Abstract
Background:
Mesenchymal stem cells (MSCs) transplantation has been proven to have therapeutic potential for acute liver failure (ALF). However, the mechanism remains controversial. Recently, modulation of inflammation by MSCs has been regarded as a crucial mechanism. The aim of the present study was to explore the soluble cytokines secreted by MSCs and their therapeutic effects in ALF.
Methods:
MSCs isolated from Sprague-Dawley rats were identified by fluorescence-activated cell sorting analysis. Conditioned medium derived from MSCs (MSCs-CM) was collected and analyzed by a cytokine microarray. MSCs and MSCs-CM were transplanted into rats with D-galactosamine-induced ALF. Liver function, survival rate, histology, and inflammatory factors were determined. Exogenous recombinant rat interleukin (IL)-10, anti-rat IL-10 antibody, and AG490 (signal transducer and activator of transcription 3 [STAT3] signaling pathway inhibitor) were administered to explore the therapeutic mechanism of MSCs-CM. Statistical analysis was performed with SPSS version 19.0, and all data were analyzed by the independent-sample t-test.
Results:
There are statistical differences of the survival curve between ALF+MSCs group and ALF+Dulbecco's modified Eagle's medium (DMEM) group, as well as ALF+MSCs-CM group and ALF+DMEM group (all P < 0.05). Serum alanine aminotransferase (ALT) level in the ALF+MSCs and ALF+MSCs-CM groups was lower than that in the ALF+DMEM group (865.53±52.80 vs. 1709.75±372.12 U/L and 964.72±414.59 vs. 1709.75±372.12 U/L, respectively, all P < 0.05); meanwhile, serum aspartate aminotransferase (AST) level in the ALF+MSCs and ALF+MSCs-CM groups was lower than that in the ALF+DMEM group (2440.83±511.94 vs. 4234.35±807.30 U/L and 2739.83±587.33 vs. 4234.35±807.30 U/L, respectively, all P < 0.05). Furthermore, MSCs or MSCs-CM treatment significantly reduced serum interferon-γ (IFN-γ), IL-1β, IL-6 levels and increased serum IL-10 level compared with DMEM (all P < 0.05). Proteome profile analysis of MSCs-CM indicated the presence of anti-inflammatory factors and IL-10 was the most distinct. Blocking of IL-10 confirmed the therapeutic significance of this cytokine. Phosphorylated STAT3 was upregulated after IL-10 infusion and inhibition of STAT3 by AG490 reversed the therapeutic effect of IL-10.
Conclusions:
The factors released by MSCs, especially IL-10, have the potential for therapeutic recovery of ALF, and the STAT3 signaling pathway may mediate the anti-inflammatory effect of IL-10.
Keywords: Conditioned Medium, Immunoregulation, Liver Disease, Signal Transducer and Activator of Transcription 3 Signaling Pathway, Stem Cell Transplantation
INTRODUCTION
Acute liver failure (ALF) is a severe disease with a high mortality rate characterized by short-term loss of liver function as well as necrosis of a large number of hepatocytes. Liver transplantation is the most commonly used therapy but limited due to organ rejection, lack of donors, and high costs. Recently, cell-based therapies have focused on the use of mesenchymal stem cells (MSCs) for ALF.[1,2,3,4]
When liver is damaged by various factors such as xenobiotics, drugs, and viruses, cellular debris released by necrotic cells will increase vascular permeability and infiltration of mononuclear macrophages.[5,6,7] Moreover, phagocytosis induced by necrotic cells will cause a cascading release of pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, chemokines, and leukotrienes. The uncontrolled immune response plays an essential role in the pathological process of ALF.[8]
Our previous study confirmed the therapeutic effect of MSCs for ALF,[9,10] but we did not explore the relevant mechanism. Currently, the prevailing view is that the therapeutic effect of MSCs is mediated by regulating the inflammatory microenvironment and secreting growth factors.[11,12,13,14] However, only a few studies have shown the benefit of factors secreted by MSCs on damaged liver in vivo.[15]
In this study, we collected conditioned medium derived from MSCs (MSCs-CM) and investigated its therapeutic effect in ALF. In addition, we performed a cytokine microarray analysis to identify the detailed inflammatory factors secreted by MSCs. We found that IL-10 secreted by MSCs had a significant benefit in the treatment of ALF.
METHODS
Animals
Male, 7-week-old Sprague-Dawley rats were purchased from the Laboratory Animal Center of the Affiliated Drum Tower Hospital of Nanjing University Medical School, and housed individually, placed in a ventilated cabinet under controlled air pressure and temperature conditions, under daily cycles of alternating 12-h light/dark. They had sterile water and sterile standard pelleted rodent diet. The rats were killed by cervical spinal dislocation. All procedures in rats were approved by the Institutional Animal Care and Use Committee of Nanjing University under the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Mesenchymal stem cells isolation and characterization
MSCs were isolated by bone marrow aspirates from the femur of Sprague-Dawley rats. Mononuclear cells were collected by gradient centrifugation over a Ficoll Histopaque layer (20 min, 400 × g, density 1.077 g/ml) and seeded at a density of 1 × 106 cells/cm2 in growth medium containing low-glucose Dulbecco's modified Eagle's medium (DMEM, Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 mg/ml). The nonadherent cells were removed after the first 24 h and changed every 3–4 days thereafter. When the cells reached 80% confluence, they were detached using 0.25% (w/v) trypsin-ethylene diamine tetraacetic acid and replated at a density of 1 × 104 cells/cm2 for expansion. Surface marker identification of the cultured MSCs was performed with an FACScan flow cytometer (Becton Dickinson, San Diego, CA, USA) using phycoerythrin-labeled monoclonal antibody to CD29, CD44, CD45, and CD90 (Antigenix America, Huntington Station, NY, USA). Isotypic antibodies served as the control.
