Abstract
Background:
Few studies have addressed whether abnormalities in the lenticular nucleus (LN) are characteristic transcranial sonography (TCS) echo features in patients with primary dystonia. This study aimed to explore alterations in the basal ganglia in different forms of primary focal dystonia.
Methods:
cross-sectional observational study was performed between December 2013 and December 2014 in 80 patients with different forms of primary focal dystonia and 55 neurologically normal control subjects. TCS was performed in patients and control subjects. Multiple comparisons of multiple rates were used to compare LN hyperechogenicity ratios between control and patient groups.
Results:
Thirteen individuals were excluded due to poor temporal bone windows, and two subjects were excluded due to disagreement in evaluation by sonologists. Totally, 70 patients (cervical dystonia, n = 30; blepharospasm, n = 30; oromandibular dystonia, n = 10) and 50 normal controls were included in the final analysis. LN hyperechogenicity was observed in 51% (36/70) of patients with primary focal dystonia, compared with 12% (6/50) of controls (P < 0.001). Substantia nigra hyperechogenicity did not differ between the two groups. LN hyperechogenicity was observed in 73% (22/30) of patients with cervical dystonia, a greater prevalence than in patients with blepharospasm (33%, 10/30, P = 0.002) and oromandibular dystonia (40%, 4/10, P = 0.126). LN hyperechogenicity was more frequently observed in patients with cervical dystonia compared with controls (73% vs. 12%, P < 0.001); however, no significant difference was detected in patients with blepharospasm (33% vs. 12%, P = 0.021) or oromandibular dystonia (40% vs. 12%, P = 0.088).
Conclusions:
LN hyperechogenicity is more frequently observed in patients with primary focal dystonia than in controls. It does not appear to be a characteristic TCS echo feature in patients with blepharospasm or oromandibular dystonia.
Keywords: Basal Ganglia, Lenticular Nucleus, Primary Focal Dystonia, Transcranial Sonography
INTRODUCTION
Dystonia is the third most common movement disorder and is characterized by sustained or intermittent muscle contractions causing twisting or repetitive movements and postural abnormalities.[1,2,3,4] It is classified according to etiology as primary or secondary dystonia. Focal dystonia is the most common form of primary dystonia, which includes blepharospasm, cervical, focal hand, laryngeal, and oromandibular dystonias, among other forms.[1,5]
The specific pathophysiology of primary dystonia remains unclear, and conventional imaging using computed tomography and magnetic resonance imaging (MRI) has failed to demonstrate any neuroanatomic abnormalities in most cases of idiopathic dystonia.[6,7] In 1995, Naumann et al. performed transcranial sonography (TCS) on patients with dystonia for the first time.[6] Hyperechogenicity, primarily in the medial lenticular nucleus (LN), was detected in more than 75% of patients with spontaneous cervical or upper limb dystonia. While similar studies have been published since then,[8,9] few address whether abnormalities in the LN are characteristic echo features in patients with primary dystonia. In this study, we investigated alterations in the basal ganglia in patients with different forms of primary focal dystonia.
METHODS
Subjects
A cross-sectional observational study was performed between December 2013 and December 2014 in 80 patients with primary focal dystonia and 55 neurologically normal control subjects after obtaining informed consent. The study protocol was approved by the Local Ethics Committee.
Patients were recruited by two experienced movement disorders specialists with over 20 years of practitioner experience. Diagnosis of primary dystonia was confirmed according to the European Federation of Neurological Societies Consensus Statement of the Movement Disorders Society, based mainly on clinical evidence.[10,11,12] All subjects were carefully screened for medical history and received a full physical examination and cranial MRI to exclude: (i) secondary dystonia (the causes of which may include perinatal brain injury, infection, drug administration/use, toxin exposure, vascular lesions, neoplastic disease, brain injury, or causes of a psychogenic nature), (ii) heredodegenerative disease with dystonia (including spinocerebellar ataxia, Huntington's disease, Parkinson's disease, and Wilson's disease), and (iii) pseudodystonia. Control subjects were recruited from inpatients and outpatients of our hospital and included neurologically normal persons, without signs and symptoms of repetitive movements or postural abnormalities, and without a history of neurological disease.
