu K, Wong DK, Fung J, Huang FY, Hung IF, et al. Patterns of hepatitis B surface antigen decline and HBV DNA suppression in Asian treatment-experienced chronic hepatitis B patients after three years of tenofovir treatment. J Hepatol. 2013;59:709–16. doi: 10.1016/j.jhep.2013.06.007. doi:10.1016/j.jhep.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Wong DK, Yuen MF, Ngai VW, Fung J, Lai CL. One-year entecavir or lamivudine therapy results in reduction of hepatitis B virus intrahepatic covalently closed circular DNA levels. Antivir Ther. 2006;11:909–16. [PubMed] [Google Scholar]
- 28.Tai DI, Tsay PK, Chen WT, Chu CM, Liaw YF. Relative roles of HBsAg seroclearance and mortality in the decline of HBsAg prevalence with increasing age. Am J Gastroenterol. 2010;105:1102–9. doi: 10.1038/ajg.2009.669. doi:10.1038/ajg.2009.669. [DOI] [PubMed] [Google Scholar]
- 29.Kwak MS, Cho EJ, Jang ES, Lee JH, Yu SJ, Kim YJ, et al. Predictors of HBsAg seroclearance in HBeAg-negative chronic hepatitis B patients. Digestion. 2011;84(Suppl 1):23–8. doi: 10.1159/000333211. doi:10.1159/000333211. [DOI] [PubMed] [Google Scholar]
- 30.Wong DK, Yuen MF, Poon RT, Yuen JC, Fung J, Lai CL. Quantification of hepatitis B virus covalently closed circular DNA in patients with hepatocellular carcinoma. J Hepatol. 2006;45:553–9. doi: 10.1016/j.jhep.2006.05.014. doi:10.1016/j.jhep.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, et al. Clearance of hepatitis B surface antigen during long-term nucleot(s)ide analog treatment in chronic hepatitis B: Results from a nine-year longitudinal study. J Gastroenterol. 2013;48:930–41. doi: 10.1007/s00535-012-0688-7. doi:10.1007/s00535-012-0688-7. [DOI] [PubMed] [Google Scholar]
- 32.Chayama K, Suzuki Y, Kobayashi M, Kobayashi M, Tsubota A, Hashimoto M, et al. Emergence and takeover of YMDD motif mutant hepatitis B virus during long-term lamivudine therapy and re-takeover by wild type after cessation of therapy. Hepatology. 1998;27:1711–6. doi: 10.1002/hep.510270634. doi:10.1002/hep.510270634. [DOI] [PubMed] [Google Scholar]
- 33.Ha NB, Ha NB, Chaung KT, Trinh HN, Nguyen HA, Nguyen KK, et al. Similar response to entecavir 0.5 and 1.0 mg in treatment-naïve chronic hepatitis B patients: A case-control study. Dig Dis Sci. 2014;59:168–73. doi: 10.1007/s10620-013-2940-2. doi:10.1007/s10620-013-2940-2. [DOI] [PubMed] [Google Scholar]