Preparation of conditioned medium and cytokine expression profiling
The isolated adherent MSCs were cultured in DMEM in 35-mm plates (Thermo Fisher Scientific, Pittsburgh, PA, USA), 5 plates per array. The control dishes only contained DMEM. The CM was collected on day 7 after inoculation. To prevent any cells remaining in the CM, the medium was centrifuged for 1 h at 15,700 × g at 0°C.
The CM was used for the Raybiotech cytokine antibody array AAM-INF-G1 (OE Biotech, Shanghai, China). Membranes were blocked (blocking buffer) followed by adding the CM or control medium. After 2-h incubation, membranes were repeatedly washed and then incubated with biotin-conjugated antibodies for 2 h at room temperature. The membranes were washed followed by detection with horseradish peroxidase (HRP)-conjugated streptavidin for 1 h, followed by several washes. The membranes were processed followed by exposure to hyper film (GE Healthcare, Little Chalfont, Bucks, UK). The film was processed and scanned, and the densitometric value of each spot on the array was measured using the UVP GelDoc system (UVP, Cambridge, UK). Two independent experiments were performed and analyzed.
In vivo animal studies
An ALF model was generated by intraperitoneal administration of one dose of 1.0 g/kg D-galactosamine (D-Gal) into Sprague-Dawley rats. Twenty-four hours later, the rats were divided into three groups using random number method and underwent intravenous tail vein transplantation of (1) 1 × 106 MSCs dissolved in 1 ml DMEM (n = 20); (2) 1.0 ml MSCs-CM (n = 20); or (3) 1.0 ml DMEM (n = 20). In each group, ten mice were picked randomly for survival analysis. Serum was collected on day 3 for biochemical analyses and inflammatory factors detection. The livers were dissected out and fixed in 4% formaldehyde on day 14 for histology.
To explore the hepatoprotective effect of IL-10, 10 μg/kg recombinant rat IL-10 (R & D Systems, Minneapolis, MN, USA) dissolved in DMEM (n = 10) and 20 mg/kg antirat IL-10 antibody (R & D Systems) combined with 1 ml MSCs-CM (n = 10) were administered intravenously to rats 24 h after injection of D-Gal. Three days after injection of D-Gal, serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were detected.
For the signal transducer and activator of transcription 3 (STAT3) experiment, 10 μmol/L AG490 (PHZ1204; Invitrogen, Carlsbad, CA, USA) (n = 10) was administered 30 min before D-Gal injection. Twenty-four hours later, 10 μg/kg rat recombinant IL-10 (R & D Systems) dissolved in DMEM (n = 10) was administered intravenously to rats. Three days after injection of D-Gal, liver tissues were obtained for detection of phosphorylated STAT3 (pSTAT3) by Western blotting, and blood samples were acquired for measurement of ALT, AST, and inflammatory factors.
Liver function and histological analysis
Serum AST and ALT levels in rat were measured with an automated biochemical analyzer on day 3 after D-Gal administration. For histology, livers were fixed in 4% formaldehyde for 24 h and embedded in paraffin on day 14 after D-Gal administration. Five-micrometer-thick liver sections were deparaffinized and fixed. Sections were stained with hematoxylin and eosin (H and E, Sigma-Aldrich, St Louis, MO, USA).
Enzyme-linked immunosorbent assay for inflammatory factors
To evaluate the anti-inflammatory effect of MSCs-CM and MSCs, we measured the serum concentration of the following cytokines by enzyme-linked immunosorbent assay (ELISA) on day 3 after induction of ALF: Granulocyte colony-stimulating factor (G-CSF)/CSF-3, IL-6, eotaxin/chemokine CC ligand (CCL) 11, IL-1α, IL-1β, IL-10, IL-2, TNF-α, and chemokine CXC ligand (CXCL) 1 (R & D Systems).
To evaluate whether STAT3 signaling pathway inhibitor neutralized the anti-inflammatory effect of IL-10, we injected AG490 after infusion of IL-10 and then measured the serum concentration of the following cytokines by ELISA: TNF-α, interferon-γ (IFN-γ), and IL-6 (R & D Systems).
Western blotting
Liver tissues were collected at day 7 after injection of D-Gal. For detection of pSTAT3, liver tissue samples were weighed before homogenization. Liver tissues (100 mg) were minced and homogenized in 1 ml DMEM with a glass homogenizer on ice. The homogenates were centrifuged at 15,700 g for 10 min, and the supernatants were stored at −80°C prior to analysis. Protein extract (20 μg) was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. The membrane was blocked with 5% milk in Tris-buffer saline solution (pH 7.6) containing 0.05% Tween-20, and incubated with primary antibodies for pSTAT3 (Abcam, Cambridge, UK) overnight at 4°C. The membrane was then incubated for 1 h with HRP-conjugated secondary antibody at room temperature, washed and developed with the ECL plus kit (Millipore, Billerica, MA, USA). Band density was analyzed by ImageJ software (National Institutes of Health, USA). This software attributed each specific data point a gray value, which enabled the semiquantitative analysis of protein expression.
Statistical analysis
All experiments were replicated a minimum of three times. Statistical analysis was performed with SPSS version 19.0 (IBM, USA), and data were expressed as means ± standard deviation (SD). For survival analyses, Kaplan–Meier method was used. All other data were analyzed by the independent-sample t-test. A value of P < 0.05 was considered statistically significant.
RESULTS
Identification of mesenchymal stem cells
MSCs retained a fibroblastic morphology after repeated passages [Figure 1a], and their immunophenotypical characterization was confirmed by flow cytometry. Over 90% of the isolated MSCs were positive for CD29, CD44, and CD90, but not for the hematopoietic marker CD45 [Figure 1b].
Figure 1.