Transcranial sonography
A regular TCS team with more than 5 years of practitioner experience and fixed ultrasonic diagnostic equipment were utilized to ensure reliability. TCS was performed according to standardized procedures established at the Ninth Meeting of the European Society of Neurosonology and Cerebral Hemodynamics.[13] A phase-array ultrasound system equipped with a 2.5-MHz transducer (Sequoia 512, 4V1C transducer, Siemens Medical Solutions USA, Inc., USA) was used, with a penetration depth of 14–16 cm, dynamic range of 45–55 dB, and time gain compensation and image brightness adjusted automatically.[14]
One sonologist blinded to subject diagnosis carried out all TCS examinations. Regions of interest were evaluated on standardized axial mesencephalic and diencephalic planes. The mesencephalic plane was identified as having a butterfly-shaped hyperechogenic area, surrounded by the hyperechogenic signal of the basal cisterns [Figure 1a]. In healthy adults, the substantia nigra (SN) is usually displayed as a weakly echogenic structure within the anterior parts of the midbrain.[15] An area of ≥0.20 cm2 was used as the cut-off value for SN hyperechogenicity [Figure 1b].[13] SN hyperechogenic regions were calculated automatically after manual selection. Tilting the probe approximately 10° enabled visualization of the diencephalic plane. The hyperechogenic regions of the pineal gland and third ventricle served as orientation landmarks.[13] Echogenicity of the LN was classified as hyperechogenic if it was more intense than that of the surrounding white matter [Figure 2].[16,17] LN hyperechogenic regions were measured planimetrically, in a manner similar to measurements of SN hyperechogenicity.[13] Evaluations were performed by two experienced sonologists blinded to subject diagnosis. Subjects over whom there was disagreement between the two sonologists were excluded.
Figure 1.
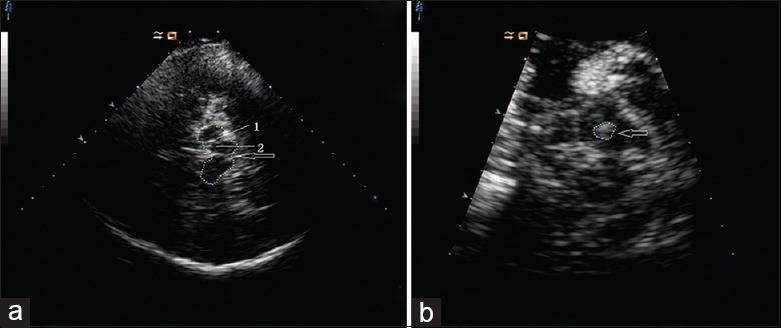
Transcranial sonography images at axial midbrain transections. (a) Image from control subject showing the raphe (arrow). 1: Normal substantia nigra signal; 2: Normal red nucleus signal. (b) Image from patient showing hyperechogenicity of substantia nigra (arrow).
Figure 2.
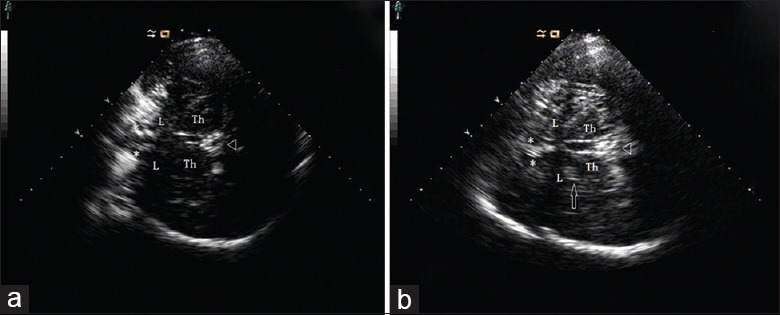
Transcranial sonography images at axial thalamus transections. (a) Image from control subject. (b) Image from patient with cervical dystonia; medial lenticular nucleus is depicted (arrow). Δ: Pineal gland; Th: Thalamus; L: Lenticular nucleus; *frontal horn of lateral ventricle.