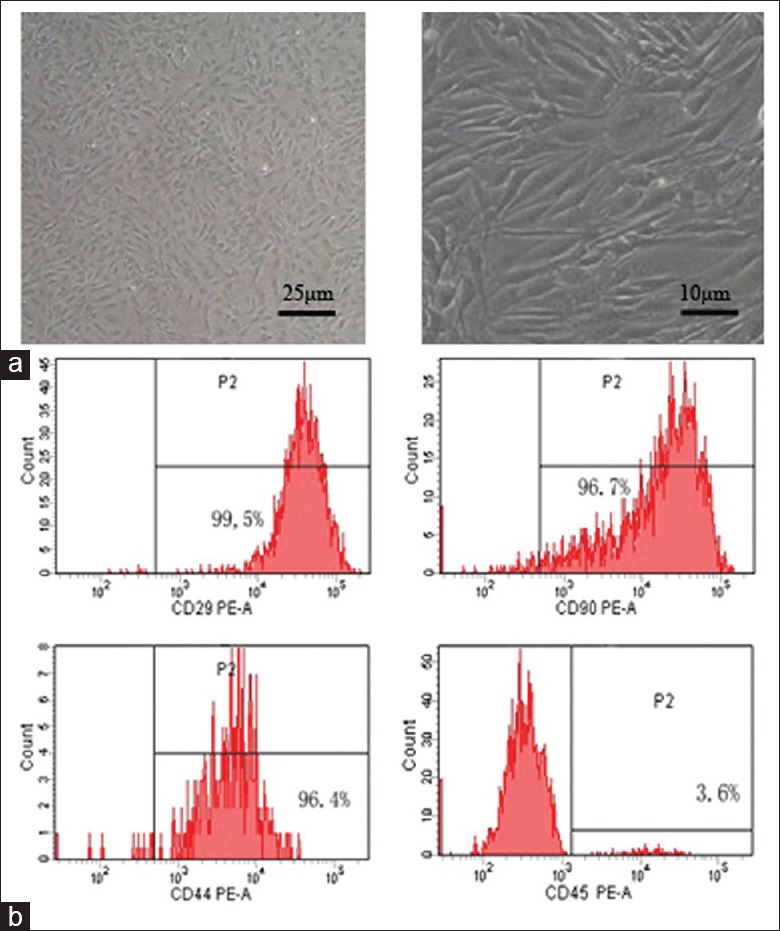
Identification of MSCs. (a) Microscope images of mice bone marrow MSCs. MSCs retained a fibroblastic morphology. (b) Flow cytometry analysis of MSCs. MSCs: Mesenchymal stem cells; PE: Phycoerythrin.
Mesenchymal stem cells-conditioned medium improves survival rate and liver histopathology and inhibits liver enzyme release
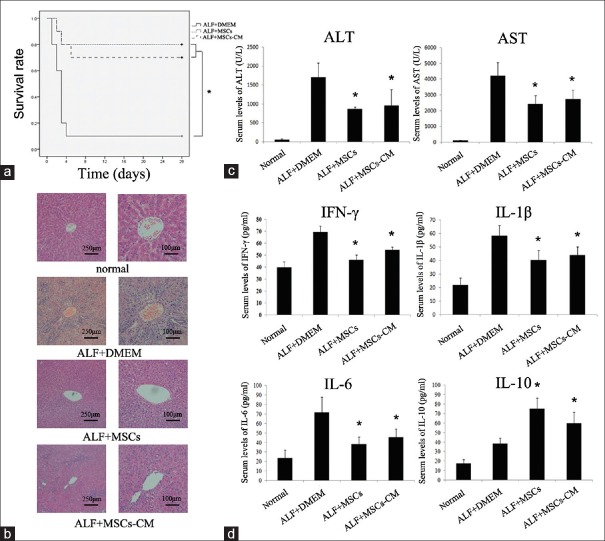
We randomly selected 10 mice from each group for survival rate analysis. During the 4 weeks after cell transplantation, only 1 of 10 rats infused with DMEM survived up to 4 weeks, and 8 of 10 rats infused with MSCs survived up to 4 weeks. Seven of 10 mice infused with MSCs-CM survived until week 4. There are statistical differences of the survival curve between ALF+MSCs group and ALF+DMEM group, as well as ALF+MSCs-CM group and ALF+DMEM group (all P < 0.05) [Figure 2a].
Figure 2.
Therapeutic efficacy was evaluated by survival rate, liver function, histological and inflammatory factors analyses. (a) Survival analysis of D-Gal-treated mice treated with MSCs, MSCs-CM, or DMEM. (b) Liver H and E staining of each group at 2nd week after cell transplantation. (c) Serum ALT and AST levels collected at 3rd day after administration of D-Gal. (d) Serum levels of IFN-γ, IL-1β, IL-6, and IL-10 of each group at 3rd day after D-Gal injection. *P < 0.05 versus ALF + DMEM group. D-Gal: D-galactosamine; ALF: Acute liver failure; MSCs: Mesenchymal stem cells; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; IFN-γ: Interferon-γ; IL: Interleukin; DMEM: Dulbecco's modified Eagle's medium.
To investigate the liver histology of rats with ALF after transplantation of MSCs or MSCs-CM, H and E staining was conducted [Figure 2b]. The liver histology of the normal rats showed uniform cellular morphology. In contrast, failing liver in the ALF + DMEM group showed extensive hepatocyte necrosis with hemorrhaging involving entire lobules, and the hepatocytes had swollen cytoplasm. However, in the rats infused with MSCs or MSCs-CM, most of the tissues returned to normal, and there were only a few of necrotic areas, indicating repair of hepatocytes.
In our preliminary study for ALF model, our data showed that on day 3 after D-Gal injection, ALT reached the highest level and then decreased gradually whereas AST reached peak on day 2 [Supplementary Figure 1 (249.1KB, tif) ]. For the overall consideration, we detected ALT and AST levels on day 3 after D-Gal injection as markers for liver condition in this study. Results showed that, serum ALT level in the ALF+MSCs and ALF+MSCs-CM groups was lower than that in the ALF+DMEM group (865.53±52.80 vs. 1709.75±372.12 U/L and 964.72±414.59 vs. 1709.75±372.12 U/L, respectively, all P < 0.05); meanwhile, serum AST level in the ALF+MSCs and ALF+MSCs-CM groups was lower than that in the ALF+DMEM group (2440.83±511.94 vs. 4234.35±807.30 U/L and 2739.83±587.33 vs. 4234.35±807.30 U/L, respectively, all P < 0.05) [Figure 2c].