Statistical analysis
Analyses were performed using the statistical software package, SAS 9.2 (SAS Institute Inc., USA). Descriptive statistics of areas of SN and LN hyperechogenicity are presented as mean ± standard deviation (SD). For comparison of means, a Student's t- test for two independent samples was performed. For categorical data, the Chi-square test was used. Results with P value < 0.05 and/or α' < 0.0083 were considered statistically significant. Pearson's correlation coefficient was calculated to determine potential correlation(s) between region of LN hyperechogenicity and age or disease duration.
RESULTS
Clinical characteristics
Thirteen individuals (9.6%, 13/135, including eight patients and five controls) were excluded due to poor temporal bone windows. Two subjects were excluded due to disagreement in evaluation by sonologists. A total of 70 patients (cervical dystonia, n = 30; blepharospasm, n = 30; oromandibular dystonia, n = 10) and 50 controls were included in the final analysis. Patients and controls were aged 49.6 ± 12.7 years and 54.6 ± 15.5 years, respectively (t = −1.93, P = 0.056). A female predominance was noted among patients with cervical dystonia and blepharospasm. Male:female ratios were 1:4 and 1:2.75, respectively. Clinical demographic data are summarized in Table 1.
Table 1.
Clinical demographic data of patients with different forms of primary focal dystonia and normal controls
| Variables | Primary focal dystonia subtypes | Controls (n = 50) | ||
|---|---|---|---|---|
| Cervical dystonia (n = 30) | Blepharospasm (n = 30) | Oromandibular dystonia (n = 10) | ||
| Age (years) | 41.1 ± 11.0 | 54.4 ± 10.8 | 41.1 ± 7.8 | 54.6 ± 15.5 |
| Gender (female/male, n) | 24/6 | 22/8 | 4/6 | 26/24 |
| Disease duration (years) | 2.5 ± 1.6 | 3.0 ± 2.8 | 1.9 ± 1.1 | Not applicable |
Values are n or mean ± standard deviation.
Alterations in the lenticular nucleus observed using transcranial sonography
LN hyperechogenicity was detected in 51% of patients (36/70) and in 12% of controls (6/50, χ2 = 19.931, P < 0.001). Among these subjects, bilateral LN hyperechogenicity was observed in ten patients and one control subject, while unilateral LN hyperechogenicity was observed in the rest. LN hyperechogenicity was observed in 73% (22/30) of patients with cervical dystonia, a prevalence higher than that of patients with blepharospasm (33%, 10/30, P = 0.002) and oromandibular dystonia (40%, 4/10, P = 0.126). LN hyperechogenicity was more frequently detected in the cervical dystonia subgroup (73% vs. 12%, χ2 = 31.004, P < 0.001) compared with controls. No significant differences in LN hyperechogenicity were detected in patients with blepharospasm (33% vs. 12%, χ2 = 5.333, P = 0.021) and oromandibular dystonia (40% vs. 12%, χ2 = 2.904, P = 0.088) compared with controls.
No correlation between lenticular nucleus hyperechogenicity and age or disease duration
The mean area of LN hyperechogenicity was 35.3 ± 13.4 mm2 in the cervical dystonia subgroup, 46.8 ± 9.0 mm2 in the blepharospasm subgroup, and 33.0 ± 13.0 mm2 in the oromandibular dystonia subgroup. The area of LN hyperechogenicity was not significantly correlated with either patient age (r = 0.345, P = 0.161) or disease duration (r = −0.141, P = 0.577).
Alterations in the substantia nigra observed using transcranial sonography
The prevalence of SN hyperechogenicity was 17% (12/70) among patients and 8% (4/50) among control subjects, which was not significantly different (χ2 =2.110, P = 0.146).
DISCUSSION
Previous studies on patients with primary focal dystonia utilizing TCS have shown a higher prevalence of LN hyperechogenicity in patients compared with controls,[6,8,9] which may be the result of moderate copper or manganese accumulation.[17,18] The results of the present study regarding the prevalence of LN hyperechogenicity are consistent with these findings. LN hyperechogenicity was consistently observed herein in patients with cervical dystonia, in line with past reports,[6] but there were no differences in the frequency of LN hyperechogenicity in patients with blepharospasm or oromandibular dystonia compared with controls.