ALT and AST levels of ALF rats (n = 6). *P < 0.05. ALT: Alanine aminotransferase; AST: Aspar tate aminotransferase; ALF: Acute liver failure.
Down regulation of systemic inflammation by mesenchymal stem cells-conditioned medium treatment
We detected the serum levels of inflammatory factors (G-CSF/CSF-3, IFN-γ, IL-6, eotaxin/CCL 11, IL-1α, IL-1β, IL-10, IL-2, TNF-α, and CXCL1) by ELISA on day 3 after infusion of MSCs or MSCs-CM. ELISA showed a decrease in the expression of pro-inflammatory molecules such as IFN-γ, IL-1β, and IL-6 after administration of MSCs or MSCs-CM compared to the DMEM, while expression of the anti-inflammatory cytokine IL-10 was significantly increased (all P < 0.05) [Figure 2d]. These data indicate that MSCs have an anti-inflammatory role that is exerted through secretion of anti-inflammatory cytokines.
Detection of cytokines in mesenchymal stem cells-conditioned medium
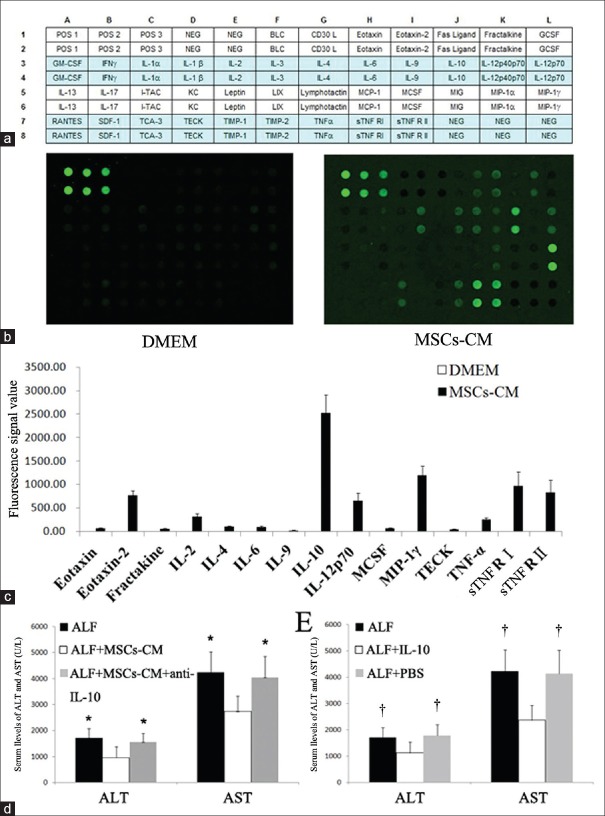
To determine the molecular mediators of MSC therapy in ALF, we examined MSCs-CM using a 40-cytokine antibody microarray chip for soluble factors [Figure 3a]. DMEM was used as a control. Of the numerous factors represented on the array, 15 were significantly abundant in MSCs-CM while none was detected in DMEM [Figure 3b and 3c]. Some of the identified cytokines such as IL-2, IL-6, IL-10, and macrophage CSF are important in the immune response, as well as in cell proliferation and differentiation. Some factors such as eotaxin, fractalkine, macrophage inflammatory protein-1γ, and thymus-expressed chemokine show chemokine activity and are involved in cell migration and adhesion. Analysis of these 15 factors for molecular and cellular functions is shown in Table 1.
Figure 3.
MSCs-CM proteome analysis and blocking of IL-10 in MSCs-CM prevented recovery of acute hepatic failure. (a) Map of 40 cytokines. (b) Proteome profile image for cytokines in MSCs-CM. (c) Expression levels of cytokines related to inflammation in MSCs-CM. (d) Serum ALT and AST levels after transplantation of MSCs-CM or MSCs-CM with anti-IL-10 antibody. (e) Serum ALT and AST after administration of recombinant rat IL-10 or PBS. *P < 0.05 versus ALF + MSCs-CM group. †P < 0.05 versus ALF + IL-10 group. POS: Positive control; NEG: Negative control; BLC: B lymphocyte chemokine; CD30L: Cluster of differentiation 30 ligand; GCSF: Granulocyte colony-stimulating factor; GM-CSF: Granulocyte macrophage colony-stimulating factor; IFN-γ: Interferon-γ; IL: Interleukin; I-TAC: Interferon induction of T-cell chemokine; KC: Keratinocyte-derived cytokine; LIX: Lipopolysaccharide induced CXC chemokine; MCP-1: Monocyte chemotactic protein 1; MCSF: Macrophage colony-stimulating factor; MIG: Monokine inducible by γ-interferon; MIP: Macrophage inflammatory protein; SDF-1: Stromal cell-derived factor 1; TCA-3: CCL1/I-309, eosinophils chemotactic protein; TECK: Thymus expressed chemokine; TIMP: Tissue inhibitor of metalloproteinase; TNF-α: Tumor necrosis factor-α; sTNF R: Soluble tumor necrosis factor receptor; MSCs: Mesenchymal stem cells; MSCs-CM: Mesenchymal stem cells-conditioned medium; ALF: Acute liver failure; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; PBS: Phosphate buffer solution.
Table 1.