Several studies using specialized imaging techniques classify primary dystonia as a neurodevelopmental circuit disorder, involving the cortico-striato-pallido-thalamo-cortical and cerebello-thalamo-cortical pathways.[19,20] Certain abnormalities have been detected in specific forms of focal dystonia,[21] providing potential insight into the pathogenic mechanisms of these dystonias. Based on our present results and previous literature,[5,6,8,9] we now hypothesize that primary defects associated with different focal dystonia subtypes may localize to different regions of the brain. Therefore, LN hyperechogenicity may not necessarily be a universal sonographic feature of all primary dystonias. It is thus imperative to characterize different patient sonographic features in future TCS studies.
We report herein a marked female predominance among patients with cervical dystonia and blepharospasm. This finding is consistent with data from western countries,[22,23,24] and these gender differences may reflect the effects of hormones.[25] For example, estrogen has been reported to act as either a neurotrophic factor (preventing or modulating insults to the dopaminergic system) or an antagonist (suppressing and blocking central dopaminergic activity).[26] Differences in LN hyperechogenicity between dystonia subtypes could not be explained by the effects of hormones in the present study.
LN hyperechogenicity was found in 12% of controls in the present study while SN hyperechogenicity has been reported in approximately 10% of healthy subjects.[27] At present, it is accepted that the frequency of SN hyperechogenicity in patients with primary focal dystonia is comparable to that of healthy controls; our present findings support these observations. As with the SN hyperechogenicity observed in healthy subjects,[28] one may reasonably speculate that the LN hyperechogenicity reported herein could indicate subclinical impairment of sensory-motor circuits. Functional neuroimaging techniques, visualization of basal ganglia circuits, and follow-up studies with these subjects may provide further insight on this hypothesis.
Our data show no correlation between quantification of LN hyperechogenicity and the age or duration of disease, consistent with findings in previous studies.[8,9,29] Considering the relatively short disease duration of the subjects included in this study and the small sample sizes of previous reports, more follow-up studies using larger sample sizes should be conducted to further assess these correlations.
The main limitation of our study was the relatively small sample size used. In addition, the control group was not gender-matched with the patient group, and we were obliged to exclude some subjects due to poor temporal bone windows.
In conclusion, there is a greater prevalence of LN hyperechogenicity, as detected by TCS, in patients with primary focal dystonia than in neurologically normal populations. LN hyperechogenicity may not necessarily be a universal echo feature in patients with blepharospasm or oromandibular dystonia. Further studies utilizing larger sample sizes and other subtypes of primary focal dystonia should be conducted.
Financial support and sponsorship
This work was supported by grants from the Second Affiliated Hospital of Soochow University Preponderant Clinic Discipline Group (No. XKQ2015002), the Research and Innovation Program for College Graduates of Jiangsu Province (No. SJLX15-0578), and the Suzhou Science and Technology Development Program (No. SYSD2015082).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Geyer HL, Bressman SB. The diagnosis of dystonia. Lancet Neurol. 2006;5:780–90. doi: 10.1016/S1474-4422(06)70547-6. doi:10.1016/S1474-4422(06)70547-6. [DOI] [PubMed] [Google Scholar]
- 2.Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–34. doi: 10.1038/nrn2337. doi:10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- 3.Vidailhet M, Grabli D, Roze E. Pathophysiology of dystonia. Curr Opin Neurol. 2009;22:406–13. doi: 10.1097/WCO.0b013e32832d9ef3. doi:10.1097/WCO.0b013e32832d9ef3. [DOI] [PubMed] [Google Scholar]
- 4.Tanabe LM, Kim CE, Alagem N, Dauer WT. Primary dystonia: Molecules and mechanisms. Nat Rev Neurol. 2009;5:598–609. doi: 10.1038/nrneurol.2009.160. doi:10.1038/nrneurol.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoons E, Booij J, Nederveen AJ, Dijk JM, Tijssen MA. Structural, functional and molecular imaging of the brain in primary focal dystonia –A review. Neuroimage. 2011;56:1011–20. doi: 10.1016/j.neuroimage.2011.02.045. doi:10.1016/j.neuroimage.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 6.Naumann M, Becker G, Toyka KV, Supprian T, Reiners K. Lenticular nucleus lesion in idiopathic dystonia detected by transcranial sonography. Neurology. 1996;47:1284–90. doi: 10.1212/wnl.47.5.1284. doi:10.1212/WNL.47.5.1284. [DOI] [PubMed] [Google Scholar]
- 7.Becker G, Naumann M, Scheubeck M, Hofmann E, Deimling M, Lindner A, et al. Comparison of transcranial sonography, magnetic resonance imaging, and single photon emission computed tomography findings in idiopathic spasmodic torticollis. Mov Disord. 1997;12:79–88. doi: 10.1002/mds.870120114. doi:10.1002/mds.870120114. [DOI] [PubMed] [Google Scholar]
- 8.Walter U, Buttkus F, Benecke R, Grossmann A, Dressler D, Altenmüller E. Sonographic alteration of lenticular nucleus in focal task-specific dystonia of musicians. Neurodegener Dis. 2012;9:99–103. doi: 10.1159/000330712. doi:10.1159/000330712. [DOI] [PubMed] [Google Scholar]
- 9.Walter U, Blitzer A, Benecke R, Grossmann A, Dressler D. Sonographic detection of basal ganglia abnormalities in spasmodic dysphonia. Eur J Neurol. 2014;21:349–52. doi: 10.1111/ene.12151. doi:10.1111/ene.12151. [DOI] [PubMed] [Google Scholar]
- 10.Albanese A, Barnes MP, Bhatia KP, Fernandez-Alvarez E, Filippini G, Gasser T, et al. A systematic review on the diagnosis and treatment of primary (idiopathic) dystonia and dystonia plus syndromes: Report of an EFNS/MDS-ES task force. Eur J Neurol. 2006;13:433–44. doi: 10.1111/j.1468-1331.2006.01537.x. doi:10.1111/j.1468-1331.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 11.Dong Q, Wang Z, Du Y, Shen F, Yu L, Li Y. EFNS guidelines on diagnosis and treatment of primary dystonias. J Neurol Neurorehabil. 2011;8:200–7. doi: 10.1111/j.1468-1331.2010.03042.x. [DOI] [PubMed] [Google Scholar]
- 12.Albanese A, Asmus F, Bhatia KP, Elia AE, Elibol B, Filippini G, et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur J Neurol. 2011;18:5–18. doi: 10.1111/j.1468-1331.2010.03042.x. doi:10.1111/j.1468-1331.2010.03042.x. [DOI] [PubMed] [Google Scholar]
- 13.Walter U, Behnke S, Eyding J, Niehaus L, Postert T, Seidel G, et al. Transcranial brain parenchyma sonography in movement disorders: State of the art. Ultrasound Med Biol. 2007;33:15–25. doi: 10.1016/j.ultrasmedbio.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Walter U, Školoudík D. Transcranial sonography (TCS) of brain parenchyma in movement disorders: Quality standards, diagnostic applications and novel technologies. Ultraschall Med. 2014;35:322–31. doi: 10.1055/s-0033-1356415. doi:10.1055/s-0033-1356415. [DOI] [PubMed] [Google Scholar]
- 15.Go CL, Frenzel A, Rosales RL, Lee LV, Benecke R, Dressler D, et al. Assessment of substantia nigra echogenicity in German and Filipino populations using a portable ultrasound system. J Ultrasound Med. 2012;31:191–6. doi: 10.7863/jum.2012.31.2.191. [DOI] [PubMed] [Google Scholar]