Molecular and cellular functions of the factors in conditional medium
| Name | Molecular functions | Cellular functions |
|---|---|---|
| Eotaxin | Chemokine activity | Wound healing Inflammatory response Cell migration |
| Eotaxin-2 | Chemokine activity | Wound healing Inflammatory response Angiogenesis |
| Fractakine | Chemokine activity | Cell migration Cell adhesion |
| IL-2 | Cytokine activity | T-cell proliferation Immune modulation Hematopoiesis |
| IL-4 | Cytokine activity | B-cell activation Inhibiting Th1 cell |
| IL-6 | Cytokine activity | T-cell proliferation B-cell proliferation Immune response |
| IL-9 | Cytokine activity | Stimulating Th2 cell and mastocyte |
| IL-10 | Cytokine activity Growth factor activity | B-cell proliferation Cell signaling Cytokine synthesis inhibition Inhibiting macrophage |
| IL-12p70 | Cytokine activity | Activation of NK cells Induction of Th1 cell differentiation |
| MCSF | Cytokine activity Growth factor activity | Immune response Cell proliferation and differentiation |
| MIP-1γ | Chemokine activity | Inflammatory response Wound healing Cell adhesion |
| TECK | Chemokine activity | Lymphocyte migration Immune response |
| TNF-α | Cytokine activity | Inflammatory response Activation of endothelial cells |
| sTNF RI | Cytokine activity | Receptor of TNF-α Immune modulation |
| sTNF RII | Cytokine activity | Receptor of TNF-α Immune modulation |
IL: Interleukin; MCSF: Macrophage colony-stimulating factor; MIP-1γ: Macrophage inflammatory protein-1γ; TECK: Thymus expressed chemokine; TNF-α: Tumor necrosis factor-α; sTNF R: Soluble tumor necrosis factor receptor; Th: T helper; NK: Natural killer.
Hepatoprotective role of interleukin-10 in acute liver failure
Cytokine microarray analysis revealed that IL-10 in MSCs-CM was the most distinct among the anti-inflammatory cytokines [Figure 3c], indicating that this molecule plays a pivotal role in the therapeutic effect in ALF. Hence, we investigated the potential therapeutic role of IL-10. ALF rats were treated intravenously with 20 mg/kg anti-rat IL-10 antibody combined with 1 ml MSCs-CM. Three days later, ALT and AST were not significantly improved in the presence of IL-10-blocked MSCs-CM. However, administration of MSCs-CM resulted in a significant decrease in ALT and AST levels [Figure 3d]. The hepatoprotective role of IL-10 was also evaluated following intravenous administration of 10 μg/kg IL-10 to ALF rats. There was a significant decrease in the levels of ALT and AST in the ALF + IL-10 group when compared with the ALF or ALF + phosphate buffer solution group [Figure 3e], reversing the hepatoprotective effect of IL-10.
Signal transducer and activator of transcription 3 mediated the therapeutic effect of interleukin-10 in acute liver failure
The STAT3 signaling pathway involves in anti-inflammatory response (AIR). We previously detected STAT3 expression in normal rats and ALF rats injected with/without MSCs [Supplementary Figure 2 (193.4KB, tif) ]. When compared with normal rats, pSTAT3 expression in ALF rats had no significant change (P > 0.05) whereas MSCs could enhance pSTAT3 expression significantly (P < 0.05). We supposed that upregulation of pSTAT3 by MSCs was mediated by IL-10. Therefore, we assayed the effect of IL-10 implantation on the activation of STAT3 signaling pathways by examining pSTAT3 via western blotting analysis. As shown in Figure 4a, IL-10 injection resulted in significantly increased pSTAT3 while the increases were remarkably attenuated by AG490 (P < 0.05). We detected serum transaminases and some inflammatory cytokines (G-CSF/CSF-3, IFN-γ, IL-6, eotaxin/CCL 11, IL-1α, IL-1β, IL-10, IL-2, TNF-α, and CXCL1) after administration of AG490. Consistent with our expectations, liver function indexes (ALT and AST) and proinflammatory factors (TNF-α, IFN-γ, and IL-6) increased again after administration of AG490 [Figure 4b and 4c]. These data demonstrated that AG490 reversed the therapeutic effect and anti-inflammatory effect of IL-10, suggesting that the STAT3 signaling pathway mediates the therapeutic effect and anti-inflammatory effect of IL-10.
Figure 4.
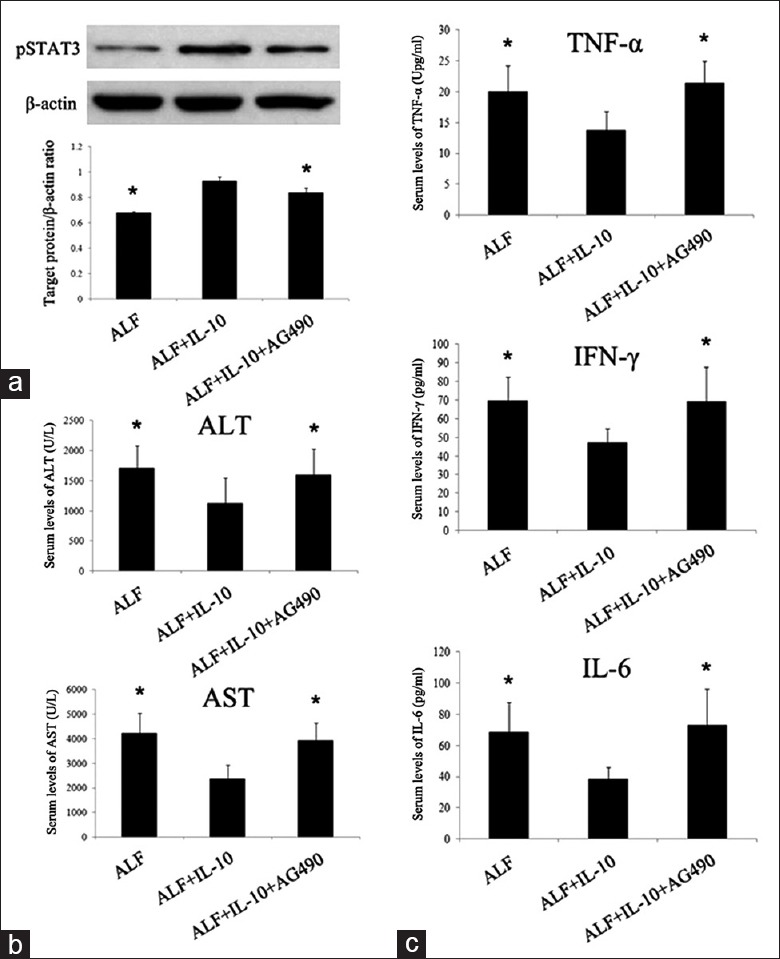
Inhibition of STAT3 neutralized the therapeutic and anti-inflammatory effect of IL-10 on ALF. (a) Western blotting analysis of liver tissues for pSTAT3 at 7th day after injecting D-Gal. (b) Concentrations of ALT and AST in blood serum at 3rd day after D-Gal injection. (c) Serum levels of TNF-α, IFN-γ, and IL-6 of each group at 3rd day after D-Gal injection. *P < 0.05 versus ALF + IL-10 group. STAT3: Signal transducer and activator of transcription 3; pSTAT3: Phosphorylated signal transducer and activator of transcription 3; D-Gal: D-galactosamine; ALF: Acute liver failure; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; TNF-α: Tumor necrosis factor α; IFN-γ: Interferon-γ; IL: Interleukin.
STAT3 expression in normal rats and ALF rats injected with/without MSCs. (a) Western blotting analysis of liver tissues at 7th day after injecting D-Gal. (b) The band intensities of scanning densitometry. *P < 0.05 versus ALF group. STAT3: Signal transducer and activator of transcription 3; pSTAT3: Phosphorylated STAT3; MSCs: Mesenchymal stem cells; ALF: Acute liver failure.
DISCUSSION
The mechanism of MSCs-based liver repair remains controversial. Some researchers have reported that stem cells facilitate liver regeneration by transdifferentiation into primary hepatocytes.[16,17] However, this property has been challenged in subsequent reports. Some researchers reported that only limited donor-derived cells were detected in vivo.[18,19] Such limited cells were not enough to replace the damaged hepatocytes. Therefore, there must be other mechanisms mediating the effect of MSCs. Recently, the concept of stem cell transplantation secreting cytokines and exerting an immunoregulatory effect is gaining support. The released factors are known to be important for cell survival, proliferation, and immunoregulation during liver repair.[20,21,22,23,24] Cervelló et al.[25] showed that CD133(+) bone marrow-derived stem cells induced proliferation of endometrial surrounding cells through paracrine molecules such as thrombospondin 1 and insulin-like growth factor 1. Yoo et al.[26] demonstrated human adipose-tissue-derived stem cells decreased proliferation of antigen-specific T helper (Th) 1/Th17 cells and induced production of anti-inflammatory cytokine IL-10 in splenocytes. To verify the main therapeutic effect of MSCs, we examined whether delivery of MSCs-CM could improve liver function.
Our study demonstrated that MSCs and MSCs-CM both improved the liver function of ALF rats, as well as survival rate. Serum levels of ALT and AST in the ALF + MSCs or ALF + MSCs-CM group were significantly lower than in the ALF + DMEM group [Figure 2c]. Although MSCs showed more improvement than MSCs-CM, there were no significant differences between the two therapies. This result reminded us that the main therapeutic mechanism of MSCs for ALF is secreting some soluble factors rather than transdifferentiation. Moreover, we evaluated the anti-inflammatory effect of MSCs and MSCs-CM. We measured the serum concentration of the following cytokines at day 3 after ALF by ELISA: IFN-γ, IL-1β, IL-6, and IL-10. When compared with the ALF + DMEM group, IFN-γ, IL-1β, and IL-6 levels in the ALF + MSCs or ALF + MSCs-CM group were significantly lower whereas IL-10 levels were significantly higher. However, no differences of IFN-γ, IL-1β, IL-6, and IL-10 levels were observed between the ALF + MSC and ALF + MSCs-CM groups [Figure 2d]. These data suggested that the factors in MSCs-CM were capable of reducing the inflammatory response.
Some other studies also investigated the therapeutic effect of CM from stem cells for protection against liver injury.[27,28,29] These studies were consistent with our results, but the detailed secreted factors from stem cells were not explored. We found a significant reduction of inflammation in the rats treated with MSCs-CM suggesting that the factors in MSCs-CM are capable of reducing the inflammatory response. This supposition was investigated by using a 40-cytokine antibody microarray chip, which showed that 15 factors were significantly abundant in MSCs-CM. Our analysis demonstrated that IL-10 in MSCs-CM was the most distinct among the anti-inflammatory cytokines, suggesting the crucial role of IL-10 in the treatment of ALF with MSCs. Subsequently, we confirmed the hepatoprotective role of IL-10 in ALF by administration of anti-IL-10 antibody and exogenous recombinant rat IL-10. It is clear that the paracrine effects and secreted molecules, especially IL-10, from donor cells may be the key issue for the rapid improvement in the liver.
We preliminarily explored the relevant signaling pathway mediating the therapeutic effect of IL-10 for ALF. STAT3 is activated by immune effector cells and has emerged over the past few years as an important negative regulator of inflammatory responses.[30] STAT3 is involved in a great diversity of cellular functions, including metabolism, development, and in orchestrating responses to environmental stimuli, particularly the anti-inflammatory effect. According to other studies, IL-10 initiates the AIR upon binding to its cognate receptor (IL-10R) expressed in macrophages, natural killer cells, T-cells, dendritic cells, B-cells, and mast cells, and then activates Janus kinase 1 (JAK1), activated JAK1 pSTAT3. Upon entering the nucleus, STAT3 activates specific target genes and in turns repress the expression of pro-inflammatory genes partly at the transcriptional level.[31,32] STAT3 not only mediates the AIR of IL-10 but also is activated by other cytokines (e.g., IL-6 and IL-22). STAT3 is the only obligate factor required for IL-10-mediated anti-inflammatory signaling, namely IL-10 receptor only combined with STAT3.[31,33] These studies were consistent with our results.