- 16.Becker G, Berg D, Francis M, Naumann M. Evidence for disturbances of copper metabolism in dystonia: From the image towards a new concept. Neurology. 2001;57:2290–4. doi: 10.1212/wnl.57.12.2290. doi:10.1212/WNL.57.12.2290. [DOI] [PubMed] [Google Scholar]
- 17.Walter U, Krolikowski K, Tarnacka B, Benecke R, Czlonkowska A, Dressler D. Sonographic detection of basal ganglia lesions in asymptomatic and symptomatic Wilson disease. Neurology. 2005;64:1726–32. doi: 10.1212/01.WNL.0000161847.46465.B9. doi:10.1212/01.WNL.0000161847.46465.B9. [DOI] [PubMed] [Google Scholar]
- 18.Becker G, Berg D, Rausch WD, Lange HK, Riederer P, Reiners K. Increased tissue copper and manganese content in the lentiform nucleus in primary adult-onset dystonia. Ann Neurol. 1999;46:260–3. doi: 10.1002/1531-8249(199908)46:2<260::aid-ana18>3.0.co;2-6. doi:10.1002/1531-8249(199908) 46:2%2C260:AID-ANA18%3E3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Niethammer M, Carbon M, Argyelan M, Eidelberg D. Hereditary dystonia as a neurodevelopmental circuit disorder: Evidence from neuroimaging. Neurobiol Dis. 2011;42:202–202. doi: 10.1016/j.nbd.2010.10.010. doi:10.1016/j.nbd.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehéricy S, Tijssen MA, Vidailhet M, Kaji R, Meunier S. The anatomical basis of dystonia: Current view using neuroimaging. Mov Disord. 2013;28:944–57. doi: 10.1002/mds.25527. doi:10.1002/mds.25527. [DOI] [PubMed] [Google Scholar]
- 21.Defazio G, Berardelli A, Hallett M. Do primary adult-onset focal dystonias share aetiological factors? Brain. 2007;130(Pt 5):1183–93. doi: 10.1093/brain/awl355. doi:10.1093/brain/awl355. [DOI] [PubMed] [Google Scholar]
- 22.Epidemiological Study of Dystonia in Europe (ESDE) Collaborative Group. A prevalence study of primary dystonia in eight European countries. J Neurol. 2000;247:787–92. doi: 10.1007/s004150070094. [DOI] [PubMed] [Google Scholar]
- 23.Asgeirsson H, Jakobsson F, Hjaltason H, Jonsdottir H, Sveinbjornsdottir S. Prevalence study of primary dystonia in Iceland. Mov Disord. 2006;21:293–8. doi: 10.1002/mds.20674. doi:10.1002/mds.20674. [DOI] [PubMed] [Google Scholar]
- 24.Papantonio AM, Beghi E, Fogli D, Zarrelli M, Logroscino G, Bentivoglio A, et al. Prevalence of primary focal or segmental dystonia in adults in the district of Foggia, Southern Italy:A service-based study. Neuroepidemiology. 2009;33:117–23. doi: 10.1159/000226124. doi:10.1159/000226124. [DOI] [PubMed] [Google Scholar]
- 25.Jamora RD, Tan AK, Tan LC. A 9-year review of dystonia from a movement disorders clinic in Singapore. Eur J Neurol. 2006;13:77–81. doi: 10.1111/j.1468-1331.2006.01150.x. doi:10.1111/j.1468-1331.2006.01150.x. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto S, Nishimura M, Shibasaki H, Kaji R. Epidemiology of primary dystonias in Japan: Comparison with western countries. Mov Disord. 2003;18:1196–8. doi: 10.1002/mds.10480. doi:10.1002/mds.10480. [DOI] [PubMed] [Google Scholar]
- 27.Godau J, Schweitzer KJ, Liepelt I, Gerloff C, Berg D. Substantia nigra hypoechogenicity: Definition and findings in restless legs syndrome. Mov Disord. 2007;22:187–92. doi: 10.1002/mds.21230. doi:10.1002/mds.21230. [DOI] [PubMed] [Google Scholar]
- 28.Berg D, Seppi K, Behnke S, Liepelt I, Schweitzer K, Stockner H, et al. Enlarged substantia nigra hyperechogenicity and risk for Parkinson disease: A 37-month 3-center study of 1847 older persons. Arch Neurol. 2011;68:932–7. doi: 10.1001/archneurol.2011.141. doi:10.1001/archneurol.2011.141. [DOI] [PubMed] [Google Scholar]
- 29.Hagenah J, König IR, Kötter C, Seidel G, Klein C, Brüggemann N. Basal ganglia hyperechogenicity does not distinguish between patients with primary dystonia and healthy individuals. J Neurol. 2011;258:590–5. doi: 10.1007/s00415-010-5795-x. doi:10.1007/s00415-010-5795-x. [DOI] [PubMed] [Google Scholar]