STAT3 also has an important role in liver diseases. For instance, in alcoholic liver injury, ethanol-fed endothelial cell-specific STAT3 knockout (STAT3 [E-/-]) mice displayed greater hepatic inflammation and hepatic injury compared with wild-type mice.[34] Moreover, deletion of STAT3 in myeloid cells results in markedly enhanced liver inflammation after CCl4 injection,[35] and hepatocyte-specific STAT3 knockout mice with an ethanol-containing diet showed greater hepatic steatosis, hypertriglyceridemia, and hepatic expression of lipogenic genes.[36] The STAT3 pathway is closely related to hepatocyte damage and inflammation. However, whether the STAT3 signaling pathway is involved in the inhibitory effect of MSCs on inflammation in ALF rats has not yet been reported. In this study, we discovered that IL-10 activated STAT3 in ALF rats [Figure 4a]. In addition, inhibition of the STAT3 pathway by AG490 reversed the therapeutic effect of IL-10 [Figure 4b and 4c]. These results indicate that the STAT3 pathway mediates the anti-inflammatory effect of IL-10 secreted by MSCs in ALF.
Currently, exploiting inflammation-related signaling pathways mediated by MSCs in ALF has been less characterized. Our future work will focus on exploring the molecular mechanism and gene transcription of anti-inflammatory effects exerted by STAT3 in ALF.
In summary, we have shown that factors secreted by MSCs in CM, especially IL-10, have anti-inflammatory and tissue repair properties. Activation of the STAT3 signaling pathway by MSCs in the liver may trigger anti-inflammatory activity and wound healing. These data provide the impetus for further preclinical studies to improve stem cell replacement strategies as an attractive alternative approach to liver repair and regeneration.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
This study was supported by grants from the National Natural Science Foundation of China (No. 81170418, No. 81500478), and the Natural Science Foundation of Jiangsu Province, China (No. BK20131084).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–70. doi: 10.1126/science.284.5417.1168. doi:10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 2.Moore JK, Stutchfield BM, Forbes SJ. Systematic review: The effects of autologous stem cell therapy for patients with liver disease. Aliment Pharmacol Ther. 2014;39:673–85. doi: 10.1111/apt.12645. doi:10.1111/apt.12645. [DOI] [PubMed] [Google Scholar]
- 3.Levine P, McDaniel K, Francis H, Kennedy L, Alpini G, Meng F. Molecular mechanisms of stem cell therapy in alcoholic liver disease. Dig Liver Dis. 2014;46:391–7. doi: 10.1016/j.dld.2013.11.015. doi:10.1016/j.dld.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Salomone F, Barbagallo I, Puzzo L, Piazza C, Li Volti G. Efficacy of adipose tissue-mesenchymal stem cell transplantation in rats with acetaminophen liver injury. Stem Cell Res. 2013;11:1037–44. doi: 10.1016/j.scr.2013.07.003. doi:10.1016/j.scr.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Osaki M, et al. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2009;24:70–7. doi: 10.1111/j.1440-1746.2008.05496.x. doi:10.1111/j.1440-1746.2008.05496.x. [DOI] [PubMed] [Google Scholar]
- 6.Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2:e941. doi: 10.1371/journal.pone.0000941. doi:10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, et al. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47:1634–43. doi: 10.1002/hep.22236. doi:10.1002/hep. 22236. [DOI] [PubMed] [Google Scholar]
- 8.Krysko DV, Denecker G, Festjens N, Gabriels S, Parthoens E, D'Herde K, et al. Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell Death Differ. 2006;13:2011–22. doi: 10.1038/sj.cdd.4401900. doi:10.1038/sj.cdd.4401900. [DOI] [PubMed] [Google Scholar]
- 9.Xiao JQ, Shi XL, Ma HC, Tan JJ, Lin-zhang, Xu Q, et al. Administration of IL-1Ra chitosan nanoparticles enhances the therapeutic efficacy of mesenchymal stem cell transplantation in acute liver failure. Arch Med Res. 2013;44:370–9. doi: 10.1016/j.arcmed.2013.06.004. doi:10.1016/j.arcmed.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Ma HC, Shi XL, Ren HZ, Yuan XW, Ding YT. Targeted migration of mesenchymal stem cells modified with CXCR4 to acute failing liver improves liver regeneration. World J Gastroenterol. 2014;20:14884–94. doi: 10.3748/wjg.v20.i40.14884. doi:10.3748/wjg.v20.i40.14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stucky EC, Schloss RS, Yarmush ML, Shreiber DI. Alginate micro-encapsulation of mesenchymal stromal cells enhances modulation of the neuro-inflammatory response. Cytotherapy. 2015;17:1353–64. doi: 10.1016/j.jcyt.2015.05.002. doi:10.1016/j.jcyt.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li D, Wang N, Zhang L, Hanyu Z, Xueyuan B, Fu B, et al. Mesenchymal stem cells protect podocytes from apoptosis induced by high glucose via secretion of epithelial growth factor. Stem Cell Res Ther. 2013;4:103. doi: 10.1186/scrt314. doi:10.1186/scrt314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Wang Y, Wang Z, Gutkind JS, Wang Z, Wang F, et al. Engineered mesenchymal stem cells with enhanced tropism and paracrine secretion of cytokines and growth factors to treat traumatic brain injury. Stem Cells. 2015;33:456–67. doi: 10.1002/stem.1878. doi:10.1002/stem.1878. [DOI] [PubMed] [Google Scholar]
- 14.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–36. doi: 10.1038/nri2395. doi:10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 15.Zagoura DS, Roubelakis MG, Bitsika V, Trohatou O, Pappa KI, Kapelouzou A, et al. Therapeutic potential of a distinct population of human amniotic fluid mesenchymal stem cells and their secreted molecules in mice with acute hepatic failure. Gut. 2012;61:894–906. doi: 10.1136/gutjnl-2011-300908. doi:10.1136/gutjnl-2011-300908. [DOI] [PubMed] [Google Scholar]
- 16.Thorgeirsson SS, Grisham JW. Hematopoietic cells as hepatocyte stem cells: A critical review of the evidence. Hepatology. 2006;43:2–8. doi: 10.1002/hep.21015. doi:10.1002/hep.21015. [DOI] [PubMed] [Google Scholar]
- 17.Almeida-Porada G, Porada CD, Chamberlain J, Torabi A, Zanjani ED. Formation of human hepatocytes by human hematopoietic stem cells in sheep. Blood. 2004;104:2582–90. doi: 10.1182/blood-2004-01-0259. doi:10.1182/blood-2004-01-0259. [DOI] [PubMed] [Google Scholar]
- 18.Puppi J, Strom SC, Hughes RD, Bansal S, Castell JV, Dagher I, et al. Improving the techniques for human hepatocyte transplantation: Report from a consensus meeting in London. Cell Transplant. 2012;21:1–10. doi: 10.3727/096368911X566208. doi:10.3727/096368911X566208. [DOI] [PubMed] [Google Scholar]
- 19.Khan AA, Shaik MV, Parveen N, Rajendraprasad A, Aleem MA, Habeeb MA, et al. Human fetal liver-derived stem cell transplantation as supportive modality in the management of end-stage decompensated liver cirrhosis. Cell Transplant. 2010;19:409–18. doi: 10.3727/096368910X498241. doi:10.3727/096368910X498241. [DOI] [PubMed] [Google Scholar]
- 20.Cho JW, Lee CY, Ko Y. Therapeutic potential of mesenchymal stem cells overexpressing human forkhead box A2 gene in the regeneration of damaged liver tissues. J Gastroenterol Hepatol. 2012;27:1362–70. doi: 10.1111/j.1440-1746.2012.07137.x. doi:10.1111/j.1440-1746.2012.07137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yagi H, Soto-Gutierrez A, Navarro-Alvarez N, Nahmias Y, Goldwasser Y, Kitagawa Y, et al. Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1. Mol Ther. 2010;18:1857–64. doi: 10.1038/mt.2010.155. doi:10.1038/mt.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ionescu L, Byrne RN, van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ, et al. Stem cell conditioned medium improves acute lung injury in mice: In vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303:967–77. doi: 10.1152/ajplung.00144.2011. doi:10.1152/ajplung.00144.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the P13K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25:2363–70. doi: 10.1634/stemcells.2006-0686. doi:10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]
- 24.Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7:e35685. doi: 10.1371/journal.pone.0035685. doi:10.1371/journal.pone.0035685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CervellóI Gil-Sanchis C, Santamaría X, Cabanillas S, Díaz A, Faus A, et al. Human CD133(+) bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome. Fertil Steril. 2015;104:1552–60.e3. doi: 10.1016/j.fertnstert.2015.08.032. doi:10.1016/j.fertnstert.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 26.Yoo TJ, Du X, Zhou B. The paracrine effect of mesenchymal human stem cells restored hearing in ß-tubulin induced autoimmune sensorineural hearing loss. Hear Res. 2015;330(Pt A):57–61. doi: 10.1016/j.heares.2015.07.021. doi:10.1016/j.heares.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Lee SK, Lee SC, Kim SJ. A novel cell-free strategy for promoting mouse liver regeneration: Utilization of a conditioned medium from adipose-derived stem cells. Hepatol Int. 2015;9:310–20. doi: 10.1007/s12072-014-9599-4. doi:10.1007/s12072-014-9599-4. [DOI] [PubMed] [Google Scholar]
- 28.Chen YX, Zeng ZC, Sun J, Zeng HY, Huang Y, Zhang ZY. Mesenchymal stem cell-conditioned medium prevents radiation-induced liver injury by inhibiting inflammation and protecting sinusoidal endothelial cells. J Radiat Res. 2015;56:700–8. doi: 10.1093/jrr/rrv026. doi:10.1093/jrr/rrv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SC, Kim JO, Kim SJ. Secretome from human adipose-derived stem cells protects mouse liver from hepatic ischemia-reperfusion injury. Surgery. 2015;157:934–43. doi: 10.1016/j.surg.2014.12.016. doi:10.1016/j.surg.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. doi:10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutchins AP, Diez D, Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: Recent developments and future challenges. Brief Funct Genomics. 2013;12:489–98. doi: 10.1093/bfgp/elt028. doi:10.1093/bfgp/elt028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray PJ. The JAK-STAT signaling pathway: Input and output integration. J Immunol. 2007;178:2623–9. doi: 10.4049/jimmunol.178.5.2623. doi:10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 33.Murray PJ. STAT3-mediated anti-inflammatory signalling. 2006;34(Pt 6):1028–31. doi: 10.1042/BST0341028. doi:10.1042/BST0341028. [DOI] [PubMed] [Google Scholar]
- 34.Miller AM, Wang H, Park O, Horiguchi N, Lafdil F, Mukhopadhyay P, et al. Anti-inflammatory and anti-apoptotic roles of endothelial cell STAT3 in alcoholic liver injury. Alcohol Clin Exp Res. 2010;34:719–25. doi: 10.1111/j.1530-0277.2009.01141.x. doi:10.1111/j.1530-0277.2009.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horiguchi N, Lafdil F, Miller AM, Park O, Wang H, Rajesh M, et al. Dissociation between liver inflammation and hepatocellular damage induced by carbon tetrachloride in myeloid cell-specific signal transducer and activator of transcription 3 gene knockout mice. Hepatology. 2010;51:1724–34. doi: 10.1002/hep.23532. doi:10.1002/hep.23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horiguchi N, Wang L, Mukhopadhyay P, Park O, Jeong WI, Lafdil F, et al. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134:1148–58. doi: 10.1053/j.gastro.2008.01.016. doi:10.1053/j.gastro.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ALT and AST levels of ALF rats (n = 6). *P < 0.05. ALT: Alanine aminotransferase; AST: Aspar tate aminotransferase; ALF: Acute liver failure.
STAT3 expression in normal rats and ALF rats injected with/without MSCs. (a) Western blotting analysis of liver tissues at 7th day after injecting D-Gal. (b) The band intensities of scanning densitometry. *P < 0.05 versus ALF group. STAT3: Signal transducer and activator of transcription 3; pSTAT3: Phosphorylated STAT3; MSCs: Mesenchymal stem cells; ALF: Acute liver failure.